Adipose and Muscle Cell Co-Culture System: A Novel In Vitro Tool to Mimic the In Vivo Cellular Environment
Abstract
Simple Summary
Abstract
1. Introduction
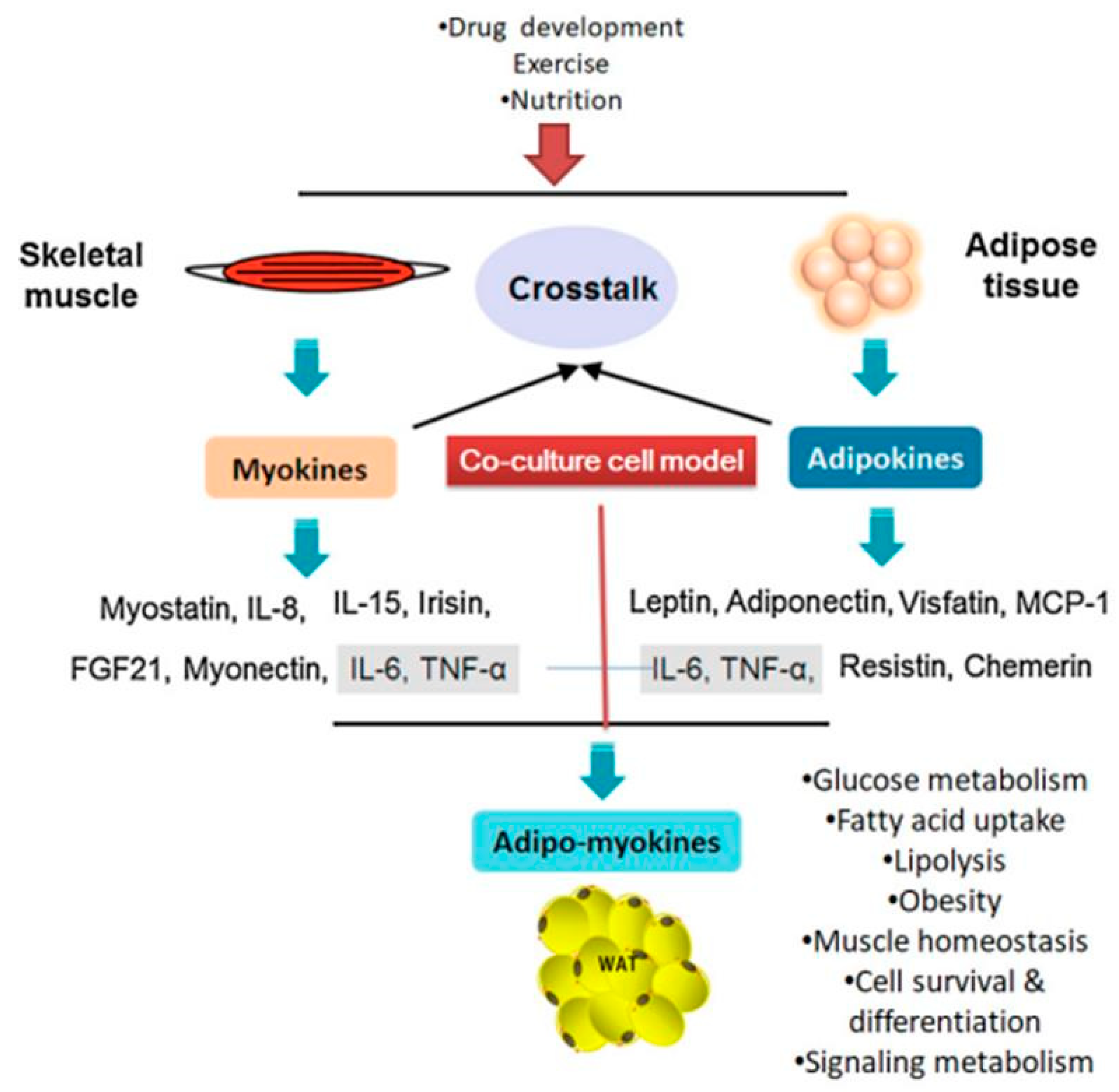
2. Adipocytes/Muscle Cells Co-Culture Models
| Co-Culture Model | Compounds Used | Study Findings | Ref. |
|---|---|---|---|
| Pre-adipocytes-myoblasts | Arginine and/or trans 10, cis-12 CLA | Increased adipogenic gene expression in myoblasts | [7] |
| Bovine adipocytes and pre-adipocytes | Adipogenic induction medium | Increase lipolytic response and glycerol release | [20] |
| 3T3-L1 adipocyte–C2C12 cells | Ferulic acid | Increase lipolytic profile and glycerol release | [21] |
| C2C12 myoblasts-3T3-L1 adipocytes | Adipocytes medium induced IL-6 | Suppress the differentiation of C2C12 cells | [22] |
| Differentiated C2C12 with 3T3-L1 cells | Calcitriol | Decreased anti-inflammatory cytokines production | [23] |
| 3T3-L1 (adipocyte)-L6 muscle cell line | Differentiation media without additives | Co-culture adipocyte cells increased GPDH activity | [24] |
| Human fat and skeletal muscle cells | Differentiation medium with 1 pmol/L insulin | adipocyte induce a paracrine perturbation in muscle cells | [25] |
| 3T3-L1 preadipocytes-differentiated C2C12 | DMEM differentiation medium | C2C12 suppressed the mRNA, protein expression of glucocorticoids receptor | [11] |
| C2C12 myocytes and 3T3-L1 adipocytes | Adipocyte conditioned medium with Leucine | Modulation of muscle and adipocyte energy metabolism | [26] |
| C2C12 myocytes and 3T3-L1 pre-adipocytes | Zinc oxide nanoparticles | Increased expression of antioxidant enzymes and mRNA expression | [27] |
| 3T3-L1 adipocytes with RAW 264 macrophage | Dietary calcium | Reduce the inflammatory cytokine and oxidative stress in adipocytes | [28] |
| 3T3-L1 adipocytes with splenocytes cells | Lipopolysaccharides (LPS) | Elevated cytokine secretion (TNF-a, IL-6, MCP-1) | [29] |
| Murine adipocytes-C2C12 cells | Leucine and calcitriol | Decrease energy storage in adipocytes and increasing fatty acid utilization in C2C12 | [30] |
| 3T3-L1 pre-adipocytes and C2C12 muscle cells | DMEM/FBS growth medium | Promote the mitochondrial biogenesis bydirect activation of SIRT1 in both cells | [31] |
| 3T3-L1 pre-adipocytes and L6 muscle cells | DMEM/F12 supplemented with BSA | Oxygen species production and level of Glut1 mRNA and protein increased in L6 cells | [32] |
| Primary human adipocyte and skeletal myotubes | Low serum differentiation medium | Understating the metabolic function of intra muscular adipogenesis (lipolytic activity) | [17] |
| 3T3-L1 cells with J-6 cells | Defined Medium for co-cultured cells | Low level of IGF-1 IGF-II are not likely to play a role in intercellular communication between these cells | [1] |
| Porcine pre-adipocytes and muscle satellite cells | DMEM/F12 medium | Induce cell growth and proliferation meanwhile, inhibited the cell differentiation | [33] |
| Skeletal muscle (L6)-adipocyte (3T3-L1) | Specific differentiation medium for both cells | IL-6 cytokine plays main role in cross-talk between these cells | [34] |
| 3T3-L1 and L6 cell line | Differentiation medium containing 5% HS | Adipocyte differentiation inhibited and suppress the lipogenic gene expression | [35] |
3. Monoculture vs. Co-Culture
3.1. Monoculture Techniques
3.2. Co-Culture Techniques
4. Co-Culture System Advantages and Disadvantages
5. Secreted Factors in Co-Culture Model
6. Models for Co-Culture System
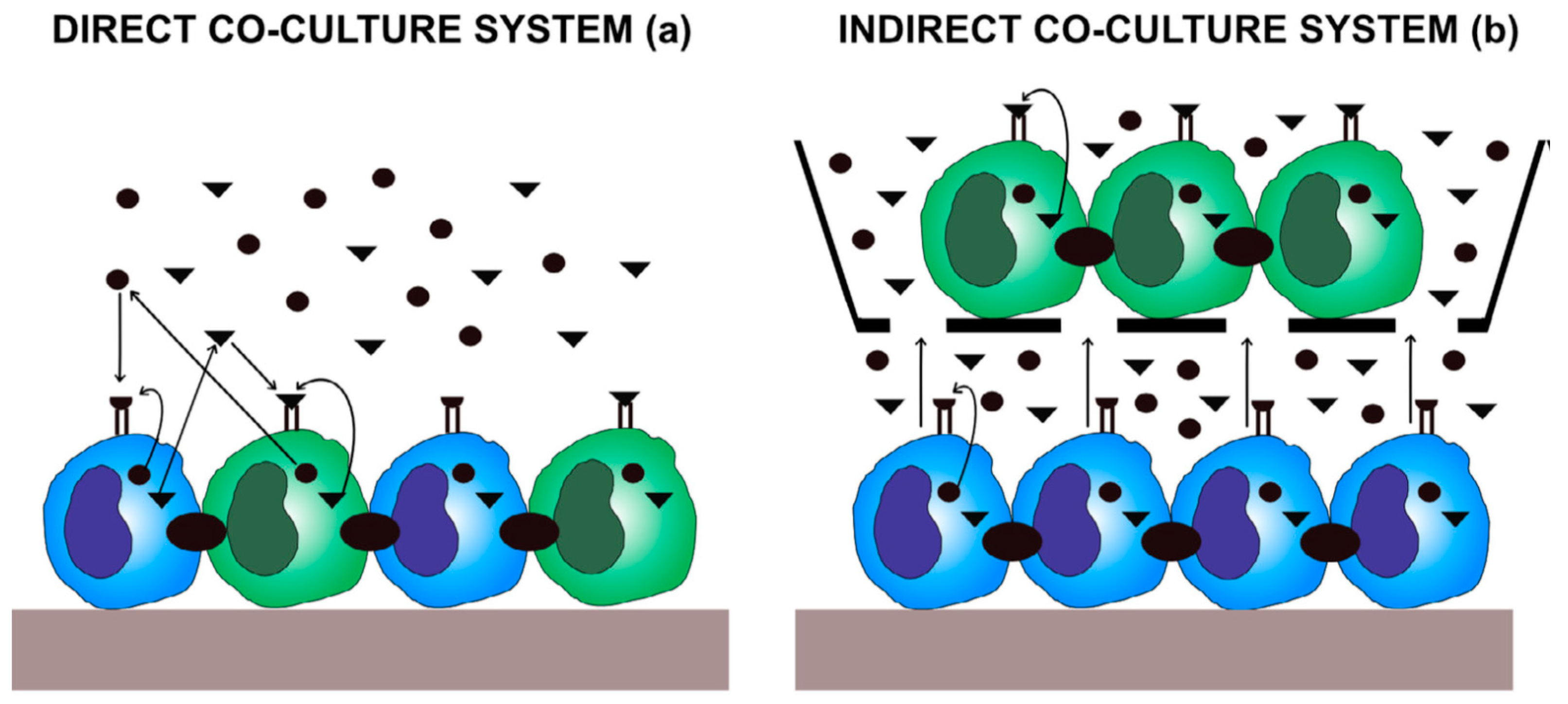
6.1. Direct Co-Culture Models
6.2. Indirect Co-Culture Models
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dodson, M.V.; Vierck, J.L.; Hossner, K.L.; Byrne, K.; McNamara, J.P. The development and utility of a defined muscle and fat co-culture system. Tissue Cell 1997, 29, 517–524. [Google Scholar] [CrossRef]
- Levorson, E.J.; Santoro, M.; Kasper, F.K.; Mikos, A.G. Direct and indirect co-culture of chondrocytes and mesenchymal stem cells for the generation of polymer/extracellular matrix hybrid constructs. Acta Biomater. 2014, 10, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Niesler, C. A triple co-culture method to investigate the effect of macrophages and fibroblasts on myoblast proliferation and migration. Biotechniques 2018, 64, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Goodyear, L.J. Muscle-Adipose Tissue Cross-Talk. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef]
- Gupta, R.K. Adipocytes. Curr. Biol. 2014, 24, R988–R993. [Google Scholar] [CrossRef][Green Version]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef]
- Choi, S.H.; Chung, K.Y.; Johnson, B.J.; Go, G.W.; Kim, K.H.; Choi, C.W.; Smith, S.B. Co-culture of bovine muscle satellite cells with preadipocytes increases PPARγ and C/EBPβ gene expression in differentiated myoblasts and increases GPR43 gene expression in adipocytes. J. Nutr. Biochem. 2013, 24, 539–543. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; You, W.; Shan, T. Myokines mediate the cross-talk between skeletal muscle and other organs. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Oh, K.-J.; Lee, D.S.; Kim, W.K.; Han, B.S.; Lee, S.C.; Bae, K.-H. Metabolic Adaptation in Obesity and Type II Diabetes: Myokines, Adipokines and Hepatokines. Int. J. Mol. Sci. 2016, 18, 8. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharm. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Chu, W.; Wei, W.; Yu, S.; Han, H.; Shi, X.; Sun, W.; Gao, Y.; Zhang, L.; Chen, J. C2C12 myotubes inhibit the proliferation and differentiation of 3T3-L1 preadipocytes by reducing the expression of glucocorticoid receptor gene. Biochem. Biophys. Res. Commun. 2016, 472, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Shahin-Shamsabadi, A.; Selvaganapathy, P.R. A 3D self-assembled in vitro model to simulate direct and indirect interactions between adipocytes and skeletal muscle cells. Adv. Biosyst. 2020, 4, 2000034. [Google Scholar] [CrossRef] [PubMed]
- Anan, M.; Uchihashi, K.; Aoki, S.; Matsunobu, A.; Ootani, A.; Node, K.; Toda, S. A promising culture model for analyzing the interaction between adipose tissue and cardiomyocytes. Endocrinology 2011, 152, 1599–1605. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Duan, Y.; Hu, C.-A.A.; Tang, Y.; Yin, Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. 2017, 33, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.A.; MacSween, J.M.; Robson, D.A. Growth of B cell colonies independent of T cell contact. Immunol. Lett. 1989, 22, 167–171. [Google Scholar] [CrossRef]
- Dyck, D.J.; Heigenhauser, G.J.; Bruce, C.R. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol. 2006, 186, 5–16. [Google Scholar] [CrossRef]
- Kovalik, J.-P.; Slentz, D.; Stevens, R.D.; Kraus, W.E.; Houmard, J.A.; Nicoll, J.B.; Lea-Currie, Y.R.; Everingham, K.; Kien, C.L.; Buehrer, B.M.; et al. Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes 2011, 60, 1882–1893. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. AMS 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Signalling role of adipose tissue: Adipokines and inflammation in obesity. Biochem. Soc. Trans. 2005, 33, 1078–1081. [Google Scholar] [CrossRef]
- Strieder-Barboza, C.; Thompson, E.; Thelen, K.; Contreras, G.A. Technical note: Bovine adipocyte and preadipocyte co-culture as an efficient adipogenic model. J. Dairy Sci. 2019, 102, 3622–3629. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Soundharrajan, I.; Hwang, I.; Kim, D.; Choi, K.C.; National Institute of Animal Science, Rural Development Ad-ministration, Cheonan, Korea. Unpublished work. 2020.
- Seo, K.; Suzuki, T.; Kobayashi, K.; Nishimura, T. Adipocytes suppress differentiation of muscle cells in a co-culture system. Anim. Sci. J. 2019, 90, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Myung, K. Calcitriol enhances fat synthesis factors and calpain activity in co-cultured cells. Cell Biol. Int. 2014, 38, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-W.; Cho, W.-M.; Yeon, S.-H.; HwangBo, S.; Song, M.-K.; Park, S.; Baek, K.-H. Comparison between Single and Co-culture of Adipocyte and Muscle Cell Lines in Cell Morphology and Cytosolic Substances. J. Anim. Sci. Technol. 2012, 54, 103–109. [Google Scholar] [CrossRef][Green Version]
- Dietze, D.; Koenen, M.; Röhrig, K.; Horikoshi, H.; Hauner, H.; Eckel, J. Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes 2002, 51, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zemel, M.B. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr. Metab. 2009, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, M.; Veerappan, M.; Kim, D.H. Cytotoxicity of zinc oxide nanoparticles on antioxidant enzyme activities and mRNA expression in the cocultured C2C12 and 3T3-L1 cells. Appl. Biochem. Biotechnol. 2015, 175, 1270–1280. [Google Scholar] [CrossRef]
- Sun, X.; Zemel, M.B. Calcitriol and calcium regulate cytokine production and adipocyte-macrophage cross-talk. J. Nutr. Biochem. 2008, 19, 392–399. [Google Scholar] [CrossRef]
- Nitta, C.F.; Orlando, R.A. Crosstalk between immune cells and adipocytes requires both paracrine factors and cell contact to modify cytokine secretion. PLoS ONE 2013, 8, e77306. [Google Scholar] [CrossRef]
- Sun, X.; Zemel, M.B. Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells. Lipids 2007, 42, 297–305. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Zemel, M.B. Effects of dairy consumption on SIRT1 and mitochondrial biogenesis in adipocytes and muscle cells. Nutr. Metab. 2011, 8, 91. [Google Scholar] [CrossRef]
- Kudoh, A.; Satoh, H.; Hirai, H.; Watanabe, T.; Shimabukuro, M. Preliminary Evidence for Adipocytokine Signals in Skeletal Muscle Glucose Uptake. Front Endocrinol. 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gan, L.; Yang, H.; Sun, C. The proliferation and differentiation characteristics of co-cultured porcine preadipocytes and muscle satellite cells in vitro. Mol. Biol. Rep. 2013, 40, 3197–3202. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, B.; Fite, A.; Abou-Samra, A.B. Effects of 3T3 adipocytes on interleukin-6 expression and insulin signaling in L6 skeletal muscle cells. Biochem. Biophys. Res. Commun. 2011, 410, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baek, K.; Choi, C. Suppression of adipogenic differentiation by muscle cell-induced decrease in genes related to lipogenesis in muscle and fat co-culture system. Cell Biol. Int. 2013, 37, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Hausman, G.J.; Poulos, S.P. A method to establish co-cultures of myotubes and preadipocytes from collagenase digested neonatal pig semitendinosus muscles. J. Anim. Sci. 2005, 83, 1010–1016. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, J.; Hu, X.; Dai, L.; Fu, X.; Zhang, J.; Duan, X.; Ao, Y. A co-culture system of rat synovial stem cells and meniscus cells promotes cell proliferation and differentiation as compared to mono-culture. Sci. Rep. 2018, 8, 7693. [Google Scholar] [CrossRef]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef]
- Wu, M.H.; Huang, S.B.; Lee, G.B. Microfluidic cell culture systems for drug research. Lab Chip 2010, 10, 939–956. [Google Scholar] [CrossRef]
- Kelley, D.E.; Goodpaster, B.H. Stewing in Not-So-Good Juices: Interactions of Skeletal Muscle with Adipose Secretions. Diabetes 2015, 64, 3055–3057. [Google Scholar] [CrossRef] [PubMed]
- Wohlers, L.M.; Sweeney, S.M.; Ward, C.W.; Lovering, R.M.; Spangenburg, E.E. Changes in contraction-induced phosphorylation of AMP-activated protein kinase and mitogen-activated protein kinases in skeletal muscle after ovariectomy. J. Cell. Biochem. 2009, 107, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Tishinsky, J.M.; De Boer, A.A.; Dyck, D.J.; Robinson, L.E. Modulation of visceral fat adipokine secretion by dietary fatty acids and ensuing changes in skeletal muscle inflammation. Appl. Physiol. Nutr. Metab. 2014, 39, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Romanazzo, S.; Forte, G.; Morishima, K.; Taniguchi, A. IL-12 involvement in myogenic differentiation of C2C12 in vitro. Biomater. Sci. 2015, 3, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.J.; Chamberlain, L.M.; Grainger, D.W. Cell–cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials 2010, 31, 9382–9394. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Nelson, J. Structure and Function in Cell Signall; Wiley Publisher: Hoboken, NJ, USA, 2008; Chapter 1–3, pp. 1–95. [Google Scholar]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med Sci. AMS 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Andersen, M.L.; Winter, L.M.F. Animal models in biological and biomedical research—experimental and ethical concerns. An. Acad. Bras. Ciênc. 2019, 91, e20170238. [Google Scholar] [CrossRef]
- Li, F.; Yang, H.; Duan, Y.; Yin, Y. Myostatin regulates preadipocyte differentiation and lipid metabolism of adipocyte via ERK1/2. Cell Biol. Int. 2011, 35, 1141–1146. [Google Scholar] [CrossRef]
- Hauner, H. Secretory factors from human adipose tissue and their functional role. Proc. Nutr. Soc. 2005, 64, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Drevon, C.A.; Eckel, J. Secreted proteins from adipose tissue and skeletal muscle—Adipokines, myokines and adipose/muscle cross-talk. Arch. Physiol. Biochem. 2011, 117, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Cohen, R.N.; Brey, E.M. Optimization of Co-Culture Conditions for a Human Vascularized Adipose Tissue Model. Bioengineering 2020, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.X.; Guo, L.P.; Zhao, G.P.; Liu, R.R.; Li, Q.H.; Zheng, M.Q.; Wen, J. Method using a co-culture system with high-purity intramuscular preadipocytes and satellite cells from chicken pectoralis major muscle. Poult. Sci. 2018, 97, 3691–3697. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Caruso, C.; Candore, G. The Role of Adipose Tissue and Adipokines in Obesity-Related Inflammatory Diseases. Mediat. Inflamm. 2010, 2010, 802078. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- De Boer, A.A.; Monk, J.M.; Robinson, L.E. Docosahexaenoic acid decreases pro-inflammatory mediators in an in vitro murine adipocyte macrophage co-culture model. PLoS ONE 2014, 9, e85037. [Google Scholar] [CrossRef]
- Kokta, T.A.; Dodson, M.V.; Gertler, A.; Hill, R.A. Intercellular signaling between adipose tissue and muscle tissue. Domest. Anim. Endocrinol. 2004, 27, 303–331. [Google Scholar] [CrossRef]
- Pandurangan, M.; Hwang, I. Application of cell co-culture system to study fat and muscle cells. Appl Microbiol. Biotechnol. 2014, 98, 7359–7364. [Google Scholar] [CrossRef]
- Yang, X.; Bi, P.; Kuang, S. Fighting obesity: When muscle meets fat. Adipocyte 2014, 3, 280–289. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co–culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vashisht, A.A.; O’Rourke, J.; Corbel, S.Y.; Moran, R.; Romero, A.; Miraglia, L.; Zhang, J.; Durrant, E.; Schmedt, C.; et al. The microprotein Minion controls cell fusion and muscle formation. Nat. Commun. 2017, 8, 15664. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Cao, T.; Lee, E.H. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells 2004, 22, 1152–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bu, S.; Sun, J.; Xu, M.; Yao, X.; He, K.; Lai, D. Paracrine effects of human amniotic epithelial cells protect against chemotherapy-induced ovarian damage. Stem. Cell Res. 2017, 8, 270. [Google Scholar] [CrossRef]
- Zuo, Q.; Cui, W.; Liu, F.; Wang, Q.; Chen, Z.; Fan, W. Co-cultivated mesenchymal stem cells support chondrocytic differentiation of articular chondrocytes. Int. Orthop. 2013, 37, 747–752. [Google Scholar] [CrossRef]
- Huang, C.P.; Lu, J.; Seon, H.; Lee, A.P.; Flanagan, L.A.; Kim, H.Y.; Putnam, A.J.; Jeon, N.L. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip 2009, 9, 1740–1748. [Google Scholar] [CrossRef]
- Ou, D.B.; Zeng, D.; Jin, Y.; Liu, X.T.; Teng, J.W.; Guo, W.G.; Wang, H.T.; Su, F.F.; He, Y.; Zheng, Q.S. The long-term differentiation of embryonic stem cells into cardiomyocytes: An indirect co-culture model. PLoS ONE 2013, 8, e55233. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clément, K.; Butler-Browne, G.S.; Lacasa, D. Human Adipocytes Induce Inflammation and Atrophy in Muscle Cells During Obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef]
- Grellier, M.; Bordenave, L.; Amedee, J. Cell-to-cell communication between osteogenic and endothelial lineages: Implications for tissue engineering. Trends Biotechnol. 2009, 27, 562–571. [Google Scholar] [CrossRef]
- Renaud, J.; Martinoli, M.G. Development of an Insert Co-culture System of Two Cellular Types in the Absence of Cell–cell Contact. J. Vis. Exp. 2016, e54356. [Google Scholar] [CrossRef]
- Lee, D.; Chambers, M. A co-culture model of the bovine alveolus. F1000Res 2019, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.A.; Rivera, C.; Voipio, J.; Kaila, K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003, 26, 199–206. [Google Scholar] [CrossRef]
- Saldaña, L.; Vallés, G.; Bensiamar, F.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Paracrine interactions between mesenchymal stem cells and macrophages are regulated by 1,25-dihydroxyvitamin D3. Sci. Rep. 2017, 7, 14618. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuppusamy, P.; Kim, D.; Soundharrajan, I.; Hwang, I.; Choi, K.C. Adipose and Muscle Cell Co-Culture System: A Novel In Vitro Tool to Mimic the In Vivo Cellular Environment. Biology 2021, 10, 6. https://doi.org/10.3390/biology10010006
Kuppusamy P, Kim D, Soundharrajan I, Hwang I, Choi KC. Adipose and Muscle Cell Co-Culture System: A Novel In Vitro Tool to Mimic the In Vivo Cellular Environment. Biology. 2021; 10(1):6. https://doi.org/10.3390/biology10010006
Chicago/Turabian StyleKuppusamy, Palaniselvam, Dahye Kim, Ilavenil Soundharrajan, Inho Hwang, and Ki Choon Choi. 2021. "Adipose and Muscle Cell Co-Culture System: A Novel In Vitro Tool to Mimic the In Vivo Cellular Environment" Biology 10, no. 1: 6. https://doi.org/10.3390/biology10010006
APA StyleKuppusamy, P., Kim, D., Soundharrajan, I., Hwang, I., & Choi, K. C. (2021). Adipose and Muscle Cell Co-Culture System: A Novel In Vitro Tool to Mimic the In Vivo Cellular Environment. Biology, 10(1), 6. https://doi.org/10.3390/biology10010006






