Susceptibility of Asialoglycoprotein Receptor-Deficient Mice to LPS/Galactosamine Liver Injury and Protection by Betaine Administration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Serum Transaminase
2.3. Serum Cytokine Levels
2.4. Histopatholog
2.5. TUNEL Assay
2.6. Caspase-3 Activation Assay
2.7. Statistical Analyses
3. Results
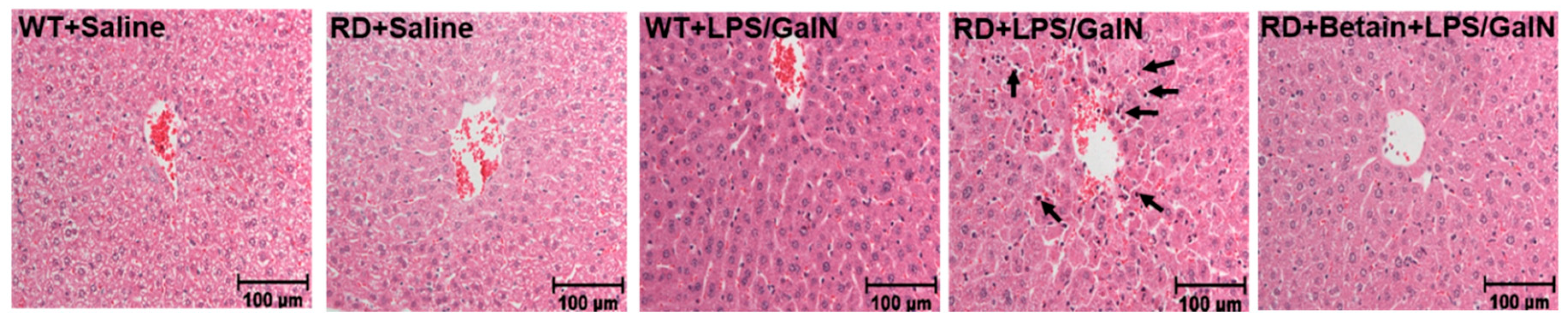
3.1. Histological Changes
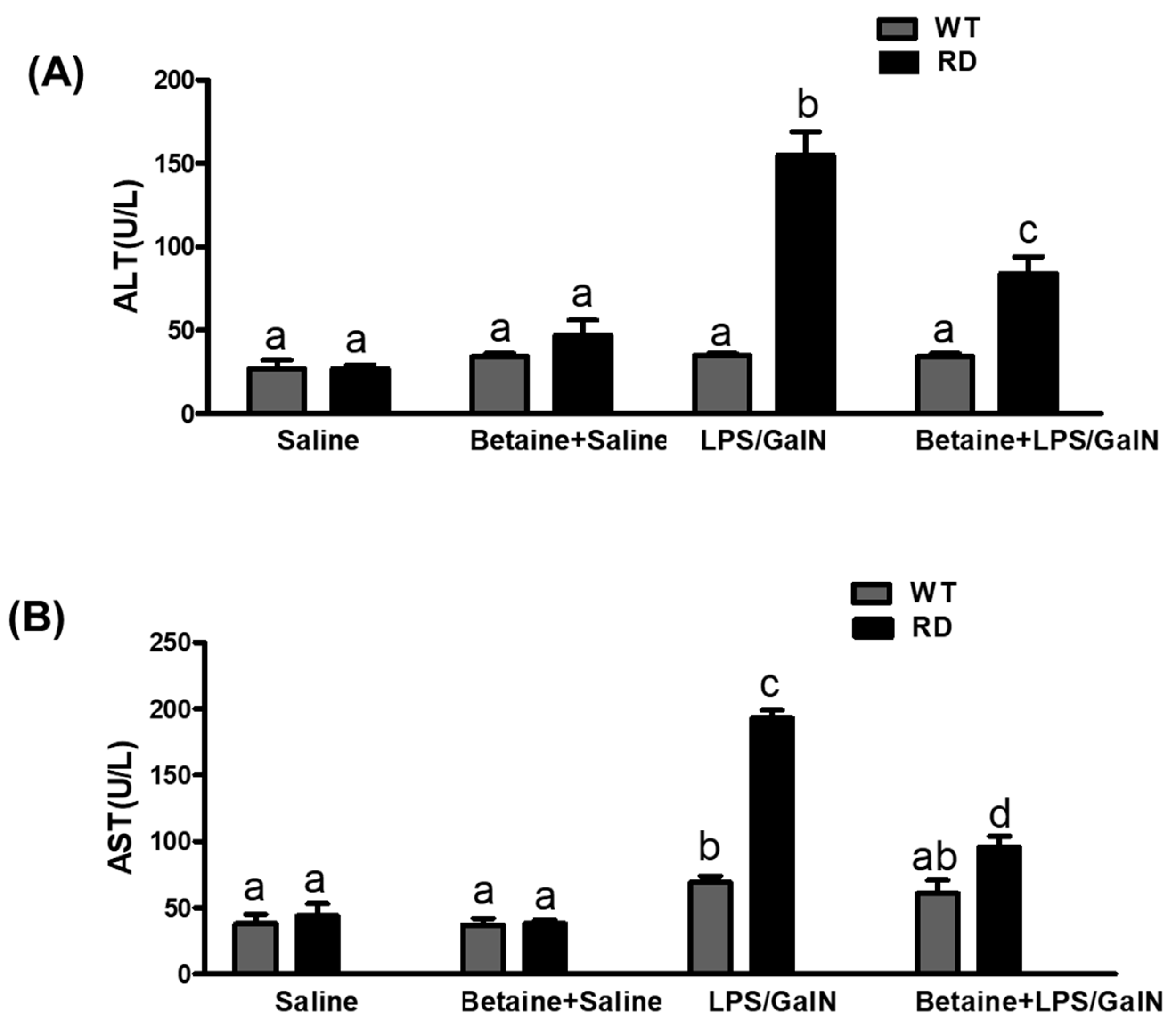
3.2. Serum ALT and AST Levels
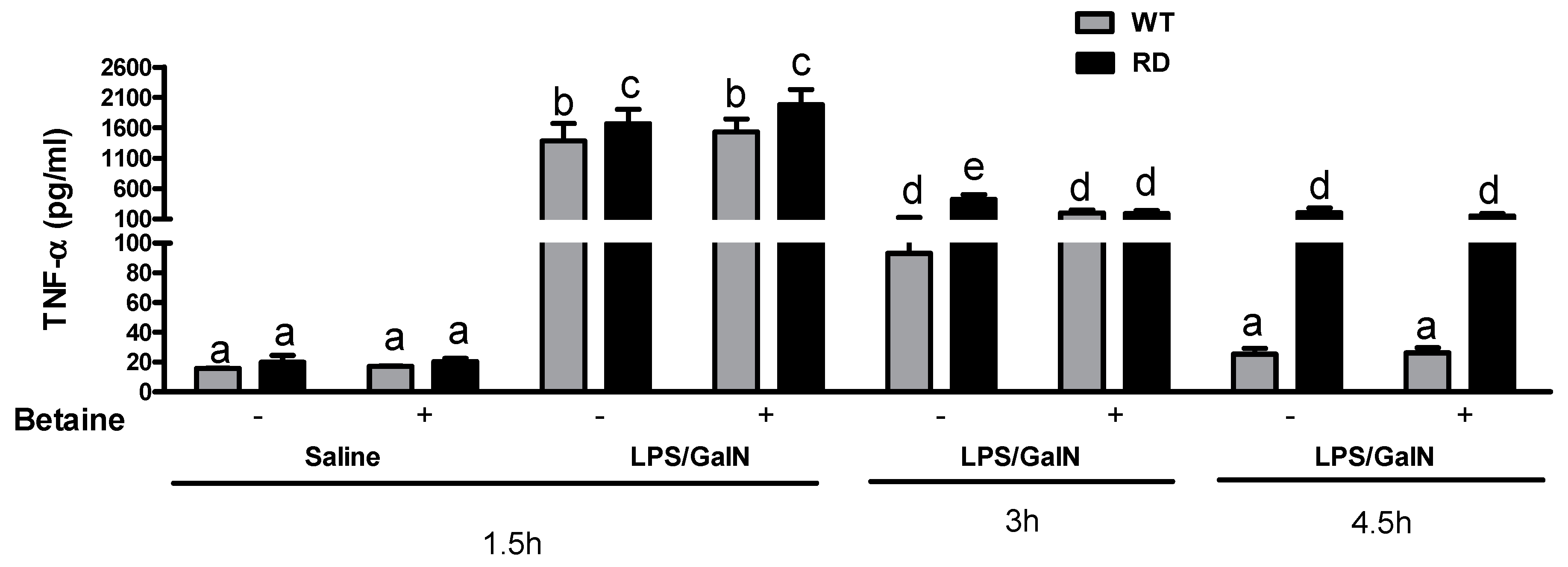
3.3. Cytokine Levels
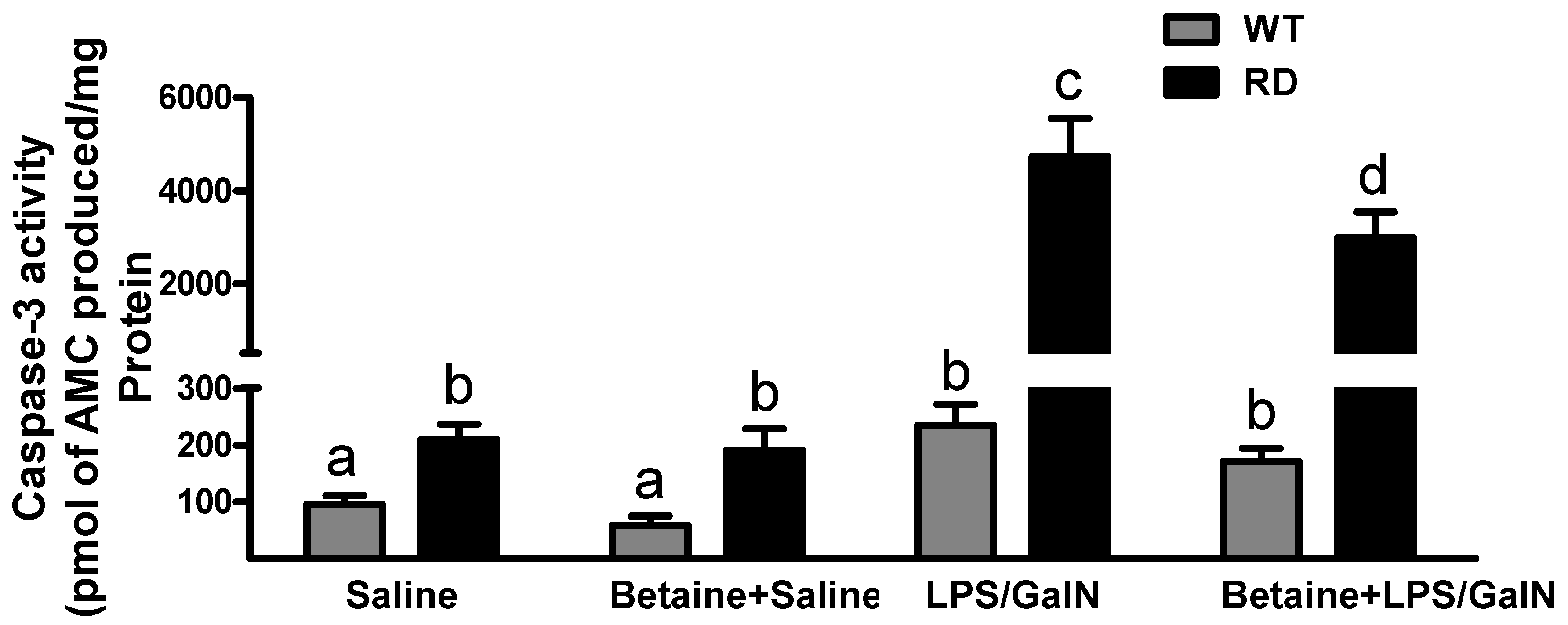
3.4. Hepatocyte Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Grek, A.; Arasi, L. Acute liver failure. AACN Adv. Crit. Care 2016, 27, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.E.; McKiernan, P.; Squires, R.H. Acute liver failure: An update. Clin. Liver Dis. 2018, 22, 773–805. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; Squires, R.H., Jr.; Nyberg, S.L.; Doo, E.; Hoofnagle, J.H. Acute liver failure: Summary of a workshop. Hepatology 2008, 47, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; Stravitz, R.T.; Larson, A.M. Introduction to the revised american association for the study of liver diseases position paper on acute liver failure 2011. Hepatology 2012, 55, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.I.; Hong, J.M.; Choi, J.W.; Choi, H.S.; Kwak, J.H.; Lee, D.U.; Lee, S.K.; Lee, S.M. Beta-caryophyllene alleviates d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the tlr4 and rage signaling pathways. Eur. J. Pharmacol. 2015, 764, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Song, W.; Xu, R. Autophagy and er stress in lps/galninduced acute liver injury. Mol. Med. Rep. 2017, 16, 7001–7005. [Google Scholar] [CrossRef]
- Galanos, C.; Freudenberg, M.A.; Reutter, W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA 1979, 76, 5939–5943. [Google Scholar] [CrossRef]
- Leist, M.; Gantner, F.; Bohlinger, I.; Tiegs, G.; Germann, P.G.; Wendel, A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am. J. Pathol. 1995, 146, 1220–1234. [Google Scholar]
- Li, X.; Gou, C.; Yang, H.; Qiu, J.; Gu, T.; Wen, T. Echinacoside ameliorates d-galactosamine plus lipopolysaccharide-induced acute liver injury in mice via inhibition of apoptosis and inflammation. Scand. J. Gastroenterol. 2014, 49, 993–1000. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, L.; Jiang, K. Propofol attenuates inflammatory response and apoptosis to protect d-galactosamine/lipopolysaccharide induced acute liver injury via regulating tlr4/nf-kappab/nlrp3 pathway. Int. Immunopharmacol. 2019, 77, 105974. [Google Scholar] [CrossRef]
- Kmiec, Z.; Smolenski, R.T.; Zych, M.; Mysliwski, A. The effects of galactosamine on utp levels in the livers of young, adult and old rats. Acta Biochim. Pol. 2000, 47, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Grun, B.R.; Berger, U.; Oberdorfer, F.; Hull, W.E.; Ostertag, H.; Keppler, D. In vivo metabolism and utp-depleting action of 2-deoxy-2-fluoro-d-galactose. Adv. Enzym. Regul. 1990, 30, 231–242. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, N.; Ni, S.; Li, W.; Xu, L.; Dong, P.; Lu, M. Pretreatment of lipopolysaccharide (lps) ameliorates d-galn/lps induced acute liver failure through tlr4 signaling pathway. Int. J. Clin. Exp. Pathol. 2014, 7, 6626–6634. [Google Scholar]
- Su, G.L. Lipopolysaccharides in liver injury: Molecular mechanisms of kupffer cell activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G256–G265. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.B.; Ganey, P.E.; Roth, R.A. The role of kupffer cells and tnf-alpha in monocrotaline and bacterial lipopolysaccharide-induced liver injury. Toxicol. Sci. 2003, 71, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Dorman, R.B.; Gujral, J.S.; Bajt, M.L.; Farhood, A.; Jaeschke, H. Generation and functional significance of cxc chemokines for neutrophil-induced liver injury during endotoxemia. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G880–G886. [Google Scholar] [CrossRef] [PubMed]
- Bantel, H.; Schulze-Osthoff, K. Mechanisms of cell death in acute liver failure. Front. Physiol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Li, Z.; Weinman, S.A. Regulation of hepatic inflammation via macrophage cell death. Semin. Liver Dis. 2018, 38, 340–350. [Google Scholar] [CrossRef]
- Maes, M.; Vinken, M.; Jaeschke, H. Experimental models of hepatotoxicity related to acute liver failure. Toxicol. Appl. Pharmacol. 2016, 290, 86–97. [Google Scholar] [CrossRef]
- Gujral, J.S.; Hinson, J.A.; Farhood, A.; Jaeschke, H. Nadph oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G243–G252. [Google Scholar] [CrossRef]
- Dini, L.; Autuori, F.; Lentini, A.; Oliverio, S.; Piacentini, M. The clearance of apoptotic cells in the liver is mediated by the asialoglycoprotein receptor. FEBS Lett. 1992, 296, 174–178. [Google Scholar] [CrossRef]
- McVicker, B.L.; Tuma, D.J.; Kubik, J.A.; Hindemith, A.M.; Baldwin, C.R.; Casey, C.A. The effect of ethanol on asialoglycoprotein receptor-mediated phagocytosis of apoptotic cells by rat hepatocytes. Hepatology 2002, 36, 1478–1487. [Google Scholar] [PubMed]
- Ji, C.; Kaplowitz, N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 2003, 124, 1488–1499. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Rogers, D.D., 2nd; Mailliard, M.E.; Siford, G.L.; Barak, A.J.; Beckenhauer, H.C.; Sorrell, M.F.; Tuma, D.J. Role of elevated s-adenosylhomocysteine in rat hepatocyte apoptosis: Protection by betaine. Biochem. Pharmacol. 2005, 70, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K.; Mailliard, M.E.; Baldwin, C.R.; Beckenhauer, H.C.; Sorrell, M.F.; Tuma, D.J. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J. Hepatol. 2007, 46, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K.; Mailliard, M.E.; Baldwin, C.R.; Sorrell, M.F.; Tuma, D.J. Accumulation of proteins bearing atypical isoaspartyl residues in livers of alcohol-fed rats is prevented by betaine administration: Effects on protein-l-isoaspartyl methyltransferase activity. J. Hepatol. 2007, 46, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Abdelmalek, M.F.; Barve, S.; Benevenga, N.J.; Halsted, C.H.; Kaplowitz, N.; Kharbanda, K.K.; Liu, Q.Y.; Lu, S.C.; McClain, C.J.; et al. Role of s-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: Summary of a symposium. Am. J. Clin. Nutr. 2007, 86, 14–24. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Todero, S.L.; Ward, B.W.; Cannella, J.J., 3rd; Tuma, D.J. Betaine administration corrects ethanol-induced defective vldl secretion. Mol. Cell. Biochem. 2009, 327, 75–78. [Google Scholar] [CrossRef]
- Kharbanda, K.K. Alcoholic liver disease and methionine metabolism. Semin. Liver Dis. 2009, 29, 155–165. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Todero, S.L.; King, A.L.; Osna, N.A.; McVicker, B.L.; Tuma, D.J.; Wisecarver, J.L.; Bailey, S.M. Betaine treatment attenuates chronic ethanol-induced hepatic steatosis and alterations to the mitochondrial respiratory chain proteome. Int. J. Hepatol. 2012, 2012, 962183. [Google Scholar] [CrossRef]
- Kharbanda, K.K. Methionine metabolic pathway in alcoholic liver injury. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K.; Ronis, M.J.J.; Shearn, C.T.; Petersen, D.R.; Zakhari, S.; Warner, D.R.; Feldstein, A.E.; McClain, C.J.; Kirpich, I.A. Role of nutrition in alcoholic liver disease: Summary of the symposium at the esbra 2017 congress. Biomolecules 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Varatharajalu, R.; Garige, M.; Leckey, L.C.; Gong, M.; Lakshman, M.R. Betaine protects chronic alcohol and omega-3 pufa-mediated down-regulations of pon1 gene, serum pon1 and homocysteine thiolactonase activities with restoration of liver gsh. Alcohol. Clin. Exp. Res. 2010, 34, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Varatharajalu, R.; Garige, M.; Leckey, L.C.; Arellanes-Robledo, J.; Reyes-Gordillo, K.; Shah, R.; Lakshman, M.R. Adverse signaling of scavenger receptor class b1 and pgc1s in alcoholic hepatosteatosis and steatohepatitis and protection by betaine in rat. Am. J. Pathol. 2014, 184, 2035–2044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Junnila, M.; Barak, A.J.; Beckenhauer, H.C.; Rahko, T. Betaine reduces hepatic lipidosis induced by carbon tetrachloride in sprague-dawley rats. Vet. Hum. Toxicol. 1998, 40, 263–266. [Google Scholar] [PubMed]
- Junnila, M.; Rahko, T.; Sukura, A.; Lindberg, L.A. Reduction of carbon tetrachloride-induced hepatotoxic effects by oral administration of betaine in male han-wistar rats: A morphometric histological study. Vet. Pathol. 2000, 37, 231–238. [Google Scholar] [CrossRef]
- Murakami, T.; Nagamura, Y.; Hirano, K. The recovering effect of betaine on carbon tetrachloride-induced liver injury. J. Nutr. Sci. Vitaminol. 1998, 44, 249–255. [Google Scholar] [CrossRef]
- Tsai, M.T.; Chen, C.Y.; Pan, Y.H.; Wang, S.H.; Mersmann, H.J.; Ding, S.T. Alleviation of carbon-tetrachloride-induced liver injury and fibrosis by betaine supplementation in chickens. Evid. Based Complement. Altern. Med. 2015, 2015, 725379. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Angulo, P.; Jorgensen, R.A.; Sylvestre, P.B.; Lindor, K.D. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: Results of a pilot study. Am. J. Gastroenterol. 2001, 96, 2711–2717. [Google Scholar] [CrossRef]
- Kathirvel, E.; Morgan, K.; Nandgiri, G.; Sandoval, B.C.; Caudill, M.A.; Bottiglieri, T.; French, S.W.; Morgan, T.R. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: A potential mechanism for hepatoprotection by betaine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1068–G1077. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bernard, T.; Kharbanda, K.; Barak, A.J.; Sorrell, M.F.; Tuma, D.J. Impact of betaine on hepatic fibrosis and homocysteine in non-alcoholic steatohepatitis-a prospective cohort study. Open Transl. J. 2011, 3, 1–4. [Google Scholar]
- Song, Z.; Deaciuc, I.; Zhou, Z.; Song, M.; Chen, T.; Hill, D.; McClain, C.J. Involvement of amp-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G894–G902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, T.; Pini, M.; Zhou, Z.; Fantuzzi, G.; Song, Z. Betaine improved adipose tissue function in mice fed a high-fat diet: A mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G634–G642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, L.W.; Wang, L.K.; Li, X.; Zhang, H.; Luo, L.P.; Song, J.C.; Gong, Z.J. Betaine protects against high-fat-diet-induced liver injury by inhibition of high-mobility group box 1 and toll-like receptor 4 expression in rats. Dig. Dis. Sci. 2013, 58, 3198–3206. [Google Scholar] [CrossRef]
- Kawakami, S.; Han, K.H.; Nakamura, Y.; Shimada, K.; Kitano, T.; Aritsuka, T.; Nagura, T.; Ohba, K.; Nakamura, K.; Fukushima, M. Effects of dietary supplementation with betaine on a nonalcoholic steatohepatitis (nash) mouse model. J. Nutr. Sci. Vitaminol. 2012, 58, 371–375. [Google Scholar] [CrossRef]
- Ji, C.; Deng, Q.; Kaplowitz, N. Role of tnf-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology 2004, 40, 442–451. [Google Scholar] [CrossRef]
- Horio, M.; Ito, A.; Matsuoka, Y.; Moriyama, T.; Orita, Y.; Takenaka, M.; Imai, E. Apoptosis induced by hypertonicity in madin darley canine kidney cells: Protective effect of betaine. Nephrol. Dial. Transplant. 2001, 16, 483–490. [Google Scholar] [CrossRef]
- Im, A.R.; Kim, Y.H.; Uddin, M.R.; Chae, S.; Lee, H.W.; Kim, Y.S.; Lee, M.Y. Betaine protects against rotenone-induced neurotoxicity in pc12 cells. Cell. Mol. Neurobiol. 2013, 33, 625–635. [Google Scholar] [CrossRef]
- Dalton, S.R.; Lee, S.M.; King, R.N.; Nanji, A.A.; Kharbanda, K.K.; Casey, C.A.; McVicker, B.L. Carbon tetrachloride-induced liver damage in asialoglycoprotein receptor-deficient mice. Biochem. Pharmacol. 2009, 77, 1283–1290. [Google Scholar] [CrossRef]
- Dalton, S.R.; Wiegert, R.L.; Baldwin, C.R.; Kassel, K.M.; Casey, C.A. Impaired receptor-mediated endocytosis by the asialoglycoprotein receptor in ethanol-fed mice: Implications for studying the role of this receptor in alcoholic apoptosis. Biochem. Pharmacol. 2003, 65, 535–543. [Google Scholar] [CrossRef]
- Okumura, A.; Saito, T.; Tobiume, M.; Hashimoto, Y.; Sato, Y.; Umeyama, T.; Nagi, M.; Tanabe, K.; Unoki-Kubota, H.; Kaburagi, Y.; et al. Alleviation of lipopolysaccharide/d-galactosamine-induced liver injury in leukocyte cell-derived chemotaxin 2 deficient mice. Biochem. Biophys. Rep. 2017, 12, 166–171. [Google Scholar] [CrossRef]
- Hishinuma, I.; Nagakawa, J.; Hirota, K.; Miyamoto, K.; Tsukidate, K.; Yamanaka, T.; Katayama, K.; Yamatsu, I. Involvement of tumor necrosis factor-alpha in development of hepatic injury in galactosamine-sensitized mice. Hepatology 1990, 12, 1187–1191. [Google Scholar] [CrossRef]
- Wu, Y.H.; Hu, S.Q.; Liu, J.; Cao, H.C.; Xu, W.; Li, Y.J.; Li, L.J. Nature and mechanisms of hepatocyte apoptosis induced by d-galactosamine/lipopolysaccharide challenge in mice. Int. J. Mol. Med. 2014, 33, 1498–1506. [Google Scholar] [CrossRef]
- Canbay, A.; Taimr, P.; Torok, N.; Higuchi, H.; Friedman, S.; Gores, G.J. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab. Investig. 2003, 83, 655–663. [Google Scholar] [CrossRef]
- Ganesan, M.; New-Aaron, M.; Dagur, R.S.; Makarov, E.; Wang, W.; Kharbanda, K.K.; Kidambi, S.; Poluektova, L.Y.; Osna, N.A. Alcohol metabolism potentiates HIV-induced hepatotoxicity: Contribution to end-stage liver disease. Biomolecules 2019, 9, 851. [Google Scholar] [CrossRef]
- Arvelo, M.B.; Cooper, J.T.; Longo, C.; Daniel, S.; Grey, S.T.; Mahiou, J.; Czismadia, E.; Abu-Jawdeh, G.; Ferran, C. A20 protects mice from d-galactosamine/lipopolysaccharide acute toxic lethal hepatitis. Hepatology 2002, 35, 535–543. [Google Scholar] [CrossRef]
- Rousta, A.M.; Mirahmadi, S.M.; Shahmohammadi, A.; Ramzi, S.; Baluchnejadmojarad, T.; Roghani, M. S-allyl cysteine, an active ingredient of garlic, attenuates acute liver dysfunction induced by lipopolysaccharide/ d-galactosamine in mouse: Underlying mechanisms. J. Biochem. Mol. Toxicol. 2020, 26, e22518. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Y.; Shen, Y.; Li, L.; Huang, J.; Tang, L.; Zhang, L. Luzindole attenuates lps/d-galactosamine-induced acute hepatitis in mice. Innate Immune 2020, 26, 319–327. [Google Scholar] [CrossRef]
- Peng, X.; Yang, Y.; Tang, L.; Wan, J.; Dai, J.; Li, L.; Huang, J.; Shen, Y.; Lin, L.; Gong, X.; et al. Therapeutic benefits of apocynin in mice with lipopolysaccharide/d-galactosamine-induced acute liver injury via suppression of the late stage pro-apoptotic ampk/jnk pathway. Biomed. Pharmacother. 2020, 125, 110020. [Google Scholar] [CrossRef]
- Lv, H.; An, B.; Yu, Q.; Cao, Y.; Liu, Y.; Li, S. The hepatoprotective effect of myricetin against lipopolysaccharide and d-galactosamine-induced fulminant hepatitis. Int. J. Biol. Macromol. 2020, 155, 1092–1104. [Google Scholar] [CrossRef]
- Gao, K.; Liu, F.; Chen, X.; Chen, M.; Deng, Q.; Zou, X.; Guo, H. Crocetin protects against fulminant hepatic failure induced by lipopolysaccharide/d-galactosamine by decreasing apoptosis, inflammation and oxidative stress in a rat model. Exp. Ther. Med. 2019, 18, 3775–3782. [Google Scholar] [CrossRef]
- Yang, C.; He, L.; Wang, C.; Huang, Y.; Wang, A.; Li, X.; Ao, J. Dexmedetomidine alleviated lipopolysaccharide/d-galactosamine-induced acute liver injury in mice. Int. Immunopharmacol. 2019, 72, 367–373. [Google Scholar] [CrossRef]
- Yoshida, T.; Abe, K.; Ikeda, T.; Matsushita, T.; Wake, K.; Sato, T.; Sato, T.; Inoue, H. Inhibitory effect of glycyrrhizin on lipopolysaccharide and d-galactosamine-induced mouse liver injury. Eur. J. Pharmacol. 2007, 576, 136–142. [Google Scholar] [CrossRef]
- Mato, J.M.; Alvarez, L.; Ortiz, P.; Pajares, M.A. S-adenosylmethionine synthesis: Molecular mechanisms and clinical implications. Pharmacol. Ther. 1997, 73, 265–280. [Google Scholar] [CrossRef]
- Ozturk, M.; Lemonnier, F.; Cresteil, D.; Lemonnier, A. Changes in methionine metabolism induced by d-galactosamine in isolated rat hepatocytes. Biochem. Pharmacol. 1986, 35, 4223–4228. [Google Scholar] [CrossRef]
- Lu, S.C.; Mato, J.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef]
- Clawson, G.A.; Sesno, J.; Milam, K.; Wang, Y.F.; Gabriel, C. The hepatocyte protein synthesis defect induced by galactosamine involves hypomethylation of ribosomal rna. Hepatology 1990, 11, 428–434. [Google Scholar] [CrossRef]
- Halsted, C.H.; Medici, V. Aberrant hepatic methionine metabolism and gene methylation in the pathogenesis and treatment of alcoholic steatohepatitis. Int. J. Hepatol. 2012, 2012, 959746. [Google Scholar] [CrossRef]
- Li, Z.; Feng, H.; Han, L.; Ding, L.; Shen, B.; Tian, Y.; Zhao, L.; Jin, M.; Wang, Q.; Qin, H.; et al. Chicoric acid ameliorate inflammation and oxidative stress in lipopolysaccharide and d-galactosamine induced acute liver injury. J. Cell. Mol. Med. 2020, 24, 3022–3033. [Google Scholar] [CrossRef]
- Zhou, R.J.; Zhao, Y.; Fan, K.; Xie, M.L. Protective effect of apigenin on d-galactosamine/lps-induced hepatocellular injury by increment of nrf-2 nucleus translocation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 929–936. [Google Scholar] [CrossRef]
- Lyu, Z.; Ji, X.; Chen, G.; An, B. Atractylodin ameliorates lipopolysaccharide and d-galactosamine-induced acute liver failure via the suppression of inflammation and oxidative stress. Int. Immunopharmacol. 2019, 72, 348–357. [Google Scholar] [CrossRef]
- Liu, T.G.; Sha, K.H.; Zhang, L.G.; Liu, X.X.; Yang, F.; Cheng, J.Y. Protective effects of alpinetin on lipopolysaccharide/d-galactosamine-induced liver injury through inhibiting inflammatory and oxidative responses. Microb. Pathog. 2019, 126, 239–244. [Google Scholar] [CrossRef]
- Thomes, P.G.; Osna, N.A.; Bligh, S.M.; Tuma, D.J.; Kharbanda, K.K. Role of defective methylation reactions in ethanol-induced dysregulation of intestinal barrier integrity. Biochem. Pharmacol. 2015, 96, 30–38. [Google Scholar] [CrossRef]
- Thomes, P.G.; Bligh, S.M.; Kharbanda, K.K. Multiple roles of betaine against alcohol-induced liver injury. In Betaine, Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; Royal Society of Chemistry: London, UK, 2015; pp. 285–310. [Google Scholar]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasineni, K.; Lee, S.M.L.; McVicker, B.L.; Osna, N.A.; Casey, C.A.; Kharbanda, K.K. Susceptibility of Asialoglycoprotein Receptor-Deficient Mice to LPS/Galactosamine Liver Injury and Protection by Betaine Administration. Biology 2021, 10, 19. https://doi.org/10.3390/biology10010019
Rasineni K, Lee SML, McVicker BL, Osna NA, Casey CA, Kharbanda KK. Susceptibility of Asialoglycoprotein Receptor-Deficient Mice to LPS/Galactosamine Liver Injury and Protection by Betaine Administration. Biology. 2021; 10(1):19. https://doi.org/10.3390/biology10010019
Chicago/Turabian StyleRasineni, Karuna, Serene M. L. Lee, Benita L. McVicker, Natalia A. Osna, Carol A. Casey, and Kusum K. Kharbanda. 2021. "Susceptibility of Asialoglycoprotein Receptor-Deficient Mice to LPS/Galactosamine Liver Injury and Protection by Betaine Administration" Biology 10, no. 1: 19. https://doi.org/10.3390/biology10010019
APA StyleRasineni, K., Lee, S. M. L., McVicker, B. L., Osna, N. A., Casey, C. A., & Kharbanda, K. K. (2021). Susceptibility of Asialoglycoprotein Receptor-Deficient Mice to LPS/Galactosamine Liver Injury and Protection by Betaine Administration. Biology, 10(1), 19. https://doi.org/10.3390/biology10010019







