Multicomponent Crystals of Phthalocyanines–A Possibility of Fine-Tuning Properties
Abstract
:1. Introduction
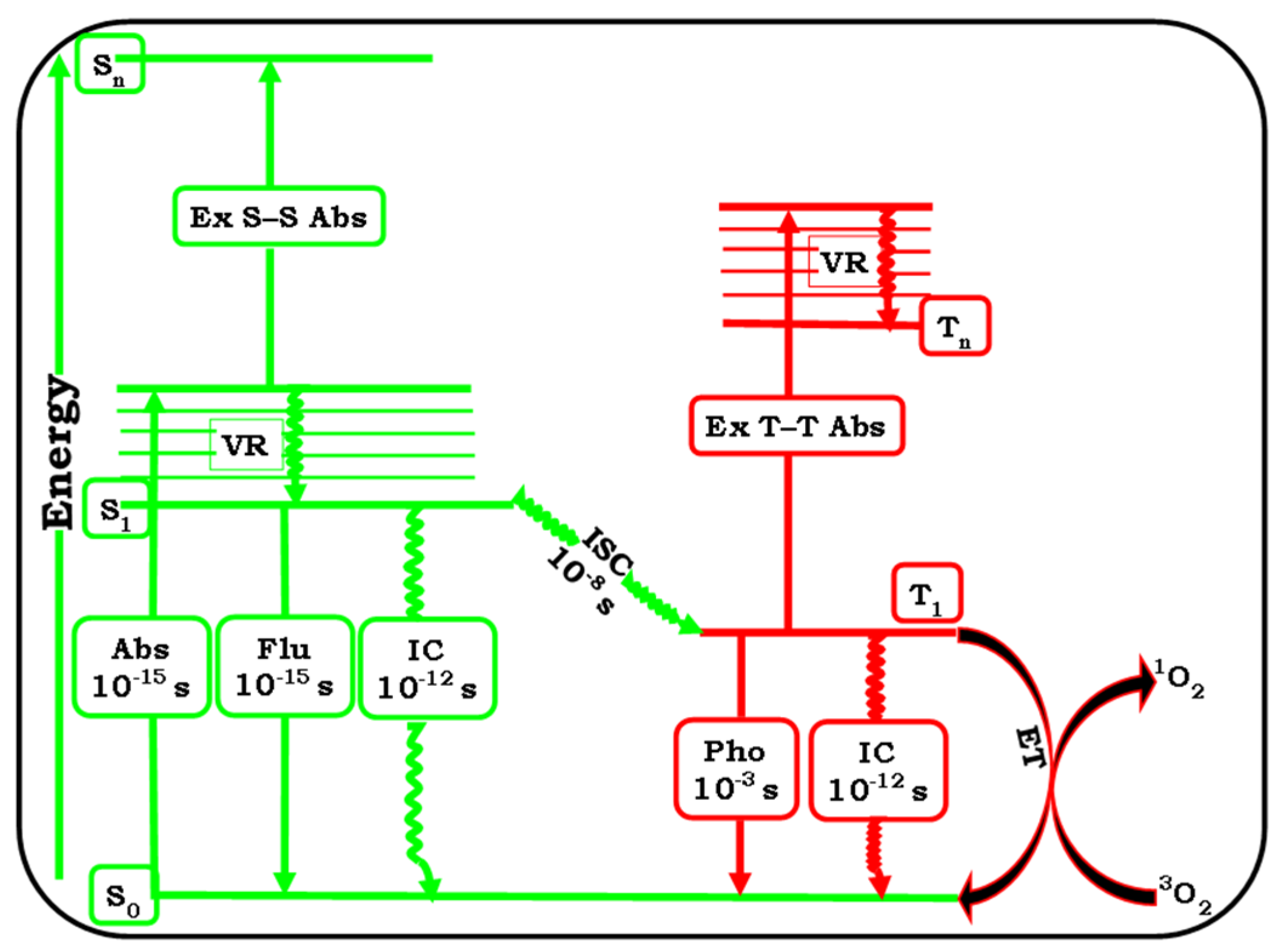
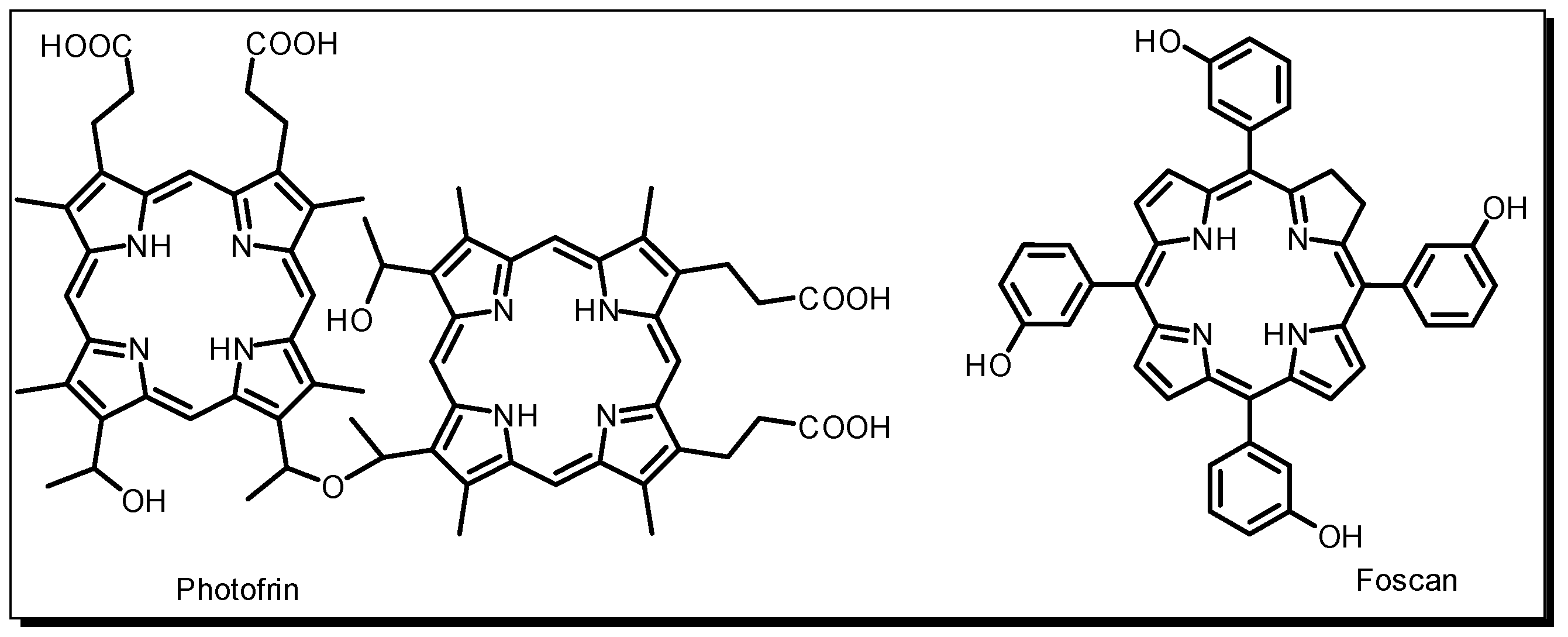
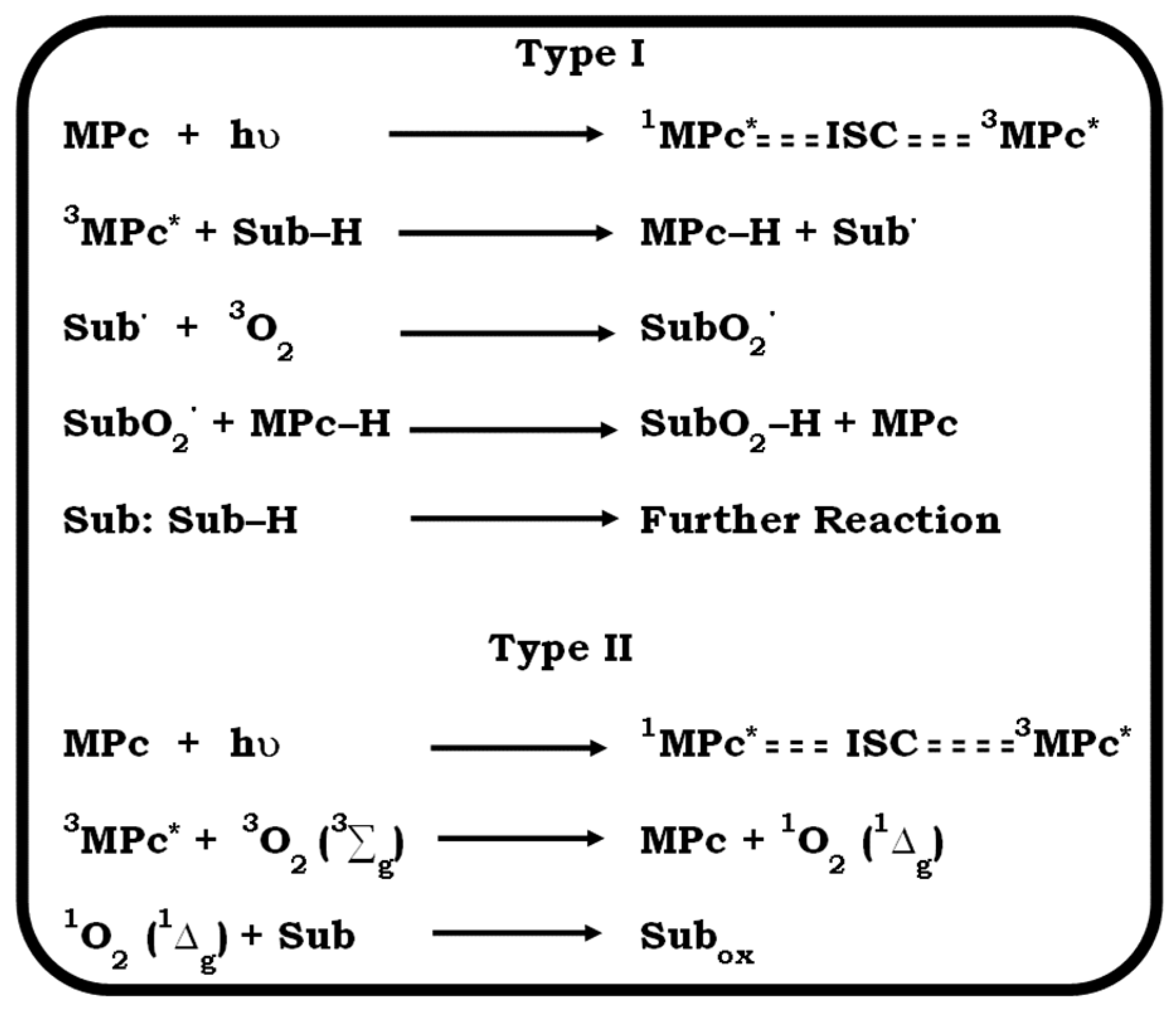
2. Photodynamic Therapy and Photosensitiser
3. Approaches for Improving Compounds’ Physicochemical Properties
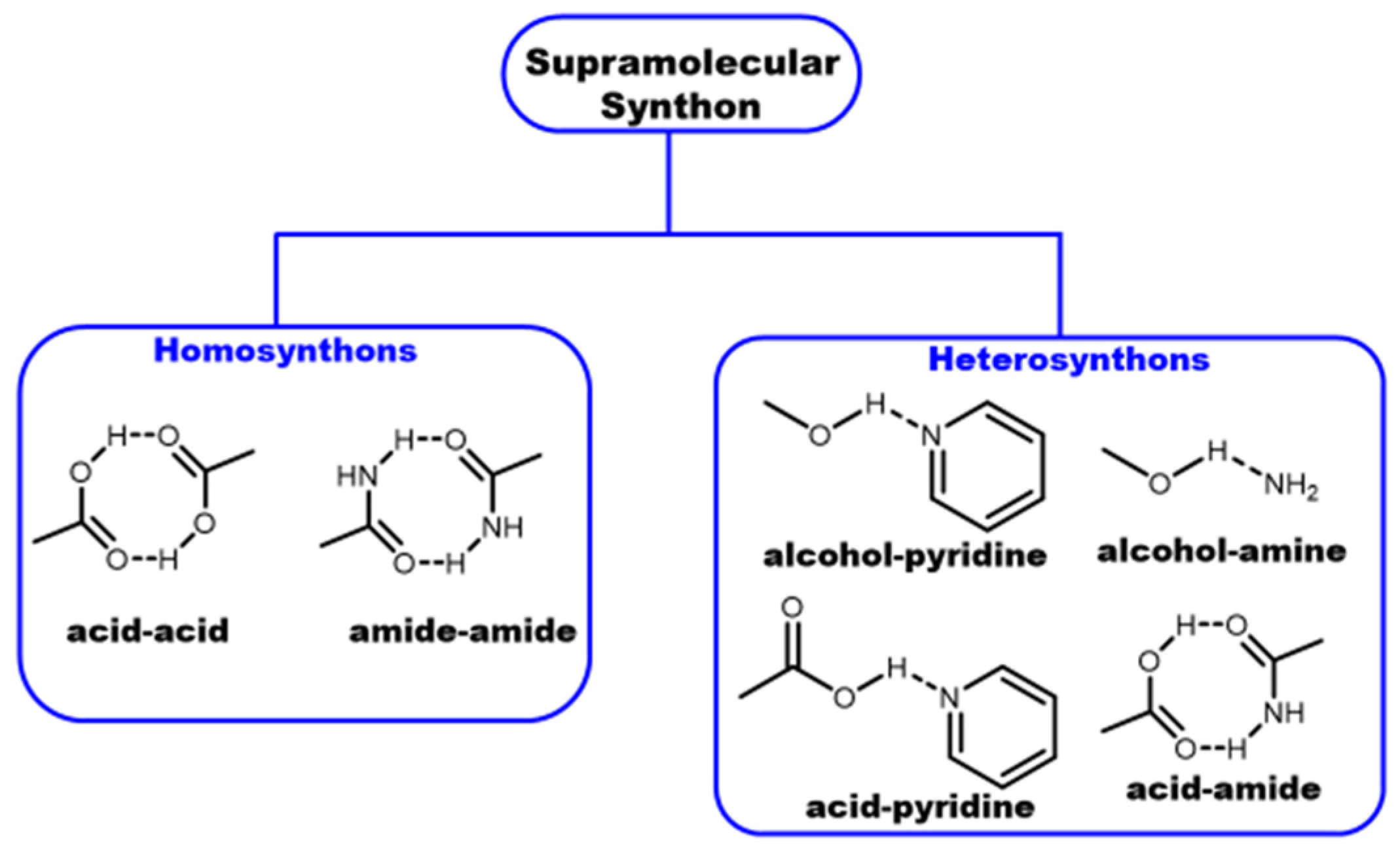
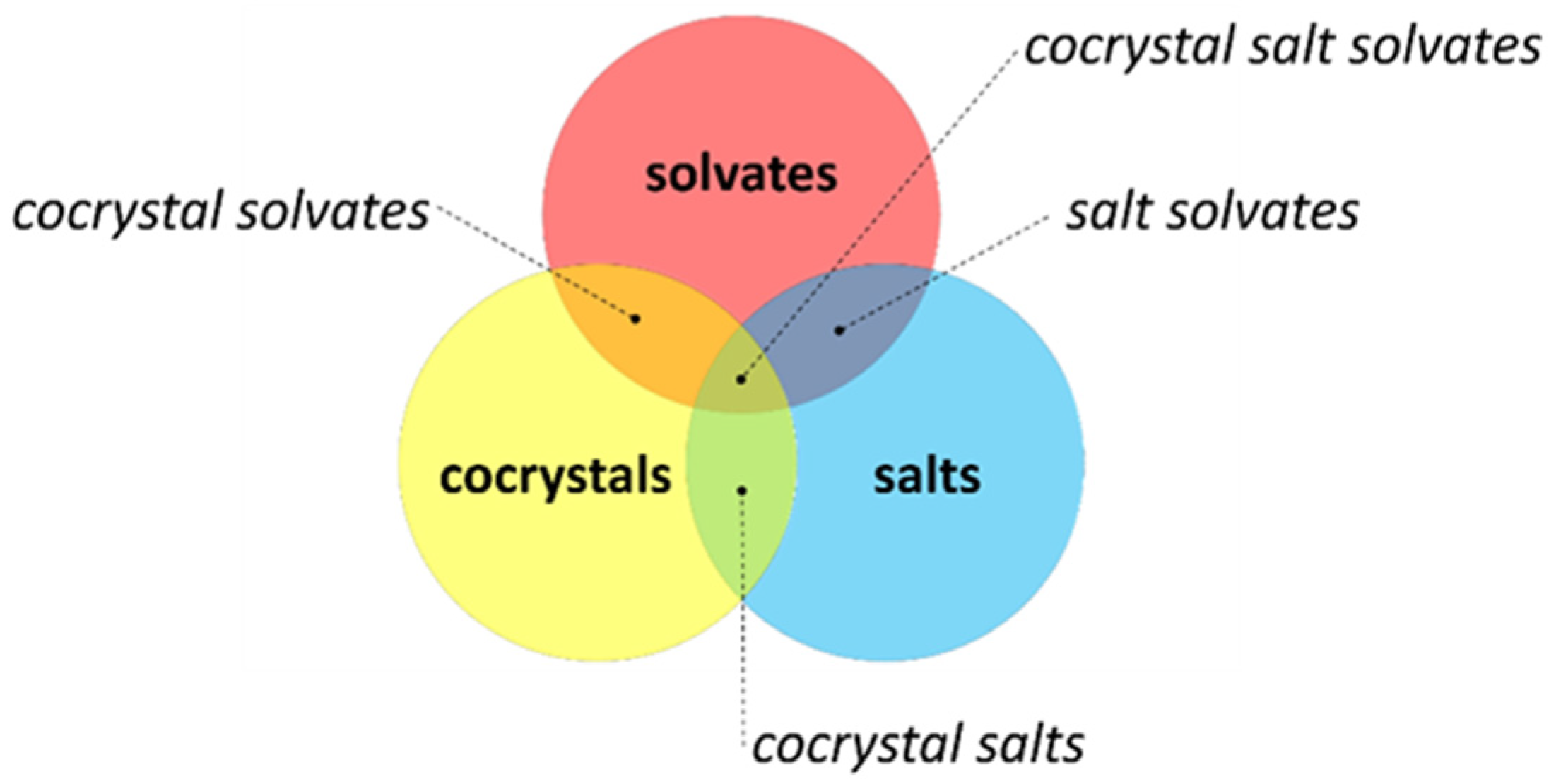
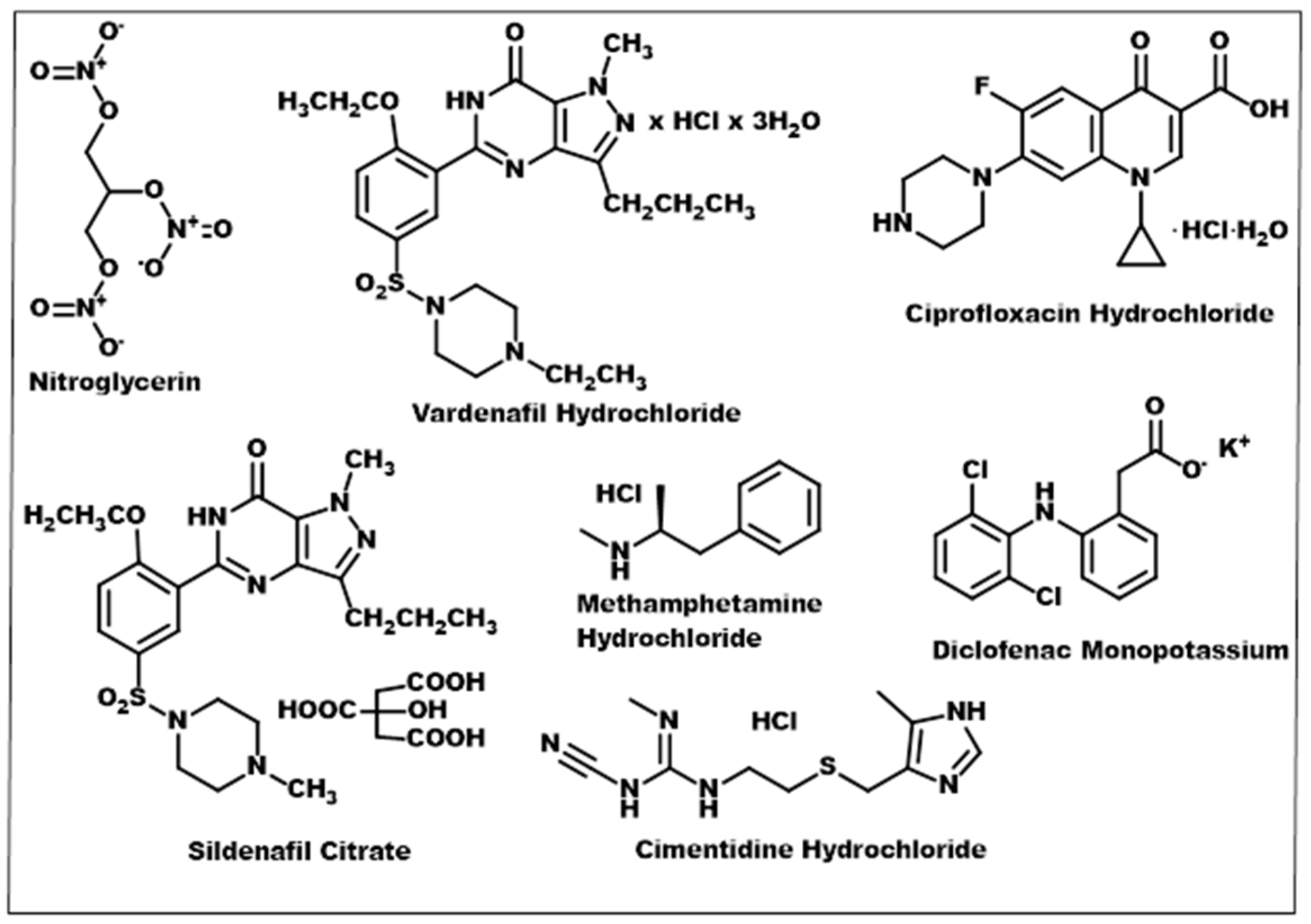
3.1. Crystal Engineering, Supramolecular Synthons, and Multicomponent Crystals
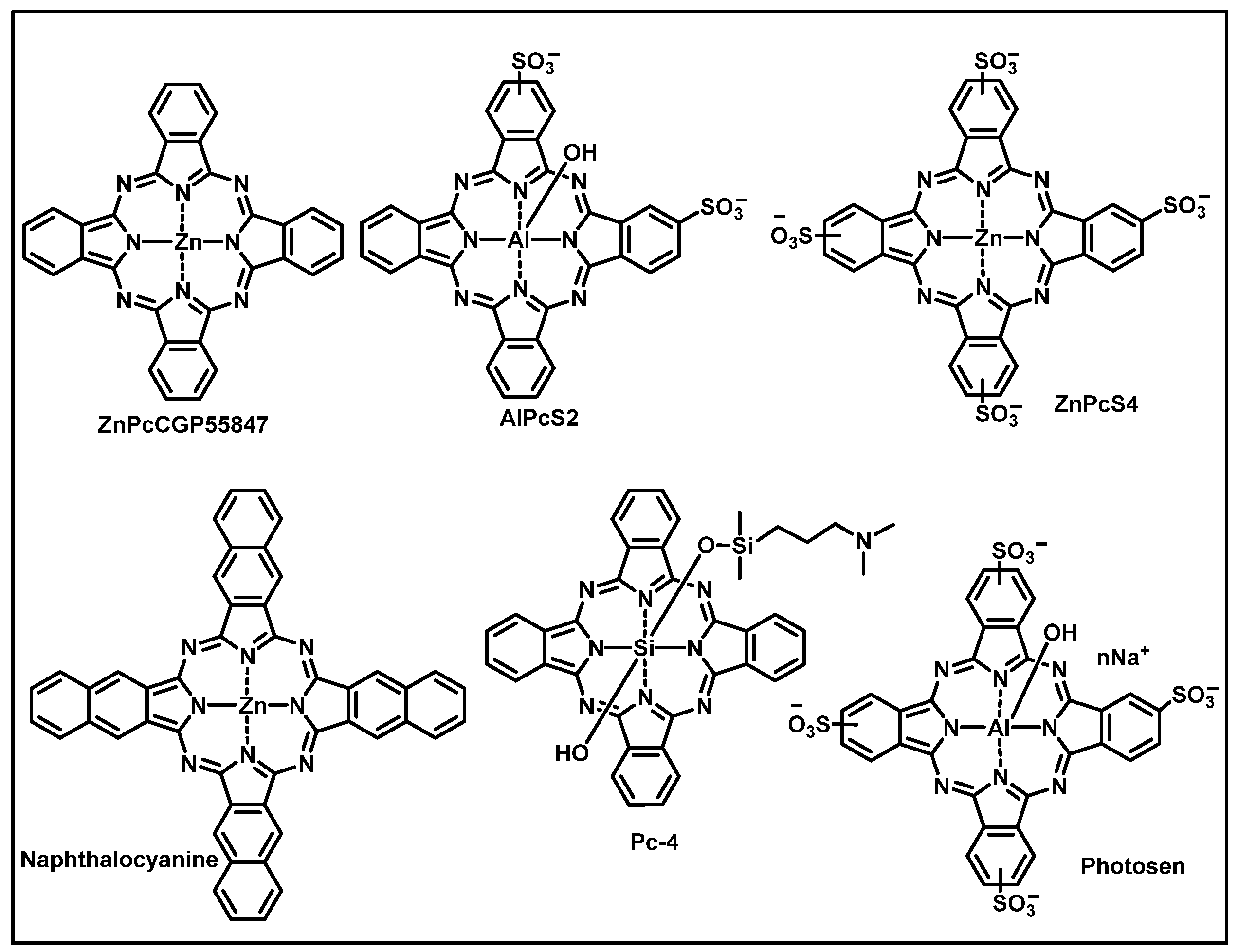
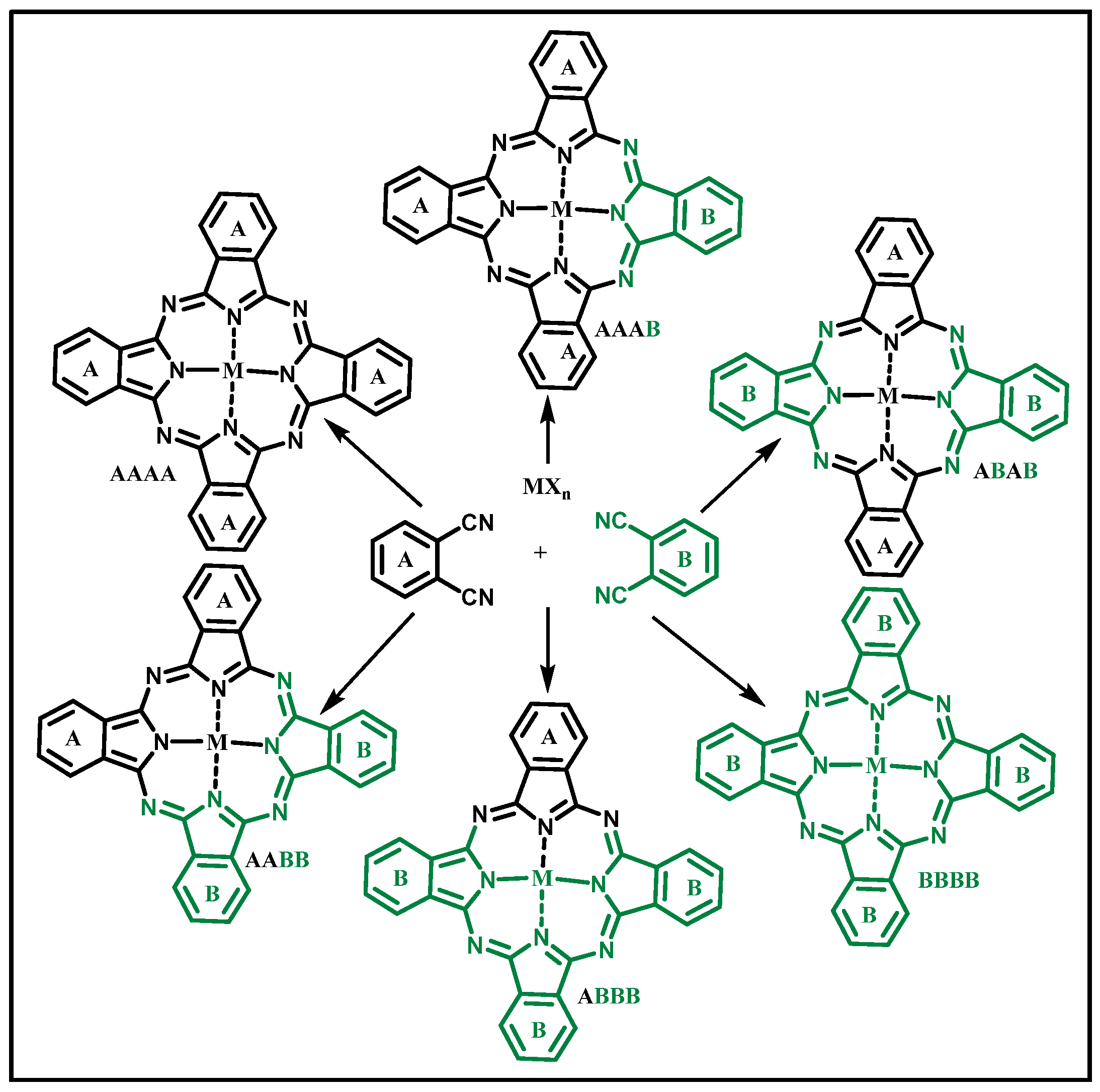
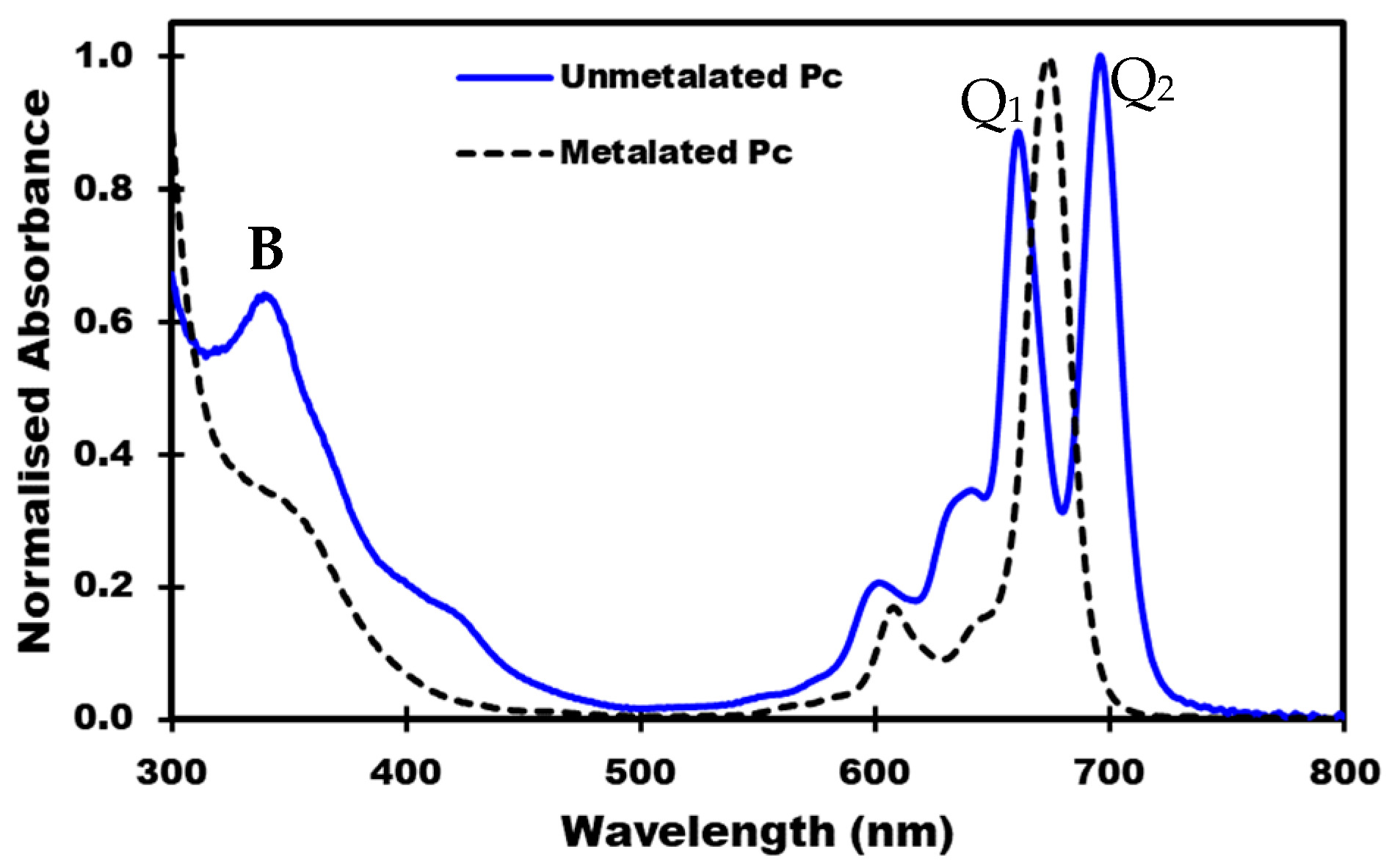
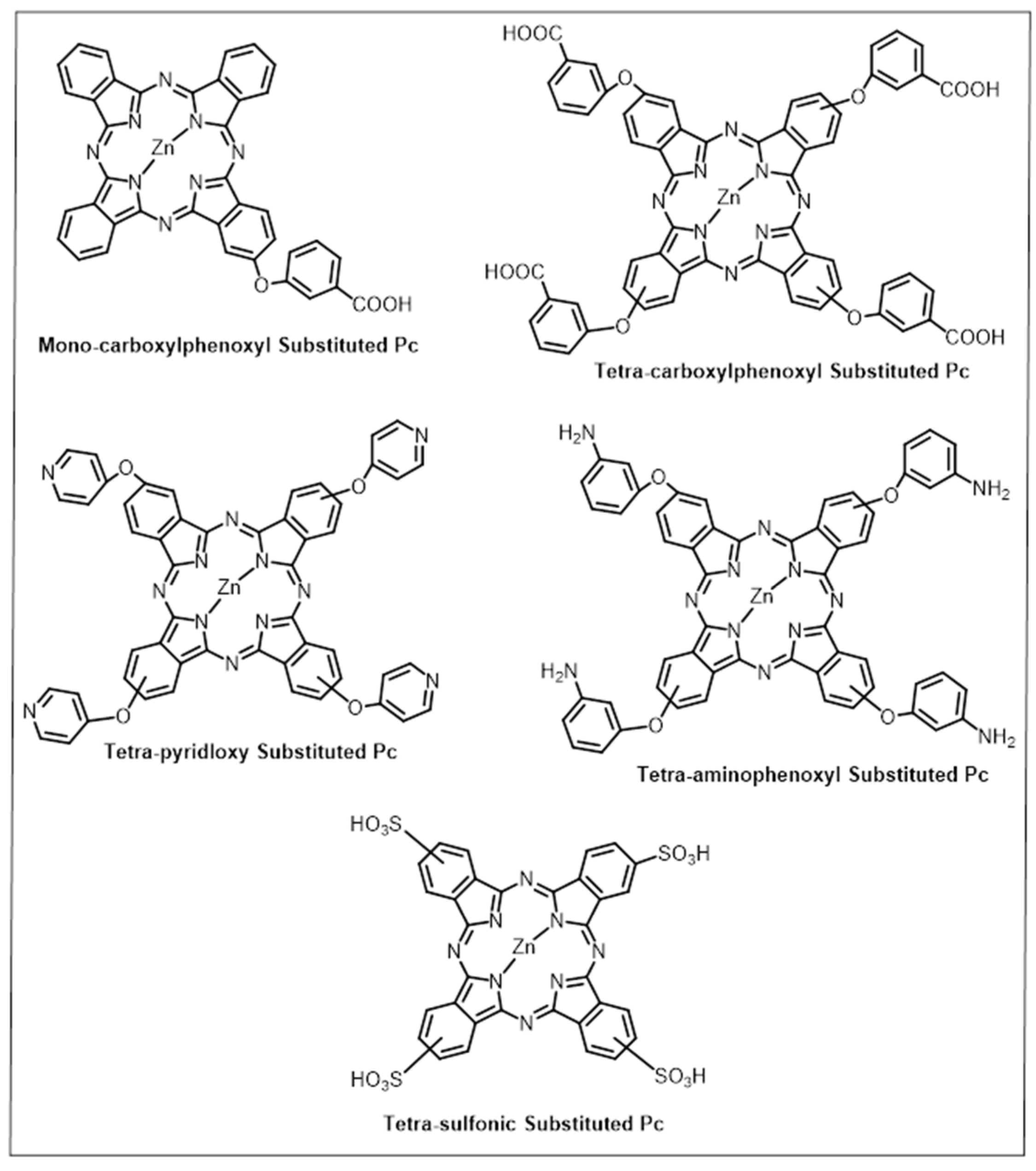
3.2. Phthalocyanines: Molecular Design and the Possibility of Fine-Tuning Their Physicochemical Properties through Crystal Engineering
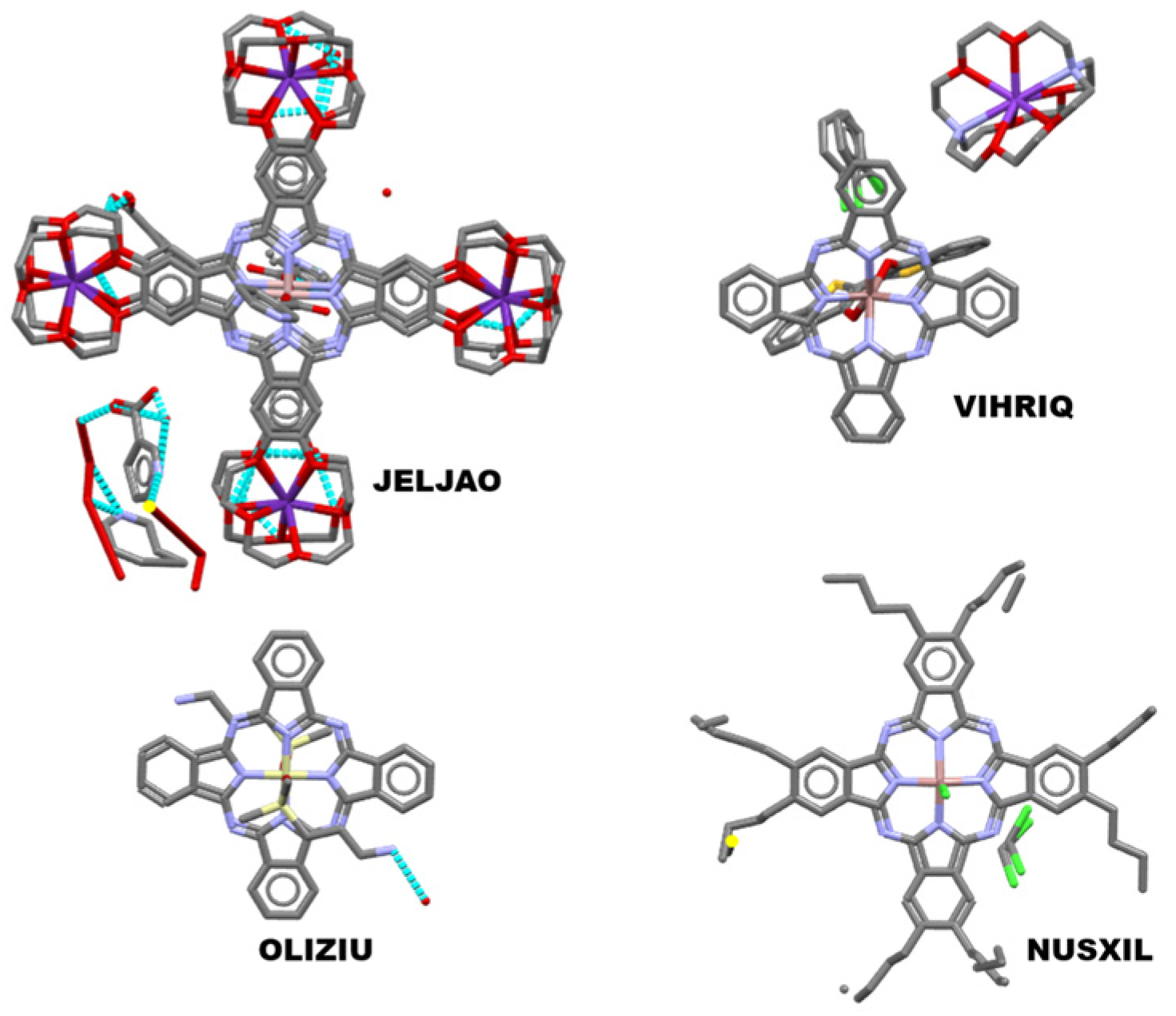
4. Cambridge Structural Database Repository Analysis of Multicomponent Crystals of Phthalocyanines
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials-are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic Therapy in Oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef]
- Huang, Z. A Review of Progress in Clinical Photodynamic Therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [Green Version]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar]
- Boutayeb, A. The Burden of Communicable and Non-Communicable Diseases in Developing Countries. In Handbook of Disease Burdens and Quality of Life; Preedy, V.R., Watson, R.R., Eds.; Springer: New York, NY, USA, 2010; pp. 531–546. [Google Scholar] [CrossRef]
- European Commission Staff Working Document. Progress Report on the Action Plan against the Rising Threats from Antimicrobial Resistance. 2015. Available online: http://ec.europa.eu/health/antimicrobial_resistance/docs/2015_amr_progress_report_en.pdf (accessed on 16 October 2020).
- ECDC Surveillance Report. Annual Epidemiological Report. Antimicrobial Resistance and Healthcare-Associated Infections. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2017/2018. Available online: https://www.ecdc.europa.eu/en/publications/EU-summary-report-antimicrobial-resistance-zoonoses-2017-2018 (accessed on 20 May 2021).
- Oluwole, D.O.; Coleman, L.; Buchanan, W.; Chen, T.; La Ragione, R.M.; Liu, L.X. Antibiotics-Free Compounds for Chronic Wound Healing. Pharmaceutics. 2022, 5, 1021. [Google Scholar] [CrossRef]
- US CDC. Available online: http://www.cdc.gov/drugresistance/pdf/carb_national_strategy.pdf (accessed on 18 May 2021).
- Lo, P.-C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2019, 49, 1041–1056. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Babilas, P.; Schreml, S.; Landthaler, M.; Szeimies, R.-M. Photodynamic therapy in dermatology: State-of-the-art. Photodermatol. Photoimmunol. Photomed. 2010, 26, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic Therapy in Dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, G.; Colin, P.; Betrouni, N.; Nevoux, P.; Ouzzane, A.; Puech, P.; Villers, A.; Mordon, S. Photodynamic therapy in urology: What can we do now and where are we heading? Photodiagnosis Photodyn. Ther. 2012, 9, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R. Chemical Aspects of Photodynamic Therapy; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 2000. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent advances in photodynamic therapy for cancer and infectious diseases. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1560. [Google Scholar] [CrossRef]
- Deng, W.; McKelvey, K.J.; Guller, A.; Fayzullin, A.; Campbell, J.M.; Clement, S.; Habibalahi, A.; Wargocka, Z.; Liang, L.; Shen, C.; et al. Application of Mitochondrially Targeted Nanoconstructs to Neoadjuvant X-ray-Induced Photodynamic Therapy for Rectal Cancer. ACS Central Sci. 2020, 6, 715–726. [Google Scholar] [CrossRef]
- Savoia, P.; Deboli, T.; Previgliano, A.; Broganelli, P. Usefulness of Photodynamic Therapy as a Possible Therapeutic Alternative in the Treatment of Basal Cell Carcinoma. Int. J. Mol. Sci. 2015, 16, 23300–23317. [Google Scholar] [CrossRef] [Green Version]
- Nyokong, T. Desired properties of new phthalocyanines for photodynamic therapy. Pure Appl. Chem. 2011, 83, 1763–1779. [Google Scholar] [CrossRef]
- Methfessel, C.D.; Volland, M.; Brunner, K.; Wibmer, L.; Hahn, U.; De La Torre, G.; Torres, T.; Hirsch, A.; Guldi, D.M. Exfoliation of Graphene by Dendritic Water-Soluble Zinc Phthalocyanine Amphiphiles in Polar Media. Chem. A Eur. J. 2018, 24, 18696–18704. [Google Scholar] [CrossRef]
- Oluwole, D.O.; Manoto, S.L.; Malabi, R.; Maphanga, C.; Ombinda-Lemboumba, S.; Mthunzi-Kufa, P.; Nyokong, T. Evaluation of the photophysicochemical properties and photodynamic therapy activity of nanoconjugates of zinc phthalocyanine linked to glutathione capped Au and Au 3 Ag 1 nanoparticles. Dye. Pigment. 2018, 150, 139–150. [Google Scholar] [CrossRef]
- Love, W.G.; Duk, S.; Biolo, R.; Jori, G.; Taylor, P.W. Liposome-Mediated Delivery of Photosensitizers: Localization of Zinc (11)-Phthalocyanine within Implanted Tumors after Intravenous Administration. Photochem. Photobiol. 1996, 63, 656–661. [Google Scholar] [CrossRef]
- Brilkina, A.A.; Dubasova, L.V.; Sergeeva, E.A.; Pospelov, A.; Shilyagina, N.Y.; Shakhova, N.M.; Balalaeva, I.V. Photobiological properties of phthalocyanine photosensitizers Photosens, Holosens and Phthalosens: A comparative in vitro analysis. J. Photochem. Photobiol. B Biol. 2018, 191, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Baron, E.D.; Scull, H.; Hsia, A.; Berlin, J.C.; McCormick, T.; Colussi, V.; Kenney, M.E.; Cooper, K.; Oleinick, N.L. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: The case experience with preclinical mechanistic and early clinical–translational studies. Toxicol. Appl. Pharmacol. 2007, 224, 290–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2019, 96, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Sobotta, L.; Kucinska, M.; Murias, M.; Mielcarek, J.; Duzgunes, N. Cellular Changes, Molecular Pathways and the Immune System Following Photodynamic Treatment. Curr. Med. Chem. 2014, 21, 4059–4073. [Google Scholar] [CrossRef]
- Chandel, N.S. Mitochondria and cancer. Cancer Metab. 2014, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Peralta, S.; Moraes, C.T. Mitochondrial Alterations During Carcinogenesis: A review of metabolic transformation and targets for anticancer treatments. Adv. Cancer Res. 2013, 119, 127–160. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Tarhouni, M.; Durand, D.; Önal, E.; Aggad, D.; Işci, Ü.; Ekineker, G.; Brégier, F.; Jamoussi, B.; Sol, V.; Gary-Bobo, M.; et al. Triphenylphosphonium-substituted phthalocyanine: Design, synthetic strategy, photoproperties and photodynamic activity. J. Porphyr. Phthalocyanines 2018, 22, 552–561. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, Y.; Yuan, G.; Zuo, K.; Huang, Y.; Chen, J.; Li, J.; Xue, J. A novel tumor and mitochondria dual-targeted photosensitizer showing ultra-efficient photodynamic anticancer activities. Chem. Commun. 2018, 55, 866–869. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, L.; Ling, X.; Shao, P.; Wang, X.; Edwards, W.B.; Bai, M. Tumor mitochondria-targeted photodynamic therapy with a translocator protein (TSPO)-specific photosensitizer. Acta Biomater. 2015, 28, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Gong, N.; Ma, X.; Ye, X.; Zhou, Q.; Chen, X.; Tan, X.; Yao, S.; Huo, S.; Zhang, T.; Chen, S.; et al. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat. Nanotechnol. 2019, 14, 379–387. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, Z.; Zhang, K.Y.; Yang, H.; Liu, S.; Xu, A.; Guo, S.; Zhao, Q.; Huang, W.A. Mitochondria-targeted photosensitizer showing improved photodynamic therapy effects under hypoxia. Angew. Chem. Int. Ed. 2016, 55, 9947–9951. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xue, J.; Dai, Y.; Liu, H.; Chen, N.; Jia, L.; Huang, J. Inhibition of human hepatocellular carcinoma HepG2 by phthalocyanine photosensitiser PHOTOCYANINE: ROS production, apoptosis, cell cycle arrest. Eur. J. Cancer. 2012, 13, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Sounik, J.R.; Rihter, B.D.; Ford, W.E.; Rodgers, M.A.J.; Kenney, M.E. Naphthalocyanines relevant to the search for second-generation PDT sensitizers. Proc. SPIE 1991, 1426, 340–349. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Uspenskiĭ, L.V.; Chistov, L.V.; Kogan, E.A.; Loshchenov, V.B.; Ablitsov, I.A.; Rybin, V.K.; Zavodnov, V.I.; Shiktorov, D.I.; Serbinenko, N.F.; Semenova, I.G. Endobronchial laser therapy in complex preoperative preparation of patients with lung diseases. Khirurgiia 2000, 2, 38–40. [Google Scholar]
- Stranadko, E.F.; Garbuzov, M.I.; Zenger, V.G.; Nasedkin, A.N.; Markichev, N.A.; Riabov, M.V.; Leskov, I.V. Photodynamic therapy of recurrent and residual oropharyngeal and laryngeal tumors. Vestn. Otorinolaringol. 2001, 3, 36–39. [Google Scholar]
- Filonenko, E.; Sokolov, V.V.; Chissov, V.I.; Lukyanets, E.A.; Vorozhtsov, G.N. Photodynamic therapy of early esophageal cancer. Photodiagnosis Photodyn. Ther. 2008, 5, 187–190. [Google Scholar] [CrossRef]
- Ghazal, B.; Machacek, M.; Shalaby, M.A.; Novakova, V.; Zimcik, P.; Makhseed, S. Phthalocyanines and Tetrapyrazinoporphyrazines with Two Cationic Donuts: High Photodynamic Activity as a Result of Rigid Spatial Arrangement of Peripheral Substituents. J. Med. Chem. 2017, 60, 6060–6076. [Google Scholar] [CrossRef]
- Huang, Z. An update on the regulatory status of PDT photosensitizers in China. Photodiagnosis Photodyn. Ther. 2008, 5, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hur, E.; Kol, R.; Marko, R.; Riklis, E.; Rosenthal, I. Combined Action of Phthalocyanine Photosensitization and Gamma-radiation on Mammalian Cells. Int. J. Radiat. Biol. 1988, 54, 21–30. [Google Scholar] [CrossRef]
- Sakamoto, K.; Okumura, E.; Hirohashi, R. Phthalocyanine as Functional Dyes; IPC: Tokyo, Japan, 2004. [Google Scholar]
- de la Torre, G.; Vázquez, P.; Agulló-López, F.; Torres, T. Role of Structural Factors in the Nonlinear Optical Properties of Phthalocyanines and Related Compounds. Chem. Rev. 2004, 104, 3723–3750. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, D.O.; Yagodin, A.V.; Mkhize, N.C.; Sekhosana, K.E.; Martynov, A.G.; Gorbunova, Y.G.; Tsivadze, A.Y.; Nyokong, T. First Example of Nonlinear Optical Materials Based on Nanoconjugates of Sandwich Phthalocyanines with Quantum Dots. Chem.—A Eur. J. 2017, 23, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Cid, J.-J.; Yum, J.-H.; Jang, S.-R.; Nazeeruddin, M.K.; Martínez-Ferrero, E.; Palomares, E.; Ko, J.; Grätzel, M.; Torres, T. Molecular Cosensitization for Efficient Panchromatic Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2007, 46, 8358–8362. [Google Scholar] [CrossRef]
- Gu, D.; Chen, Q.; Tang, X.; Gan, F.; Shen, S.; Liu, K.; Xu, H. Application of phthalocyanine thin films in optical recording. Opt. Commun. 1995, 121, 125–129. [Google Scholar] [CrossRef]
- Campidelli, S.; Ballesteros, B.; Filoramo, A.; Díaz, D.D.; de la Torre, G.; Torres, T.; Rahman, G.M.A.; Ehli, C.; Kiessling, D.; Werner, F.; et al. Facile Decoration of Functionalized Single-Wall Carbon Nanotubes with Phthalocyanines via “Click Chemistry”. J. Am. Chem. Soc. 2008, 130, 11503–11509. [Google Scholar] [CrossRef]
- Okura, I. Photosensitization of Porphyrins and Phthalocyanines; Gordon and Breach: Amsteldijk, The Netherlands, 2001; p. 151. [Google Scholar]
- Dini, D.; Hanack, M. Phthalocyanines Properties and Materials. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2003; Volume 17, p. 107. [Google Scholar]
- Ben-Hur, E.; Chan, W.S. Phthalocyanines in Photobiology and their Medical Applications. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2003; Volume 19, p. 1. [Google Scholar]
- Gregory, P. Industrial applications of phthalocyanines. J. Porphyr. Phthalocya 2000, 4, 432–437. [Google Scholar] [CrossRef]
- Sorokin, A.B. Phthalocyanine Metal Complexes in Catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: The Design of Organic Solids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Duggirala, N.K.; Perry, M.L.; Almarsson, O.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2015, 52, 640–655. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering. From molecules to materials. J. Mol. Struct. 2003, 656, 5–15. [Google Scholar] [CrossRef]
- Lipinski, C.A. Solubility in water and DMSO: Issues and potential solutions. In Pharmaceutical Profiling in Drug Discovery for Lead Selection; RBorchardt, T., Kerns, E.H., Lipinski, C.A., Thakker, D., Wang, B., Eds.; AAPS Press: Arlington, VA, USA, 2004; pp. 93–125. [Google Scholar]
- Hens, B.; Brouwers, J.; Corsetti, M.; Augustijns, P. Supersaturation and Precipitation of Posaconazole Upon Entry in the Upper Small Intestine in Humans. J. Pharm. Sci. 2016, 105, 2677–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A Provisional Biopharmaceutical Classification of the Top 200 Oral Drug Products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Whalen, K.; Feild, C.; Radhakrishnan, R. Lippincott® Illustrated Reviews: Pharmacology, 7th ed.; Lippincott Williams & Wilkins (Wolters Kluwer): Philadelphia, PA, USA, 2019. [Google Scholar]
- Babu, N.J.; Nangia, A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Shiraki, K.; Takata, N.; Takano, R.; Hayashi, Y.; Terada, K. Dissolution improvement and the mechanism of the improvement from cocrystallization of poorly water-soluble compounds. Pharm. Res. 2008, 25, 2581–2592. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Walsh, R.D.B.; Bradner, M.W.; Fleischman, S.; Morales, L.A.; Moulton, B.; Rodríguez-Hornedo, N.; Zaworotko, M.J. Crystal engineering of the composition of pharmaceutical phases. Chem. Commun. 2003, 2, 186–187. [Google Scholar] [CrossRef]
- United State Food and Drug Administration (USFDA). Generally Recognized as Safe (GRAS) Food Additive Status List; USFDA: Maryland, MD, USA, 2022. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 20 May 2021).
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Grothe, E.; Meekes, H.; Vlieg, E.; ter Horst, J.H.; de Gelder, R. Solvates, Salts, and Cocrystals: A Proposal for a Feasible Classification System. Cryst. Growth Des. 2016, 16, 3237–3243. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. Crystengcomm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Stahl, P.H.; Wermuth, C.G. Handbook of Pharmaceutical Salts: Properties, Selection, and Use, Wiley-vch. Org. Proc. Res. Dev. 2003, 7, 222–223. [Google Scholar]
- Bhogala, B.R.; Basavoju, S.; Nangia, A. Tape and layer structures in cocrystals of some di- and tricarboxylic acids with 4,4′-bipyridines and isonicotinamide. From binary to ternary cocrystals. Crystengcomm 2005, 7, 551–562. [Google Scholar] [CrossRef]
- Paulekuhn, G.S.; Dressman, J.B.; Saal, C. Trends in Active Pharmaceutical Ingredient Salt Selection based on Analysis of the Orange Book Database. J. Med. Chem. 2007, 50, 6665–6672. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Vittal, J.J.; Ramanan, A. Crystal Engineering: A Textbook; World Scientific: Singapore, 2011. [Google Scholar]
- Shan, N.; Toda, F.; Jones, W. Mechanochemistry and co-crystal formation: Effect of solvent on reaction kinetics. Chem. Commun. 2002, 20, 2372–2373. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2011, 41, 413–447. [Google Scholar] [CrossRef] [Green Version]
- Kaupp, G. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Aakeröy, C.B.; Forbes, S.; Desper, J. Using Cocrystals To Systematically Modulate Aqueous Solubility and Melting Behavior of an Anticancer Drug. J. Am. Chem. Soc. 2009, 131, 17048–17049. [Google Scholar] [CrossRef] [Green Version]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-R.; Wang, X.; Yang, Y.; Chen, X.; Mei, X. Solid-state characterization of 17β-estradiol co-crystals presenting improved dissolution and bioavailability. Crystengcomm 2016, 18, 3498–3505. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Zhang, Q.; Zhang, Q.-Q.; Chen, C.; He, M.-Y.; Chen, Q.; Song, G.-Q.; Xuan, X.-P.; Huang, X.-F. From a binary salt to salt co-crystals of antibacterial agent lomefloxacin with improved solubility and bioavailability. Acta Crystallogr. Sect. B Struct. Sci. 2015, 71, 437–446. [Google Scholar] [CrossRef] [PubMed]
- D’aertrycke, J.D.M.; Robeyns, K.; Willocq, J.; Leyssens, T. Cocrystallization as a tool to solve deliquescence issues: The case of l-lactic acid. J. Cryst. Growth 2017, 472, 3–10. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Pharmaceutical Cocrystallization: Engineering a Remedy for Caffeine Hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Musumeci, D.; Hunter, C.A.; Prohens, R.; Scuderi, S.; McCabe, J.F. Virtual cocrystal screening. Chem. Sci. 2011, 2, 883–890. [Google Scholar] [CrossRef]
- Fábián, L. Cambridge Structural Database Analysis of Molecular Complementarity in Cocrystals. Cryst. Growth Des. 2009, 9, 1436–1443. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Loschen, C.; Klamt, A. Rational Coformer or Solvent Selection for Pharmaceutical Cocrystallization or Desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef] [Green Version]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. Crystengcomm 2008, 11, 418–426. [Google Scholar] [CrossRef]
- Malapile, R.J.; Nyamayaro, K.; Nassimbeni, L.R.; Báthori, N.B. Multicomponent crystals of baclofen with acids and bases—Conformational flexibility and synthon versatility. Crystengcomm 2020, 23, 91–99. [Google Scholar] [CrossRef]
- Odounga, J.-E.O.; Báthori, N.B. Systematic comparison of racemic and enantiopure multicomponent crystals of phenylsuccinic acid—The role of chirality. Crystengcomm 2020, 22, 2208–2218. [Google Scholar] [CrossRef]
- McKeown, N.B. Phthalocyanine Materials–Synthesis, Structure and Function; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Nxele, S.R.; Oluwole, D.O.; Nyokong, T. Electrocatalytic activity of a push pull Co(II) phthalocyanine in the presence of graphitic carbon nitride quantum dots. Electrochim. Acta 2019, 326, 134978. [Google Scholar] [CrossRef]
- Nyokong, T.; Isago, H. The renaissance in optical spectroscopy of phthalocyanines and other tetraazaporphyrins. J. Phthalocya Porphyr. 2004, 8, 1083–1090. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Nyokong, T. Effects of substituents and solvents on the photochemical properties of zinc phthalocyanine complexes and their protonated derivatives. J. Mol. Struct. 2003, 689, 89–97. [Google Scholar] [CrossRef]
- Kobayashi, N.; Muranaka, A.; Ishii, K. Symmetry-Lowering of the Phthalocyanine Chromophore by a C2 Type Axial Ligand. Inorg. Chem. 2000, 39, 2256–2257. [Google Scholar] [CrossRef]
- Nyokong, T.; Antunes, E. The Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2010; Volume 7, p. 247. [Google Scholar]
- Liu, Y.; Zhu, D.; Wada, T.; Yamada, A.; Sasabe, H. Synthesis and characterization of a novel unsymmetrical metal-free phthalocyanine with donor-acceptor substituents. J. Heterocycl. Chem. 1994, 31, 1017–1020. [Google Scholar] [CrossRef]
- Oluwole, D.O.; Yagodin, A.V.; Britton, J.; Martynov, A.G.; Gorbunova, Y.G.; Tsivadze, A.Y.; Nyokong, T. Optical limiters with improved performance based on nanoconjugates of thiol substituted phthalocyanine with CdSe quantum dots and Ag nano-particles. Dalton Trans. 2017, 46, 16190–16198. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Morgade, M.S.; de la Torre, G.; Torres, T. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Science: Amsterdam, The Netherlands, 2003; Volume 15, p. 99. [Google Scholar]
- Kadish, K.; Smith, K.M.; Guilard, R. (Eds.) . The Porphyrin Handbook; Academic Press: San Diego, CA, USA, 2003; Volume 15. [Google Scholar]
- Gouterman, M. The Porphyrins, Part A. Physical Chemistry, Optical Spectra and Electronic Structure of Porphyrins and Related Rings; Dolphin, D., Ed.; Chapter 1; Academic Press: New York, NY, USA, 1978; Volume 3, pp. 1–29. [Google Scholar]
- Darwent, J.R.; McCubbin, I.; Phillips, D. Excited singlet and triplet state electron-transfer reactions of aluminium(III) sulphonated phthalocyanine. J. Chem. Soc. Faraday Trans. 2 1982, 78, 347–357. [Google Scholar] [CrossRef]
- Sosa-Sánchez, J.L.; Sosa-Sánchez, A.; Farfan, N.; Zamudio-Rivera, L.S.; López-Mendoza, G.; Flores, J.P.; Beltrán, H.I. Novel Phthalocyaninatobis(alkylcarboxylato)silicon(IV) Compounds: NMR Data and X-ray Structures To Study the Spacing Provided by Long Hydrocarbon Tails That Enhance Their Solubility. Chem.—A Eur. J. 2005, 11, 4263–4273. [Google Scholar] [CrossRef]
- Ikeue, T.; Mori, S.; Fujishiro, R.; Sonoyama, H.; Ide, Y.; Sugimori, T.; Nagai, A. Molecular Structure and Spectroscopic Properties of [2,3,9,10,16,17,23,24-Octakis(3-carboxyphenoxy)phthalocyaninato-κ4N](pyridine-κN)zinc(II) Pyridine Octasolvate. Heterocycles 2017, 94, 131–139. [Google Scholar] [CrossRef]
- Engel, M.K. Single-Crystal and Solid-State Molecular Structures of Phthalocyanine Complexes. Kawamura Rikagaku Kenkyusho Hokoku 1997, 8, 11–54. [Google Scholar]
- Tedesco, A.C.; Preto, R.; Beltrame, M. Phthalocyanines: Synthesis, Characterisation and Biological Applications of Photo-Dynamic Therapy (PDT), Nanobiotechnology, Magnetohyperthermia and Photodiagnosis (Theranostics); Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Konarev, D.V.; Khasanov, S.S.; Lyubovskaya, R.N. Fullerene complexes with coordination assemblies of metalloporphyrins and metal phthalocyanines. Coord. Chem. Rev. 2013, 262, 16–36. [Google Scholar] [CrossRef]
- Feng, X.; Liu, C.; Wang, X.; Jiang, Y.; Yang, G.; Wang, R.; Zheng, K.; Zhang, W.; Wang, T.; Jiang, J. Functional Supramolecular Gels Based on the Hierarchical Assembly of Porphyrins and Phthalocyanines. Front. Chem. 2019, 7, 336. [Google Scholar] [CrossRef] [Green Version]
- Galstyan, A.; Block, D.; Niemann, S.; Grüner, M.C.; Abbruzzetti, S.; Oneto, M.; Daniliuc, C.G.; Hermann, S.; Viappiani, C.; Schäfers, M.; et al. Labeling and Selective Inactivation of Gram-Positive Bacteria Employing Bimodal Photoprobes with Dual Readouts. Chem. A Eur. J. 2016, 22, 5243–5252. [Google Scholar] [CrossRef]
- Lapkina, L.A.; Larchenko, V.E.; Kirakosyan, G.A.; Tsivadze, A.Y.; Troyanov, S.I.; Gorbunova, Y.G. Cation-Induced Dimerization of Crown-Substituted Phthalocyanines by Complexation with Rubidium Nicotinate As Revealed by X-ray Structural Data. Inorg. Chem. 2017, 57, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Konarev, D.V.; Kuzmin, A.; Khasanov, S.S.; Fatalov, A.M.; Yudanova, E.I.; Lyubovskaya, R.N. Coordination Complexes of Titanium(IV) and Indium(III) Phthalocyanines with Carbonyl-Containing Dyes: The Formation of Singly Bonded Anionic Squarylium Dimers. Chem.—A Eur. J. 2018, 24, 8415–8423. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Chen, Y.; Fan, X.; Zeng, Q. Solid-state supramolecular chemistry of zinc tetraphenylporphyrin and zinc phthalo-cyanine with bis(pyridyl) ligands. J. Mol. Struct. 2011, 1002, 145–150. [Google Scholar] [CrossRef]
- Fujishiro, R.; Sonoyama, H.; Ide, Y.; Fujimura, T.; Sasai, R.; Nagai, A.; Mori, S.; Kaufman, N.E.M.; Zhou, Z.; Vicente, M.G.H.; et al. Synthesis, photodynamic activities, and cytotoxicity of new water-soluble cationic gallium(III) and zinc(II) phthalocyanines. J. Inorg. Biochem. 2019, 192, 7–16. [Google Scholar] [CrossRef]
- Guo, Y. Impact of solid-state characteristics to the physical stability of drug substance and drug product. In Handbook of Stability Testing in Pharmaceutical Development; Huynh-Ba, K., Ed.; Springer: New York, NY, USA, 2009; pp. 241–261. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluwole, D.O.; Báthori, N.B. Multicomponent Crystals of Phthalocyanines–A Possibility of Fine-Tuning Properties. Colorants 2023, 2, 405-425. https://doi.org/10.3390/colorants2020018
Oluwole DO, Báthori NB. Multicomponent Crystals of Phthalocyanines–A Possibility of Fine-Tuning Properties. Colorants. 2023; 2(2):405-425. https://doi.org/10.3390/colorants2020018
Chicago/Turabian StyleOluwole, David O., and Nikoletta B. Báthori. 2023. "Multicomponent Crystals of Phthalocyanines–A Possibility of Fine-Tuning Properties" Colorants 2, no. 2: 405-425. https://doi.org/10.3390/colorants2020018
APA StyleOluwole, D. O., & Báthori, N. B. (2023). Multicomponent Crystals of Phthalocyanines–A Possibility of Fine-Tuning Properties. Colorants, 2(2), 405-425. https://doi.org/10.3390/colorants2020018





