Adsorption Process of Methyl Orange Dye onto Zinc Hydroxide Nitrate: Kinetic and Thermodynamic Studies
Abstract
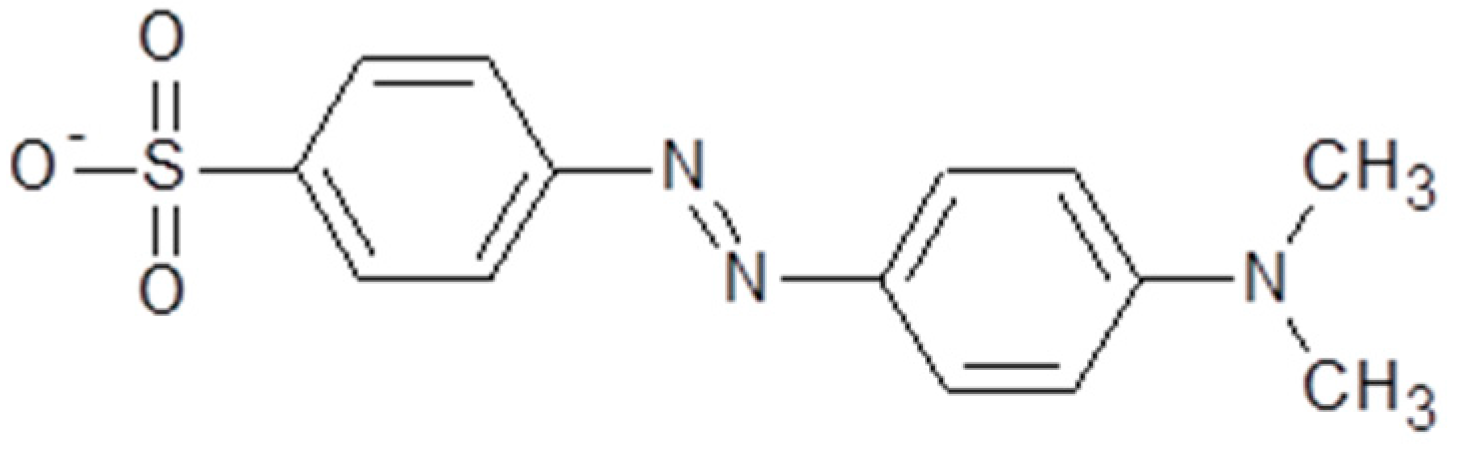
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Zinc Hydroxide Nitrate (ZHN)
2.3. Adsorption Experiments
2.4. Characterizations
3. Results and Discussion
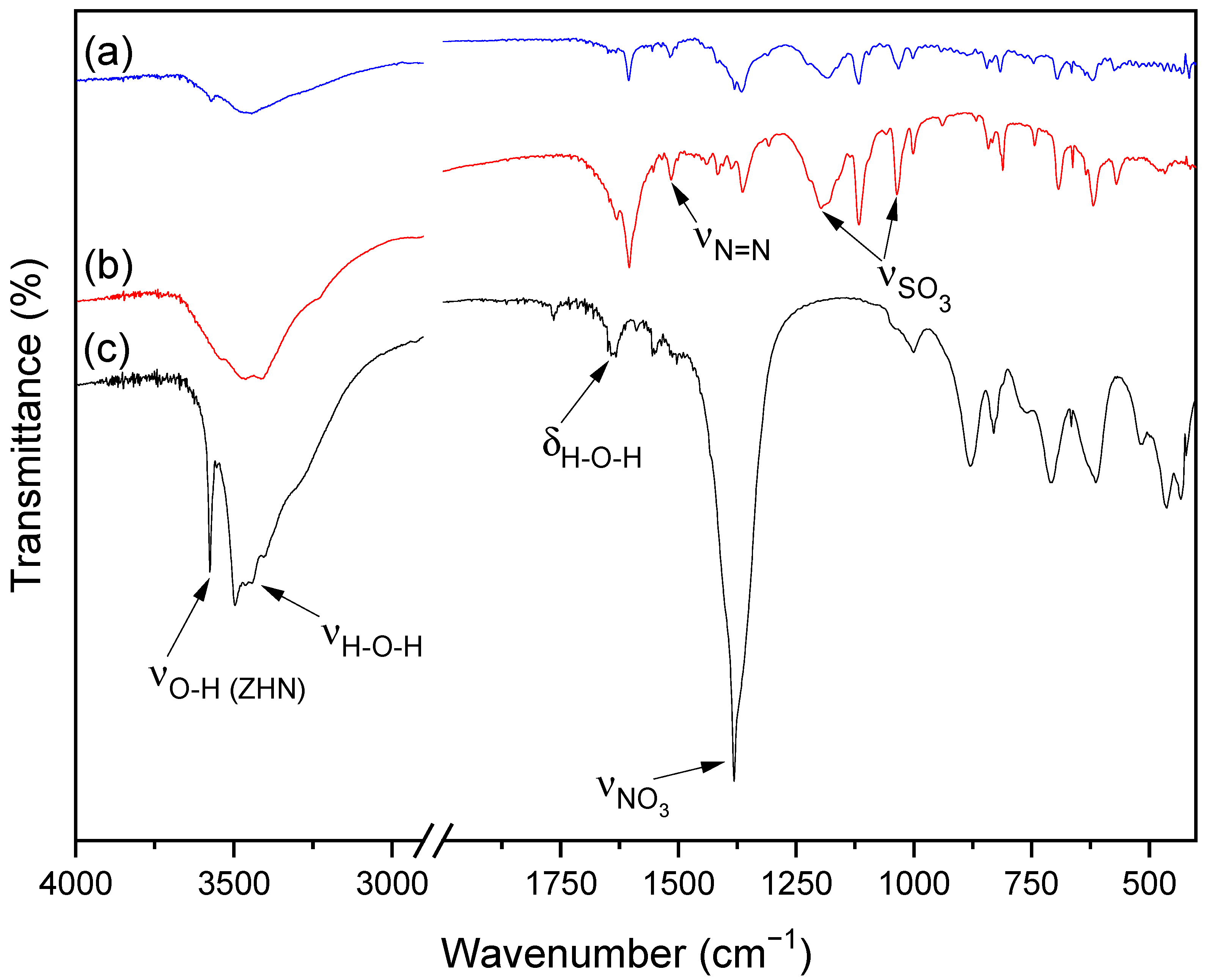
3.1. ZHN and ZHN-MO Characterizations
3.2. Adsorption Experiments
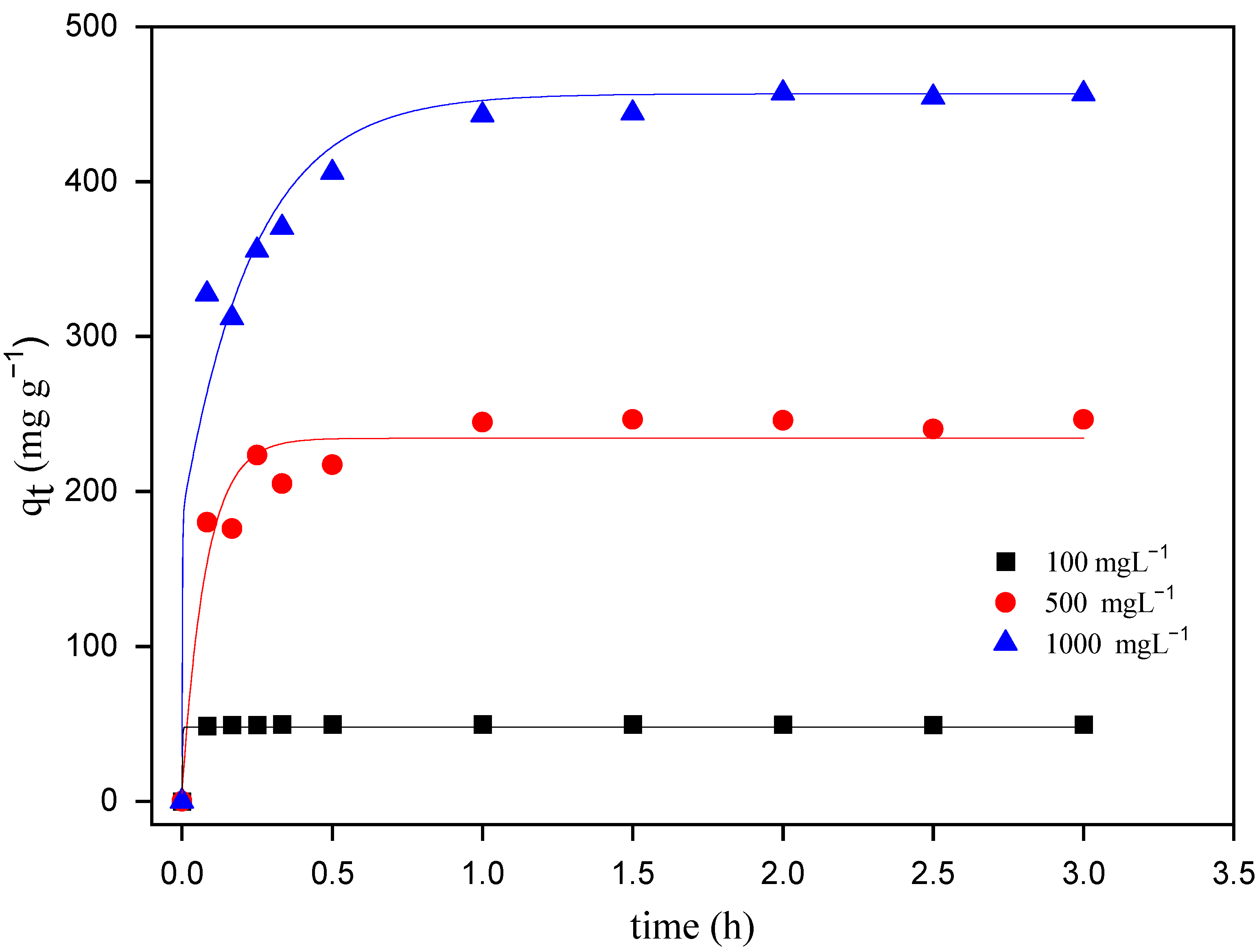
3.2.1. Effect of Initial MO Concentration
3.2.2. Effect of Contact Time
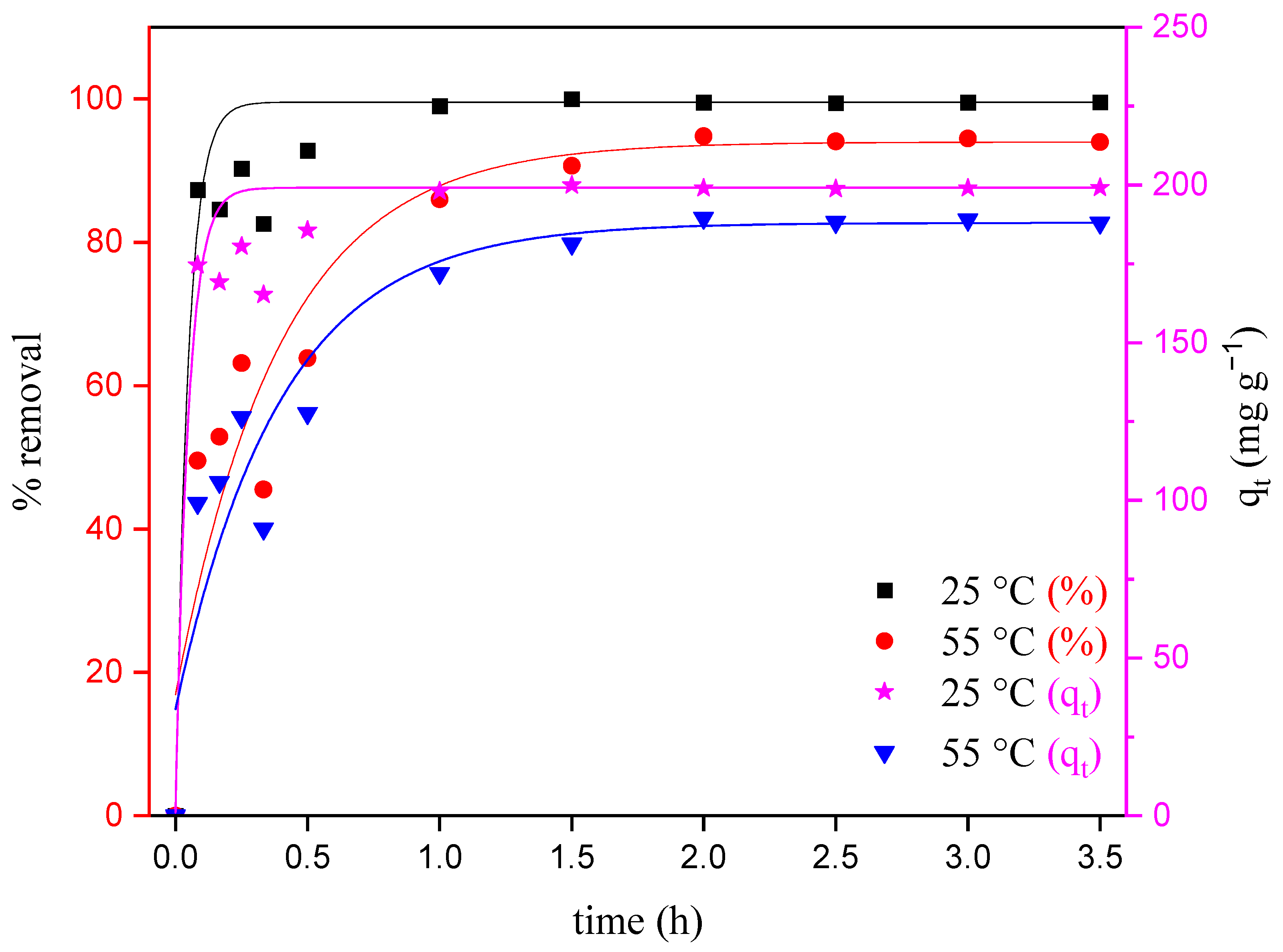
3.2.3. Effect of Temperature
3.2.4. Adsorption Isotherms
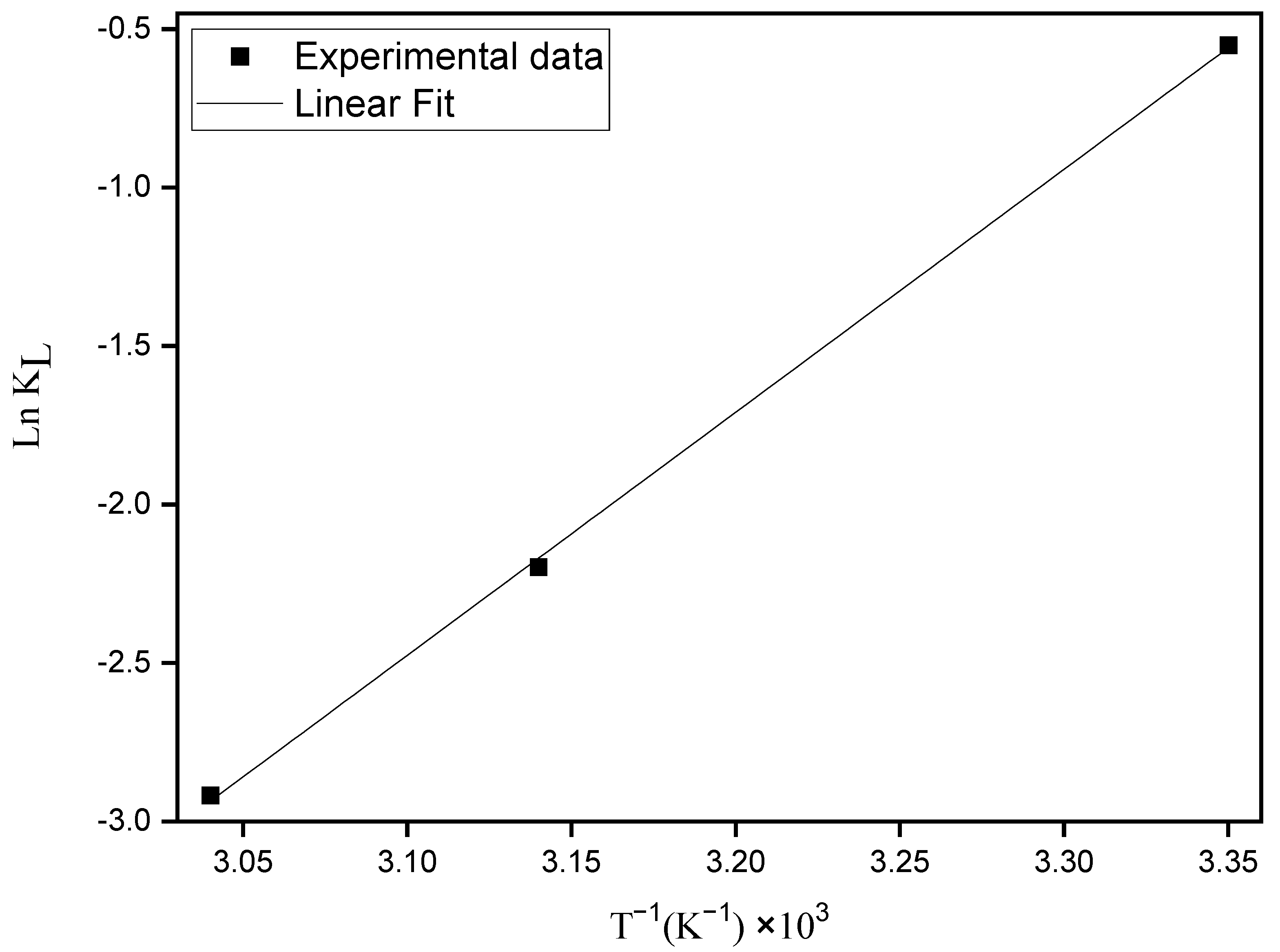
3.2.5. Thermodynamic Parameters
3.2.6. Kinetic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemai, R.; Djebbi, M.A.; Boubakri, S.; Rhaiem, H.B.; Amara, A.B.H. Effective removal of methyl orange dyes using an adsorbent prepared from porous starch aerogel and organoclay. Colorants 2023, 2, 209–229. [Google Scholar] [CrossRef]
- Brar, S.K.; Wangoo, N.; Sharma, R.K. Enhanced and selective adsorption of cationic dyes using novel biocompatible self-assembled peptide fibrils. J. Environ. Manag. 2020, 255, 109804. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.C.J.; Cavalcanti, D.L.; Silva, C.A.A.; Andrade, R.F.S.; Takaki, G.M. Decolorization of black B azo dye by Pseudomonas aeruginosa. Int. J. Microbiol. Appl. Sci. 2015, 4, 720–728. [Google Scholar]
- Meerbergen, K.; Willems, K.; Dewil, R.; Impe, J.V.; Appels, L.; Lievens, B. Isolation and screening of bacterial isolates from wastewater treatment plants to decolorize azo dyes. J. Biosci. Bioeng. 2018, 125, 448–456. [Google Scholar] [CrossRef]
- Wu, Z.; Shan, X.; Li, Z. Preparation of a porous graphene oxide/alkali lignin aerogel composite and its adsorption properties for methylene blue. Int. J. Biol. Macromol. 2019, 143, 325–333. [Google Scholar] [CrossRef]
- Shen, L.; Jiang, X.; Chen, Z.; Fu, D.; Li, Q.; Ouyang, T.; Wang, Y. Chemical reactive of novel amino acids intercalated layered double hydroxides in As(III) and As (V) adsorption. Chemosphere 2017, 176, 57–66. [Google Scholar] [CrossRef]
- Yan, J.; Wang, W.; Wu, K.; Miao, L.; Huang, Y.; Yang, Y. Dehydrogenation of methylcyclohexane over Pt-Sn supported on Mg-Al mixed metal oxides derived from layered double hydroxides. Int. J. Hydrogen Energy 2018, 43, 9343–9352. [Google Scholar] [CrossRef]
- Hou, X.-J.; Li, H.; He, P.; Sun, Z.; Li, S. Structural and electronic analysis of Li/Al layered double hydroxides and their adsorption for CO2. Appl. Surf. Sci. 2017, 416, 411–423. [Google Scholar] [CrossRef]
- Cordeiro, C.S.; Silva, F.R.; Wypych, F.; Ramos, L.P. Catalisadores heterogêneos para produção de monoésteres graxos (biodisel). Quim. Nova 2011, 34, 477–486. [Google Scholar] [CrossRef]
- Liao, C.; Liu, X.; Ren, Y.; Gong, D.; Zhang, Z. Catalytic deoxygenation of vanillin over layered double hydroxide supported Pd catalyst. J. Ind. Eng. Chem. 2018, 68, 380–386. [Google Scholar] [CrossRef]
- Deng, X.; Huang, J.; Wan, H.; Chen, F.; Lin, Y.; Xu, X.; Ma, R.; Sasaki, T. Recent progress in functionalized layered double hydroxides and their application in efficient electrocatalytic water oxidation. J. Energy Chem. 2019, 32, 93–104. [Google Scholar] [CrossRef]
- Arizaga, G.G.C.; Satyanarayana, K.G.; Wypych, F. Layered hydroxide salts: Synthesis, properties, and potential application. Solid State Ion. 2007, 178, 1143–1162. [Google Scholar] [CrossRef]
- Takei, T.; Fuse, H.; Miura, A.; Kumara, N. Topotactic transformation of Ni-based layered double hydroxide film to layered metal oxide and hydroxide. Appl. Clay Sci. 2016, 124–125, 236–242. [Google Scholar] [CrossRef]
- Wang, L.; Dou, Y.; Wang, J.; Han, J.; Liu, L.; Wei, M. Layer-by-layer assembly of layered double hydroxide/rubber multilayer films with excellent gas barrier property. Compos. Part A Appl. Sci. Manuf. 2017, 102, 314–321. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Shamsayei, M. Highly selective and efficient removal of arsenic (V) chromium(VI) and selenium (VI) oxyanions by layered double hydroxide intercalated with zwitterionic glycine. J. Hazard. Mater. 2017, 339, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, S.; Kodama, T.; Fujii, Y.; Kikuch, H.; Fujita, W. Preparation, crystal structure, and magnetic properties of a copper hydroxy salt with diamond chain magnetic network. CrystEngComm 2014, 16, 10385–10388. [Google Scholar] [CrossRef]
- Lee, A.-H.; Tanaka, M.; Takahashi, Y.; Kim, K.-W. Enhanced adsorption of arsenate and antimonate by calcined Mg/Al layered bouble hydroxide: Investigation of comparative adsorption mechanism by surface characterization. Chemosphere 2018, 211, 903–911. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Al-Rashdi, A.A.; Barakat, M.A. Hydrothermal synthesis of structurally variable binary CuAl, MnAl and ternary CuMnAl hydroxides for oxytetracycline antibiotic adsorption. J. Environ. Chem. Eng. 2019, 8, 103535. [Google Scholar] [CrossRef]
- Hidekazu, T.; Kaneda, R.; Fujioka, A.; Ishikwa, T. Synthesis of Ti (IV) substituted calcium hydroxydeapatite microparticles by hydrolysis of phenyl phosphate. J. Adv. Powder Technol. 2010, 21, 169–170. [Google Scholar]
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505. [Google Scholar] [CrossRef]
- Haque, M.M.; Haque, M.A.; Mosharaf, M.K.; Marcus, P.K. Decolorizarion, degragation and detoxification of carcinogenic sulfonated azo dye methyl orange by newly developed biofilm consortia. Saudi J. Biol. Sci. 2021, 28, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, X.; Lv, G.; Zhu, R.; Tian, L.; Liu, M.; Li, W.; Rao, W.; Liu, T.; Liao, L. Study on the adsorption Properties of methyl Orange by natural one-dimensional nano-mineral materials with different structures. Sci. Rep. 2021, 11, 10640. [Google Scholar] [CrossRef] [PubMed]
- Jaerger, S.; Nogueira, D.A.R.; Oliveira, D.S.; Machado, M.V.; Marangoni, R. Study of different morphology of zinc hydroxides salts as adsorbant of azo dyes. ChemistrySelect 2021, 6, 4354–4367. [Google Scholar] [CrossRef]
- Hallajiqomi, M.; Hossein, E. Adsorption of manganese ion using polyaniline and it’s nanocomposite: Kinetics and isotherm studies. J. Ind. Eng. Chem. 2017, 55, 191–197. [Google Scholar] [CrossRef]
- Oliveira, H.B.; Wypych, F. Comparative sorption studies of chromate by nano-and-micro sized Fe2O3 particles. J. Solid State Chem. Sci. 2016, 243, 136–145. [Google Scholar]
- Louer, D.; Louer, M.J. Methode d’essais et erreurs pour l’indexation automatique des diagrammes de poudre. J. Appl. Crystallogr. 1972, 5, 271–275. [Google Scholar] [CrossRef]
- Marangoni, R.; Ramos, L.P.; Wypych, F. New multifunctional materials obtained by the intercalation of anionic dyes into layered zinc hydroxide nitrate followed by dispersion into poly(vinyl alcohol)(PVA). J. Colloid Interface Sci. 2009, 330, 303–309. [Google Scholar] [CrossRef]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Collet, L.; Mello, C.A.D.J. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Asif Tahir, M.; Bhati, H.N.; Lqbal, J.M. Solar Red britlle blue direct dye adsorption onto eucalyptus angophoroides bark: Equilibrium, kinetics and thermodynamic studies. J. Environ. Chem. Eng. 2016, 4, 2431–2439. [Google Scholar] [CrossRef]
- Cui, M.; Johandersson, K.H. Comparison of tungstate and tetrathiotungstate adsorption onto pyrite. Chem. Geol. 2017, 464, 57–68. [Google Scholar] [CrossRef]
- Manzoor, Q.; Nadeem, R.; Lqbal, M.; Saeed, R.; Ansari, T.M. Organic acids pretreatment effect on rosa brourbonia phyto-bomass for removal of Pb (II) and Cu(II) from aqueous media. Bioresour. Technol. 2013, 132, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Badawi, M.A.; Negm, N.A.; Kanac, M.T.H.; Hefnib, H.H.; Moneema, M.M.A. Adsorption of aluminum and lead from wastewater by chitosan-tannic acid modified biopolymers: Isotherms, kinetics, thermodynamics and process mechanics. Int. J. Biol. Macromol. 2017, 99, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.N.; Almeida, C.P.A.; Menezes, C.T.B.; Debacher, N.A.; Sierra, M.M.D. Removal of methylene blue from aqueous solution by peat. J. Hazard. Mater. 2007, 144, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.A.P.; Santos, A.; Jaeger, S.; Debacher, N.A.; Hankins, N.P. Mineral waste from coal mining for removal of astrazon red dye from aqueous solution. Desalination 2010, 264, 181–187. [Google Scholar] [CrossRef]
- Qiu, M.; Huang, C. Removal of dyes from aqueous solution by activated carbon from sewage sludge of the municipal wastewater treatment plant. Water Treat. 2015, 53, 3641–3648. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F.Z. Uber die adsorpition in losungen. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Mahmound, M.E.; Nabil, G.M.; El-Mallah, N.M.; Bassiouny, H.I.; Kumar, S.; Abdel-Fattah, T.M. Kinetics, isotherms, and thermodynamics studies of the adsorpition of reactive red 195 a dye from water by modified swichgrass biochass adsorbent. J. Ind. Eng. Chem. 2016, 37, 156–167. [Google Scholar] [CrossRef]
- Ozer, A.; Akkaya, G.; Turabit, M. Biosorption of acid red 274(Ar274) on enteromorpha in a batch system. J. Hazard. Mater. 2005, 126, 119–127. [Google Scholar] [CrossRef]
- Tay, C.C.; Redwan, G.; Liew, H.H.; Young, S.K.; Surif, S.; Adbuk, T.S. Copper(II) biosorption characteristic of Pleurotus spent mushroom compost. In Proceedings of the International Conference on Science and Social Research (CSSR), Kuala Lumpur, Malaysia, 5–7 December 2010; pp. 6–10. [Google Scholar]
- Hawary, A.; Khraisheh, M.; Al-Ghouth, M.A. Characteristics of olive mill solid residue and its application in remediation of Pb2+ CU2+ and Ni2+ from aqueous solution: Mechanistic studies. Chem. Eng. J. 2014, 251, 329–336. [Google Scholar] [CrossRef]
- Bai, C.; Wang, L.; Zhu, Z. Adsorption of Cr(III) and Pb(II) by graphene oxide/alginate hydrogel membrane: Characterization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2020, 147, 898–910. [Google Scholar] [CrossRef]
- Ho, Y.S. Citation review of langergrem kinetic rate equation on adsorption reaction. Scientometrics 2004, 59, 171–177. [Google Scholar]
- Ho, Y.S.; Mckay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss pear. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Q.; Liu, M.; Daí, Y.; Chen, J.; Huang, H.; Wen, Y.; Zhu, X.; Zhang, X.; Wei, Y. Synthesis of functionalized MgAl-Layered double hydroxides via modified mussel inspired chemistry and their application in organic dye adsorption. J. Colloid Interface Sci. 2017, 505, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.R.; Boaventura, R.A.R. Adsorption of cationic and anionic azo dyes on sepiolite clay: Equilibrium and kinetic studies in batch mode. J. Environ. Chem. Eng. 2016, 4, 1473–1483. [Google Scholar] [CrossRef]


| T (°C) | Initial Concentration of MO in mg L−1 (C0) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 900 | 1000 | |
| Amount of MO Mass Adsorbed by ZHN in mg g−1 (qe) | ||||||||||
| 25 | 49.9 | 97.2 | 148.0 | 197.3 | 243.1 | 280.4 | 288.4 | 361.5 | 413.2 | 413.2 |
| 45 | 49.1 | 99.0 | 148.8 | 198.0 | 247.9 | 296.4 | 336.0 | 357.4 | 405.2 | 431.6 |
| 55 | 48.3 | 96.0 | 144.8 | 185.8 | 240.3 | 240.3 | 299.1 | 301.9 | 316.1 | 346.4 |
| Percentage of Removal of MO by ZHN (%) | ||||||||||
| 25 | 99.4 | 99.4 | 99.8 | 99.5 | 98.5 | 97.5 | 98.9 | 94.3 | 91.2 | 91.3 |
| 45 | 97.8 | 99.0 | 99.1 | 99.5 | 99.4 | 99.3 | 98.6 | 97.2 | 96.7 | 90.8 |
| 55 | 96.9 | 96.0 | 96.1 | 94.5 | 96.1 | 92.8 | 91.9 | 75.5 | 72.6 | 71.2 |
| T (°C) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL (L mg−1) | Q (mg g−1) | r | 1/n | KF | r | |
| (L g−1) | ||||||
| 25 | 0.576 | 333.3 | 0.97063 | 0.297 | 101.955 | 0.80239 |
| 45 | 0.111 | 500.0 | 0.98597 | 0.348 | 90.357 | 0.66985 |
| 55 | 0.054 | 357.1 | 0.99548 | 0.384 | 52.558 | 0.57379 |
| T (°C) | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| qexp (mg g−1) | k1 (min−1) | qcalc (mg g−1) | r1 | k2 (g mg−1 min−1) | qcalc (mg g−1) | r2 | |
| 25 | 413.21 | −4.15 | 63.91 | 0.372 | 0.122 | 411.52 | 0.999 |
| 45 | 405.22 | 0.04 | −0.453 | 0.790 | 0.040 | 429.18 | 0.999 |
| 55 | 316.11 | 0.15 | −0.858 | 0.823 | 0.050 | 320.51 | 0.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, D.A.d.R.; Zanela, T.M.P.; Machado, M.V.; Almeida, C.A.P.; Marangoni, R. Adsorption Process of Methyl Orange Dye onto Zinc Hydroxide Nitrate: Kinetic and Thermodynamic Studies. Colorants 2023, 2, 565-577. https://doi.org/10.3390/colorants2030028
Nogueira DAdR, Zanela TMP, Machado MV, Almeida CAP, Marangoni R. Adsorption Process of Methyl Orange Dye onto Zinc Hydroxide Nitrate: Kinetic and Thermodynamic Studies. Colorants. 2023; 2(3):565-577. https://doi.org/10.3390/colorants2030028
Chicago/Turabian StyleNogueira, Daiane Amaral de Ramos, Tânia Marina Palhano Zanela, Monielly Viomar Machado, Carlos Alberto Policiano Almeida, and Rafael Marangoni. 2023. "Adsorption Process of Methyl Orange Dye onto Zinc Hydroxide Nitrate: Kinetic and Thermodynamic Studies" Colorants 2, no. 3: 565-577. https://doi.org/10.3390/colorants2030028
APA StyleNogueira, D. A. d. R., Zanela, T. M. P., Machado, M. V., Almeida, C. A. P., & Marangoni, R. (2023). Adsorption Process of Methyl Orange Dye onto Zinc Hydroxide Nitrate: Kinetic and Thermodynamic Studies. Colorants, 2(3), 565-577. https://doi.org/10.3390/colorants2030028






