BODIPY Dyes: A New Frontier in Cellular Imaging and Theragnostic Applications
Abstract
1. Introduction
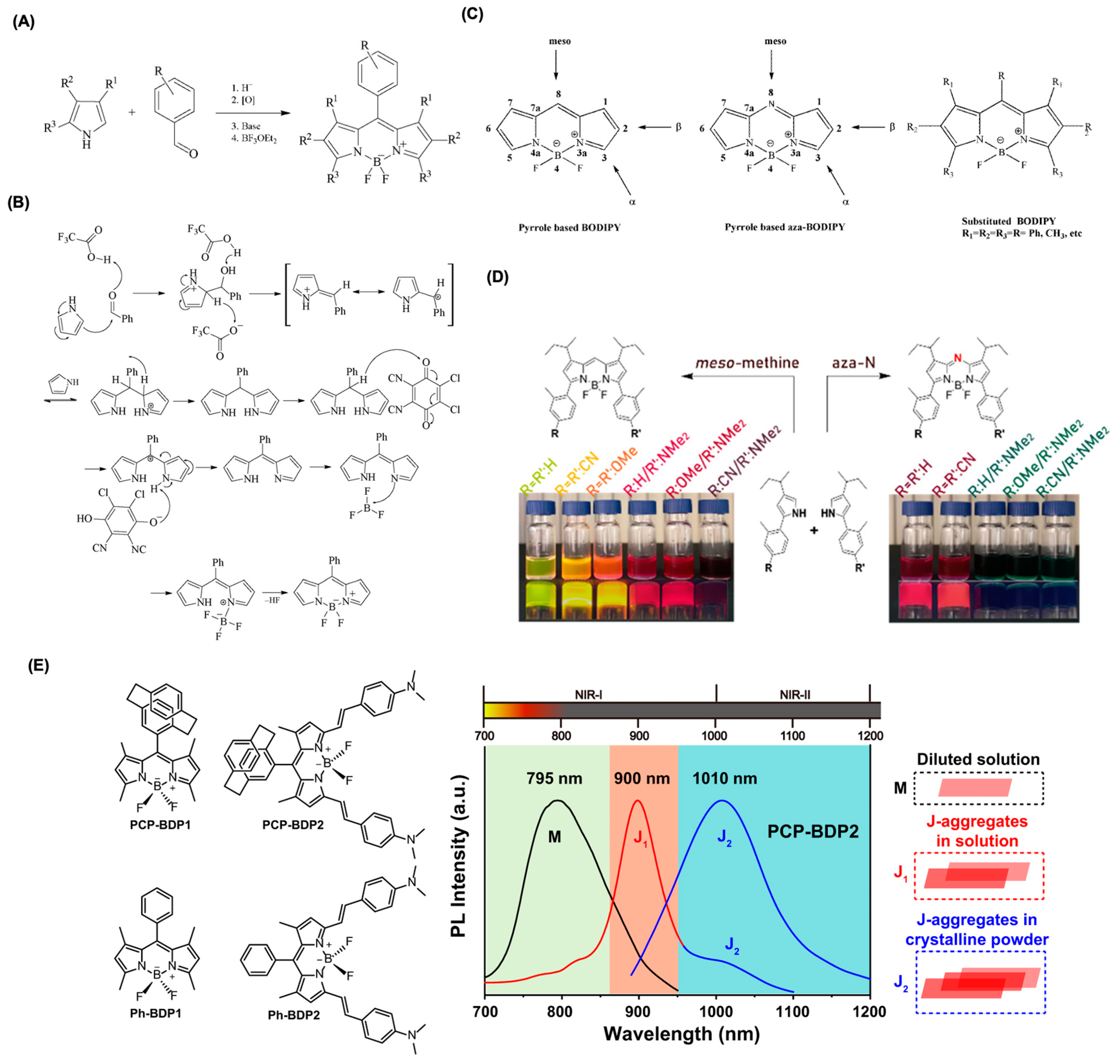
2. Mechanisms of Intracellular Distribution of BODIPY Dyes
2.1. Passive Diffusion
2.2. Endocytosis
2.3. Specific Interactions with Cellular Components
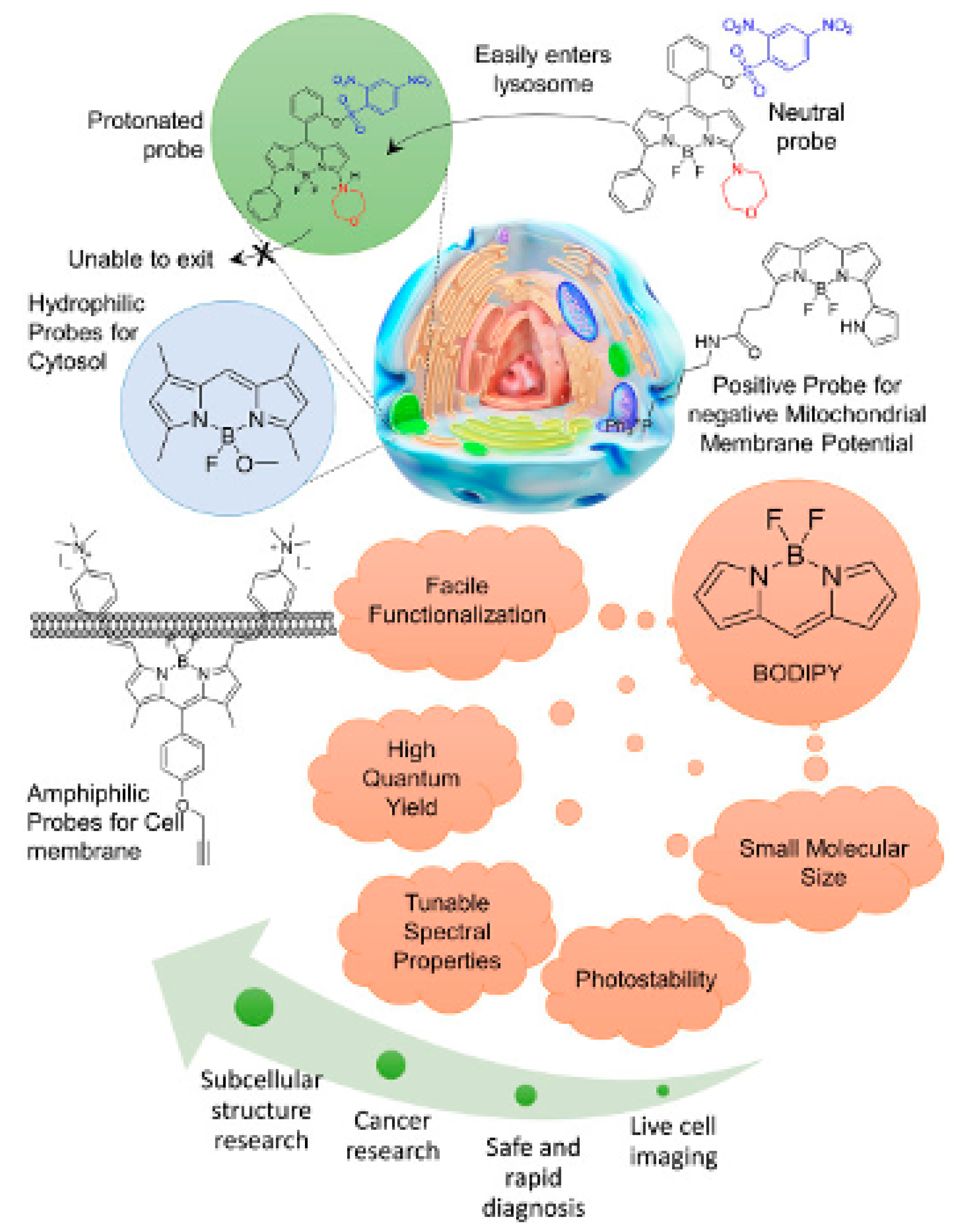
3. Intracellular Distribution of BODIPY Dyes
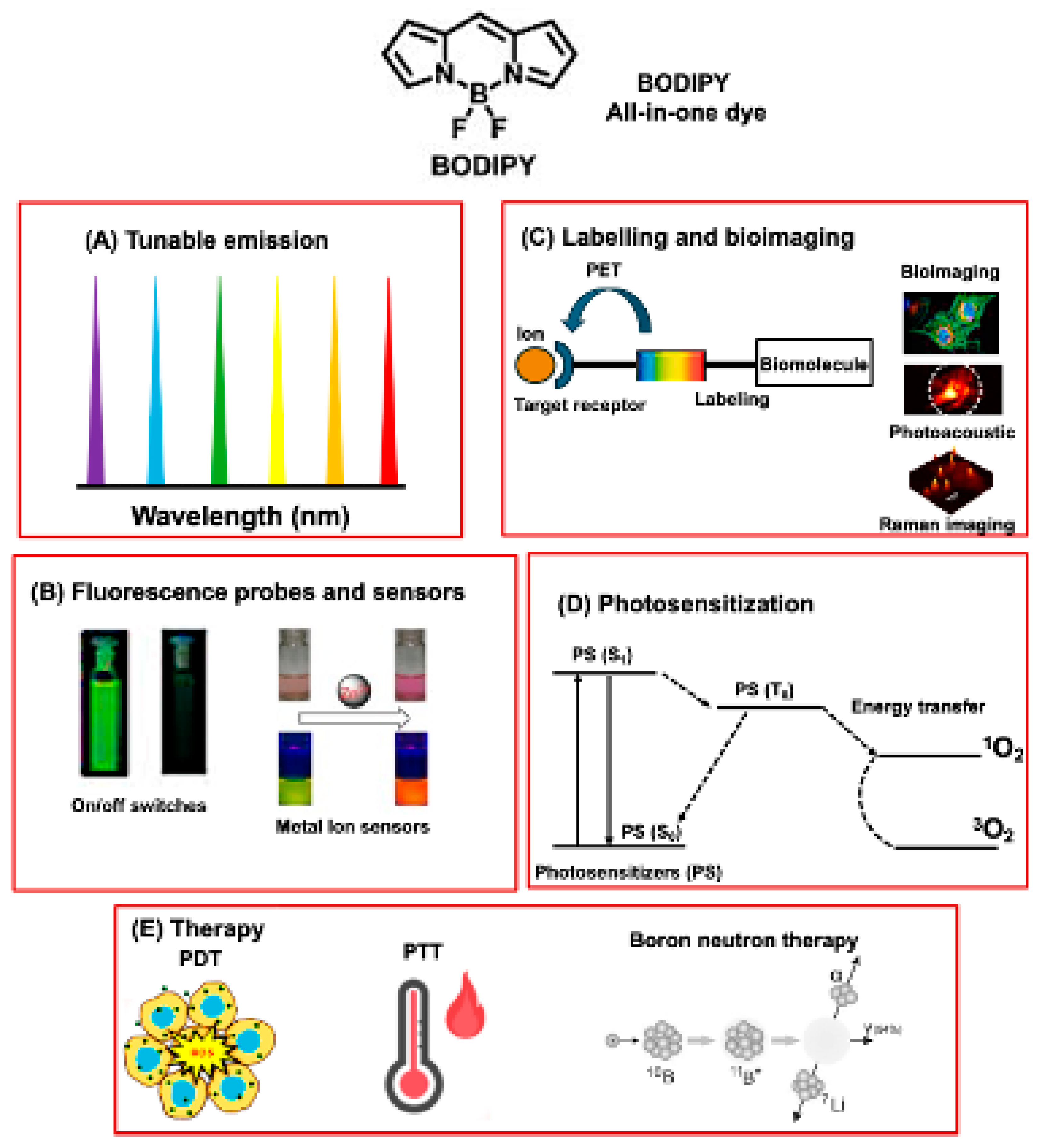
4. BODIPY for Bioimaging Applications
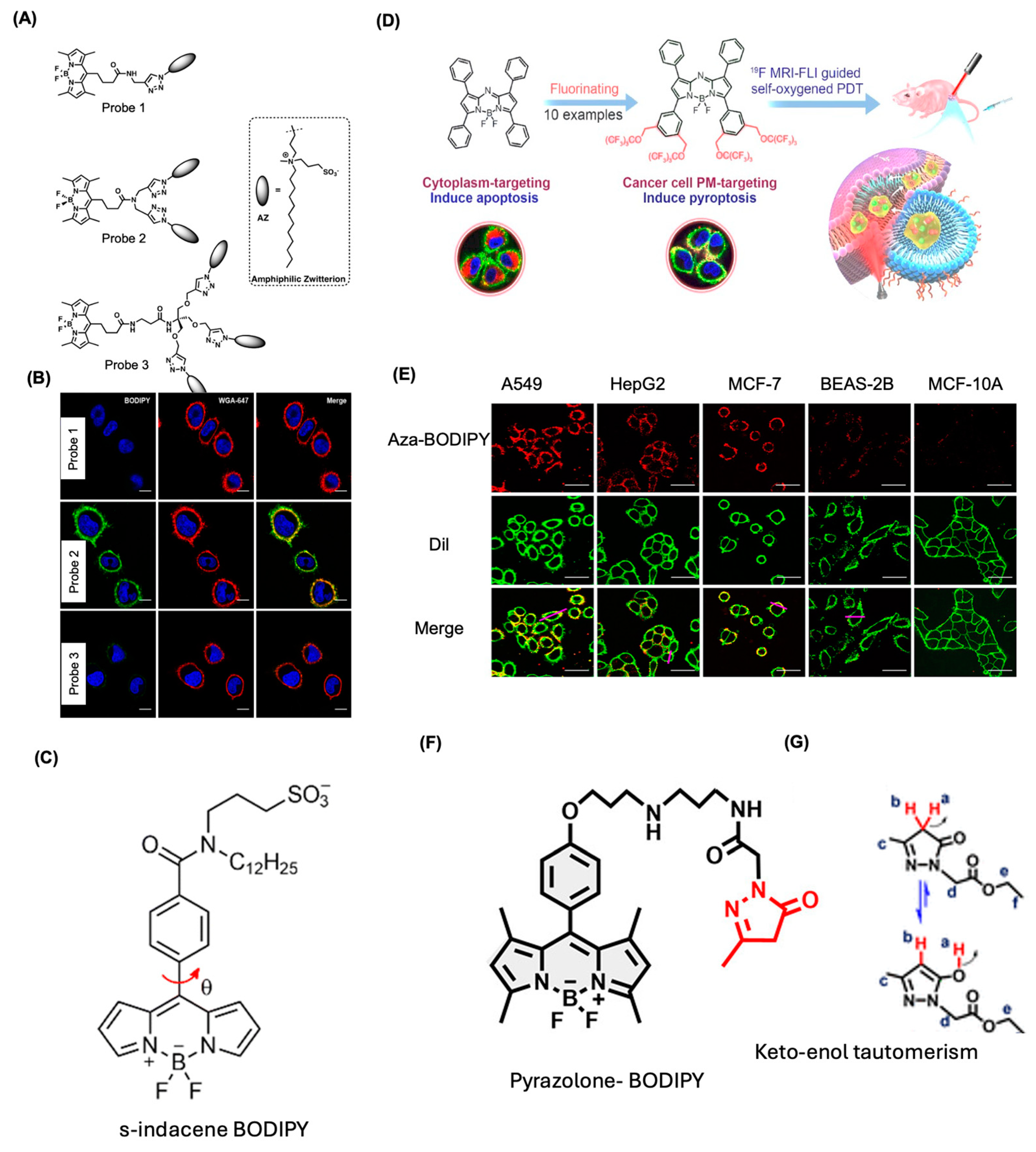
4.1. BODIPY Dyes for Membrane Imaging
4.2. BODIPY Dyes for Cytosol Imaging
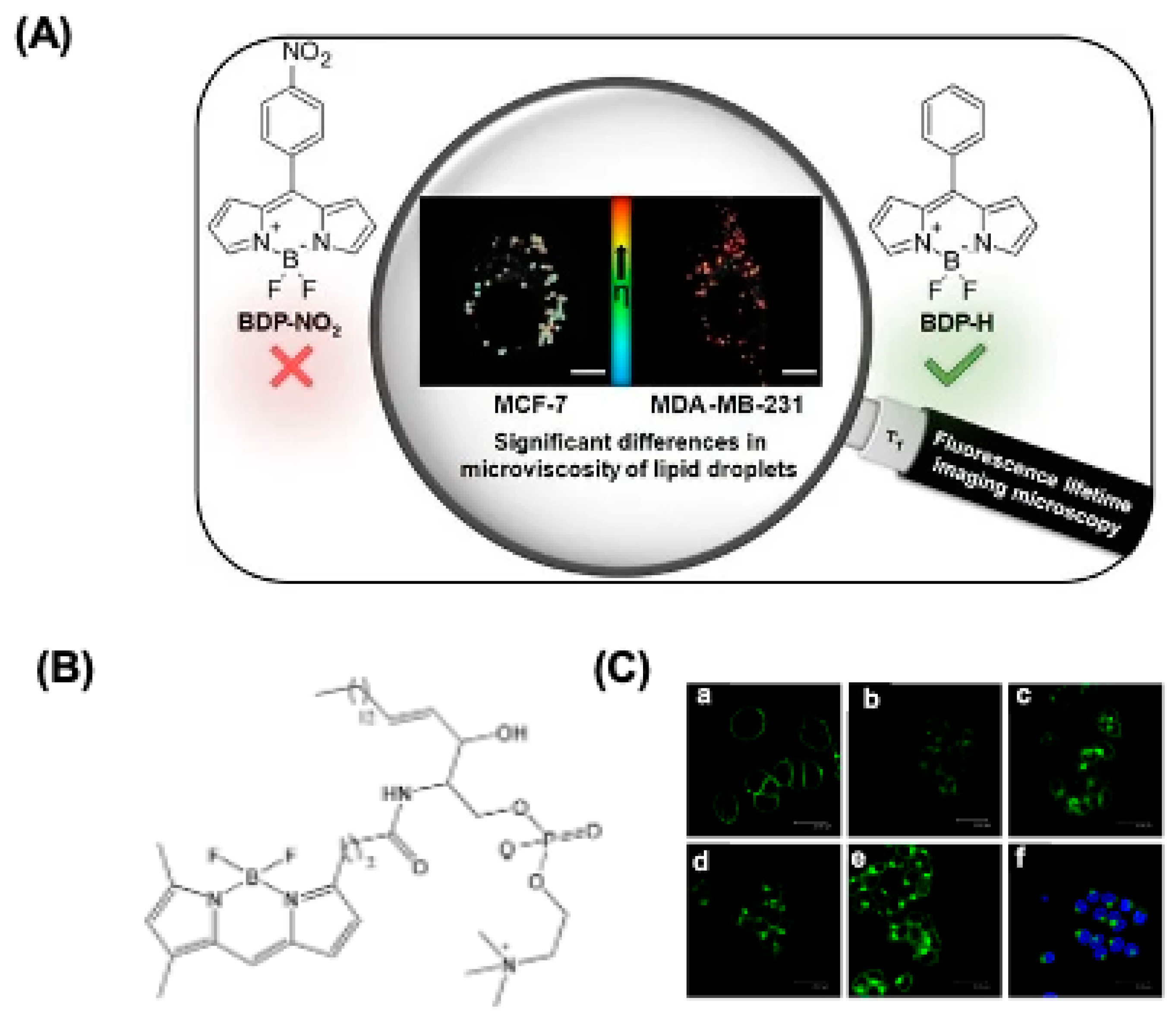
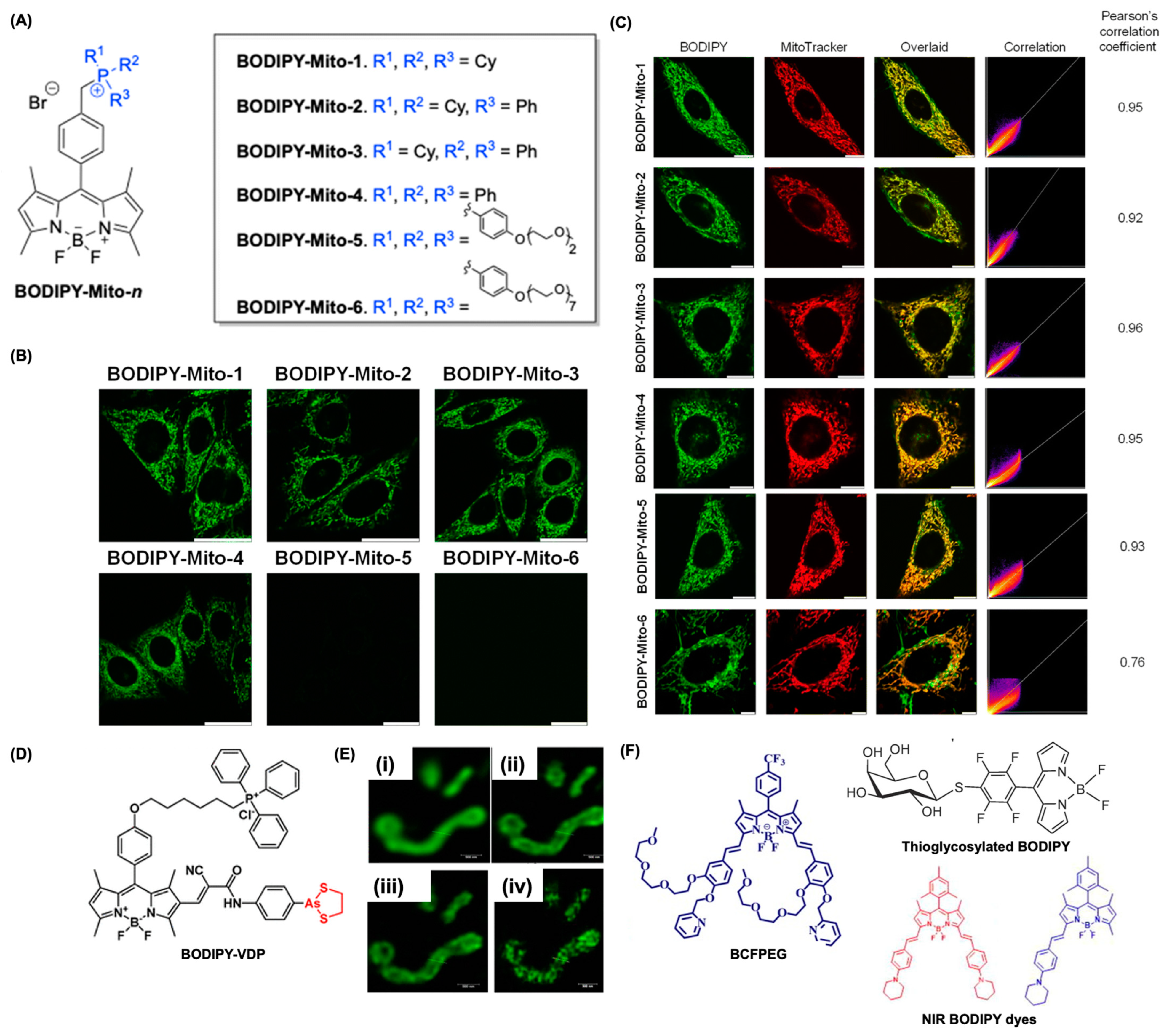
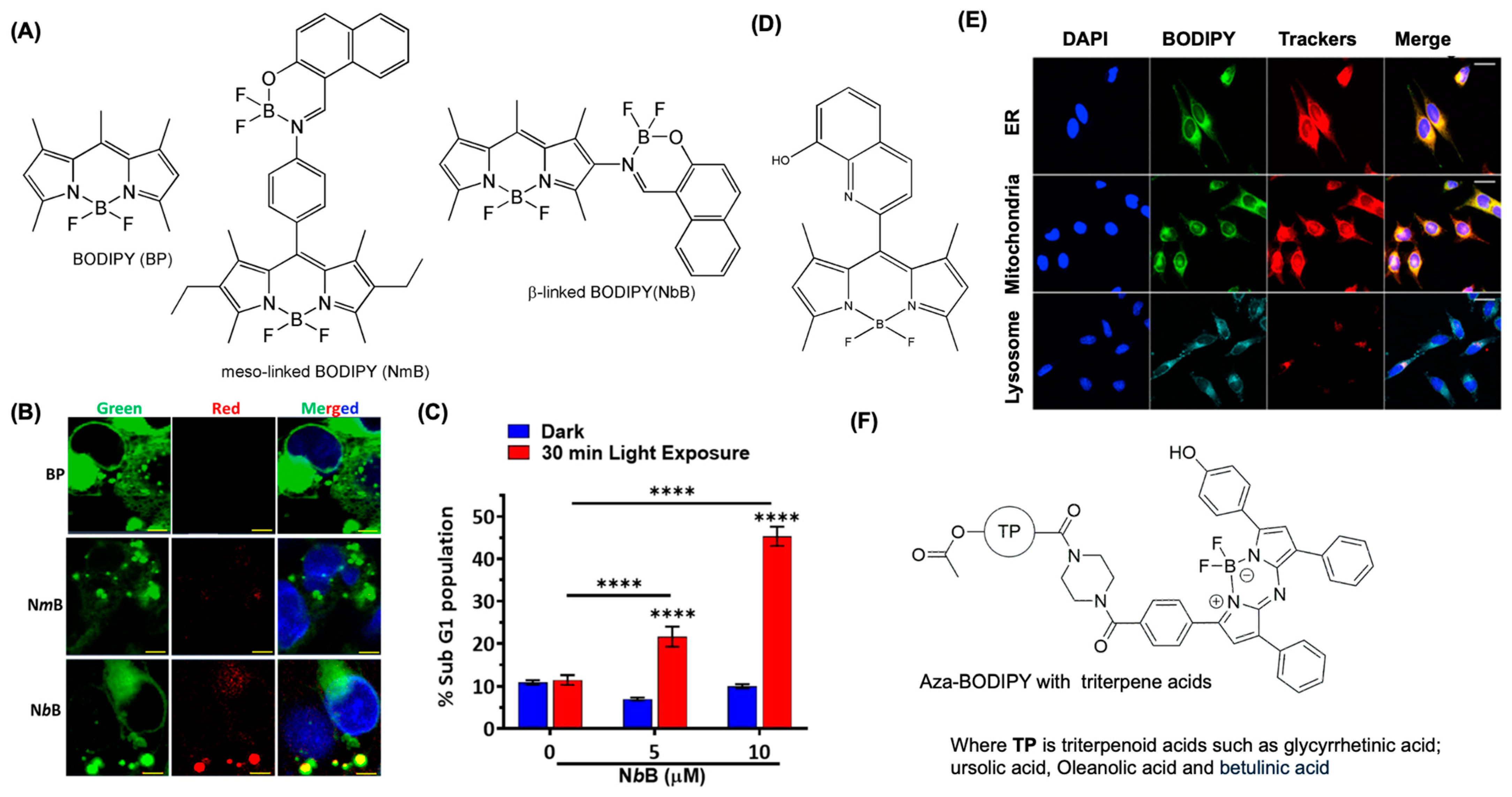
4.3. BODIPY for Mitochondria Imaging
4.4. BODIPY Dyes for Endoplasmic Reticulum Imaging
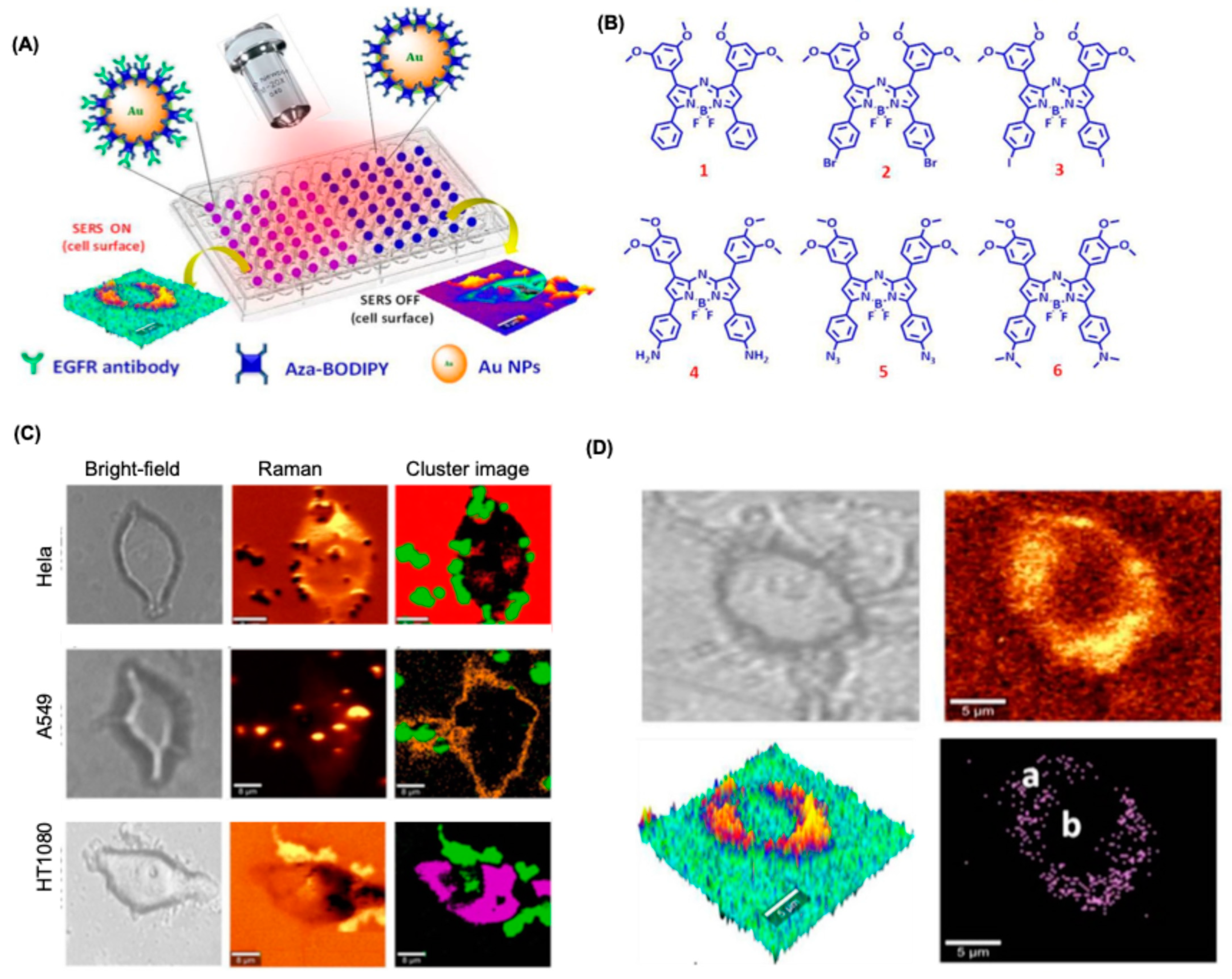
4.5. BODIPY Dyes for SERS-Based Bioimaging
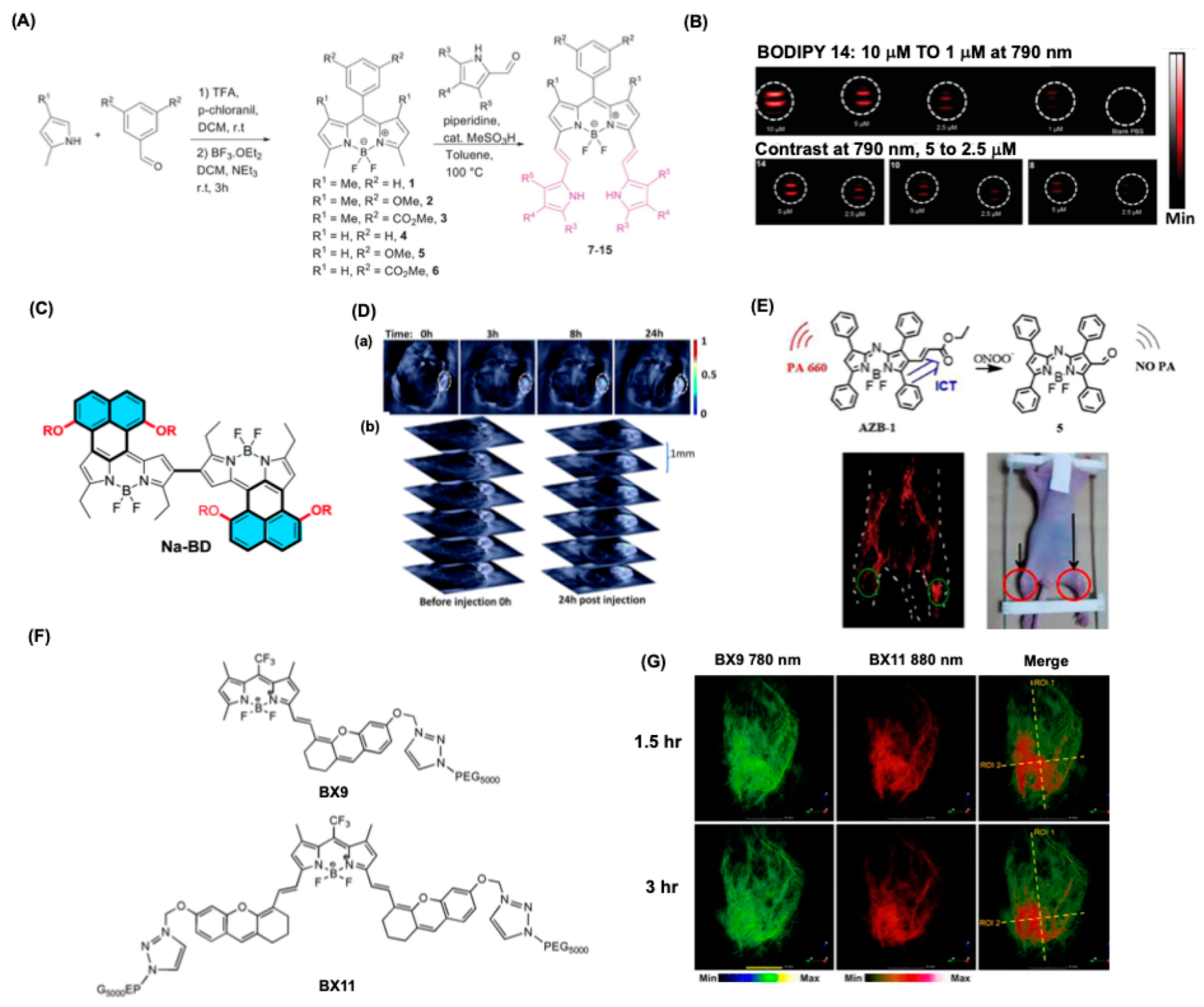
4.6. BODIPY Dyes for Photoacoustic-Based Bioimaging
5. Therapeutical Application of BODIPY
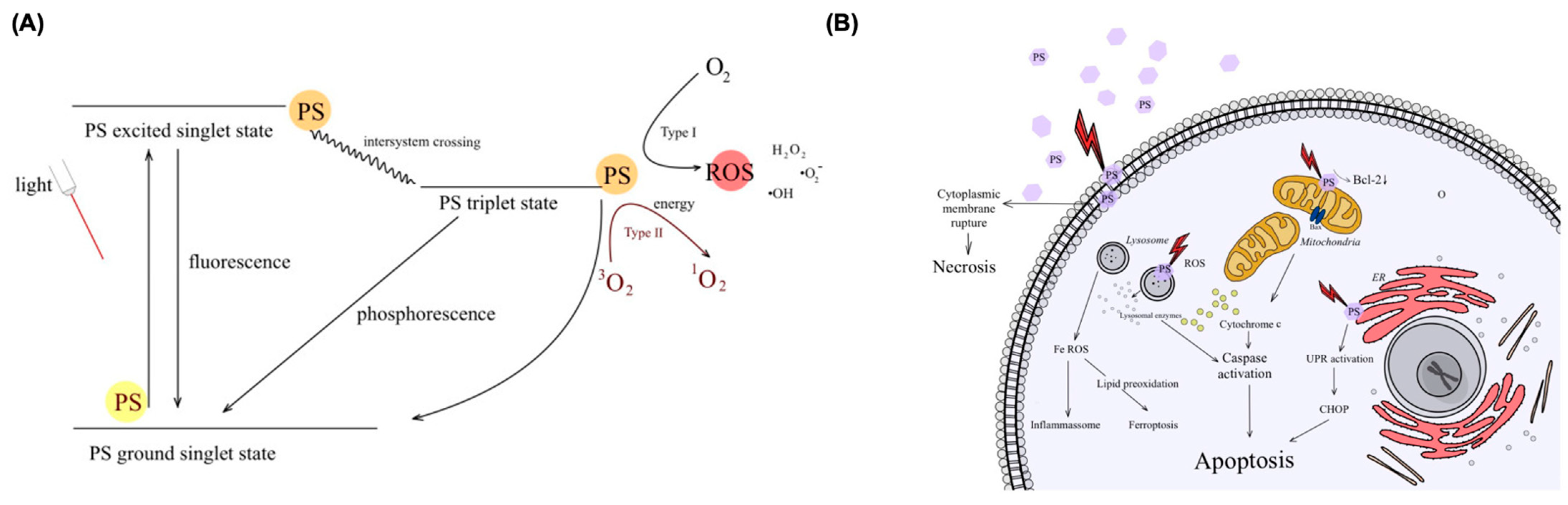
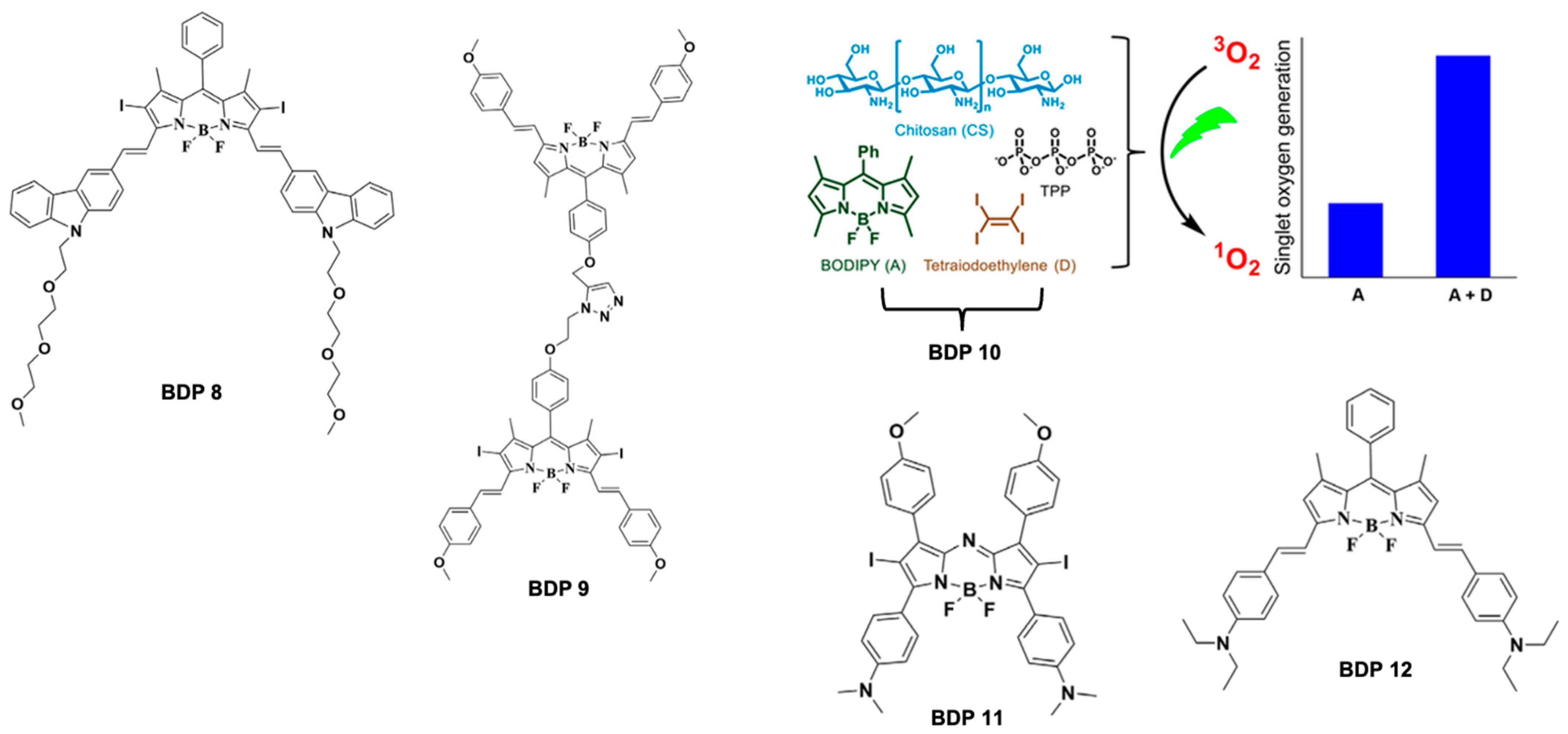
5.1. BODIPY Dyes in Photodynamic Therapy (PDT)
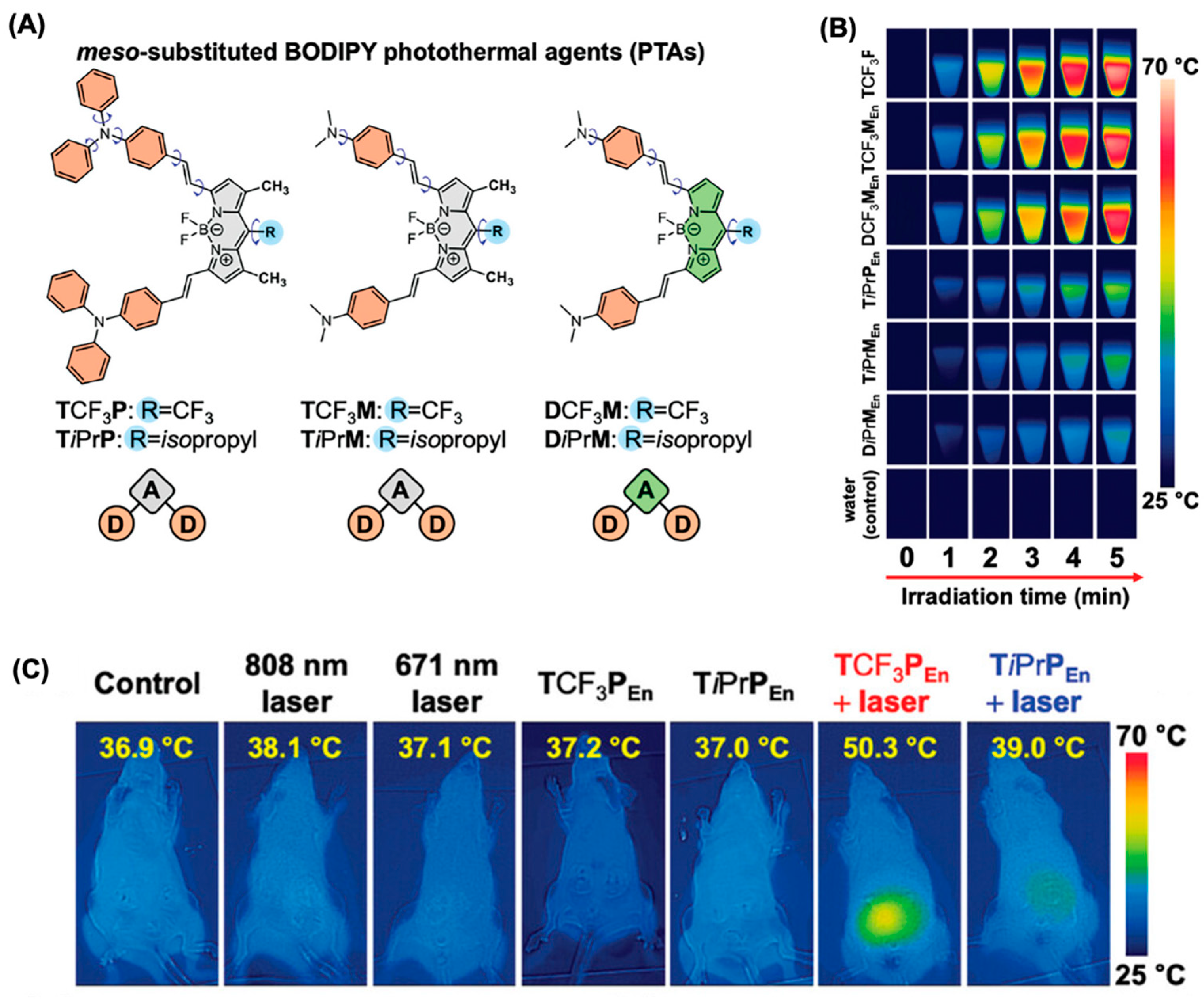
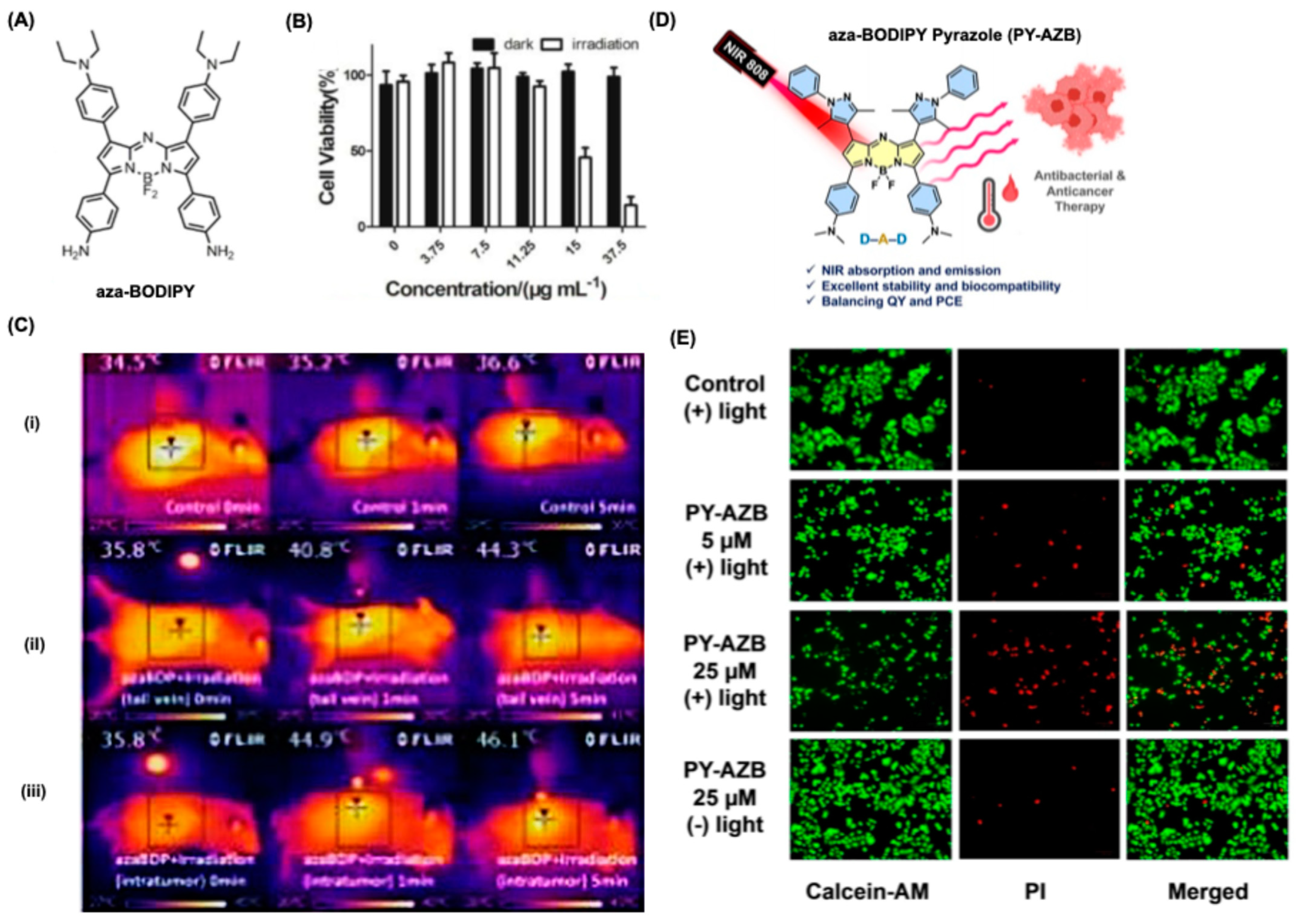
5.2. BODIPY Dyes in Photothermal Therapy (PTT)
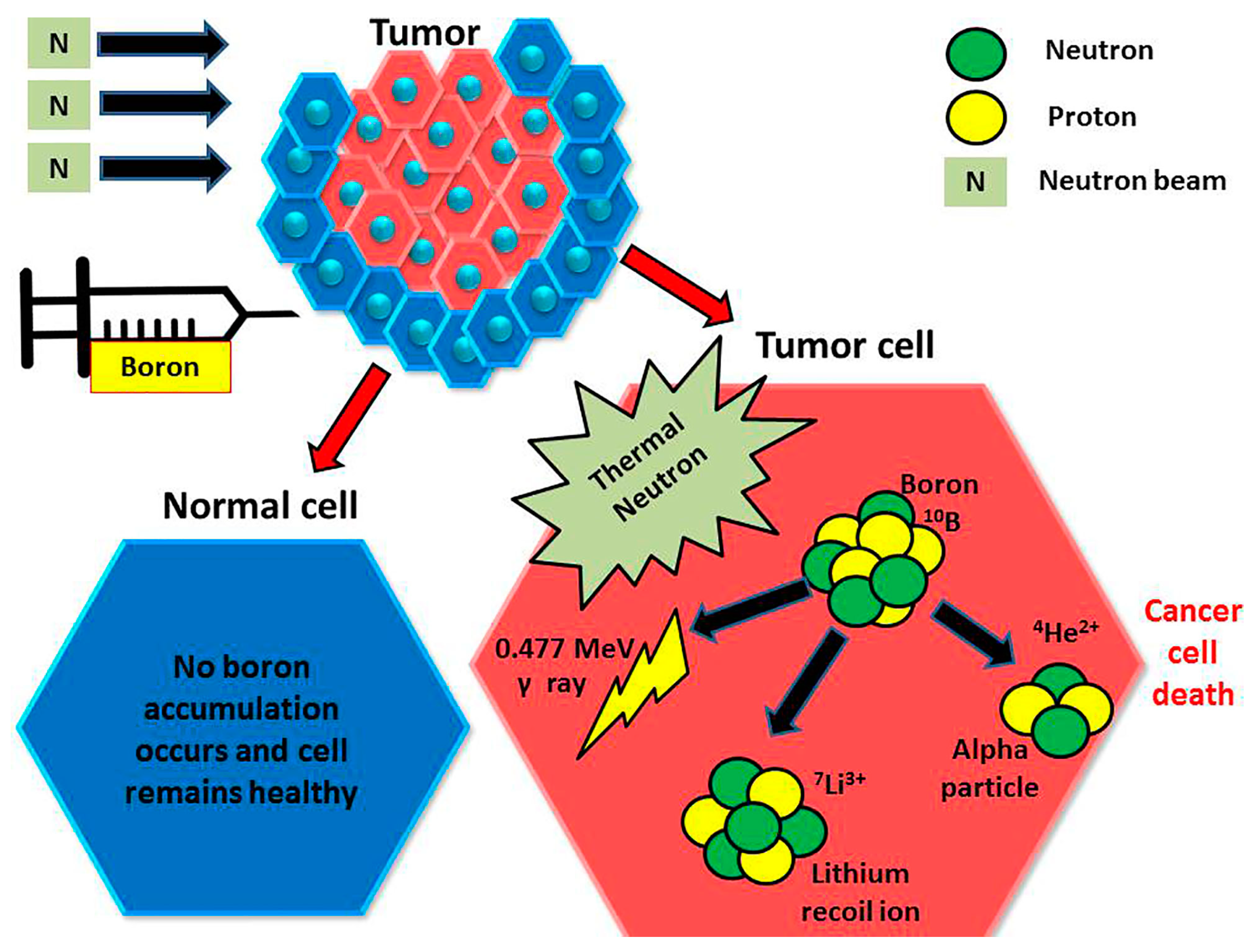
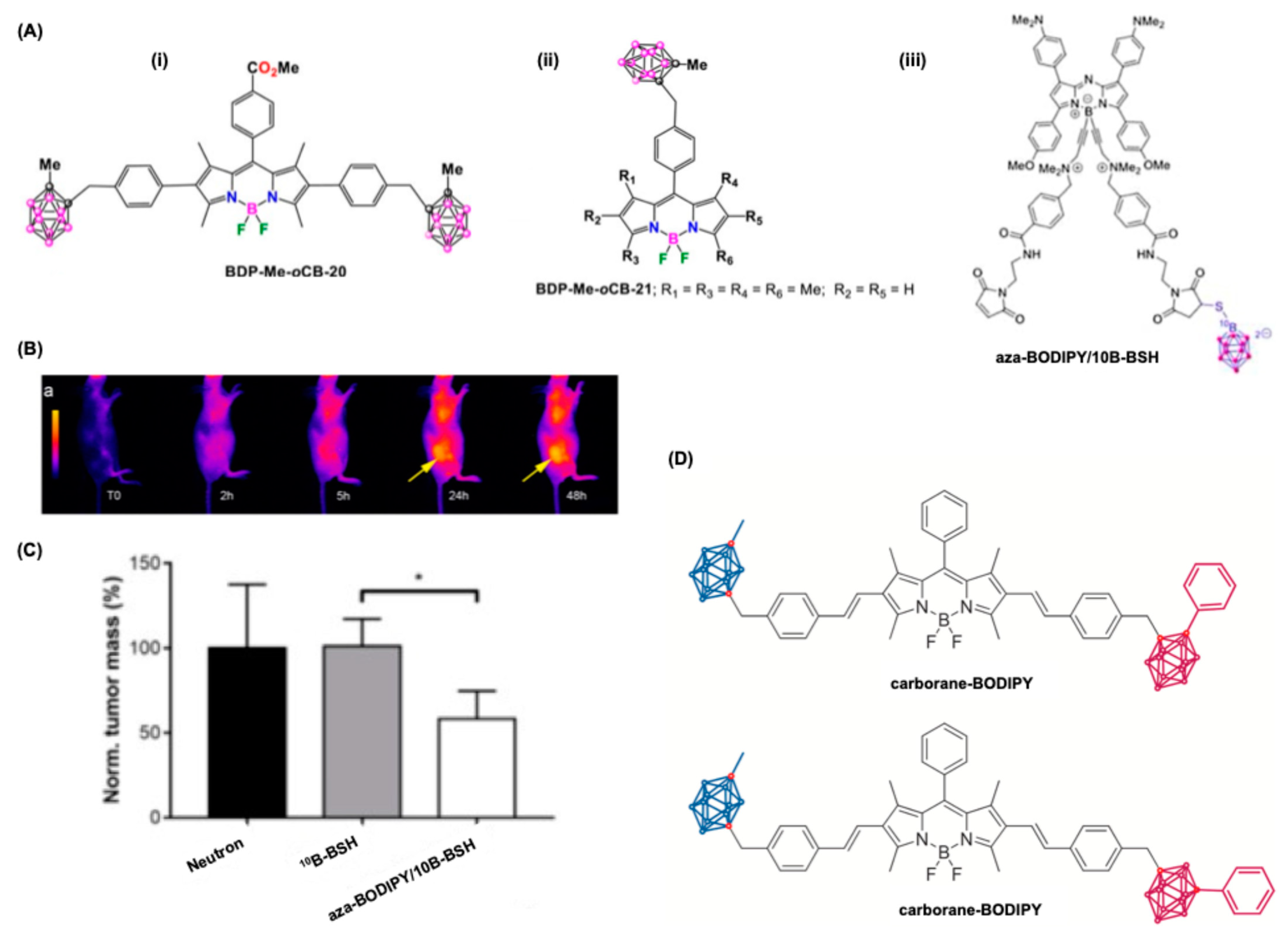
5.3. BODIPY Dyes in Boron Neutron Capture Therapy (BNCT)
6. BODIPY Based Nanomedicines for Theragnostic Applications
6.1. Self-Assembled BDPNPs for Bioimaging and Therapeutical Applications

6.2. Physical Encapsulation of BODIPY to Form BDNPs
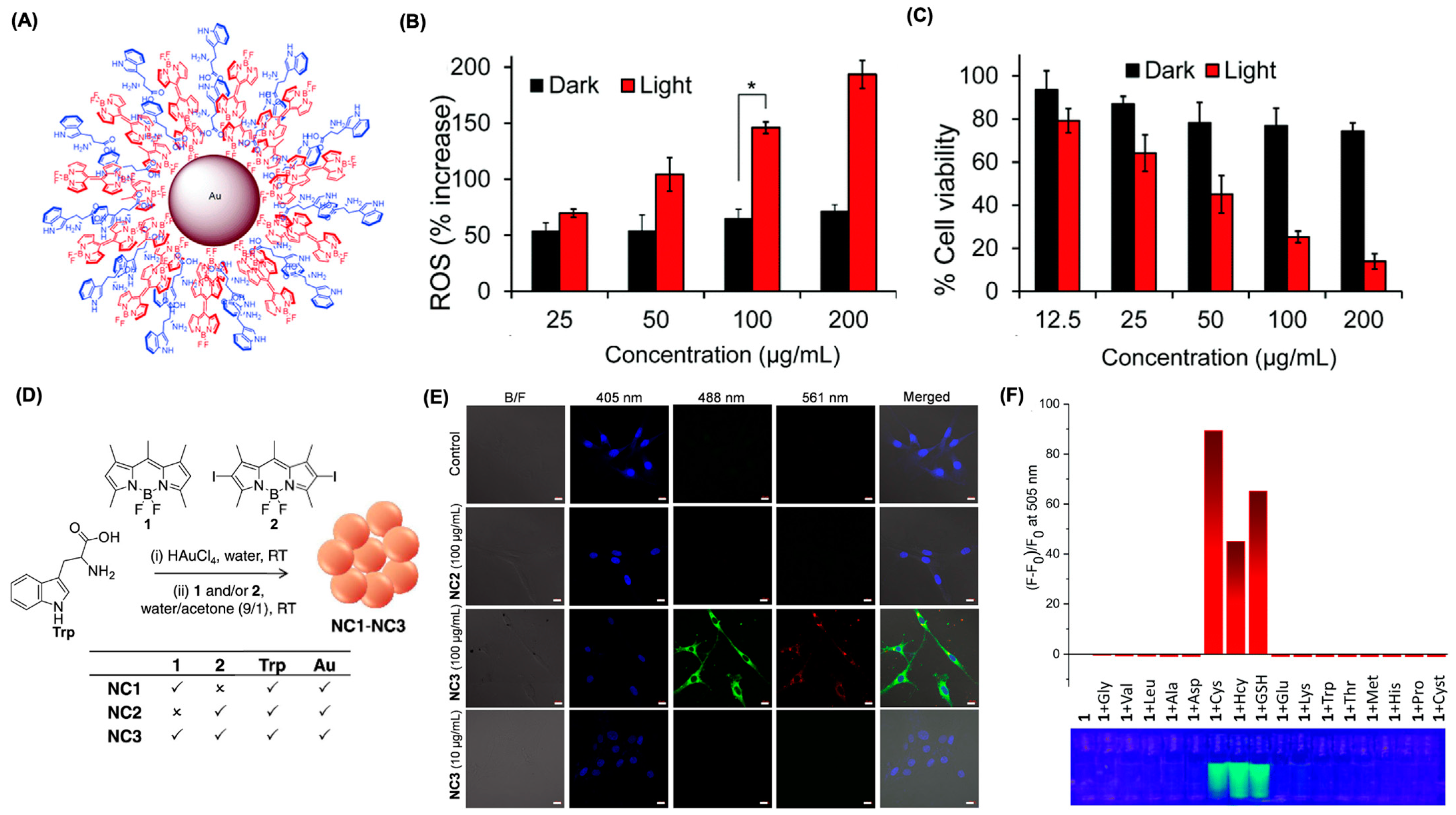
6.3. Supramolecular Approach Using Metallic Nanoparticles
7. Summary and Conclusions
8. Future Outlook for BODIPY Dyes and Nanoparticles in Cancer Imaging and Therapy
8.1. Enhanced Targeting and Delivery Mechanisms
8.2. Integration of Theranostic Applications
8.3. Innovations in Photodynamic Therapy (PDT)
8.4. Development of Novel BODIPY Derivatives
8.5. Utilization of BODIPY in Combination with Nanotechnology
8.6. Addressing Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Samanta, S.; Lai, K.; Wu, F.; Liu, Y.; Cai, S.; Yang, X.; Qu, J.; Yang, Z. Xanthene, Cyanine, Oxazine and BODIPY: The Four Pillars of the Fluorophore Empire for Super-Resolution Bioimaging. Chem. Soc. Rev. 2023, 52, 7197–7261. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural Modification Strategies for the Rational Design of Red/NIR Region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [PubMed]
- Treibs, A.; Kreuzer, F. Difluorboryl-Komplexe von Di- Und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Krasnopyorov, A.I.; Larkina, E.A. Features of F2-BODIPY Synthesis (A Review). Russ. J. Org. Chem. 2024, 60, 789–805. [Google Scholar] [CrossRef]
- Das, S.; Dey, S.; Patra, S.; Bera, A.; Ghosh, T.; Prasad, B.; Sayala, K.D.; Maji, K.; Bedi, A.; Debnath, S. BODIPY-Based Molecules for Biomedical Applications. Biomolecules 2023, 13, 1723. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent Advances in the Application of BODIPY in Bioimaging and Chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Ahmad, H.; Muhammad, S.; Mazhar, M.; Farhan, A.; Iqbal, M.S.; Hiria, H.; Yu, C.; Zhang, Y.; Guo, B. Unveiling Cellular Mysteries: Advances in BODIPY Dyes for Subcellular Imaging. Coord. Chem. Rev. 2025, 526, 216383. [Google Scholar] [CrossRef]
- Yadav, I.S.; Misra, R. Design, Synthesis and Functionalization of BODIPY Dyes: Applications in Dye-Sensitized Solar Cells (DSSCs) and Photodynamic Therapy (PDT). J. Mater. Chem. C 2023, 11, 8688–8723. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Schäfer, C.; Mony, J.; Olsson, T.; Börjesson, K. Effect of the Aza-N-Bridge and Push–Pull Moieties: A Comparative Study between BODIPYs and Aza-BODIPYs. J. Org. Chem. 2022, 87, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Han, X.; Hu, W.; Bai, H.; Peng, B.; Ji, L.; Fan, Q.; Li, L.; Huang, W. Bioapplications of Small Molecule Aza-BODIPY: From Rational Structural Design to in Vivo Investigations. Chem. Soc. Rev. 2020, 49, 7533–7567. [Google Scholar] [CrossRef]
- Dutta, D.; Nair, R.R.; Mangalath, S.; Nair, S.A.; Joseph, J.; Gogoi, P.; Ramaiah, D. Biocompatible Aza-BODIPY-Biotin Conjugates for Photodynamic Therapy of Cancer. ACS Omega 2023, 8, 26180–26190. [Google Scholar] [CrossRef]
- Elgun, T.; Yurttas, A.G.; Cinar, K.; Ozcelik, S.; Gul, A. Effect of Aza-BODIPY-Photodynamic Therapy on the Expression of Carcinoma-Associated Genes and Cell Death Mode. Photodiagnosis Photodyn. Ther. 2023, 44, 103849. [Google Scholar] [CrossRef]
- Hlogyik, T.; Laczkó-Rigó, R.; Bakos, É.; Poór, M.; Kele, Z.; Özvegy-Laczka, C.; Mernyák, E. Synthesis and in Vitro Photodynamic Activity of Aza-BODIPY-Based Photosensitizers. Org. Biomol. Chem. 2023, 21, 6018–6027. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Duan, X.; Jiang, Z.; Ding, D.; Chen, Y.; Zhang, G.-Q.; Liu, Z. J-Aggregates of Meso-[2.2]Paracyclophanyl-BODIPY Dye for NIR-II Imaging. Nat. Commun. 2021, 12, 2376. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Fang, Z.; Zhu, C.; Yang, Y.; Yang, Z.; Huang, W. Crucial Breakthrough of BODIPY-Based NIR-II Fluorescent Emitters for Advanced Biomedical Theranostics. Adv. Funct. Mater. 2024, 34, 2401325. [Google Scholar] [CrossRef]
- Bai, L.; Sun, P.; Liu, Y.; Zhang, H.; Hu, W.; Zhang, W.; Liu, Z.; Fan, Q.; Li, L.; Huang, W. Novel Aza-BODIPY Based Small Molecular NIR-II Fluorophores for in Vivo Imaging. Chem. Commun. 2019, 55, 10920–10923. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Tian, Y.; Sun, Q.; Sun, D.; Wang, F.; Xu, H.; Ying, G.; Wang, J.; Yetisen, A.K.; et al. Near-Infrared-II Nanoparticles for Cancer Imaging of Immune Checkpoint Programmed Death-Ligand 1 and Photodynamic/Immune Therapy. ACS Nano 2021, 15, 515–525. [Google Scholar] [CrossRef]
- Yang, N.; Song, S.; Liu, C.; Ren, J.; Wang, X.; Zhu, S.; Yu, C. An Aza-BODIPY-Based NIR-II Luminogen Enables Efficient Phototheranostics. Biomater. Sci. 2022, 10, 4815–4821. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, H.; Cheng, Q.; Dang, H.; Qian, H.; Teng, C.; Xie, K.; Yan, L. Stable Twisted Conformation Aza-BODIPY NIR-II Fluorescent Nanoparticles with Ultra-Large Stokes Shift for Imaging-Guided Phototherapy. J. Mater. Chem. B 2022, 10, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zheng, X.; Liu, W.; Li, J.; Gao, Z.; Ren, H.; Zhang, W.; Lee, C.-S.; Wang, P. Pyrrolopyrrole Aza-BODIPY-Based NIR-II Fluorophores for in Vivo Dynamic Vascular Dysfunction Visualization of Vascular-Targeted Photodynamic Therapy. Biomaterials 2023, 298, 122130. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yu, Y.; Zhang, J.; Dong, X.; Liu, C.; Xiao, H. Self-Sacrificially Degradable Pseudo-Semiconducting Polymer Nanoparticles That Integrate NIR-II Fluorescence Bioimaging, Photodynamic Immunotherapy, and Photo-Activated Chemotherapy. Adv. Mater. 2022, 34, 2203820. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, X.; Tang, D.; Liu, T.; Xiao, H. Biodegradable NIR-II Pseudo Conjugate Polymeric Nanoparticles Amplify Photodynamic Immunotherapy via Alleviation of Tumor Hypoxia and Tumor-Associated Macrophage Reprogramming. Adv. Mater. 2023, 35, 2209799. [Google Scholar] [CrossRef]
- Bu, F.; Kang, X.; Tang, D.; Liu, F.; Chen, L.; Zhang, P.; Feng, W.; Yu, Y.; Li, G.; Xiao, H.; et al. Enhancing Near-Infrared II Photodynamic Therapy with Nitric Oxide for Eradicating Multidrug-Resistant Biofilms in Deep Tissues. Bioact. Mater. 2024, 33, 341–354. [Google Scholar] [CrossRef]
- Tian, Y.; Yin, D.; Cheng, Q.; Dang, H.; Teng, C.; Yan, L. Supramolecular J-Aggregates of Aza-BODIPY by Steric and π–π Nteractions for NIR-II Phototheranostics. J. Mater. Chem. B 2022, 10, 1650–1662. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, P.; Liu, S.; Yang, J.; Wu, F.; Cao, W.; Yang, Y.; Zheng, B.; Xiong, H. Electron-Withdrawing Substituents Allow Boosted NIR-II Fluorescence in J-Type Aggregates for Bioimaging and Information Encryption. Angew. Chem. Int. Ed. 2023, 62, e202313166. [Google Scholar] [CrossRef]
- Dang, H.; Yin, D.; Tian, Y.; Cheng, Q.; Teng, C.; Xu, Y.; Yan, L. In Situ Formation of J-Aggregate in the Tumor Microenvironment Using Acidity Responsive Polypeptide Nanoparticle Encapsulating Galactose-Conjugated BODIPY Dye for NIR-II Phototheranostics. J. Mater. Chem. B 2022, 10, 5279–5290. [Google Scholar] [CrossRef]
- Li, F.-Z.; Wu, Z.; Lin, C.; Wang, Q.; Kuang, G.-C. Photophysical Properties Regulation and Applications of BODIPY-Based Derivatives with Electron Donor-Acceptor System. Results Chem. 2022, 4, 100384. [Google Scholar] [CrossRef]
- Bumagina, N.A.; Antina, E.V. Review of Advances in Development of Fluorescent BODIPY Probes (Chemosensors and Chemodosimeters) for Cation Recognition. Coord. Chem. Rev. 2024, 505, 215688. [Google Scholar] [CrossRef]
- Mao, Z.; Kim, J.H.; Lee, J.; Xiong, H.; Zhang, F.; Kim, J.S. Engineering of BODIPY-Based Theranostics for Cancer Therapy. Coord. Chem. Rev. 2023, 476, 214908. [Google Scholar] [CrossRef]
- Kim, G.; Luo, Y.; Shin, M.; Bouffard, J.; Bae, J.; Kim, Y. Making the Brightest Ones Dim: Maximizing the Photothermal Conversion Efficiency of BODIPY-Based Photothermal Agents. Adv. Healthc. Mater. 2024, 13, 2400885. [Google Scholar] [CrossRef] [PubMed]
- Koczorowski, T.; Glowacka-Sobotta, A.; Sysak, S.; Mlynarczyk, D.T.; Lesyk, R.; Goslinski, T.; Sobotta, L. BODIPY-Based Nanomaterials—Sensing and Biomedical Applications. Appl. Sci. 2022, 12, 7815. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, T.; Dong, X.; Mou, X. BODIPY Based Nanomedicine for Cancer Imaging and Phototherapy. Colloid. Interface Sci. Commun. 2025, 64, 100816. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, N.K.; Kikuchi, K.; Kaur, A.; New, E.J. BODIPY-based Fluorescent Indicators for Lipid Droplets. Anal. Sens. 2024, 4, e202300049. [Google Scholar] [CrossRef]
- Fan, W.; Li, X. Using BODIPY FL-Sphingolipid Analogs to Study Sphingolipid Metabolism in Mouse Embryonic Stem Cells. Bio-Protocol 2022, 12, e4555. [Google Scholar] [CrossRef]
- Jurgutis, D.; Jarockyte, G.; Poderys, V.; Dodonova-Vaitkuniene, J.; Tumkevicius, S.; Vysniauskas, A.; Rotomskis, R.; Karabanovas, V. Exploring BODIPY-Based Sensor for Imaging of Intracellular Microviscosity in Human Breast Cancer Cells. IJMS 2022, 23, 5687. [Google Scholar] [CrossRef]
- Nusshold, C.; Uellen, A.; Bernhart, E.; Hammer, A.; Damm, S.; Wintersperger, A.; Reicher, H.; Hermetter, A.; Malle, E.; Sattler, W. Endocytosis and Intracellular Processing of BODIPY-Sphingomyelin by Murine CATH.a Neurons. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2013, 1831, 1665–1678. [Google Scholar] [CrossRef]
- Qiu, B.; Simon, M. BODIPY 493/503 Staining of Neutral Lipid Droplets for Microscopy and Quantification by Flow Cytometry. Bio-Protocol 2016, 6, e1912. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Brown, D.A. Fluorescent Detection of Lipid Droplets and Associated Proteins. CP Cell Biol. 2007, 35, 24.2.1–24.2.11. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Lin, Q.; Buccella, D. Highly Selective, Red Emitting BODIPY-Based Fluorescent Indicators for Intracellular Mg2+ Imaging. J. Mater. Chem. B 2018, 6, 7247–7256. [Google Scholar] [CrossRef] [PubMed]
- Kand, D.; Liu, P.; Navarro, M.X.; Fischer, L.J.; Rousso-Noori, L.; Friedmann-Morvinski, D.; Winter, A.H.; Miller, E.W.; Weinstain, R. Water-Soluble BODIPY Photocages with Tunable Cellular Localization. J. Am. Chem. Soc. 2020, 142, 4970–4974. [Google Scholar] [CrossRef]
- Spangenburg, E.E.; Pratt, S.J.P.; Wohlers, L.M.; Lovering, R.M. Use of BODIPY (493/503) to Visualize Intramuscular Lipid Dropletsin Skeletal Muscle. BioMed Res. Int. 2011, 2011, 598358. [Google Scholar] [CrossRef]
- Miao, W.; Yu, C.; Hao, E.; Jiao, L. Functionalized BODIPYs as Fluorescent Molecular Rotors for Viscosity Detection. Front. Chem. 2019, 7, 825. [Google Scholar] [CrossRef]
- Ordóñez-Hernández, J.; Ferrusca-Martínez, F.; Jiménez-Sánchez, A. BODIPY-Derived Fluorescent Probes for Targeting and Tracking Lipid Droplets Dynamics. Tetrahedron 2024, 168, 134354. [Google Scholar] [CrossRef]
- Tatenaka, Y.; Kato, H.; Ishiyama, M.; Sasamoto, K.; Shiga, M.; Nishitoh, H.; Ueno, Y. Monitoring Lipid Droplet Dynamics in Living Cells by Using Fluorescent Probes. Biochemistry 2019, 58, 499–503. [Google Scholar] [CrossRef]
- Chen, J.; Yue, F.; Kuang, S. Labeling and Analyzing Lipid Droplets in Mouse Muscle Stem Cells. STAR Protoc. 2022, 3, 101849. [Google Scholar] [CrossRef]
- Walter, E.R.H.; Lee, L.C.-C.; Leung, P.K.-K.; Lo, K.K.-W.; Long, N.J. Mitochondria-Targeting Biocompatible Fluorescent BODIPY Probes. Chem. Sci. 2024, 15, 4846–4852. [Google Scholar] [CrossRef]
- Polita, A.; Stancikaitė, M.; Žvirblis, R.; Maleckaitė, K.; Dodonova-Vaitkūnienė, J.; Tumkevičius, S.; Shivabalan, A.P.; Valinčius, G. Designing a Green-Emitting Viscosity-Sensitive 4,4-Difluoro-4-Bora-3a,4a-Diaza-s-Indacene (BODIPY) Probe for Plasma Membrane Viscosity Imaging. RSC Adv. 2023, 13, 19257–19264. [Google Scholar] [CrossRef]
- Collot, M.; Boutant, E.; Lehmann, M.; Klymchenko, A.S. BODIPY with Tuned Amphiphilicity as a Fluorogenic Plasma Membrane Probe. Bioconjugate Chem. 2019, 30, 192–199. [Google Scholar] [CrossRef]
- Li, A.; Wang, F.; Li, Y.; Peng, X.; Liu, Y.; Zhu, L.; He, P.; Yu, T.; Chen, D.; Duan, M.; et al. Fluorination of Aza-BODIPY for Cancer Cell Plasma Membrane-Targeted Imaging and Therapy. ACS Appl. Mater. Interfaces 2025, 17, acsami.4c17943. [Google Scholar] [CrossRef]
- Xiong, T.; Chen, Y.; Peng, Q.; Li, M.; Lu, S.; Chen, X.; Fan, J.; Wang, L.; Peng, X. Pyrazolone-Protein Interaction Enables Long-Term Retention Staining and Facile Artificial Biorecognition on Cell Membranes. J. Am. Chem. Soc. 2024, 146, 24158–24166. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.N.; D’Avila, H.; Wan, H.-C.; Bozza, P.T.; Dvorak, A.M.; Weller, P.F. Lipid Bodies in Inflammatory Cells: Structure, Function, and Current Imaging Techniques. J. Histochem. Cytochem. 2011, 59, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Courtis, A.M.; Santos, S.A.; Guan, Y.; Hendricks, J.A.; Ghosh, B.; Szantai-Kis, D.M.; Reis, S.A.; Shah, J.V.; Mazitschek, R. Monoalkoxy BODIPYs—A Fluorophore Class for Bioimaging. Bioconjugate Chem. 2014, 25, 1043–1051. [Google Scholar] [CrossRef]
- Ni, Y.; Zeng, L.; Kang, N.; Huang, K.; Wang, L.; Zeng, Z.; Chang, Y.; Wu, J. Meso -Ester and Carboxylic Acid Substituted BODIPYs with Far-Red and Near-Infrared Emission for Bioimaging Applications. Chem. A Eur. J. 2014, 20, 2301–2310. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Gao, R.; Yue, Y.; Sun, G.-T.; Zhao, W. A NIR BODIPY Dye Bearing 3,4,4a-Trihydroxanthene Moieties. Org. Biomol. Chem. 2012, 10, 6861. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, D.H.; Lee, H.; Shin, J.; Yan, W.; Rathore, B.; Kim, H.-R.; Kim, S.J.; Singh, H.; Liu, L.; et al. A Fluorescent Probe for Stimulated Emission Depletion Super-Resolution Imaging of Vicinal-Dithiol-Proteins on Mitochondrial Membrane. Bioconjugate Chem. 2018, 29, 1446–1453. [Google Scholar] [CrossRef]
- Gayathri, T.; Karnewar, S.; Kotamraju, S.; Singh, S.P. High Affinity Neutral Bodipy Fluorophores for Mitochondrial Tracking. ACS Med. Chem. Lett. 2018, 9, 618–622. [Google Scholar] [CrossRef]
- Kesavan, P.E.; Pandey, V.; Raza, M.K.; Mori, S.; Gupta, I. Water Soluble Thioglycosylated BODIPYs for Mitochondria Targeted Cytotoxicity. Bioorganic Chem. 2019, 91, 103139. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Zhao, Y.; Tian, J.; Wang, S.; Gros, C.P.; Xu, H. Red/NIR Neutral BODIPY-Based Fluorescent Probes for Lighting up Mitochondria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119199. [Google Scholar] [CrossRef]
- Chauhan, N.; Koli, M.; Ghosh, R.; Majumdar, A.G.; Ghosh, A.; Ghanty, T.K.; Mula, S.; Patro, B.S. A BODIPY-Naphtholimine-BF2 Dyad for Precision Photodynamic Therapy, Targeting, and Dual Imaging of Endoplasmic Reticulum and Lipid Droplets in Cancer. JACS Au 2024, 4, 2838–2852. [Google Scholar] [CrossRef]
- De Jong, F.; Pokorny, J.; Manshian, B.; Daelemans, B.; Vandaele, J.; Startek, J.B.; Soenen, S.; Van Der Auweraer, M.; Dehaen, W.; Rocha, S.; et al. Development and Characterization of BODIPY-Derived Tracers for Fluorescent Labeling of the Endoplasmic Reticulum. Dye. Pigment. 2020, 176, 108200. [Google Scholar] [CrossRef]
- Hoenke, S.; Brandes, B.; Csuk, R. Non-Cytotoxic Aza-BODIPY Triterpene Conjugates to Target the Endoplasmic Reticulum. Eur. J. Med. Chem. Rep. 2023, 7, 100099. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Kaushal, S.; Lim, D.-K. Recent Advances in Nano/Microfabricated Substrate Platforms and Artificial Intelligence for Practical Surface-Enhanced Raman Scattering-Based Bioanalysis. TrAC Trends Anal. Chem. 2023, 168, 117341. [Google Scholar] [CrossRef]
- Lim, D.-K.; Kumar, P.P.P. Recent Advances in SERS-Based Bioanalytical Applications: Live Cell Imaging. Nanophotonics 2024, 13, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Faulds, K.; McKenzie, F.; Smith, W.E.; Graham, D. Quantitative Simultaneous Multianalyte Detection of DNA by Dual-Wavelength Surface-Enhanced Resonance Raman Scattering. Angew. Chem. Int. Ed. 2007, 46, 1829–1831. [Google Scholar] [CrossRef]
- Adarsh, N.; Ramya, A.N.; Maiti, K.K.; Ramaiah, D. Unveiling NIR Aza-Boron-Dipyrromethene (BODIPY) Dyes as Raman Probes: Surface-Enhanced Raman Scattering (SERS)-Guided Selective Detection and Imaging of Human Cancer Cells. Chem. A Eur. J. 2017, 23, 14286–14291. [Google Scholar] [CrossRef]
- Klapper, M.; Ehmke, M.; Palgunow, D.; Böhme, M.; Matthäus, C.; Bergner, G.; Dietzek, B.; Popp, J.; Döring, F. Fluorescence-Based Fixative and Vital Staining of Lipid Droplets in Caenorhabditis Elegans Reveal Fat Stores Using Microscopy and Flow Cytometry Approaches. J. Lipid Res. 2011, 52, 1281–1293. [Google Scholar] [CrossRef]
- Xia, J.; Yao, J.; Wang, L.H.V. Photoacoustic tomography: Principles and advances (Invited Review). Prog. Electromagn. Res. 2014, 147, 1–22. [Google Scholar] [CrossRef]
- Farooq, A.; Sabah, S.; Dhou, S.; Alsawaftah, N.; Husseini, G. Exogenous Contrast Agents in Photoacoustic Imaging: An In Vivo Review for Tumor Imaging. Nanomaterials 2022, 12, 393. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Lim, D.-K. Photothermal Effect of Gold Nanoparticles as a Nanomedicine for Diagnosis and Therapeutics. Pharmaceutics 2023, 15, 2349. [Google Scholar] [CrossRef]
- Ni, Y.; Kannadorai, R.K.; Peng, J.; Yu, S.W.-K.; Chang, Y.-T.; Wu, J. Naphthalene-Fused BODIPY near-Infrared Dye as a Stable Contrast Agent for in Vivo Photoacoustic Imaging. Chem. Commun. 2016, 52, 11504–11507. [Google Scholar] [CrossRef]
- Ni, Y.; Kannadorai, R.K.; Yu, S.W.-K.; Chang, Y.-T.; Wu, J. Push–Pull Type Meso-Ester Substituted BODIPY near-Infrared Dyes as Contrast Agents for Photoacoustic Imaging. Org. Biomol. Chem. 2017, 15, 4531–4535. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Lyu, Y.; Ding, D.; Pu, K. Semiconducting Oligomer Nanoparticles as an Activatable Photoacoustic Probe with Amplified Brightness for In Vivo Imaging of pH. Adv. Mater. 2016, 28, 3662–3668. [Google Scholar] [CrossRef]
- Merkes, J.M.; Lammers, T.; Kancherla, R.; Rueping, M.; Kiessling, F.; Banala, S. Tuning Optical Properties of BODIPY Dyes by Pyrrole Conjugation for Photoacoustic Imaging. Adv. Opt. Mater. 2020, 8, 1902115. [Google Scholar] [CrossRef]
- Ma, D.; Hou, S.; Bae, C.; Pham, T.C.; Lee, S.; Zhou, X. Aza-BODIPY Based Probe for Photoacoustic Imaging of ONOO− In Vivo. Chin. Chem. Lett. 2021, 32, 3886–3889. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, H.; Chen, B.; Xu, X.; Zhang, Q.; Chen, H.; Zhang, X.; Song, G. Simultaneous In Vivo Imaging of Neutrophil Elastase and Oxidative Stress in Atherosclerotic Plaques Using a Unimolecular Photoacoustic Probe. Angew. Chem. Int. Ed. 2024, 63, e202411840. [Google Scholar] [CrossRef]
- Xu, M.; Sun, Q.; Wang, X.; Gao, H.; Liu, Z. Near-Infrared Absorbing BODIPY-Xanthene Hybrids for Multiplexed Photoacoustic Imaging. Org. Lett. 2024, 26, 3750–3755. [Google Scholar] [CrossRef]
- Malacarne, M.C.; Gariboldi, M.B.; Caruso, E. BODIPYs in PDT: A Journey through the Most Interesting Molecules Produced in the Last 10 Years. IJMS 2022, 23, 10198. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol.Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Awuah, S.G.; You, Y. Boron Dipyrromethene (BODIPY)-Based Photosensitizers for Photodynamic Therapy. RSC Adv. 2012, 2, 11169. [Google Scholar] [CrossRef]
- Caruso, E.; Gariboldi, M.; Sangion, A.; Gramatica, P.; Banfi, S. Synthesis, Photodynamic Activity, and Quantitative Structure-Activity Relationship Modelling of a Series of BODIPYs. J. Photochem. Photobiol. B Biol. 2017, 167, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.; Malacarne, M.C.; Marras, E.; Papa, E.; Bertato, L.; Banfi, S.; Gariboldi, M.B. New BODIPYs for Photodynamic Therapy (PDT): Synthesis and Activity on Human Cancer Cell Lines. Bioorganic Med. Chem. 2020, 28, 115737. [Google Scholar] [CrossRef]
- Kim, B.; Sui, B.; Yue, X.; Tang, S.; Tichy, M.G.; Belfield, K.D. In Vitro Photodynamic Studies of a BODIPY-Based Photosensitizer. Eur. J. Org. Chem. 2017, 2017, 25–28. [Google Scholar] [CrossRef]
- Durantini, A.M.; Greene, L.E.; Lincoln, R.; Martínez, S.R.; Cosa, G. Reactive Oxygen Species Mediated Activation of a Dormant Singlet Oxygen Photosensitizer: From Autocatalytic Singlet Oxygen Amplification to Chemicontrolled Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 1215–1225. [Google Scholar] [CrossRef]
- Epelde-Elezcano, N.; Martínez-Martínez, V.; Peña-Cabrera, E.; Gómez-Durán, C.F.A.; Arbeloa, I.L.; Lacombe, S. Modulation of Singlet Oxygen Generation in Halogenated BODIPY Dyes by Substitution at Their Meso Position: Towards a Solvent-Independent Standard in the Vis Region. RSC Adv. 2016, 6, 41991–41998. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, J.; Ji, X.; Lin, G.; Xu, S.; Dong, X.; Zhao, W. Discovery of a Monoiodo Aza-BODIPY Near-Infrared Photosensitizer: In Vitro and in Vivo Evaluation for Photodynamic Therapy. J. Med. Chem. 2020, 63, 9950–9964. [Google Scholar] [CrossRef]
- Nguyen, V.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.M.K.; Kim, G.; Kwon, N.; Kim, C.; Park, S.; Yoon, J. Molecular Design of Highly Efficient Heavy-Atom-Free Triplet BODIPY Derivatives for Photodynamic Therapy and Bioimaging. Angew. Chem. Int. Ed. 2020, 59, 8957–8962. [Google Scholar] [CrossRef]
- Wang, L.; Bai, J.; Qian, Y. Synthesis of a Triphenylamine BODIPY Photosensitizer with D–A Configuration and Its Application in Intracellular Simulated Photodynamic Therapy. New J. Chem. 2019, 43, 16829–16834. [Google Scholar] [CrossRef]
- Wang, C.; Qian, Y. A Water Soluble Carbazolyl-BODIPY Photosensitizer with an Orthogonal D–A Structure for Photodynamic Therapy in Living Cells and Zebrafish. Biomater. Sci. 2020, 8, 830–836. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Yan, Y.; El-Zohry, A.M.; Toffoletti, A.; Zhao, J.; Barbon, A.; Dick, B.; Mohammed, O.F.; Han, G. Elucidation of the Intersystem Crossing Mechanism in a Helical BODIPY for Low-Dose Photodynamic Therapy. Angew. Chem. 2020, 132, 16248–16255. [Google Scholar] [CrossRef]
- Filatov, M.A. Heavy-Atom-Free BODIPY Photosensitizers with Intersystem Crossing Mediated by Intramolecular Photoinduced Electron Transfer. Org. Biomol. Chem. 2020, 18, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cui, X.; Zhao, J. Hetero Bodipy-Dimers as Heavy Atom-Free Triplet Photosensitizers Showing a Long-Lived Triplet Excited State for Triplet–Triplet Annihilation Upconversion. Chem. Commun. 2013, 49, 9009. [Google Scholar] [CrossRef]
- Cakmak, Y.; Kolemen, S.; Duman, S.; Dede, Y.; Dolen, Y.; Kilic, B.; Kostereli, Z.; Yildirim, L.T.; Dogan, A.L.; Guc, D.; et al. Designing Excited States: Theory-Guided Access to Efficient Photosensitizers for Photodynamic Action. Angew. Chem. Int. Ed. 2011, 50, 11937–11941. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef]
- Zhang, W.; Ahmed, A.; Cong, H.; Wang, S.; Shen, Y.; Yu, B. Application of Multifunctional BODIPY in Photodynamic Therapy. Dye. Pigment. 2021, 185, 108937. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent Advances in Selective Photothermal Therapy of Tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Zhang, D.; Cui, T.; Xu, Z.; Jiang, X.-D. Synthesis, Structure and Photochemical Properties of Asymmetric NMe2-Bearing Aza-BODIPYs as Novel Photothermal Agents. Dye. Pigment. 2022, 199, 110092. [Google Scholar] [CrossRef]
- Liu, Y.; Song, N.; Chen, L.; Liu, S.; Xie, Z. Synthesis of a Near-Infrared BODIPY Dye for Bioimaging and Photothermal Therapy. Chem. Asian J. 2018, 13, 989–995. [Google Scholar] [CrossRef]
- Pewklang, T.; Saiyasombat, W.; Chueakwon, P.; Ouengwanarat, B.; Chansaenpak, K.; Kampaengsri, S.; Lai, R.; Kamkaew, A. Revolutionary Pyrazole-based Aza-BODIPY: Harnessing Photothermal Power Against Cancer Cells and Bacteria. ChemBioChem 2024, 25, e202300653. [Google Scholar] [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A Realistic Appraisal of Boron Neutron Capture Therapy as a Cancer Treatment Modality. Cancer Commun. 2018, 38, 36. [Google Scholar] [CrossRef]
- Jin, W.H.; Seldon, C.; Butkus, M.; Sauerwein, W.; Giap, H.B. A Review of Boron Neutron Capture Therapy: Its History and Current Challenges. Int. J. Part. Ther. 2022, 9, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 601820. [Google Scholar] [CrossRef]
- Li, X.; Golberg, D. Boron Nitride Nanotubes as Drug Carriers. In Boron Nitride Nanotubes in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 79–94. ISBN 978-0-323-38945-7. [Google Scholar]
- Xuan, S.; Zhao, N.; Zhou, Z.; Fronczek, F.R.; Vicente, M.G.H. Synthesis and in Vitro Studies of a Series of Carborane-Containing Boron Dipyrromethenes (BODIPYs). J. Med. Chem. 2016, 59, 2109–2117. [Google Scholar] [CrossRef]
- Gibbs, J.H.; Wang, H.; Bhupathiraju, N.V.S.D.K.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Synthesis and Properties of a Series of Carboranyl-BODIPYs. J. Organomet. Chem. 2015, 798, 209–213. [Google Scholar] [CrossRef]
- Nakata, E.; Koizumi, M.; Yamashita, Y.; Onaka, K.; Sakurai, Y.; Kondo, N.; Ono, K.; Uto, Y.; Hori, H. Design, Synthesis and Destructive Dynamic Effects of BODIPY-Containing and Curcuminoid Boron Tracedrugs for Neutron Dynamic Therapy. Anticancer. Res. 2011, 31, 2477–2481. [Google Scholar]
- Kalot, G.; Godard, A.; Busser, B.; Pliquett, J.; Broekgaarden, M.; Motto-Ros, V.; Wegner, K.D.; Resch-Genger, U.; Köster, U.; Denat, F.; et al. Aza-BODIPY: A New Vector for Enhanced Theranostic Boron Neutron Capture Therapy Applications. Cells 2020, 9, 1953. [Google Scholar] [CrossRef]
- Bellomo, C.; Zanetti, D.; Cardano, F.; Sinha, S.; Chaari, M.; Fin, A.; Maranzana, A.; Núñez, R.; Blangetti, M.; Prandi, C. Red Light-Emitting Carborane-BODIPY Dyes: Synthesis and Properties of Visible-Light Tuned Fluorophores with Enhanced Boron Content. Dye. Pigment. 2021, 194, 109644. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Sun, T.; Xie, Z. Unadulterated BODIPY Nanoparticles for Biomedical Applications. Coord. Chem. Rev. 2019, 390, 76–85. [Google Scholar] [CrossRef]
- Lin, W.; Colombani-Garay, D.; Huang, L.; Duan, C.; Han, G. Tailoring Nanoparticles Based on Boron Dipyrromethene for Cancer Imaging and Therapy. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1627. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Z.; Liang, Z.; Wang, T.; Chen, Y.; Liu, Z. Discovery of BODIPY J-Aggregates with Absorption Maxima beyond 1200 Nm for Biophotonics. Sci. Adv. 2022, 8, eadd5660. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Yan, M.; Wang, X. Recent Progress of BODIPY Dyes With Aggregation-Induced Emission. Front. Chem. 2019, 7, 712. [Google Scholar] [CrossRef]
- Descalzo, A.B.; Ashokkumar, P.; Shen, Z.; Rurack, K. On the Aggregation Behaviour and Spectroscopic Properties of Alkylated and Annelated Boron-Dipyrromethene (BODIPY) Dyes in Aqueous Solution. ChemPhotoChem 2020, 4, 120–131. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Yadav, P.; Shanavas, A.; Neelakandan, P.P. Aggregation Enhances Luminescence and Photosensitization Properties of a Hexaiodo-BODIPY. Mater. Chem. Front. 2020, 4, 965–972. [Google Scholar] [CrossRef]
- Salis, F.; Descalzo, A.B.; Benito-Peña, E.; Moreno-Bondi, M.C.; Orellana, G. Highly Fluorescent Magnetic Nanobeads with a Remarkable Stokes Shift as Labels for Enhanced Detection in Immunoassays. Small 2018, 14, 1703810. [Google Scholar] [CrossRef]
- Rahman, A.; Praveen Kumar, P.P.; Yadav, P.; Goswami, T.; Shanavas, A.; Ghosh, H.N.; Neelakandan, P.P. Gold–BODIPY Nanoparticles with Luminescence and Photosensitization Properties for Photodynamic Therapy and Cell Imaging. ACS Appl. Nano Mater. 2022, 5, 6532–6542. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Rahman, A.; Goswami, T.; Ghosh, H.N.; Neelakandan, P.P. Fine-Tuning Plasmon-Molecule Interactions in Gold-BODIPY Nanocomposites: The Role of Chemical Structure and Noncovalent Interactions. ChemPlusChem 2021, 86, 87–94. [Google Scholar] [CrossRef]
- Kumar, P.P.P. A Multimode Detection Platform for Biothiols Using BODIPY Dye-Conjugated Gold Nanoparticles. Colorants 2024, 3, 214–228. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Yadav, P.; Shanavas, A.; Thurakkal, S.; Joseph, J.; Neelakandan, P.P. A Three-Component Supramolecular Nanocomposite as a Heavy-Atom-Free Photosensitizer. Chem. Commun. 2019, 55, 5623–5626. [Google Scholar] [CrossRef]
- Praveen Kumar, P.P.; Kaur, N.; Shanavas, A.; Neelakandan, P.P. Nanomolar Detection of Biothiols via Turn-ON Fluorescent Indicator Displacement. Analyst 2020, 145, 851–857. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Guan, X.; Xie, Z.; Huang, Y.; Jing, X. Unadulterated BODIPY-Dimer Nanoparticles with High Stability and Good Biocompatibility for Cellular Imaging. Nanoscale 2014, 6, 5662–5665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, N.; Chen, L.; Xie, Z. Triple-BODIPY Organic Nanoparticles with Particular Fluorescence Emission. Dye. Pigment. 2017, 147, 241–245. [Google Scholar] [CrossRef]
- Lin, W.; Sun, T.; Xie, Z.; Gu, J.; Jing, X. A Dual-Responsive Nanocapsule via Disulfide-Induced Self-Assembly for Therapeutic Agent Delivery. Chem. Sci. 2016, 7, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, W.; Pei, Q.; Hu, X.; Xie, Z.; Jing, X. Redox-Hypersensitive Organic Nanoparticles for Selective Treatment of Cancer Cells. Chem. Mater. 2016, 28, 4440–4446. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, W.; Sun, T.; Gu, J.; Xie, Z.; Jing, X. Effect of Molecular Structure on Stability of Organic Nanoparticles Formed by Bodipy Dimers. Langmuir 2016, 32, 9575–9581. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Zhao, Y.; Zhang, Y.; Wu, S.; Zhao, J.; Han, G. Ultralow-Power Near Infrared Lamp Light Operable Targeted Organic Nanoparticle Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 14586–14591. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Zhao, Y.; Yang, J.; Yang, Y.; Pendharkar, A.I.; Zhang, Y.; Kelmar, S.; Chen, L.; Wu, W.; et al. Enhancing Photodynamic Therapy through Resonance Energy Transfer Constructed Near-Infrared Photosensitized Nanoparticles. Adv. Mater. 2017, 29, 1604789. [Google Scholar] [CrossRef]
- Naim, K.; Nair, S.T.; Yadav, P.; Shanavas, A.; Neelakandan, P.P. Supramolecular Confinement within Chitosan Nanocomposites Enhances Singlet Oxygen Generation. ChemPlusChem 2018, 83, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Xiao, W.; Huang, C.; Si, W.; Shao, J.; Huang, W.; Chen, P.; Zhang, Q.; Dong, X. pH-Triggered and Enhanced Simultaneous Photodynamic and Photothermal Therapy Guided by Photoacoustic and Photothermal Imaging. Chem. Mater. 2017, 29, 5216–5224. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, W.; Liu, S.; Li, Z.; Hu, X.; Xie, Z.; Duan, C.; Han, G. Engineering pH-Responsive BODIPY Nanoparticles for Tumor Selective Multimodal Imaging and Phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 43928–43935. [Google Scholar] [CrossRef]
- Zou, J.; Wang, P.; Wang, Y.; Liu, G.; Zhang, Y.; Zhang, Q.; Shao, J.; Si, W.; Huang, W.; Dong, X. Penetration Depth Tunable BODIPY Derivatives for pH Triggered Enhanced Photothermal/Photodynamic Synergistic Therapy. Chem. Sci. 2019, 10, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Villalobos Gutiérrez, P.; Muñoz Carrillo, J.; Sandoval Salazar, C.; Viveros Paredes, J.; Gutiérrez Coronado, O. Functionalized Metal Nanoparticles in Cancer Therapy. Pharmaceutics 2023, 15, 1932. [Google Scholar] [CrossRef]
- Malacarne, M.C.; Caruso, E.; Gariboldi, M.B.; Marras, E.; Della Bitta, G.; Santoro, O.; Simm, A.; Li, R.; Ferguson, C.T.J. Evaluation of Nanoparticles Covalently Bound with BODIPY for Their Photodynamic Therapy Applicability. Int. J. Mol. Sci. 2024, 25, 3187. [Google Scholar] [CrossRef] [PubMed]
- Topel, S.D.; Topel, Ö.; Bostancıoğlu, R.B.; Koparal, A.T. Synthesis and Characterization of Bodipy Functionalized Magnetic Iron Oxide Nanoparticles for Potential Bioimaging Applications. Colloids Surf. B Biointerfaces 2015, 128, 245–253. [Google Scholar] [CrossRef]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.-J.; Menichetti, S.; Rotello, V.M. Entrapment of Hydrophobic Drugs in Nanoparticle Monolayers with Efficient Release into Cancer Cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Sig Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current Advance of Nanotechnology in Diagnosis and Treatment for Malignant Tumors. Sig Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]

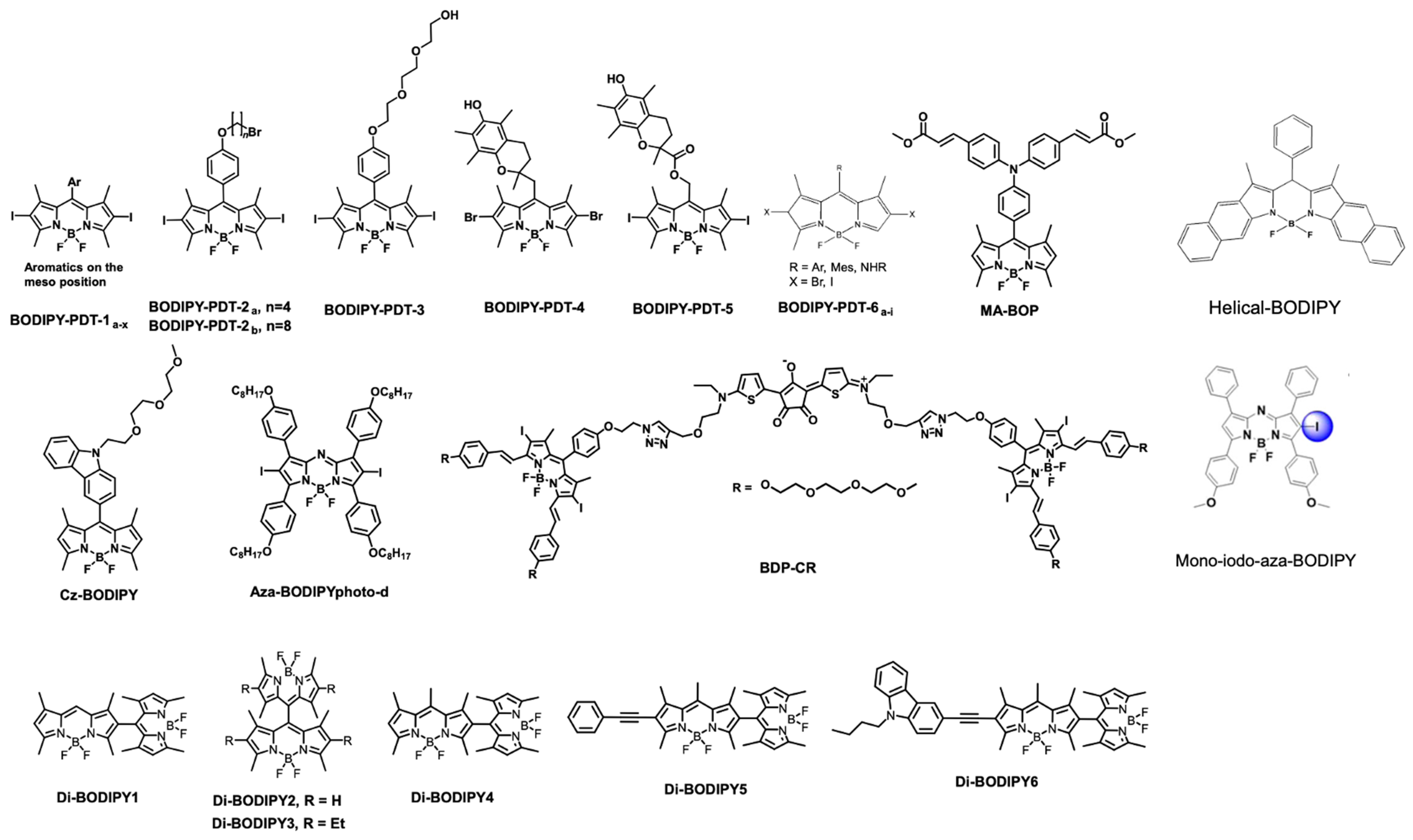
| Theranostics | Photophysics | Light Source | Therapeutic Modalities | In Vitro/In Vivo Tumor | Ref. |
|---|---|---|---|---|---|
| BODIPY-PDT-1a-x | Φfluo = 0.01 to 0.04 1O2 = 0.90 to 2.67 | Green LED fluence: 25.2 J/cm2 | PDT | SKOV 3 cells, | [85] |
| BODIPY-PDT-2a, n = 4 | Φfluo = 0.01 1O2 = 1.10 | Green LED | PDT | HCT116, SKOV3 and MCF7 | [84] |
| BODIPY-PDT-2b, n = 8 | Φfluo = 0.01 1O2 = 1.11 | Green LED | PDT | HCT116, SKOV3 and MCF7 | [86] |
| BODIPY-PDT-3 | Φfluo = 0.02 1O2 = 0.93 | Hg light; 0.2 mW/cm2 | PDT | LLC cells | [87] |
| BODIPY-PDT-4 | Φfluo = 0.008 1O2 = 0.80 | Nd: YAG laser; 2.6 mW/cm2 | PDT | Gram negative E. coli strain | [87] |
| BODIPY-PDT-5 | Φfluo = 0.009 1O2 = 0.57 | Nd: YAG laser; 2.6 mW/cm2 | PDT | Gram negative E. coli strain | [87] |
| BODIPY-PDT-6a-i | Φfluo = 0.01 to 0.51 1O2 = 0.09 to 0.96 | xenon flash lamp; 450 W | PDT | Not performed | [88] |
| MA-BOP | Φfluo = 0.56 1O2 = 0.2 | monochrome light; 12 W | PDT | A-549 cells | [91] |
| Cz-BODIPY | Φfluo = 0.10 1O2 = 0.68 | White light; 4 mW cm−2 | PDT/FL | Bel-7402 cells and Zebra fish | [92] |
| Mono-iodo-aza-BODIPY | Φfluo = 0.08 1O2 = 0.52 | 75 W halogen lampi; 54 J/cm2 for in vitro. 90 mW/cm2 for in vivo | PDT | HeLa, (MCF-7), SW480; HeLa tumor-bearing nude mice | [89] |
| Helical BODIPY | Φfluo = 0.21 1O2 = 0.36 | Irradiation by 656 nm LED, light dose: 6 J cm−2 | PDT augmented checkpoint blockade immunotherapy | CT26 tumor cells and CT26 tumors on BALB/c mice | [93] |
| Di-BODIPY 1–3 | Φfluo = 0.03, 0.31, 0.49, respectively 1O2 = 0.51, 0.46, 0.21, respectively. Di-BODIPY 6 showed a lower value for detection | LEDarrayat 2.5 mW cm−2 | PDT | K562 | [97] |
| Di-BODIPY 4–6 | Φfluo = 0.02, 0.17, 0.023, respectively 1O2 = 0.64, 0.42, respectively. Di-BODIPY 6 showed a lower value for detection | Spectrofluorometer light | Not performed | Not performed | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.P.P.; Saxena, S.; Joshi, R. BODIPY Dyes: A New Frontier in Cellular Imaging and Theragnostic Applications. Colorants 2025, 4, 13. https://doi.org/10.3390/colorants4020013
Kumar PPP, Saxena S, Joshi R. BODIPY Dyes: A New Frontier in Cellular Imaging and Theragnostic Applications. Colorants. 2025; 4(2):13. https://doi.org/10.3390/colorants4020013
Chicago/Turabian StyleKumar, Panangattukara Prabhakaran Praveen, Shivanjali Saxena, and Rakesh Joshi. 2025. "BODIPY Dyes: A New Frontier in Cellular Imaging and Theragnostic Applications" Colorants 4, no. 2: 13. https://doi.org/10.3390/colorants4020013
APA StyleKumar, P. P. P., Saxena, S., & Joshi, R. (2025). BODIPY Dyes: A New Frontier in Cellular Imaging and Theragnostic Applications. Colorants, 4(2), 13. https://doi.org/10.3390/colorants4020013







