Optimizing Thermoresponsive and Bioadhesive Systems for Local Application of Erythrosine
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Polymer Systems
2.3. Texture Profile Analysis
2.4. Rheological Analyses
2.4.1. Flow Rheometry
2.4.2. Oscillatory Rheometry
2.4.3. Determination of the Sol–Gel Transition Temperature (Tsol/gel)
2.5. Determination of In Vitro Mucoadhesion
2.6. Determination of the Ex-Vivo Bioadhesion
2.7. Photodynamic Activity
3. Results and Discussion
3.1. The Optimal Concentration of the Bioadhesive Polymer
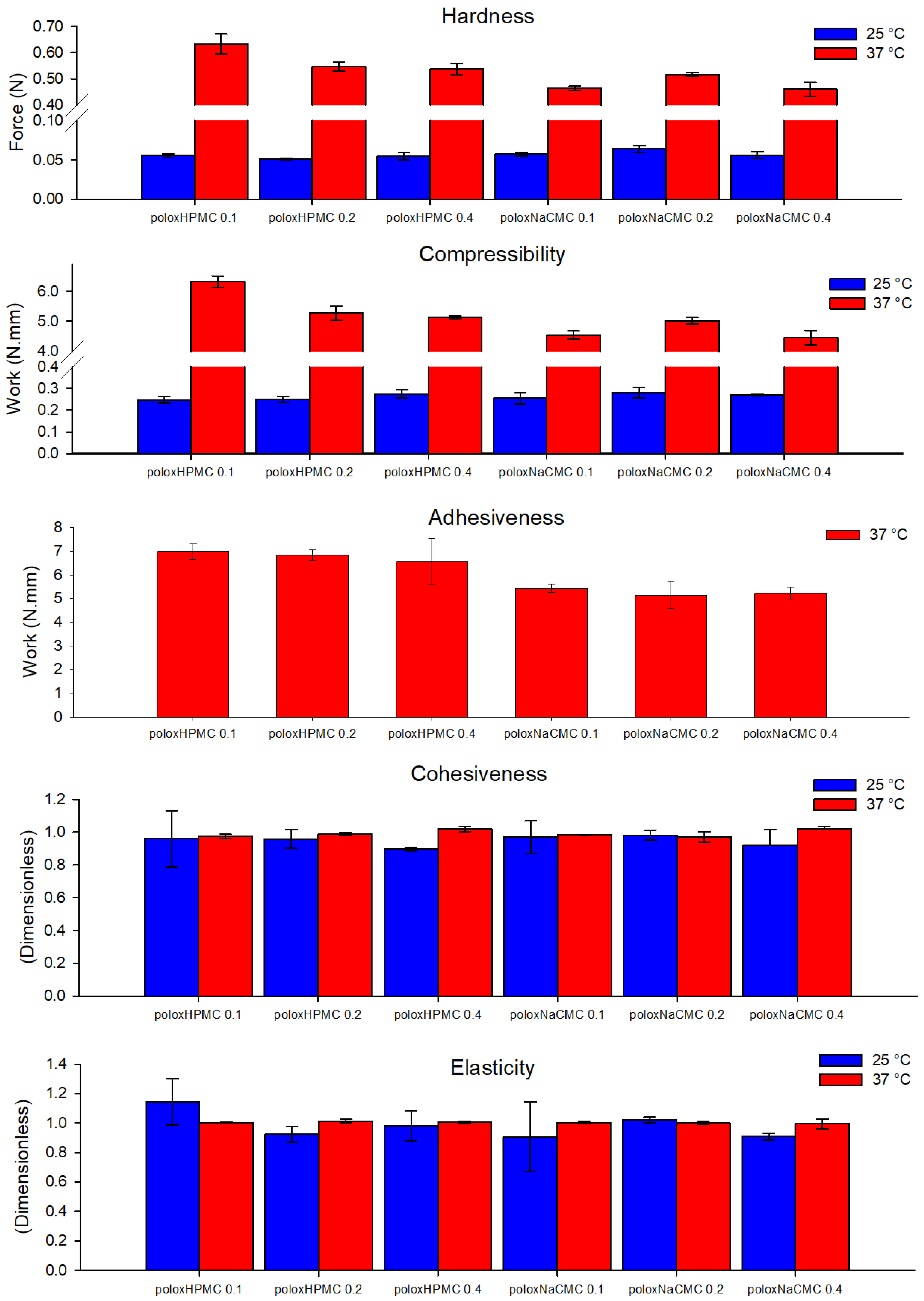
3.1.1. Texture Profile Analysis of Polymeric Formulations
3.1.2. Solution–Gel Transition Temperature Analysis
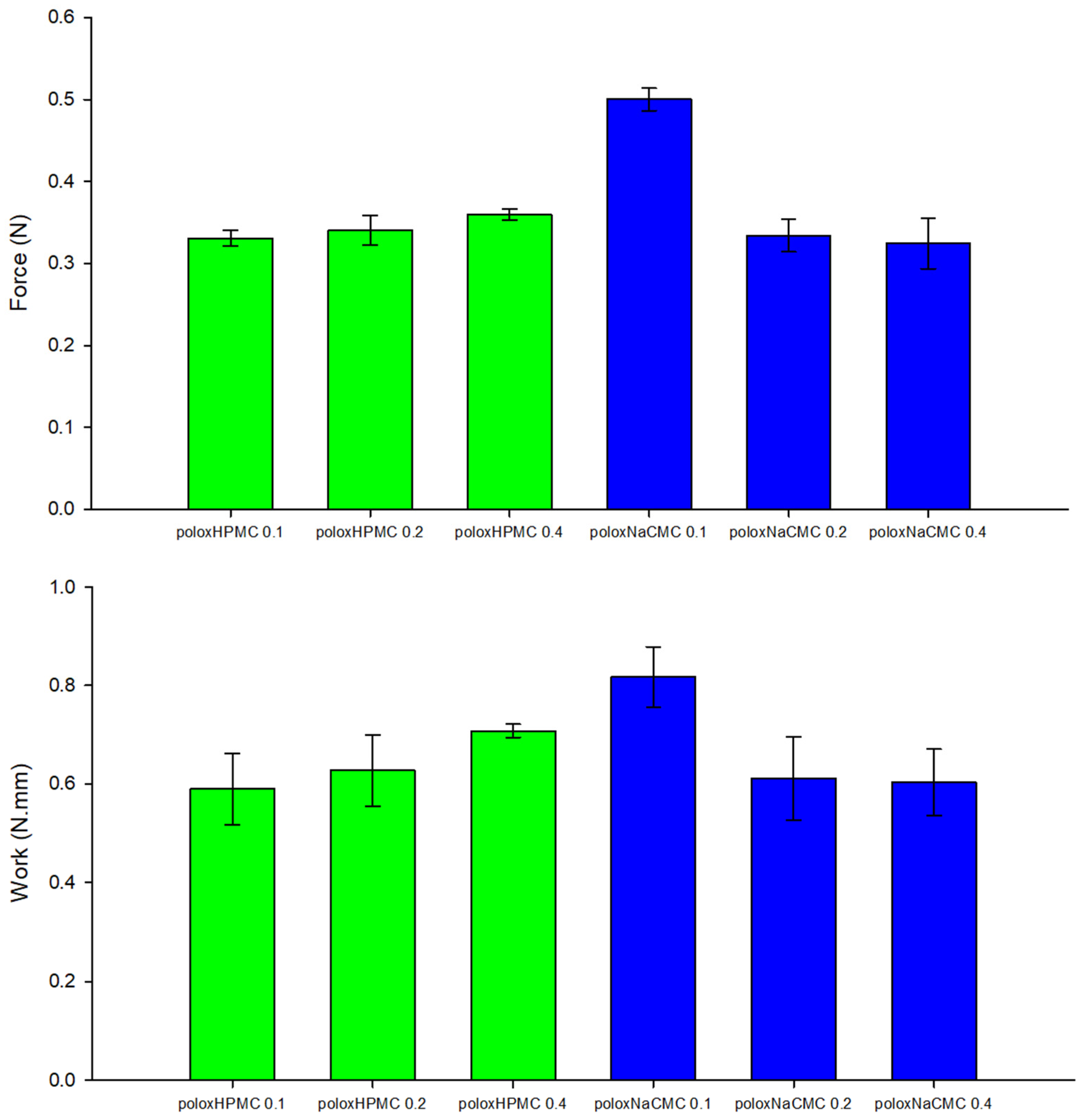
3.1.3. Mucoadhesion Determination of Polymeric Formulations
3.2. Effect of Erythrosine
3.2.1. Texture Profile Analysis of Polymeric Formulations with Erythosine
3.2.2. Solution–Gel Transition Temperature Analysis
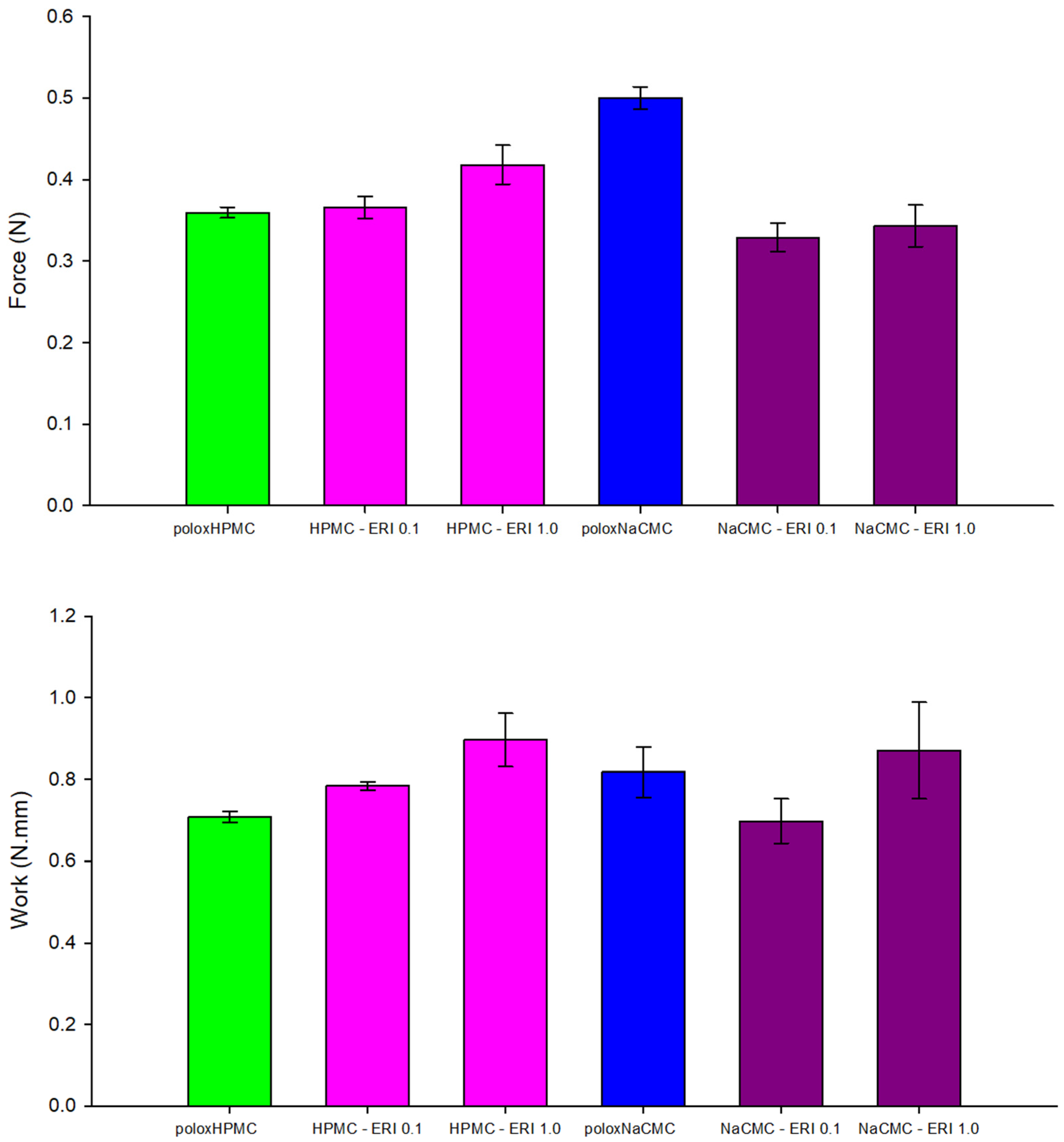
3.2.3. Mucoadhesion Determination of Polymeric Formulations with Erythosine
3.2.4. Bioadhesion Determination
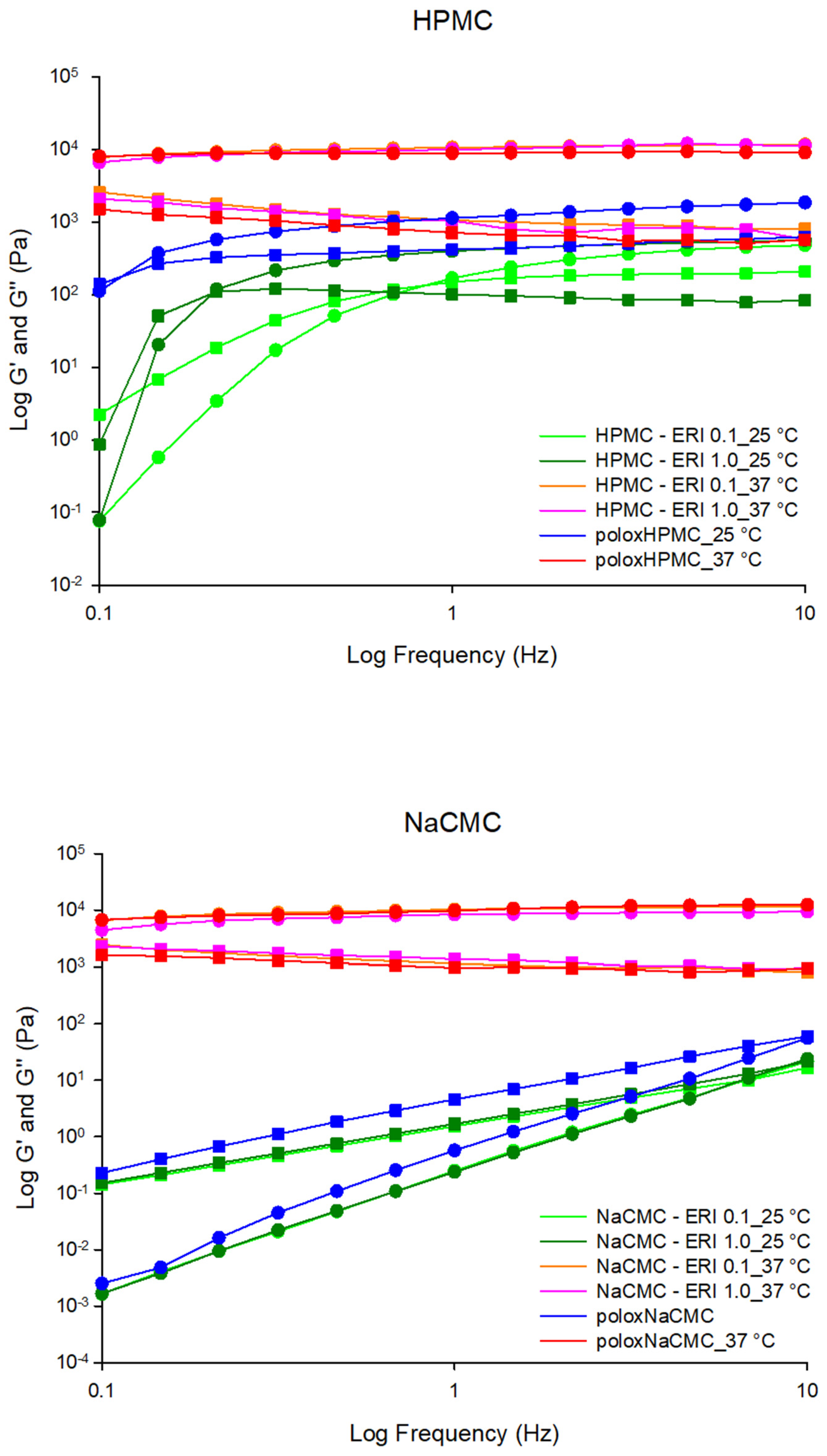
3.2.5. Rheometry
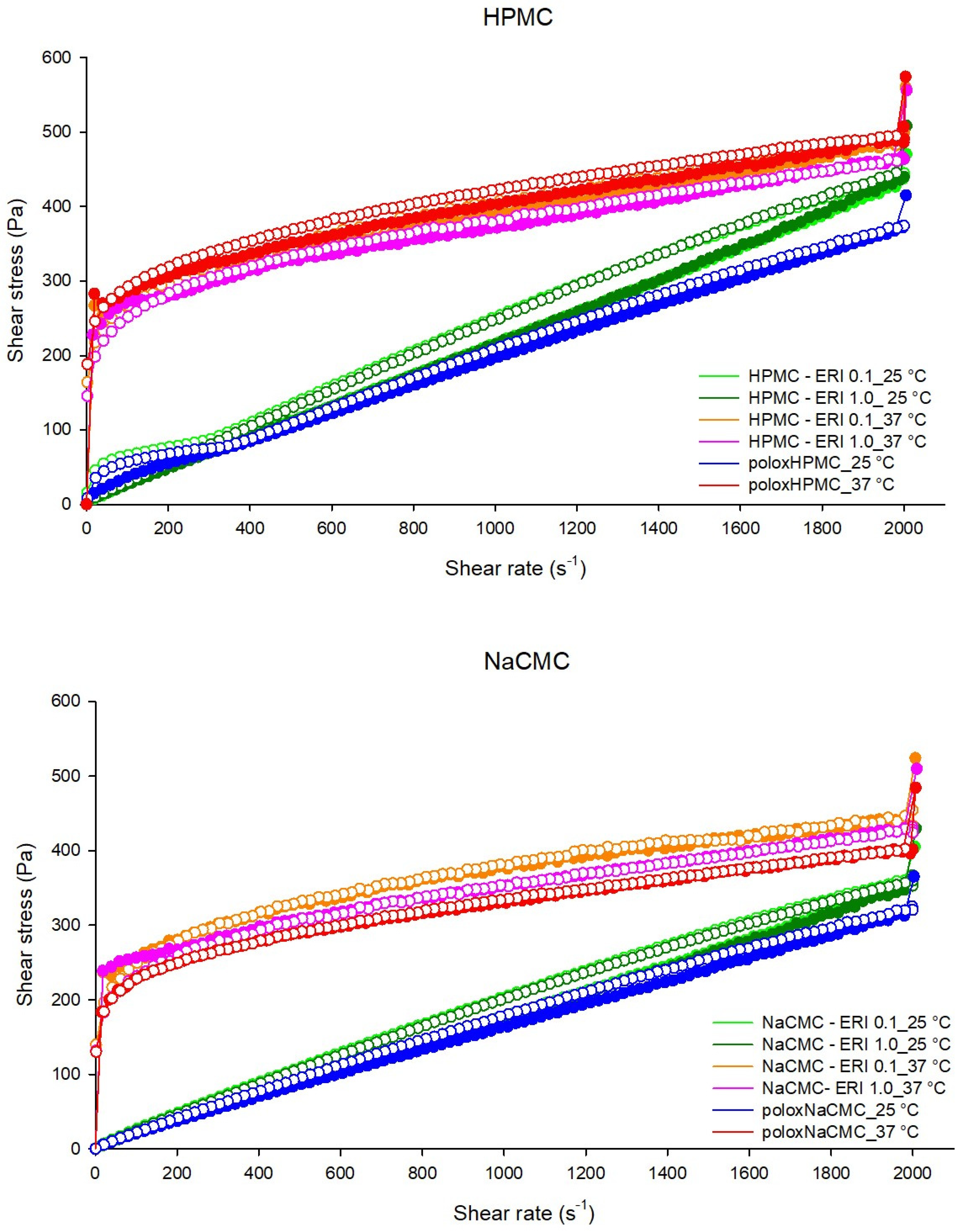
Continuous Shear (Flow) Rheology
Oscillatory Rheology
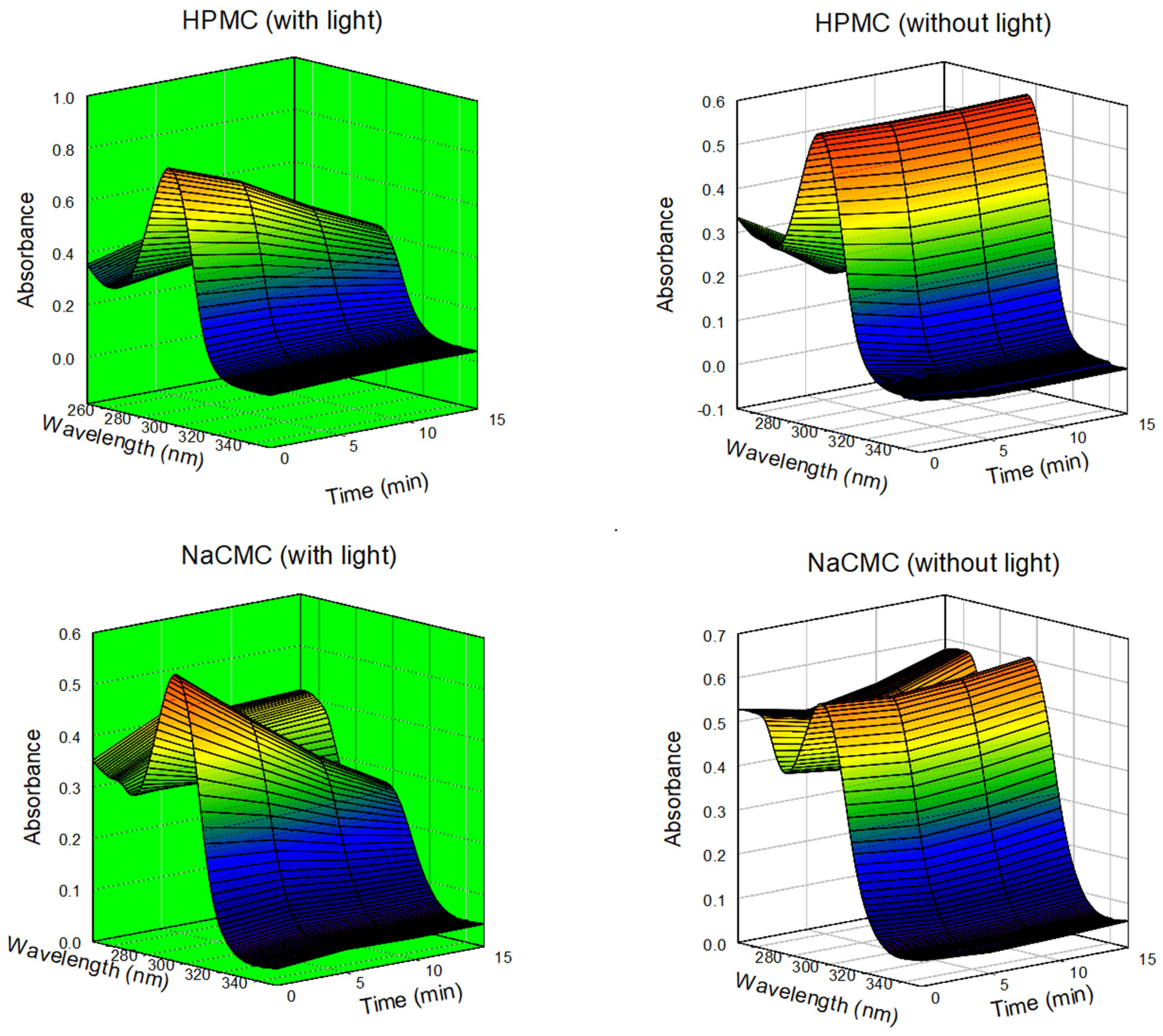
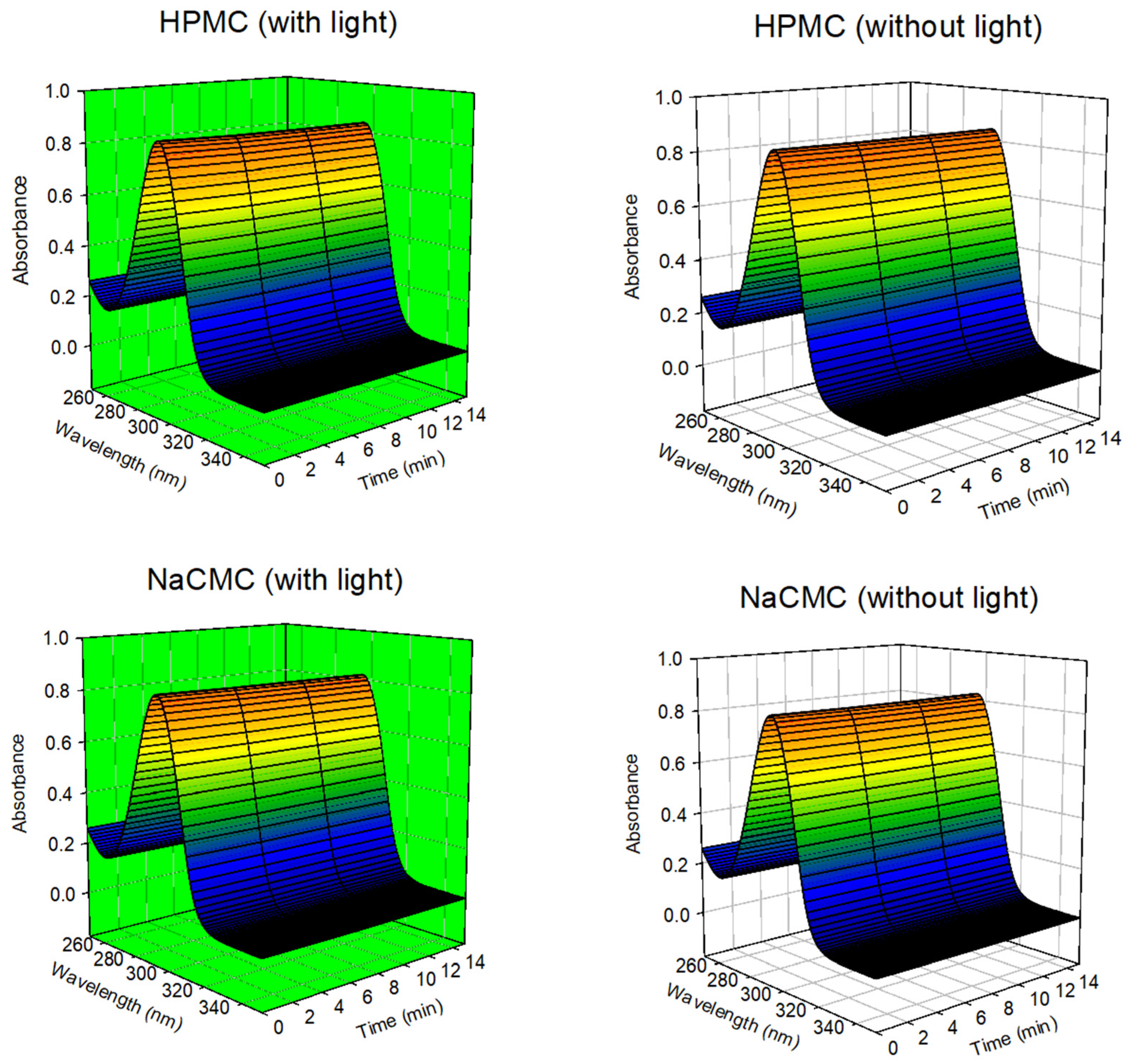
3.3. Determination of Photodynamic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Issa, M.C.A.; Manela-Azulay, M. Terapia Fotodinâmica: Revisão Da Literatura e Documentação Iconográfica. An. Bras. Dermatol. 2010, 85, 501–511. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in Photodynamic Therapy: An Emerging Paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef]
- Tokubo, L.M.; Rosalen, P.L.; de Cássia Orlandi Sardi, J.; Freires, I.A.; Fujimaki, M.; Umeda, J.E.; Barbosa, P.M.; Tecchio, G.O.; Hioka, N.; de Freitas, C.F.; et al. Antimicrobial Effect of Photodynamic Therapy Using Erythrosine/Methylene Blue Combination on Streptococcus Mutans Biofilm. Photodiagnosis Photodyn. Ther. 2018, 23, 94–98. [Google Scholar] [CrossRef]
- Wood, S.; Metcalf, D.; Devine, D.; Robinson, C. Erythrosine Is a Potential Photosensitizer for the Photodynamic Therapy of Oral Plaque Biofilms. J. Antimicrob. Chemother. 2006, 57, 680–684. [Google Scholar] [CrossRef]
- Garg, A.D.; Bose, M.; Ahmed, M.I.; Bonass, W.A.; Wood, S.R. In Vitro Studies on Erythrosine-Based Photodynamic Therapy of Malignant and Pre-Malignant Oral Epithelial Cells. PLoS ONE 2012, 7, e34475. [Google Scholar] [CrossRef]
- Song, A.; Zhang, J.; Zhang, M.; Shen, T.; Tang, J. Spectral Properties and Structure of Fluorescein and Its Alkyl Derivatives in Micelles. Colloids Surf. A Physicochem. Eng. Asp. 2000, 167, 253–262. [Google Scholar] [CrossRef]
- da Silva, J.B.; dos Santos, R.S.; Vecchi, C.F.; da Silva Souza Campanholi, K.; da Silva Junior, R.C.; de Castro Hoshino, L.V.; Caetano, W.; Baesso, M.L.; Simas, F.F.; Cook, M.T.; et al. Boosting the Photodynamic Activity of Erythrosine B by Using Thermoresponsive and Adhesive Systems Containing Cellulose Derivatives for Topical Delivery. Int. J. Biol. Macromol. 2023, 245, 125491. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Kumari, A. Polymers in Drug Delivery. J. Biosci. Med. 2016, 4, 69–84. [Google Scholar] [CrossRef]
- Sosnik, A.; Das Neves, J.; Sarmento, B. Mucoadhesive Polymers in the Design of Nano-Drug Delivery Systems for Administration by Non-Parenteral Routes: A Review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Opara, E. Controlled Drug Delivery Systems, 1st ed.; Taylor & Francis Group: Abingdon, UK, 2020. [Google Scholar]
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive Systems Composed of Poloxamer 407 and HPMC or NaCMC: Mechanical, Rheological and Sol-Gel Transition Analysis. Carbohydr. Polym. 2020, 240, 116268. [Google Scholar] [CrossRef]
- Uma, K. Bioadhesives for Clinical Applications—A Mini Review. Mater. Adv. 2023, 4, 2062–2069. [Google Scholar] [CrossRef]
- Palacio, M.L.B.; Bhushan, B. Bioadhesion: A Review of Concepts and Applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 2321–2347. [Google Scholar] [CrossRef]
- da Silva, J.B.; dos Santos, R.S.; da Silva, M.B.; Braga, G.; Cook, M.T.; Bruschi, M.L. Interaction between Mucoadhesive Cellulose Derivatives and Pluronic F127: Investigation on the Micelle Structure and Mucoadhesive Performance. Mater. Sci. Eng. C 2021, 119, 111643. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Chorilli, M.; Gremião, M.P.D. Plataformas Bio(Muco) Adesivas Poliméricas Baseadas Em Nanotecnologia Para Liberação Controlada de Fármacos—Propriedades, Metodologias e Aplicações. Polímeros 2014, 24, 203–213. [Google Scholar] [CrossRef]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef]
- Hosny, K.M.; Alkhalidi, H.M.; Alharbi, W.S.; Md, S.; Sindi, A.M.; Ali, S.A.; Bakhaidar, R.B.; Almehmady, A.M.; Alfayez, E.; Kurakula, M. Recent Trends in Assessment of Cellulose Derivatives in Designing Novel and Nanoparticulate-Based Drug Delivery Systems for Improvement of Oral Health. Polymers 2022, 14, 92. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; da Silva Souza Campanholi, K.; Braga, G.; de Souza, P.R.; Caetano, W.; Cook, M.T.; Bruschi, M.L. The Effect of Erythrosine-B on the Structuration of Poloxamer 407 and Cellulose Derivative Blends: In Silico Modelling Supporting Experimental Studies. Mater. Sci. Eng. C 2021, 130, 112440. [Google Scholar] [CrossRef]
- Schmolka, I. Artificial Skin. I. Preparation and Properties of Pluronic F-127 Gels for Treatment of Burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F.; Coulter, W.A.; McClelland, C.; Irwin, C.R. Design, Characterisation and Preliminary Clinical Evaluation of a Novel Mucoadhesive Topical Formulation Containing Tetracycline for the Treatment of Periodontal Disease. J. Control Release 2000, 67, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M. Texture Profile Analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Bruschi, M.L.; Jones, D.S.; Panzeri, H.; Gremião, M.P.D.; de Freitas, O.; Lara, E.H.G. Semisolid Systems Containing Propolis for the Treatment of Periodontal Disease: In Vitro Release Kinetics, Syringeability, Rheological, Textural, and Mucoadhesive Properties. J. Pharm. Sci. 2007, 96, 2074–2089. [Google Scholar] [CrossRef]
- Fischer, F.; Graschew, G.; Lorenz, W.J.; Schlag, P.M. A Chemical Dosimeter for the Determination of the Photodynamic Activity of Photosensitizers. Clin. Chim. Acta 1998, 274, 89–104. [Google Scholar] [CrossRef]
- Longer, M.A.; Robinson, J.R. Fundamental Aspects of Bioadhesion. Pharm. Int. 1986, 7, 114–117. [Google Scholar]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A Promising Approach in Drug Delivery System. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; de Ferreira, S.B.S.; de Freitas, O.; Bruschi, M.L. A Critical Review about Methodologies for the Analysis of Mucoadhesive Properties of Drug Delivery Systems. Drug Dev. Ind. Pharm. 2017, 43, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive Polymeric Platforms for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Noiray, M.; Briand, E.; Woodward, A.M.; Argüeso, P.; Martínez, I.T.M.; Herrero-Vanrell, R.; Ponchel, G. Interfacial Interaction between Transmembrane Ocular Mucins and Adhesive Polymers and Dendrimers Analyzed by Surface Plasmon Resonance. Pharm. Res. 2012, 29, 2329–2340. [Google Scholar] [CrossRef]
- Pagano, C.; Giovagnoli, S.; Perioli, L.; Tiralti, M.C.; Ricci, M. Development and Characterization of Mucoadhesive-Thermoresponsive Gels for the Treatment of Oral Mucosa Diseases. Eur. J. Pharm. Sci. 2020, 142, 105125. [Google Scholar] [CrossRef]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Guneri, T. Rheological and Mechanical Properties of Poloxamer Mixtures as a Mucoadhesive Gel Base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic Property in Pharmaceutical Formulations. J. Control Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Wei, G.; Lu, W. Effect of Carrageenan on Poloxamer-Based in Situ Gel for Vaginal Use: Improved in Vitro and in Vivo Sustained-Release Properties. Eur. J. Pharm. Sci. 2009, 37, 306–312. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. In Situ Gelling Systems: A Strategy to Improve the Bioavailability of Ophthalmic Pharmaceutical Formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef]
- Andrews, G.P.; Gorman, S.P.; Jones, D.S. Rheological Characterisation of Primary and Binary Interactive Bioadhesive Gels Composed of Cellulose Derivatives Designed as Ophthalmic Viscosurgical Devices. Biomaterials 2005, 26, 571–580. [Google Scholar] [CrossRef]
- Andrews, G.P.; Jones, D.S. Rheological Characterization of Bioadhesive Binary Polymeric Systems Designed as Platforms for Drug Delivery Implants. Biomacromolecules 2006, 7, 899–906. [Google Scholar] [CrossRef]
- Pellosi, D.S.; Estevão, B.M.; Freitas, C.F.; Tsubone, T.M.; Caetano, W.; Hioka, N. Photophysical Properties of Erythrosin Ester Derivatives in Ionic and Non-Ionic Micelles. Dye. Pigment. 2013, 99, 705–712. [Google Scholar] [CrossRef]


| Formulation | P407 (%, w/w) | HPMC (%, w/w) | NaCMC (%, w/w) | Erythrosine (%, w/w) |
|---|---|---|---|---|
| poloxHPMC 0.1 | 17.5 | 0.1 | - | - |
| poloxHPMC 0.2 | 17.5 | 0.2 | - | - |
| poloxHPMC 0.4 | 17.5 | 0.4 | - | - |
| HPMC–ERI 0.1 | 17.5 | 0.4 | - | 0.1 |
| HPMC–ERI 1.0 | 17.5 | 0.4 | - | 1.0 |
| poloxNaCMC 0.1 | 17.5 | - | 0.1 | - |
| poloxNaCMC 0.2 | 17.5 | - | 0.2 | - |
| poloxNaCMC 0.4 | 17.5 | - | 0.4 | - |
| NaCMC–ERI 0.1 | 17.5 | - | 0.1 | 0.1 |
| NaCMC–ERI 1.0 | 17.5 | - | 0.1 | 1.0 |
| Formulation | Gelation Temperature (°C) |
|---|---|
| poloxHPMC 0.1 | 31.987 ± 0.055 |
| poloxHPMC 0.2 | 32.293 ± 0.376 |
| poloxHPMC 0.4 | 31.603 ± 0.493 |
| poloxNaCMC 0.1 | 35.497 ± 0.153 |
| poloxNaCMC 0.2 | 32.993 ± 0.102 |
| poloxNaCMC 0.4 | 31.563 ± 0.693 |
| Formulation | Tsol/gel (°C) |
|---|---|
| HPMC–ERI 0.1 | 32.037 ± 0.067 |
| HPMC–ERI 1.0 | 32.760 ± 0.096 |
| NaCMC–ERI 0.1 | 33.547 ± 0.482 |
| NaCMC–ERI 1.0 | 33.890 ± 0.052 |
| Formulations | n (Dimensionless) | K (Pa.s) | σy (Pa) | Hysteresis Area (Pa/s) | ||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 37 °C | 25 °C | 37 °C | 25 °C | 37 °C | 25 °C | 37 °C | |
| HPMC–ERI 0.1 | 1.016 ± 0.014 | 0.209 ± 0.011 | 0.188 ± 0.020 | 94.513 ± 8.171 | 7.475 ± 0.913 | 210.167 ± 15.701 | −56,646.667 ± 8575.834 | −20,633 ± 12,827.611 |
| HPMC–ERI 1.0 | 1.040 ± 0.007 | 0.193 ± 0.012 | 0.153 ± 0.013 | 101.433 ± 8.402 | 7.867 ± 4.032 | 205.567 ± 9.730 | −47,403.333 ± 2220.480 | −7396.667 ± 8754.705 |
| NaCMC–ERI 0.1 | 0.974 ± 0.020 | 0.184 ± 0.002 | 0.215 ± 0.034 | 106.700 ± 1.652 | 0.183 ± 0.185 | 216.600 ± 7.618 | −37570 ± 11,556.003 | −2472.583 ± 2224.258 |
| NaCMC–ERI 1.0 | 0.976 ± 0.013 | 0.181 ± 0.001 | 0.210 ± 0.020 | 103.533 ± 2.409 | 0.151 ± 0.121 | 197.067 ± 4.314 | −35,903.333 ± 4947.447 | 7656.600 ± 10,166.537 |
| poloxHPMC | 0.901 ± 0.034 | 0.184 ± 0.005 | 0.397 ± 0.091 | 115.167 ± 5.900 | 2.127 ± 1.533 | 227.133 ± 3.365 | −21,354.667 ± 10427.377 | −30,576.667 ± 9452.335 |
| poloxNaCMC | 0.950 ± 0.013 | 0.194 ± 0.003 | 0.232 ± 0.016 | 88.583 ± 3.593 | 0.420 ± 0.157 | 185.467 ± 10.433 | −21,230 ± 4497.699 | −1192.133 ± 8213.354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endrice, I.A.; Gerarduzzi, S.A.F.; de Oliveira, M.C.; Bruschi, M.L.; Bassi da Silva, J. Optimizing Thermoresponsive and Bioadhesive Systems for Local Application of Erythrosine. Colorants 2025, 4, 5. https://doi.org/10.3390/colorants4010005
Endrice IA, Gerarduzzi SAF, de Oliveira MC, Bruschi ML, Bassi da Silva J. Optimizing Thermoresponsive and Bioadhesive Systems for Local Application of Erythrosine. Colorants. 2025; 4(1):5. https://doi.org/10.3390/colorants4010005
Chicago/Turabian StyleEndrice, Igor Alves, Sandy Aline Forastieri Gerarduzzi, Mariana Carla de Oliveira, Marcos Luciano Bruschi, and Jéssica Bassi da Silva. 2025. "Optimizing Thermoresponsive and Bioadhesive Systems for Local Application of Erythrosine" Colorants 4, no. 1: 5. https://doi.org/10.3390/colorants4010005
APA StyleEndrice, I. A., Gerarduzzi, S. A. F., de Oliveira, M. C., Bruschi, M. L., & Bassi da Silva, J. (2025). Optimizing Thermoresponsive and Bioadhesive Systems for Local Application of Erythrosine. Colorants, 4(1), 5. https://doi.org/10.3390/colorants4010005






