Synergistic Effect of BiVO4/P-g-C3N4 Heterojunction with Enhanced Optoelectronic Properties on Synthetic Colorants under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Phosphorous Doped g-C3N4 Nanosheet
2.3. Synthesis of BiVO4
2.4. Preparation of BiVO4/P-g-C3N4 Composite
2.5. Characterizations
2.6. Photocatalytic and Photoelectrochemical Measurements
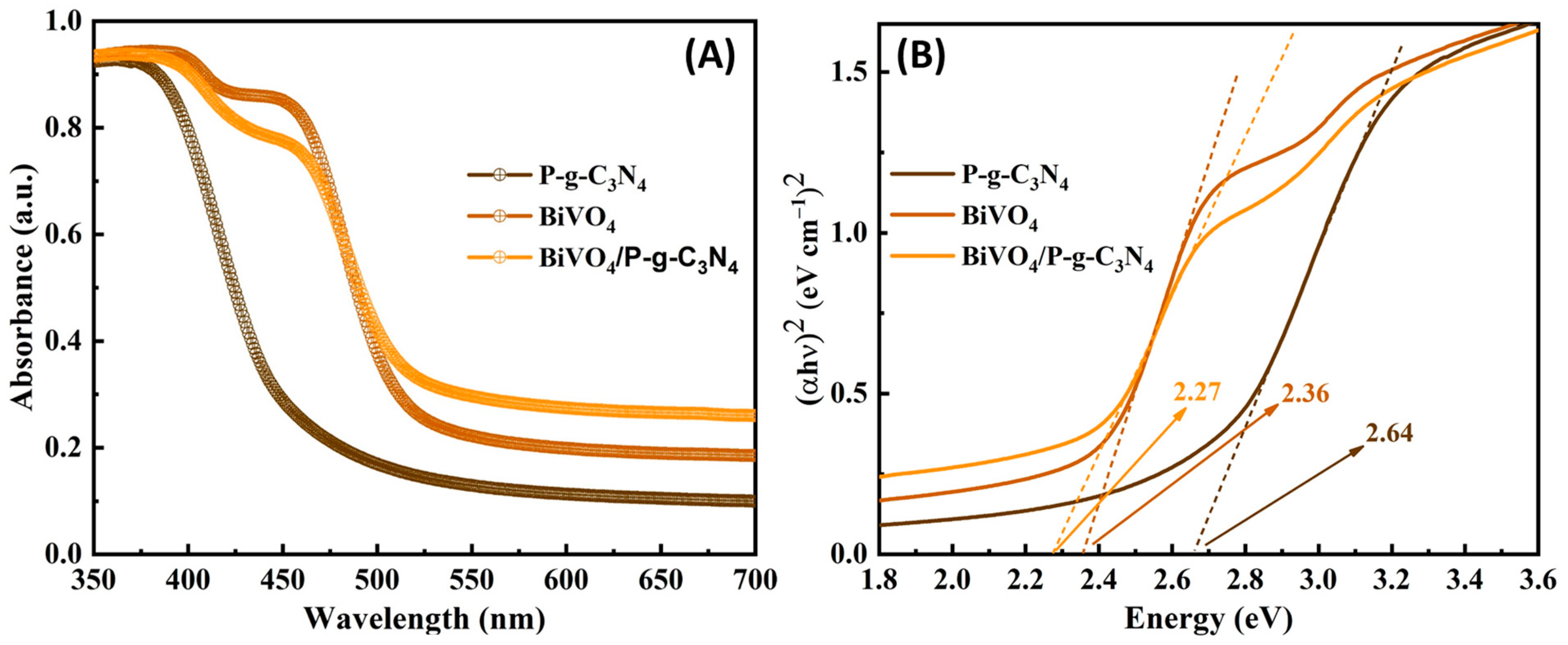
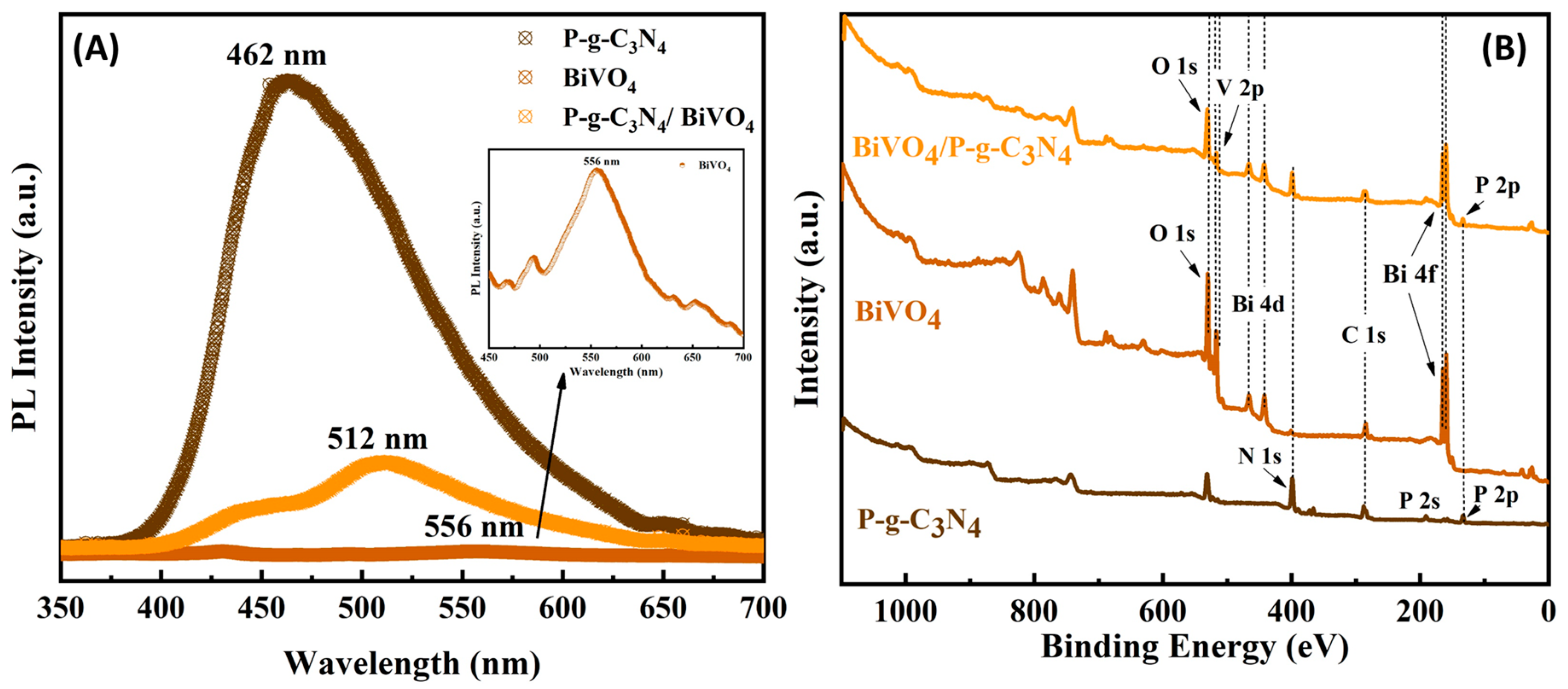
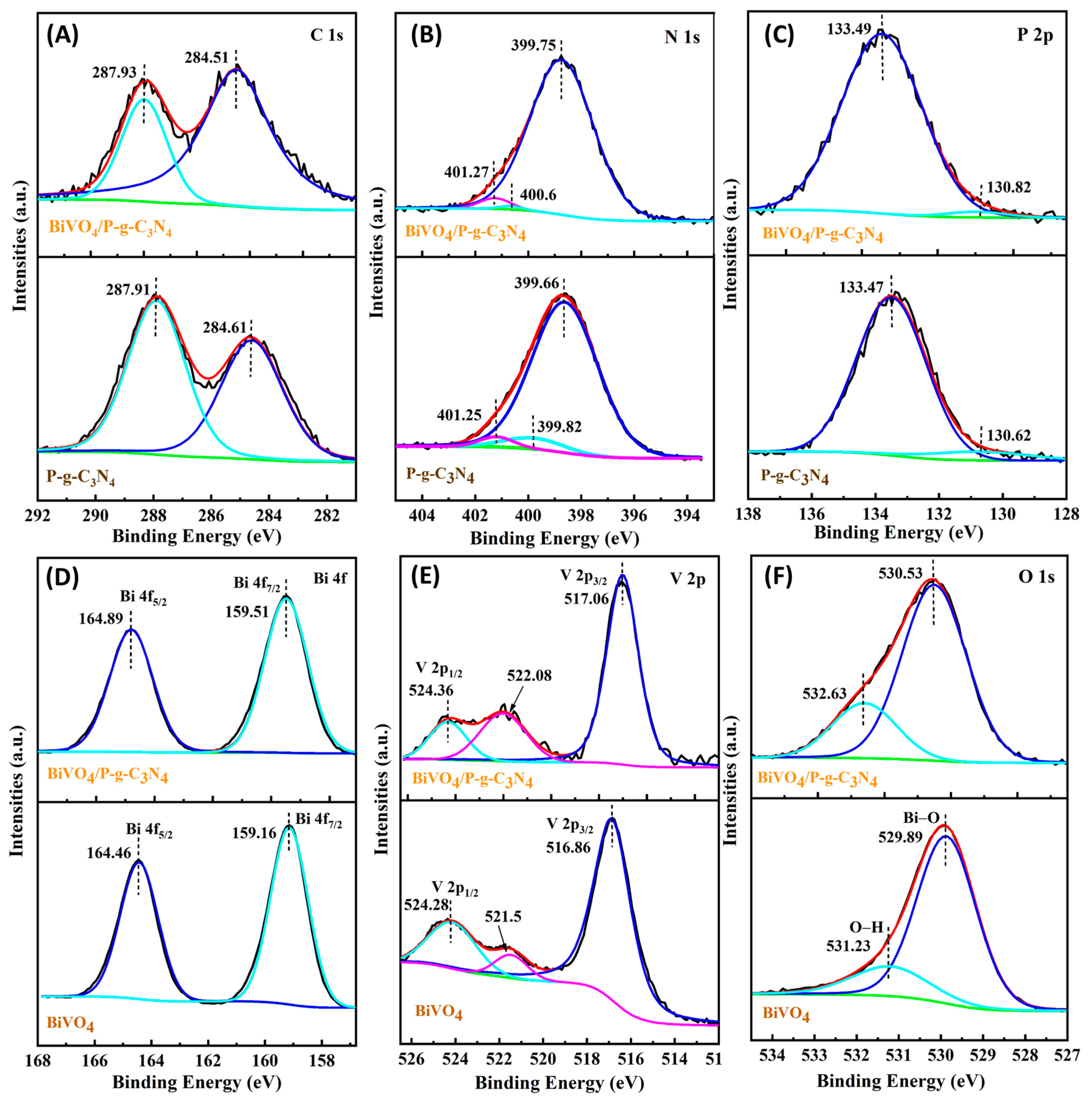
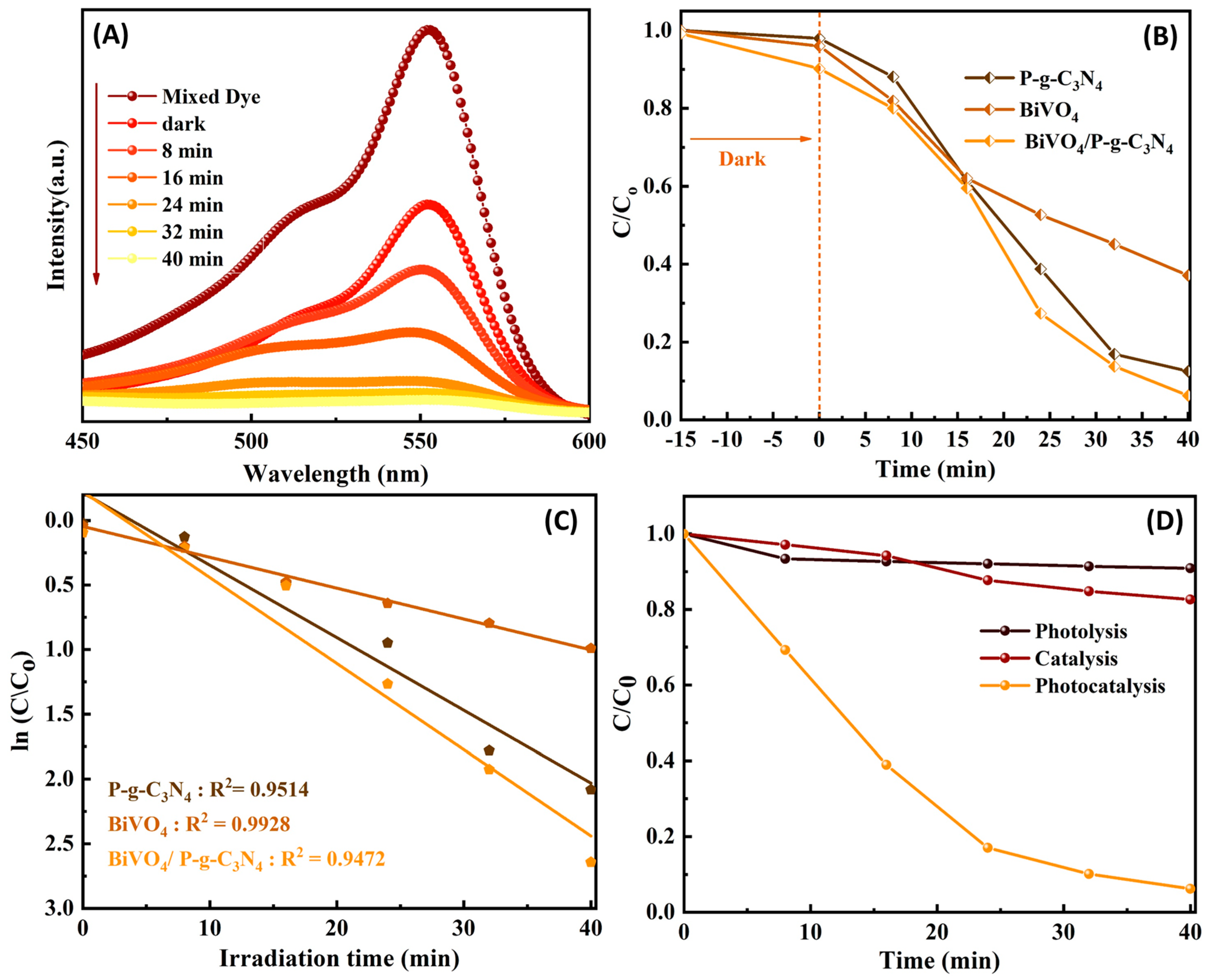
3. Results and Discussion
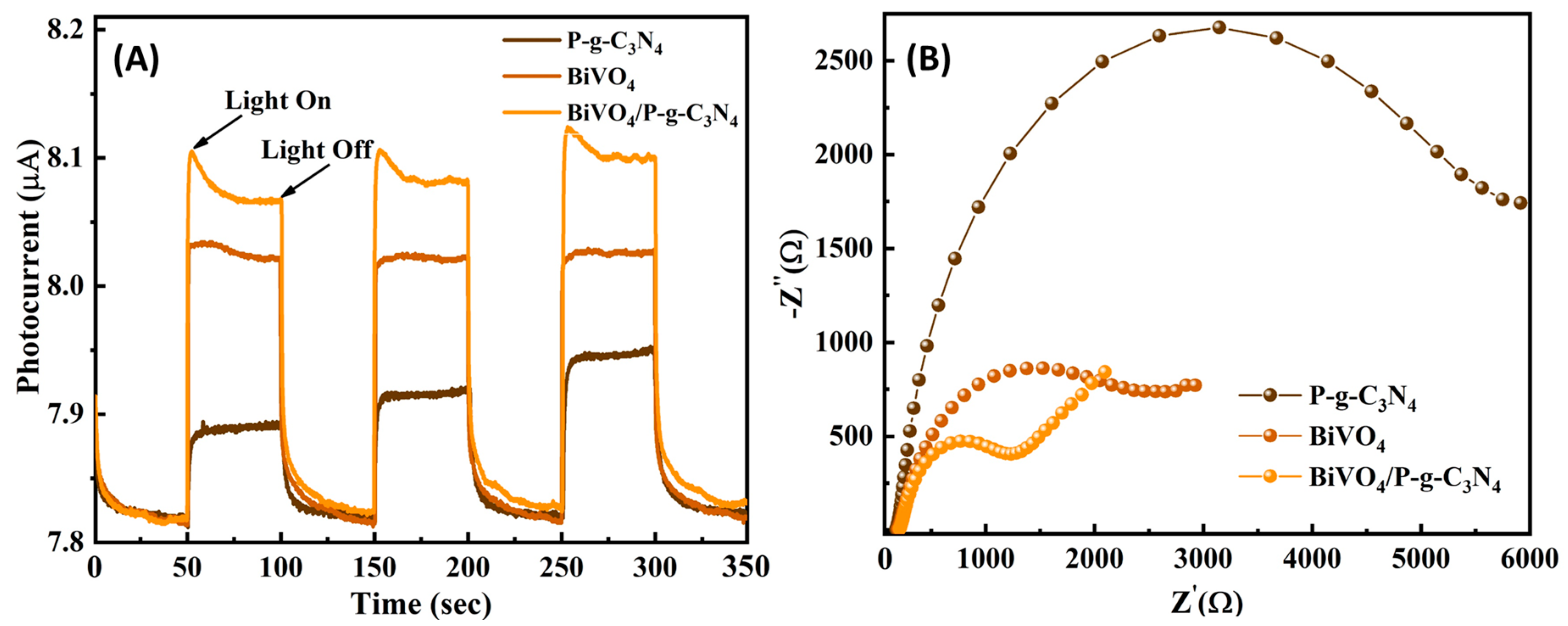
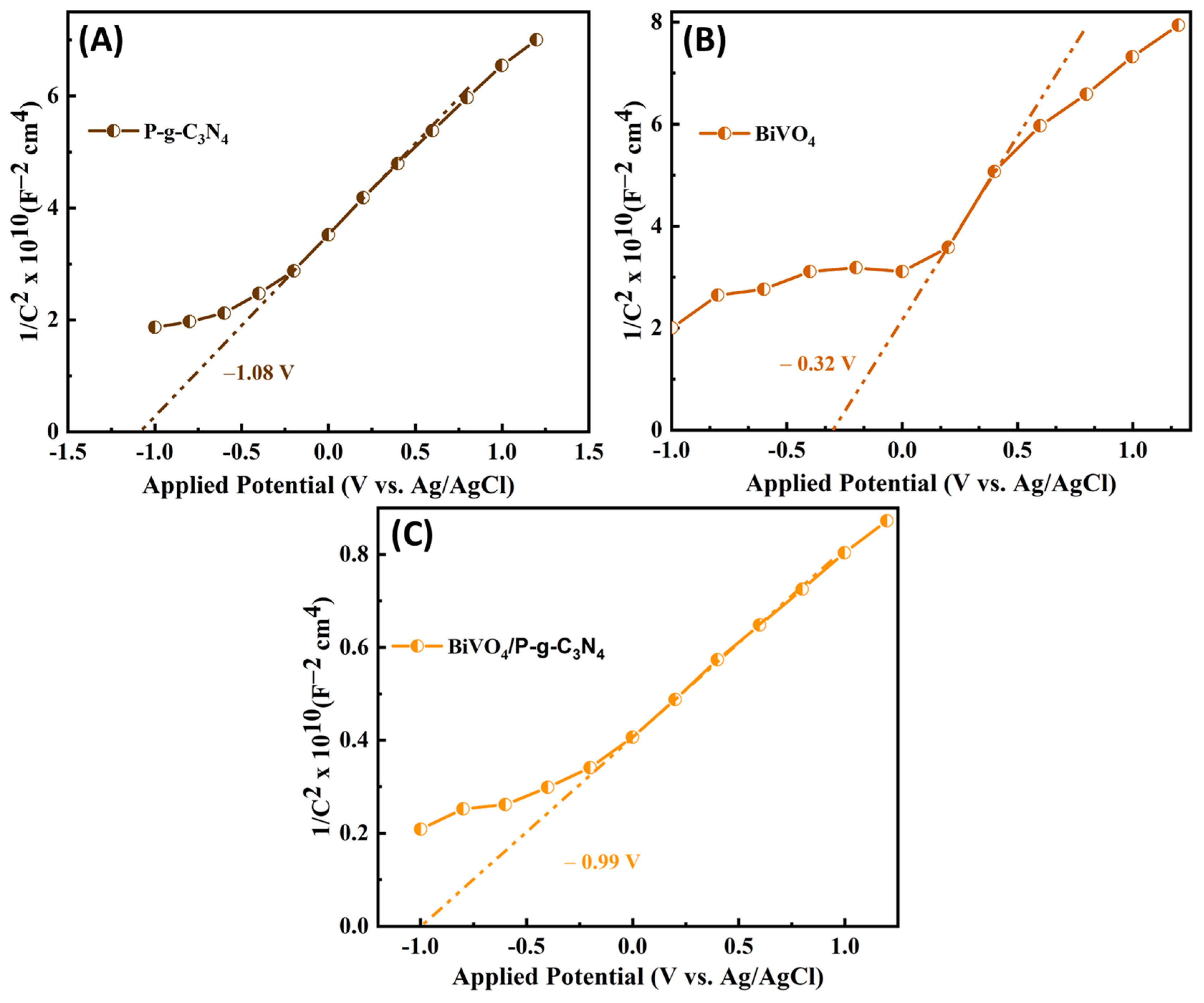
3.1. Photoelectrochemical Activities of BiVO4/P-g-C3N4 Heterojunction
| Material | Analyte | Removal (%) | Time (min) | Ref. |
|---|---|---|---|---|
| P-g-C3N4 (PCN-15) | RhB | 98 | 50 | [15] |
| P@P-g-C3N4 | RhB | 99.9 | 50 | [49] |
| m-BiVO4 NPs | RhB | 64.81 | 210 | [50] |
| P-O-C3N4 | RhB | 95 | 120 | [51] |
| ZnCo2O4/BiVO4 | RhB | 97.9 | 150 | [52] |
| Pt/Bi2MoO6 | RhB | 98.61 | 120 | [53] |
| Ag/Bi2MoO6 | RhB | 74 | 120 | [54] |
| BiVO4/P-g-C3N4 | RhB + CR | 96.94 | 40 | This work |
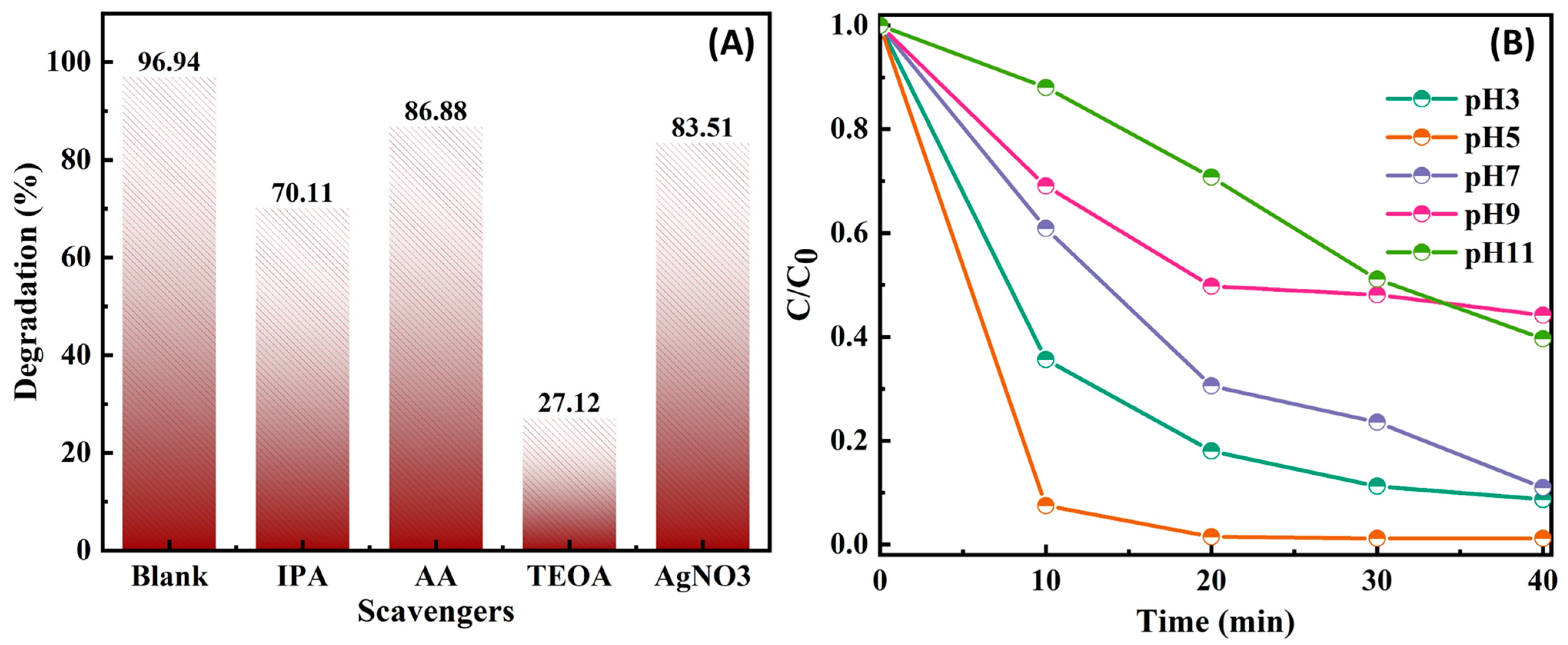
3.2. Photocatalytic Reaction Mechanism of BiVO4/P-g-C3N4 Heterojunction
3.3. Reusability and Stability of the Photocatalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santana, R.W.R.; Lima, A.E.B.; Souza, L.K.C.D.; Santos, E.C.S.; Santos, C.C.; Menezes, A.S.D.; Sharma, S.K.; Cavalcante, L.S.; da Costa, M.E.H.M.; Sales, T.O.; et al. BiOBr/ZnWO4 heterostructures: An important key player for enhanced photocatalytic degradation of rhodamine B dye and antibiotic ciprofloxacin. J. Phys. Chem. Solids 2023, 173, 111093. [Google Scholar] [CrossRef]
- Tian, H.; Wu, H.; Fang, Y.; Li, R.; Huang, Y. Hydrothermal synthesis of m-BiVO4/t-BiVO4 heterostructure for organic pollutants degradation: Insight into the photocatalytic mechanism of exposed facets from crystalline phase controlling. J. Hazard. Mater. 2020, 399, 123159. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabboh, H.S.M.; Benaissa, M.; Hamdy, M.S.; Ahmed, M.A.; Glal, M. Synthesis of an efficient, and recyclable mesoporous BiVO4/TiO2 direct Z-scheme heterojunction by sonochemical route for photocatalytic hydrogen production and photodegradation of rhodamine B dye in the visible region. Opt. Mater. 2021, 114, 110761. [Google Scholar] [CrossRef]
- Ishihara, T.; Baik, N.S.; Ono, N.; Nishiguchi, H.; Takita, Y. Effects of crystal structure on photolysis of H2O on K-Ta mixed oxide. J. Photochem. Photobiol. A Chem. 2004, 167, 149–157. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Yang, S.; Lin, X.; Shi, W. Fabrication of a ternary heterostructure BiVO4 quantum dots/C60/g-C3N4 photocatalyst with enhanced photocatalytic activity. J. Phys. Chem. Solids 2020, 136, 109164. [Google Scholar] [CrossRef]
- Long, M.; Cai, W.; Cai, J.; Zhou, B.; Chai, X.; Wu, Y. Efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under visible light irradiation. J. Phys. Chem. B 2006, 110, 20211–20216. [Google Scholar] [CrossRef]
- Long, M.; Cai, W.; Kisch, H. Visible light induced photoelectrochemical properties of n-BiVO4 and n-BiVO4/p-Co3O4. J. Phys. Chem. C 2008, 112, 548–554. [Google Scholar] [CrossRef]
- Raeisi, A.; Chermahini, A.N.; Momeni, M.M. A novel photocatalytic and photoelectrocatalytic system for oxidative desulfurization of model fuel using BiVO4@HKUST-1 composite in powder and deposited on fluorine-doped tin oxide. J. Photochem. Photobiol. A Chem. 2022, 433, 114190. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Tang, Y.; Ng, Y.H.; Li, L.; Jiang, F.; Wang, J.; Chen, W.; Li, L. Understanding photoelectrocatalytic degradation of tetracycline over three-dimensional coral-like ZnO/BiVO4 nanocomposite. Mater. Chem. Phys. 2021, 271, 124871. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Wang, X. Two-dimensional covalent carbon nitride nanosheets: Synthesis, functionalization, and applications. Energy Environ. Sci. 2015, 8, 3092–3108. [Google Scholar] [CrossRef]
- Fang, H.B.; Luo, Y.; Zheng, Y.Z.; Ma, W.; Tao, X. Facile Large-Scale Synthesis of Urea-Derived Porous Graphitic Carbon Nitride with Extraordinary Visible-Light Spectrum Photodegradation. Ind. Eng. Chem. Res. 2016, 55, 4506–4514. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Zhu, Y. Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods. J. Phys. Chem. C 2013, 117, 9952–9961. [Google Scholar] [CrossRef]
- Li, J.; Shen, B.; Hong, Z.; Lin, B.; Gao, B.; Chen, Y. A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem. Commun. 2012, 48, 12017–12019. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.B.; Zhang, X.H.; Wu, J.; Li, N.; Zheng, Y.Z.; Tao, X. Fragmented phosphorus-doped graphitic carbon nitride nanoflakes with broad sub-bandgap absorption for highly efficient visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 225, 397–405. [Google Scholar] [CrossRef]
- Meng, R.; Tang, S.; Xia, B.; Chen, X. Controllable Synthesis of Phosphorus-Doped Graphitic Carbon Nitride via a Simple Phosphorus Compound Towards Enhanced Visible-Light Photocatalytic Performance. ChemistrySelect 2020, 5, 13862–13867. [Google Scholar] [CrossRef]
- Sun, H.; Park, S.J. Phosphorus-doped g-C3N4/SnS nanocomposite for efficient photocatalytic reduction of aqueous Cr(VI) under visible light. Appl. Surf. Sci. 2020, 531, 147325. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, J.; Deng, X.; Liu, J. One-step synthesis of phosphorus-doped g-C3N4/Co3O4 quantum dots from vitamin B12 with enhanced visible-light photocatalytic activity for metronidazole degradation. Chem. Eng. J. 2019, 360, 1517–1529. [Google Scholar] [CrossRef]
- Ai, Z.; Shao, Y.; Chang, B.; Zhang, L.; Shen, J.; Wu, Y.; Huang, B.; Hao, X. Rational modulation of p-n homojunction in P-doped g-C3N4 decorated with Ti3C2 for photocatalytic overall water splitting. Appl. Catal. B Environ. 2019, 259, 118077. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Wang, F.; Liu, D.; Gui, J. A simple and efficient method to prepare a phosphorus modified g-C 3N4 visible light photocatalyst. RSC Adv. 2014, 4, 21657–21663. [Google Scholar] [CrossRef]
- Jiang, T.; Nan, F.; Zhou, J.; Zheng, F.; Weng, Y.; Cai, T.Y.; Ju, S.; Xu, B.; Fang, L. Enhanced photocatalytic and photoelectrochemical performance of g-C3N4/BiVO4 heterojunction: A combined experimental and theoretical study. AIP Adv. 2019, 9, 055225. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhang, L.; Liu, J.; Fan, X.; Wang, B.; Wang, M.; Ren, W.; Wang, J.; Li, M.; Shi, J. Brand new P-doped g-C3N4: Enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light. J. Mater. Chem. A 2015, 3, 3862–3867. [Google Scholar] [CrossRef]
- Vicas, C.S.; Keerthiraj, N.; Byrappa, N.; Byrappa, K. Hydrothermally synthesized al-doped bivo4 as a potential antibacterial agent against methicillin-resistant staphylococcus aureus. Environ. Eng. Res. 2019, 24, 566–571. [Google Scholar] [CrossRef]
- Longchin, P.; Pookmanee, P.; Satienperakul, S.; Sangsrichan, S.; Puntharod, R.; Kruefu, V.; Kangwansupamonkon, W.; Phanichphant, S. Characterization of bismuth vanadate (BiVO4) nanoparticle prepared by solvothermal method. Integr. Ferroelectr. 2016, 175, 18–24. [Google Scholar] [CrossRef]
- Zhu, Z.; Chiang, Z.X.; Wu, R.J.; Kumar, U.; Wu, C.H. A combined experimental and theoretical study of composite SnO2–BiVO4 for selective NO2 sensing. Mater. Chem. Phys. 2022, 292, 126868. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Zhou, Y.Y.; Le Cao, J.; Yuan, M.; Lv, H. Phosphorous doped g-C3N4 supported cobalt phthalocyanine: An efficient photocatalyst for reduction of CO2 under visible-light irradiation. J. Colloid Interface Sci. 2021, 594, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Chala, S.; Wetchakun, K.; Phanichphant, S.; Inceesungvorn, B.; Wetchakun, N. Enhanced visible-light-response photocatalytic degradation of methylene blue on Fe-loaded BiVO4 photocatalyst. J. Alloys Compd. 2014, 597, 129–135. [Google Scholar] [CrossRef]
- Katsumata, H.; Tateishi, I.; Furukawa, M.; Kaneco, S. Highly photocatalytic hydrogen generation over P-doped g-C3N4 with aromatic ring structure. Mater. Lett. 2021, 299, 130068. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Bui, Q.T.P.; Vo, D.V.N.; Lim, K.T.; Bach, L.G.; Do, S.T.; Van Nguyen, T.; Doan, V.D.; Nguyen, T.D.; Nguyen, T.D. Effective photocatalytic activity of sulfate-modified BiVO4 for the decomposition of methylene blue under LED visible light. Materials 2019, 12, 2681. [Google Scholar] [CrossRef] [Green Version]
- Manikantan, K.; Shanmugasundaram, K.; Thirunavukkarasu, P. Enhanced photocatalytic dye degradation and hydrogen evolution performance of Cu encapsulated BiVO4 under visible light irradiation. Chem. Phys. Impact. 2023, 6, 100178. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, L.; Tian, F.; Liu, Q.; Zhao, L.; Guan, Y.; Li, F.; Guan, Z.; Zhang, H. Fabrication of BiVO4/BiOIO3 Heterojunctions via Hydrothermal Method for Photocatalytic Activity Under Visible Light. Catal. Lett. 2018, 148, 3193–3204. [Google Scholar] [CrossRef]
- Sivakumar, V.; Suresh, R.; Giribabu, K.; Narayanan, V. Narayanan, BiVO4 nanoparticles: Preparation, characterization and photocatalytic activity. Cogent Chem. 2015, 1, 1074647. [Google Scholar] [CrossRef]
- Luo, X.L.; Chen, Z.Y.; Yang, S.Y.; Xu, Y.H. Two-step hydrothermal synthesis of peanut-shaped molybdenum diselenide/bismuth vanadate (MoSe2/BiVO4) with enhanced visible-light photocatalytic activity for the degradation of glyphosate. J. Colloid Interface Sci. 2018, 532, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Shi, J.W.; Pu, Z.; Mao, S.; Xu, X.; He, D.; Guo, R.; Chen, F. Decorating Phosphorus-Doped g-C3N4 with Zinc Porphyrin Metal–Organic Framework via an Electrostatic Self-Assembly Process: An Efficient Strategy to Boost Photocatalytic Hydrogen Evolution Performance. Sol. RRL 2022, 6, 1–11. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; Wang, X. High Efficient Photodegradation and Photocatalytic Hydrogen Production of CdS/BiVO4 Heterostructure through Z-Scheme Process. ACS Sustain. Chem. Eng. 2017, 5, 303–309. [Google Scholar] [CrossRef]
- Rahman, M.M.; Muttakin, M.; Pal, A.; Shafiullah, A.Z.; Saha, B.B. A statistical approach to determine optimal models for IUPAC-classified adsorption isotherms. Energies 2019, 12, 4565. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.M.; Shad, N.A.; Javed, Y.; Khan, S.B.; Zhang, Z.; Amin, N. Study of the interfacial charge transfer in bismuth vanadate/reduce graphene oxide (BiVO4/rGO) composite and evaluation of its photocatalytic activity. Res. Chem. Intermed. 2020, 46, 1201–1215. [Google Scholar] [CrossRef]
- Sajid, M.M.; Khan, S.B.; Shad, N.A.; Amin, N.; Zhang, Z. Visible light assisted photocatalytic degradation of crystal violet dye and electrochemical detection of ascorbic acid using a BiVO4/FeVO4 heterojunction composite. RSC Adv. 2018, 8, 23489–23498. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Geng, P.; Li, X.; Zhao, Q.; Quan, X.; Chen, G. Novel phosphorus doped carbon nitride modified TiO2 nanotube arrays with improved photoelectrochemical performance. Nanoscale 2015, 7, 16282–16289. [Google Scholar] [CrossRef]
- Qiu, Y.; Xin, L.; Jia, F.; Xie, J.; Li, W. Three-Dimensional Phosphorus-Doped Graphitic-C3N4 Self-Assembly with NH2-Functionalized Carbon Composite Materials for Enhanced Oxygen Reduction Reaction. Langmuir 2016, 32, 12569–12578. [Google Scholar] [CrossRef]
- Chen, W.; Liu, T.Y.; Huang, T.; Liu, X.H.; Yang, X.J. Novel mesoporous P-doped graphitic carbon nitride nanosheets coupled with ZnIn2S4 nanosheets as efficient visible light driven heterostructures with remarkably enhanced photo-reduction activity. Nanoscale 2016, 8, 3711–3719. [Google Scholar] [CrossRef]
- Mali, S.S.; Park, G.R.; Kim, H.; Kim, H.H.; Patil, J.V.; Hong, C.K. Synthesis of nanoporous Mo:BiVO4 thin film photoanodes using the ultrasonic spray technique for visible-light water splitting. Nanoscale Adv. 2019, 1, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.S.; Mali, M.G.; Hassan, M.A.; Patil, D.R.; Kolekar, S.S.; Ryu, S.W. One-pot in situ hydrothermal growth of BiVO4/Ag/rGO hybrid architectures for solar water splitting and environmental remediation. Sci. Rep. 2017, 7, 8404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, Y.; Xie, X.; Li, C.; Si, Y.; Zhang, M.; Yan, Q. Synthesis flower-like BiVO4/BiOI core/shell heterostructure photocatalyst for tetracycline degradation under visible-light irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 9311–9321. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Fang, W.; Yang, Y.; Liu, J.; Lu, Q.; Yan, J.; Shangguan, W. Self-assembly core-shell BixY1-xVO4@g-C3N4 as an S-scheme heterojunction photocatalyst for pure water splitting. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar] [CrossRef]
- Hong, Y.; Yang, L.; Tian, Y.; Lin, X.; Liu, E.; Sun, W.; Liu, Y.; Zhu, C.; Li, X.; Shi, J. Rational design 2D/3D MoS2/In2O3 composites for great boosting photocatalytic H2 production coupled with dye degradation. J. Taiwan Inst. Chem. Eng. 2023, 146, 104862. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X. Facile fabrication of phosphorus-doped g-C3N4 exhibiting enhanced visible light photocatalytic degradation performance toward textile dye. Solid State Sci. 2019, 89, 150–155. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.H.; Fan, L.K.; Huang, G.Q.; Gao, Z.P.; Zeng, T. Enhanced Visible-light-driven Photocatalytic Activity of Multiferroic KBiFe2O5 by Adjusting pH Value. Wuji Cailiao Xuebao/J. Inorg. Mater. 2018, 33, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zhang, D.; Zhou, H.; Pi, M.; Wang, X.; Chen, S. Coupling P Nanostructures with P-Doped g-C3N4 As Efficient Visible Light Photocatalysts for H2 Evolution and RhB Degradation. ACS Sustain. Chem. Eng. 2018, 6, 6342–6349. [Google Scholar] [CrossRef]
- Sajid, M.M.; Amin, N.; Shad, N.A.; Khan, S.B.; Javed, Y.; Zhang, Z. Hydrothermal fabrication of monoclinic bismuth vanadate (m-BiVO4) nanoparticles for photocatalytic degradation of toxic organic dyes. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 242, 83–89. [Google Scholar] [CrossRef]
- Ma, H.; Li, Y.; Li, S.; Liu, N. Novel P-O codoped g-C 3 N 4 with large specific surface area: Hydrothermal synthesis assisted by dissolution-precipitation process and their visible light activity under anoxic conditions. Appl. Surf. Sci. 2015, 357, 131–138. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, S.; Mao, Y.; Liu, X. Preparation of ZnCo2O4/BiVO4 Z-Scheme heterostructures to enhance photocatalytic performance in organic pollutant and antibiotic removal. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130165. [Google Scholar] [CrossRef]
- Pinchujit, S.; Phuruangrat, A.; Wannapop, S.; Thongtem, T.; Thongtem, S. Sonochemical-assisted synthesis of Pt/Bi2MoO6 nanocomposites for efficient photodegradation of rhodamine B. Opt. Mater. 2023, 135, 113265. [Google Scholar] [CrossRef]
- Dai, Z.X.; Zhang, L.Y.; Ruan, C.L.; Yun, Z.Q.; Zheng, G.H. Synthesis of Ag/Bi2MoO6 composite nanosheets for enhanced photocatalytic degradation of RhB solution. Dig. J. Nanomater. Biostruct. 2022, 17, 179–191. [Google Scholar] [CrossRef]
- Hemmatpour, P.; Nezamzadeh-Ejhieh, A. A Z-scheme CdS/BiVO4 photocatalysis towards Eriochrome black T: An experimental design and mechanism study. Chemosphere 2022, 307, 135925. [Google Scholar] [CrossRef] [PubMed]
- Skompska, M. Electrochimica Acta Mechanistic insight into photochemical and photoelectrochemical degradation of organic pollutants with the use of BiVO4 and BiVO4/Co-Pi. Electrochim. Acta 2022, 434, 141292. [Google Scholar] [CrossRef]
- Ren, X.; Wu, K.; Qin, Z.; Zhao, X.; Yang, H. The construction of type II heterojunction of Bi2WO6/BiOBr photocatalyst with improved photocatalytic performance. J. Alloys Compd. 2019, 788, 102–109. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Zhang, J.; Bai, F.; Zhang, H. Applied Surface Science First-principles investigation on the interfacial interaction and electronic structure of BiVO4/WO3 heterostructure semiconductor material. Appl. Surf. Sci. 2021, 549, 149309. [Google Scholar] [CrossRef]
- Anand, P.; Verma, A.; Hong, Y.; Hu, A.; Prabu, D.; Wong, M.; Fu, Y. Chemosphere Morphological and elemental tuning of BiOCl/BiVO4 heterostructure for uric acid electrochemical sensor and antibiotic photocatalytic degradation. Chemosphere 2023, 310, 136847. [Google Scholar] [CrossRef]
- Ma, C.; Din, S.T.U.; Seo, W.C.; Lee, J.; Kim, Y.; Jung, H.; Yang, W. BiVO4 ternary photocatalyst co-modified with N-doped graphene nanodots and Ag nanoparticles for improved photocatalytic oxidation: A significant enhancement in photoinduced carrier separation and broad-spectrum light absorption. Sep. Purif. Technol. 2021, 264, 118423. [Google Scholar] [CrossRef]




| Sample | BET Surface Area (m2 g−1) SBET | Pore Volume (cm3 g−1) Vpore | Pore Diameter (nm) |
|---|---|---|---|
| P-g-C3N4 | 5.7441 | 0.060259 | 416.38 |
| BiVO4 | 2.7228 | 0.027293 | 400.96 |
| BiVO4/P-g-C3N4 | 3.8993 | 0.051746 | 532.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, A.; Balu, S.; Lan, K.-W.; Wei-Chih Lee, L.; Yang, T.C.-K. Synergistic Effect of BiVO4/P-g-C3N4 Heterojunction with Enhanced Optoelectronic Properties on Synthetic Colorants under Visible Light. Colorants 2023, 2, 426-442. https://doi.org/10.3390/colorants2020019
Chowdhury A, Balu S, Lan K-W, Wei-Chih Lee L, Yang TC-K. Synergistic Effect of BiVO4/P-g-C3N4 Heterojunction with Enhanced Optoelectronic Properties on Synthetic Colorants under Visible Light. Colorants. 2023; 2(2):426-442. https://doi.org/10.3390/colorants2020019
Chicago/Turabian StyleChowdhury, Anuradha, Sridharan Balu, Kuo-Wei Lan, Louis Wei-Chih Lee, and Thomas C.-K. Yang. 2023. "Synergistic Effect of BiVO4/P-g-C3N4 Heterojunction with Enhanced Optoelectronic Properties on Synthetic Colorants under Visible Light" Colorants 2, no. 2: 426-442. https://doi.org/10.3390/colorants2020019
APA StyleChowdhury, A., Balu, S., Lan, K.-W., Wei-Chih Lee, L., & Yang, T. C.-K. (2023). Synergistic Effect of BiVO4/P-g-C3N4 Heterojunction with Enhanced Optoelectronic Properties on Synthetic Colorants under Visible Light. Colorants, 2(2), 426-442. https://doi.org/10.3390/colorants2020019









