Combined Impact of No-Till and Cover Crops with or without Short-Term Water Stress as Revealed by Physicochemical and Microbiological Indicators
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field and Experimental Conditions
2.2. Analyses of Soil Microbial Diversity
2.3. Enzymatic Activities and Soil Alteration Index
2.4. Aggregate-Sized C and N Fractions
2.5. Statistical Analyses
3. Results
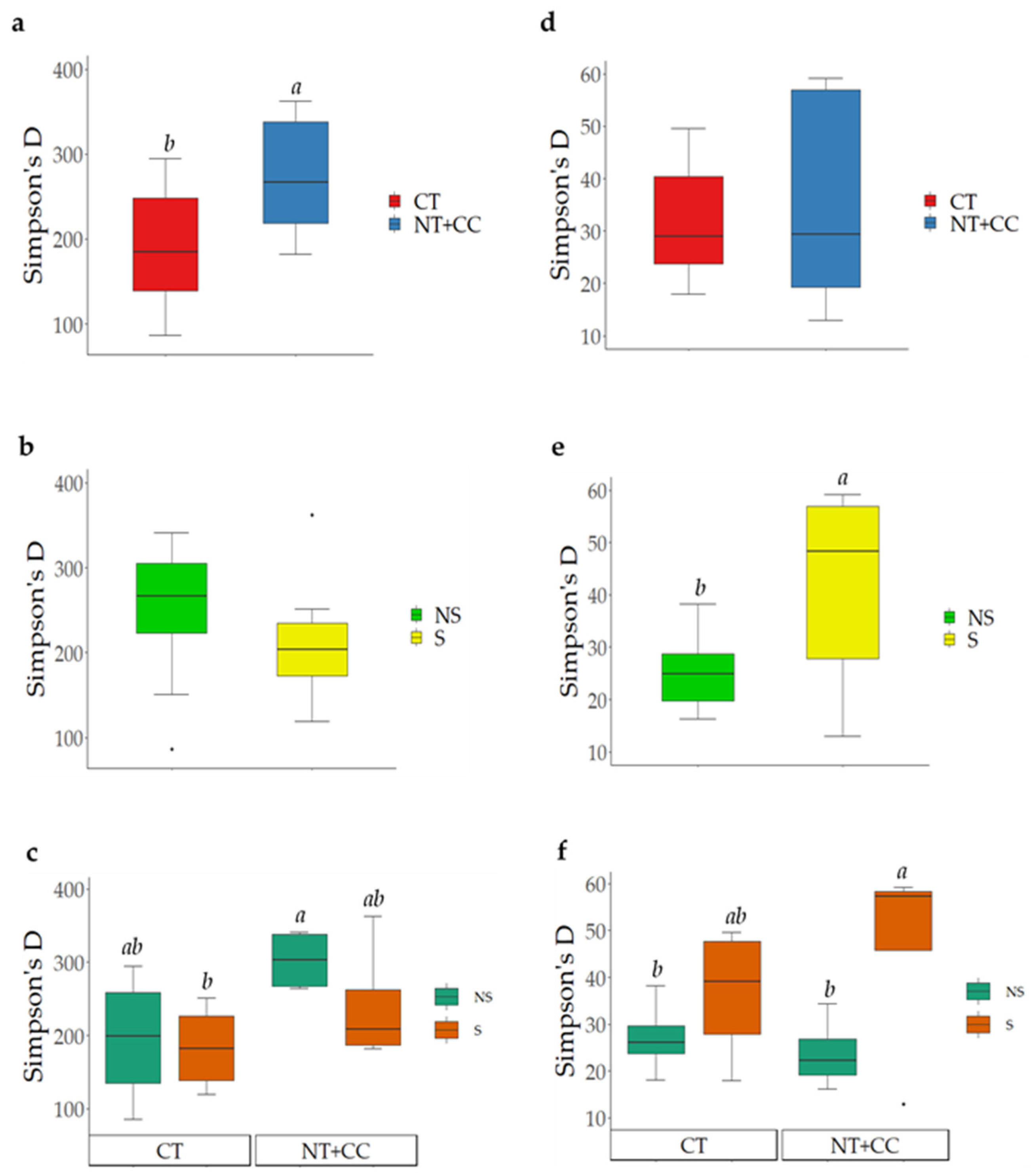
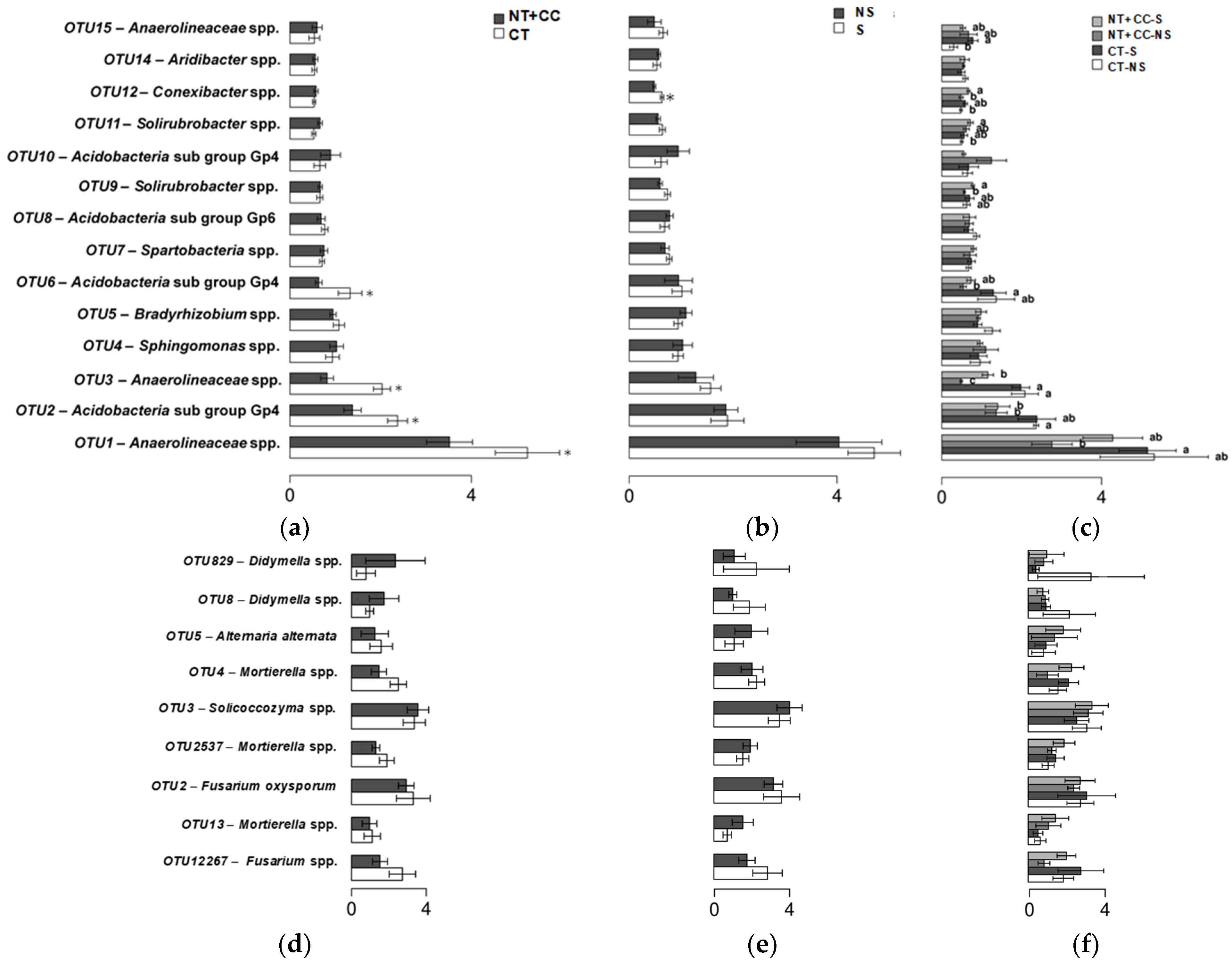
3.1. Analyses of Soil Microbial Diversity
3.2. Enzymatic Activities and Soil Alteration Index
3.3. Aggregate-Associated C and N
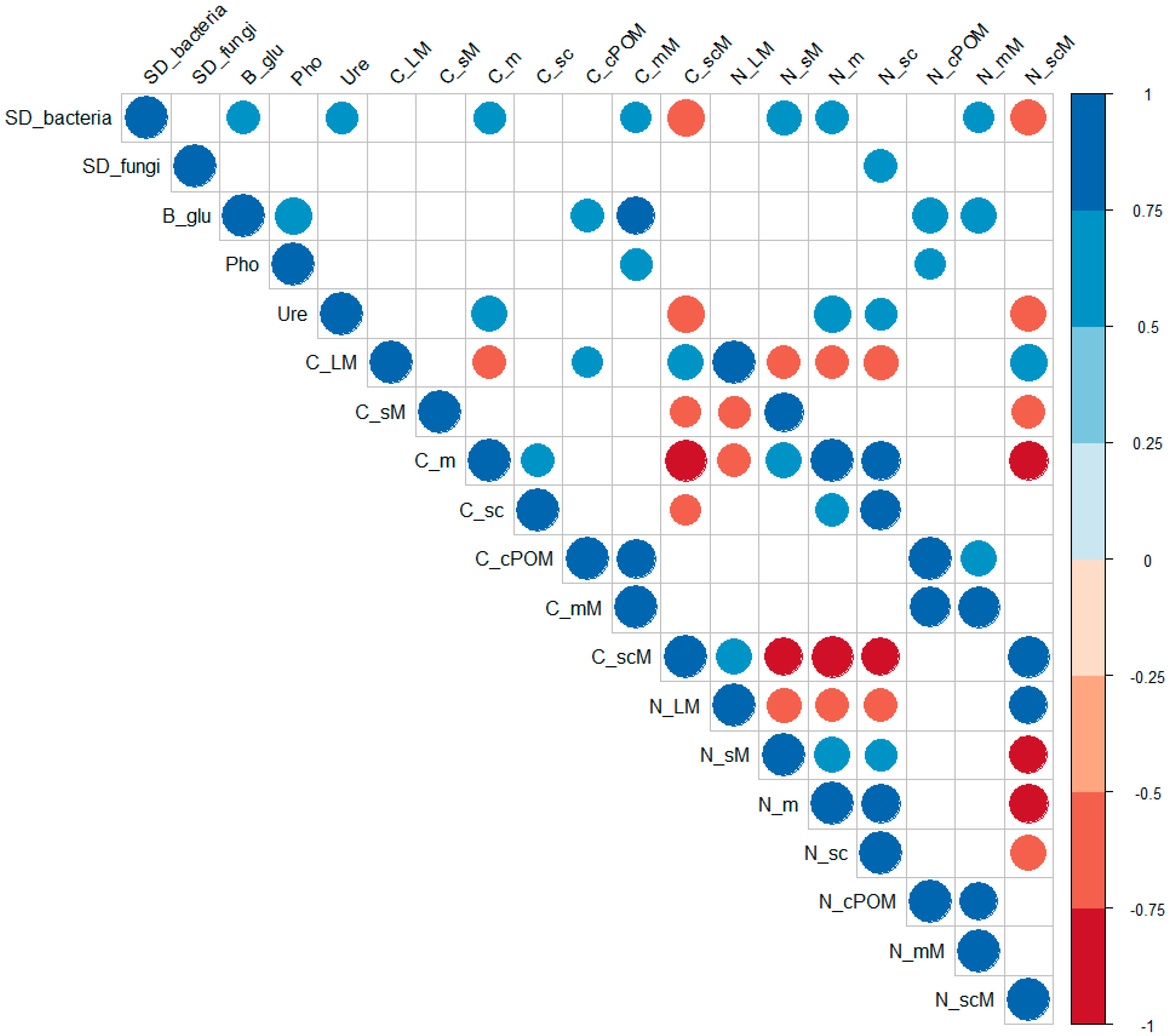
3.4. Correlations between the Microbiological and Physicochemical Properties
4. Discussion
4.1. Analyses of Soil Microbial Diversity
4.2. Enzymatic Activities and Soil Alteration Index
4.3. Aggregate-Associated C and N
4.4. Correlations between the Microbiological and Physicochemical Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Han, X.Z.; Ji, Z.J.; Li, Y.; Wang, E.T.; Xie, Z.H.; Chen, W.F. Abundance and diversity of soybean-nodulating rhizobia in black soil are impacted by land use and crop management. App. Environ. Microbiol. 2014, 80, 5394–5402. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Lal, R. A system approach to conservation agriculture. J. Soil Water Conserv. 2015, 70, 82A–88A. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, M. Using cover crops to mitigate and adapt to climate change. A review. Agron. Sustain. Dev. 2017, 37, 4. [Google Scholar] [CrossRef]
- Chivenge, P.; Mabhaudhi, T.; Modi, A.T.; Mafongoya, P. The potential role of neglected and underutilised crop species as future crops under water scarce conditions in sub-Saharan Africa. Int. J. Environ. Res. Public Health 2015, 12, 5685–5711. [Google Scholar] [CrossRef]
- Baraniya, D.; Puglisi, E.; Ceccherini, M.T.; Pietramellara, G.; Giagnoni, L.; Arenella, M.; Nannipieri, P.; Renella, G. Protease encoding microbial communities and protease activity of the rhizosphere and bulk soils of two maize lines with different N uptake efficiency. Soil Biol. Biochem. 2016, 96, 176–179. [Google Scholar] [CrossRef]
- Delgado Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome-from metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef]
- Vasileiadis, S.; Puglisi, E.; Arena, M.; Cappa, F.; Cocconcelli, P.S.; Trevisan, M. Soil bacterial diversity screening using single 16S rRNA gene V regions coupled with multi-million read generating sequencing technologies. PLoS ONE 2012, 7, e42671. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Vasileiadis, S.; Coppolecchia, D.; Puglisi, E.; Balloi, A.; Mapelli, F.; Hamon, R.E.; Daffonchio, D.; Trevisan, M. Response of ammonia oxidizing bacteria and archaea to acute zinc stress and different moisture regimes in soil. Microb. Ecol. 2012, 64, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage Changes vertical distribution of soil bacterial and fungal communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Kraut Cohen, J.; Zolti, A.; Shaltiel-Harpaz, L.; Argaman, E.; Rabinovich, R.; Green, S.J.; Minz, D. Effects of tillage practices on soil microbiome and agricultural parameters. Sci. Total Environ. 2020, 705, 135791. [Google Scholar] [CrossRef] [PubMed]
- Navarro Noya, Y.E.; Gómez Acata, S.; Montoya Ciriaco, N.; Rojas Valdez, A.; Suárez Arriaga, M.C.; Valenzuela Encinas, C.; Jiménez Bueno, N.; Verhulst, N.; Govaerts, B.; Dendooven, L. Relative impacts of tillage, residue management and crop-rotation on soil bacterial communities in a semi-arid agroecosystem. Soil Biol. Biochem. 2013, 65, 86–95. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Puglisi, E.; Del Re, A.A.M.; Rao, M.A.; Gianfreda, L. Development and validation of numerical indexes integrating enzyme activities of soils. Soil Biol. Biochem. 2006, 38, 1673–1681. [Google Scholar] [CrossRef]
- Trost, B.; Prochnow, A.; Drastig, K.; Meyer-Aurich, A.; Ellmer, F.; Baumecker, M. Irrigation, soil organic carbon and N2O emissions. A review. Agron. Sustain. Dev. 2013, 33, 733–749. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Bio. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Navarrete, A.A.; Venturini, A.M.; Meyer, K.M.; Klein, A.M.; Tiedje, J.M.; Bohannan, B.J.M.; Nüsslein, K.; Tsai, S.M.; Rodrigues, J.L.M. Differential response of acidobacteria subgroups to forest-to-pasture conversion and their biogeographic patterns in the western Brazilian Amazon. Front. Microbiol. 2015, 6, 1443. [Google Scholar] [CrossRef]
- Fiorini, A.; Boselli, R.; Amaducci, S.; Tabaglio, V. Effects of no-till on root architecture and root-soil interactions in a three-year crop rotation. Eur. J. Agron. 2018, 99, 156–166. [Google Scholar] [CrossRef]
- Wang, X.; He, T.; Gen, S.; Zhang, X.-Q.; Wang, X.; Jiang, D.; Li, C.; Li, C.; Wang, J.; Zhang, W.; et al. Soil properties and agricultural practices shape microbial communities in flooded and rainfed croplands. Appl. Soil Ecol. 2020, 147, 103449. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Bertora, C.; Pepe, O.; Giancarlo, M.; Grignani, C.; Fagnano, M. Changes in soil mineral N content and abundances of bacterial communities involved in N reactions under laboratory conditions as predictors of soil N availability to maize under field conditions. Biol. Fertil. Soil 2016, 52, 523–537. [Google Scholar] [CrossRef]
- Fiorini, A.; Boselli, R.; Maris, S.C.; Santelli, S.; Ardenti, F.; Capra, F.; Tabaglio, V. May conservation tillage enhance soil C and N accumulation without decreasing yield in intensive irrigated croplands? Results from an eight-year maize monoculture. Agric. Ecosyst. Environ. 2020, 296, 106926. [Google Scholar] [CrossRef]
- Fiorini, A.; Maris, S.C.; Abalos, D.; Amaducci, S.; Tabaglio, V. Combining no-till with rye (Secale cereale L.) cover crop mitigates nitrous oxide emissions without decreasing yield. Soil Tillage Res. 2020, 196, 104442. [Google Scholar] [CrossRef]
- Kyei Boahen, S.; Savala, C.E.N.; Chikoye, D.; Abaidoo, R. Growth and yield responses of cowpea to inoculation and phosphorus fertilization in different environments. Front. Plant. Sci 2017, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- De Mastro, F.; Traversa, A.; Cocozza, C.; Pallara, M.; Brunetti, G. Soil organic carbon stabilization: Influence of tillage on mineralogical and chemical parameters. Soil Syst. 2020, 4, 58. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Mezzapesa, G.N.; Stellacci, A.M.; Ferrara, G.; Occhiogrosso, G.; Petrelli, G.; Castellini, M.; Spagnuolo, M. Cover crop for a sustainable viticulture: Effects on soil properties and table grape production. Agronomy 2020, 10, 1334. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Sun, O.J. Can no-tillage stimulate carbon sequestration in agricultural soils? A meta-analysis of paired experiments. Agric. Ecosyst. Environ. 2010, 139, 224–231. [Google Scholar] [CrossRef]
- Powlson, D.S.; Stirling, C.M.; Jat, M.L.; Gerard, B.G.; Palm, C.A.; Sanchez, P.A.; Cassman, K.G. Limited potential of no-till agriculture for climate change mitigation. Nat. Clim. Chang. 2014, 4, 678–683. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Boselli, R.; Fiorini, A.; Santelli, S.; Ardenti, F.; Capra, F.; Maris, S.C.; Tabaglio, V. Cover crops during transition to no-till maintain yield and enhance soil fertility in intensive agro-ecosystems. Field Crop. Res. 2020, 255, 107871. [Google Scholar] [CrossRef]
- Guzzetti, L.; Fiorini, A.; Panzeri, D.; Tommasi, N.; Grassi, F.; Taskin, E.; Misci, C.; Puglisi, E.; Tabaglio, V.; Galimberti, A.; et al. Sustainability perspectives of Vigna unguiculata L. Walp. Cultivation under no tillage and water stress conditions. Plants 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, S.; Puglisi, E.; Trevisan, M.; Scheckel, K.G.; Langdon, K.A.; McLaughlin, M.J.; Lombi, E.; Donner, E. Changes in soil bacterial communities and diversity in response to long-term silver exposure. FEMS Microbiol. Ecol. 2015, 91, fiv114. [Google Scholar] [CrossRef] [PubMed]
- Bandini, F.; Misci, C.; Taskin, E.; Cocconcelli, P.S.; Puglisi, E. Biopolymers modulate microbial communities in municipal organic waste digestion. FEMS Microbiol. Ecol. 2020, 96, fiaa183. [Google Scholar] [CrossRef] [PubMed]
- Połka, J.; Rebecchi, A.; Pisacane, V.; Morelli, L.; Puglisi, E. Bacterial diversity in typical Italian salami at different ripening stages as revealed by high-throughput sequencing of 16S rRNA amplicons. Food Microbiol. 2015, 46, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, S.; Puglisi, E.; Arena, M.; Cappa, F.; Veen, J.A.; Cocconcelli, P.S.; Trevisan, M. Soil microbial diversity patterns of a lowland spring environment. FEMS Microbiol. Ecol. 2013, 86, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. App. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Keller, K.; Brodie, E.L.; Larsen, N.; Piceno, Y.M.; Phan, R.; Andersen, G.L. NAST: A multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 2006, 34, W394–W399. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D. The effects of alignment quality, distance calculation method, sequence filtering, and region on the analysis of 16S rRNA gene-based studies. PLoS Comput. Biol. 2010, 6, e1000844. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2011, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Eivazi, F.; Tabatabai, M.A. Factors affecting glucosidase and galactosidase activities in soils. Soil Biol. Biochem. 1990, 22, 891–897. [Google Scholar] [CrossRef]
- Sannino, F.; Gianfreda, L. Pesticide influence on soil enzymatic activities. Chemosphere 2001, 45, 417–425. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soil 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils1. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenhau, R.C. Aggregate Stability and Size Distribution. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Black, C.A., Ed.; ASA SSSA: Madison, WI, USA, 1965; Volume 9, pp. 511–519. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Paulson, J.N.; Pop, M.; Bravo, H.C. Metastats: An improved statistical method for analysis of metagenomic data. Genome Biol. 2011, 12, P17. [Google Scholar] [CrossRef]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Carof, M.; Chen, W.; Le Floch, G. Effect of tillage and static abiotic soil properties on microbial diversity. App. Soil Ecol. 2018, 132, 135–145. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Chen, Q.; Wen, X.; Liao, Y. Conservation tillage increases soil bacterial diversity in the dryland of northern China. Agron. Sustain. Dev. 2016, 36, 28. [Google Scholar] [CrossRef]
- Babin, D.; Deubel, A.; Jacquiod, S.; Sørensen, S.J.; Geistlinger, J.; Grosch, R.; Smalla, K. Impact of long-term agricultural management practices on soil prokaryotic communities. Soil Biol. Biochem. 2019, 129, 17–28. [Google Scholar] [CrossRef]
- Essel, E.; Xie, J.H.; Deng, C.C.; Peng, Z.K.; Wang, J.B.; Shen, J.C.; Xie, J.H.; Coulter, J.A.; Li, L.L. Bacterial and fungal diversity in rhizosphere and bulk soil under different long-term tillage and cereal/legume rotation. Soil Tillage Res. 2019, 194, 104302. [Google Scholar] [CrossRef]
- Chavez Romero, Y.; Navarro Noya, Y.E.; Reynoso Martinez, S.C.; Sarria Guzman, Y.; Govaerts, B.; Verhulst, N.; Dendooven, L.; Luna Guido, M. 16S metagenomics reveals changes in the soil bacterial community driven by soil organic C, N-fertilizer and tillage-crop residue management. Soil Tillage Res. 2016, 159, 1–8. [Google Scholar] [CrossRef]
- Kaisermann, A.; Maron, P.A.; Beaumelle, L.; Lata, J.C. Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Appl. Soil Ecol. 2015, 86, 158–164. [Google Scholar] [CrossRef]
- Sánchez Marañón, M.; Miralles, I.; Aguirre-Garrido, J.F.; Anguita-Maeso, M.; Millán, V.; Ortega, R.; García Salcedo, J.A.; Martínez-Abarca, F.; Soriano, M. Changes in the soil bacterial community along a pedogenic gradient. Sci. Rep. 2017, 7, 14593. [Google Scholar] [CrossRef]
- Mangalassery, S.; Mooney, S.J.; Sparkes, D.L.; Fraser, W.T.; Sjögersten, S. Impacts of zero tillage on soil enzyme activities, microbial characteristics and organic matter functional chemistry in temperate soils. Eur. J. Soil Biol. 2015, 68, 9–17. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Larney, F.J.; Blackshaw, R.E.; Kanashiro, D.A.; Pearson, D.C. Phospholipid fatty acid biomarkers show positive soil microbial community responses to conservation soil management of irrigated crop rotations. Soil Tillage Res. 2017, 168, 1–10. [Google Scholar] [CrossRef]
- Schutter, M.; Sandeno, J.; Dick, R. Seasonal, soil type, and alternative management influences on microbial communities of vegetable cropping systems. Biol. Fertil. Soils 2001, 34, 397–410. [Google Scholar] [CrossRef]
- Panettieri, M.; Knicker, H.; Berns, A.E.; Murillo, J.M.; Madejón, E. Moldboard plowing effects on soil aggregation and soil organic matter quality assessed by 13C CPMAS NMR and biochemical analyses. Agric. Ecosyst. Environ. 2013, 177, 48–57. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Klimkowicz-Pawlas, A.; Gałazka, A.; Grzadziel, J.; Stuczynski, T. Impact of water stress on microbial community and activity in sandy and loamy soils. Agronomy 2020, 10, 1429. [Google Scholar] [CrossRef]
- Marxsen, J.; Zoppini, A.; Wilczek, S. Microbial communities in streambed sediments recovering from desiccation. FEMS Microbiol. Ecol. 2010, 71, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Zoppini, A.; Marxsen, J. Importance of extracellular enzymes for biogeochemical processes in temporary river sediments during fluctuating dry-wet conditions. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 103–117. [Google Scholar]
- Jain, N.K.; Jat, R.S.; Meena, H.N.; Chakraborty, K. Productivity, nutrient, and soil enzymes influenced with conservation agriculture practices in peanut. Agron. J. 2018, 110, 1165–1172. [Google Scholar] [CrossRef]
- Zibilske, L.M.; Bradford, J.M. Soil aggregation, aggregate carbon and nitrogen, and moisture retention induced by conservation tillage. Soil Sci. Soc. Am. J. 2007, 71, 793–802. [Google Scholar] [CrossRef]
- Lichter, K.; Govaerts, B.; Six, J.; Sayre, K.D.; Deckers, J.; Dendooven, L. Aggregation and C and N contents of soil organic matter fractions in a permanent raised-bed planting system in the Highlands of Central Mexico. Plant Soil 2008, 305, 237–252. [Google Scholar] [CrossRef]
- Huang, S.; Sun, Y.-N.; Rui, W.-Y.; Liu, W.-R.; Zhang, W.-J. Long-term effect of no-tillage on soil organic carbon fractions in a continuous maize cropping system of northeast China. Pedosphere 2010, 20, 285–292. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Bergmann, J.; Verbruggen, E.; Veresoglou, S.D.; Lehmann, A. Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol. 2015, 205, 1385–1388. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K.; Doran, J.W. Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Denef, K.; Six, J.; Merckx, R.; Paustian, K. Carbon sequestration in microaggregates of no-tillage soils with different clay mineralogy. Soil Sci. Soc. Am. J. 2004, 68, 1935–1944. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Smith, S.F.; Brye, K.R.; Gbur, E.E.; Chen, P.Y.; Korth, K. Residue and water management effects on aggregate stability and aggregate-associated carbon and nitrogen in a wheat-soybean, double-crop system. Soil Sci. Soc. Am. J. 2014, 78, 1378–1391. [Google Scholar] [CrossRef]
- Sainju, U.M.; Caesar-TonThat, T.; Jabro, J.D. Carbon and nitrogen fractions in dryland soil aggregates affected by long-term tillage and cropping sequence. Soil Sci. Soc. Am. J. 2009, 73, 1488–1495. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.J.; Gregorich, E.G.; McLaughlin, N.B.; Zhang, X.P.; Guo, Y.F.; Liang, A.Z.; Fan, R.Q.; Sun, B.J. No-tillage with continuous maize cropping enhances soil aggregation and organic carbon storage in Northeast China. Geoderma 2018, 330, 204–211. [Google Scholar] [CrossRef]
- Wang, X.; Qi, J.Y.; Zhang, X.Z.; Li, S.S.; Virk, A.L.; Zhao, X.; Xiao, X.P.; Zhang, H.L. Effects of tillage and residue management on soil aggregates and associated carbon storage in a double paddy cropping system. Soil Tillage Res. 2019, 194, 104339. [Google Scholar] [CrossRef]
- Lopez Bellido, R.J.; Munoz Romero, V.; Fuentes Guerra, R.; Fernandez Garcia, P.; Lopez Bellido, L. No-till: A key tool for sequestering C and N in microaggregates on a Mediterranean Vertisol. Soil Tillage Res. 2017, 166, 131–137. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Oades, J.M. The role of biology in the formation, stabilization and degradation of soil structure. In Soil Structure/Soil Biota Interrelationships; Brussaard, L., Kooistra, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 377–400. [Google Scholar] [CrossRef]
- Sekaran, U.; Sagar, K.L.; Denardin, L.G.D.O.; Singh, J.; Singh, N.; Abagandura, G.O.; Kumar, S.; Farmaha, B.S.; Bly, A.; Martins, A.P. Responses of soil biochemical properties and microbial community structure to short and long-term no-till systems. Eur. J. Soil Sci. 2019, 71, 1018–1033. [Google Scholar] [CrossRef]


| Measured Soil Enzymes | |||||
|---|---|---|---|---|---|
| Source of Variation | AI3 5 | β GLU (μmol PNG g−1 h−1) | PHO (μmol PNP g−1 h−1) | URE (μg urea g−1 h−1) | |
| Tillage (T) | CT 1 | −85.35 (±5.05) b | 8.86 (±0.8) b | 18.51 (±0.48) b | 5.85 (±1.07) a |
| NT + CC 2 | −38.43 (±5.86) a | 20.85 (±2) a | 24.21 (±1.6) a | 7.05 (±1.1) a | |
| Water (W) | NS 3 | −60.72 (±4.47) a | 16.84 (±4.17) a | 23.13 (±1.85) a | 6.47 (±1.63) a |
| S 4 | −63.05 (±5.83) a | 12.86 (±1.41) a | 19.6 (±0.55) a | 6.42 (±1.12) a | |
| T × W | CT-NS | −96.14 (±3.63) b | 8.06 (±0.81) b | 19.04 (±0.53) ab | 6.18 (±1.13) a |
| CT-S | −74.56 (±4.65) ab | 9.65 (±0.85) b | 17.98 (±0.39) b | 5.51 (±1.1) a | |
| NT + CC-NS | −25.3 (±3.33) a | 25.63 (±1.51) a | 27.21 (±1.4) a | 6.77 (±2.52) a | |
| NT + CC-S | −72.09 (±5.38) ab | 16.07 (±1.14) b | 23.72 (±1.08) ab | 7.33 (±1.99) a | |
| Depth | Source of Variation | Code | Whole Soil—C Amount (g C kg−1 Soil) | Macroaggregates—C Amount (g C kg−1 Soil) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LM 1 | sM 2 | m 3 | s + c 4 | cPOM 5 | mM 6 | s + cM 7 | |||||||
| 0–5 cm | Tillage (T) | CT | 2.71 | b | 6.49 | 1.73 | 1.43 | 0.66 | b | 6.34 | b | 2.75 | |
| NT + CC | 7.37 | a | 5.67 | 1.85 | 1.66 | 2.20 | a | 8.29 | a | 3.65 | |||
| p value | 0.0093 | 0.4249 | 0.5076 | 0.2816 | 0.0039 | 0.0433 | 0.0688 | ||||||
| Water (W) | NS | 6.20 | 6.59 | 1.77 | 1.63 | 1.66 | a | 7.59 | a | 3.55 | |||
| S | 3.88 | 5.57 | 1.81 | 1.45 | 1.19 | b | 7.03 | b | 2.85 | ||||
| p value | 0.0779 | 0.2344 | 0.7912 | 0.3374 | 0.0401 | 0.0258 | 0.0676 | ||||||
| T × W | CT-NS | 3.74 | 5.52 | 1.63 | 1.57 | 0.43 | c | 6.27 | b | 2.93 | |||
| CT-S | 1.68 | 7.47 | 1.83 | 1.28 | 0.89 | bc | 6.40 | ab | 2.57 | ||||
| NT + CC-NS | 8.68 | 5.62 | 1.91 | 1.69 | 2.90 | a | 8.92 | a | 4.18 | ||||
| NT + CC-S | 6.07 | 5.72 | 1.79 | 1.62 | 1.50 | b | 7.65 | ab | 3.13 | ||||
| p value | 0.8142 | 0.2800 | 0.3219 | 0.5429 | 0.0028 | 0.0109 | 0.3187 | ||||||
| 5–20 cm | Tillage (T) | CT | 4.59 | 5.24 | 1.42 | 0.89 | 0.57 | 5.97 | 3.84 | a | |||
| NT + CC | 4.17 | 5.64 | 1.62 | 0.94 | 0.56 | 6.61 | 2.70 | b | |||||
| p value | 0.6124 | 0.4097 | 0.0636 | 0.5325 | 0.7415 | 0.0720 | 0.0396 | ||||||
| Water (W) | NS | 4.89 | 5.53 | 1.31 | 0.81 | 0.60 | 6.31 | 3.53 | |||||
| S | 3.87 | 5.35 | 1.73 | 1.01 | 0.53 | 6.27 | 3.01 | ||||||
| p value | 0.1196 | 0.5502 | 0.0698 | 0.1453 | 0.5609 | 0.8356 | 0.0688 | ||||||
| T × W | CT-NS | 4.94 | 4.92 | 1.24 | 0.83 | 0.55 | 5.91 | 4.15 | |||||
| CT-S | 4.24 | 5.57 | 1.59 | 0.94 | 0.60 | 6.02 | 3.52 | ||||||
| NT + CC-NS | 4.84 | 5.50 | 1.37 | 0.79 | 0.65 | 6.71 | 2.92 | ||||||
| NT + CC-S | 3.50 | 4.92 | 1.86 | 1.08 | 0.46 | 6.51 | 2.49 | ||||||
| p value | 0.5888 | 0.1531 | 0.6745 | 0.4593 | 0.3593 | 0.4858 | 0.6980 | ||||||
| Depth | Source of Variation | Code | Whole Soil—N Amount (g N kg−1 Soil) | Macroaggregates—N Amount (g N kg−1 Soil) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LM 1 | sM 2 | m 3 | s + c 4 | cPOM 5 | mM 6 | s + cM 7 | ||||||||
| 0–5 cm | Tillage (T) | CT | 0.38 | b | 0.76 | 0.22 | 0.14 | 0.05 | b | 0.95 | 0.34 | b | ||
| NT + CC | 0.87 | a | 0.65 | 0.21 | 0.16 | 0.17 | a | 0.79 | 0.51 | a | ||||
| p value | 0.0317 | 0.2986 | 0.9460 | 0.1566 | 0.0040 | 0.1375 | 0.0356 | |||||||
| Water (W) | NS | 0.73 | 0.78 | 0.20 | 0.16 | 0.12 | a | 0.88 | 0.45 | |||||
| S | 0.51 | 0.63 | 0.23 | 0.15 | 0.09 | b | 0.87 | 0.40 | ||||||
| p value | 0.1188 | 0.1685 | 0.3011 | 0.5377 | 0.0426 | 0.6622 | 0.3658 | |||||||
| T × W | CT-NS | 0.45 | 0.62 | 0.19 | 0.14 | 0.03 | c | 0.78 | 0.36 | |||||
| CT-S | 0.30 | 0.90 | 0.24 | 0.13 | 0.07 | bc | 0.80 | 0.32 | ||||||
| NT + CC-NS | 1.02 | 0.63 | 0.21 | 0.17 | 0.21 | a | 0.98 | 0.54 | ||||||
| NT + CC-S | 0.72 | 0.67 | 0.21 | 0.16 | 0.12 | b | 0.93 | 0.47 | ||||||
| p value | 0.5748 | 0.2753 | 0.3251 | 0.8816 | 0.0020 | 0.2588 | 0.7685 | |||||||
| 5–20 cm | Tillage (T) | CT | 0.58 | 0.62 | 0.17 | b | 0.12 | 0.04 | 0.75 | b | 0.49 | |||
| NT + CC | 0.53 | 0.71 | 0.21 | a | 0.13 | 0.05 | 0.88 | a | 0.38 | |||||
| p value | 0.6322 | 0.2829 | 0.0479 | 0.5391 | 0.1422 | 0.0213 | 0.0547 | |||||||
| Water (W) | NS | 0.62 | 0.66 | 0.22 | a | 0.11 | 0.05 | 0.81 | 0.46 | |||||
| S | 0.49 | 0.69 | 0.16 | b | 0.14 | 0.04 | 0.82 | 0.41 | ||||||
| p value | 0.1118 | 0.8928 | 0.0351 | 0.0998 | 0.4358 | 0.7583 | 0.2126 | |||||||
| T × W | CT-NS | 0.64 | 0.58 | 0.15 | 0.11 | 0.04 | 0.75 | 0.52 | ||||||
| CT-S | 0.52 | 0.68 | 0.19 | 0.12 | 0.04 | 0.75 | 0.45 | |||||||
| NT + CC-NS | 0.61 | 0.74 | 0.17 | 0.10 | 0.06 | 0.88 | 0.39 | |||||||
| NT + CC-S | 0.46 | 0.67 | 0.24 | 0.16 | 0.05 | 0.89 | 0.36 | |||||||
| p value | 0.8395 | 0.1653 | 0.5989 | 0.1403 | 0.3549 | 0.7353 | 0.6329 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taskin, E.; Boselli, R.; Fiorini, A.; Misci, C.; Ardenti, F.; Bandini, F.; Guzzetti, L.; Panzeri, D.; Tommasi, N.; Galimberti, A.; et al. Combined Impact of No-Till and Cover Crops with or without Short-Term Water Stress as Revealed by Physicochemical and Microbiological Indicators. Biology 2021, 10, 23. https://doi.org/10.3390/biology10010023
Taskin E, Boselli R, Fiorini A, Misci C, Ardenti F, Bandini F, Guzzetti L, Panzeri D, Tommasi N, Galimberti A, et al. Combined Impact of No-Till and Cover Crops with or without Short-Term Water Stress as Revealed by Physicochemical and Microbiological Indicators. Biology. 2021; 10(1):23. https://doi.org/10.3390/biology10010023
Chicago/Turabian StyleTaskin, Eren, Roberta Boselli, Andrea Fiorini, Chiara Misci, Federico Ardenti, Francesca Bandini, Lorenzo Guzzetti, Davide Panzeri, Nicola Tommasi, Andrea Galimberti, and et al. 2021. "Combined Impact of No-Till and Cover Crops with or without Short-Term Water Stress as Revealed by Physicochemical and Microbiological Indicators" Biology 10, no. 1: 23. https://doi.org/10.3390/biology10010023
APA StyleTaskin, E., Boselli, R., Fiorini, A., Misci, C., Ardenti, F., Bandini, F., Guzzetti, L., Panzeri, D., Tommasi, N., Galimberti, A., Labra, M., Tabaglio, V., & Puglisi, E. (2021). Combined Impact of No-Till and Cover Crops with or without Short-Term Water Stress as Revealed by Physicochemical and Microbiological Indicators. Biology, 10(1), 23. https://doi.org/10.3390/biology10010023









