Mathematical Approach to Optimizing the Panchromatic Absorption of Natural Dye Combinations for Dye-Sensitized Solar Cells
Abstract
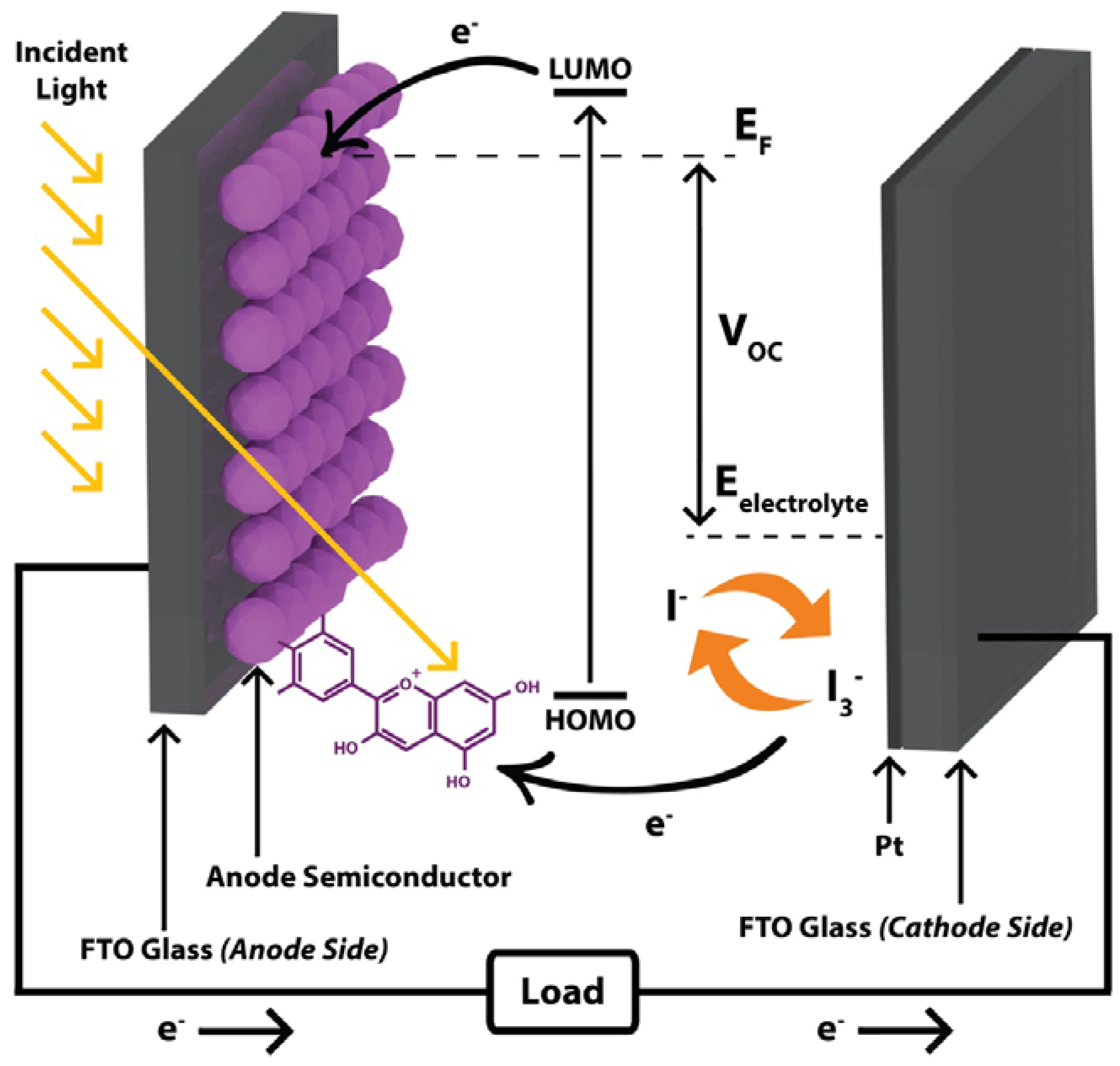
1. Introduction
2. Materials and Methods
2.1. Dye Preparation
| Dye Class (One Letter Abbreviation) | Major Chemical Species | Precursor Material | Molar Extinction Coefficient (M−1 cm−1) | Dye Concentration (μM) |
|---|---|---|---|---|
| Anthocyanins (A) | Cyanidin-3-Glucoside [20] | Fresh frozen Aroniaberries | 34,300 @ 520 nm [21] | 0.74 |
| Betalins (B) | Betanin [22] | Fresh frozen Prickly Pear | 65,000 @ 535 nm [22] | 0.03 |
| Curcuminoids (K) | Curcumin [23] | Turmeric powder | 55,000 @ 425 nm [24] | 1.88 |
| Chlorophyll (C) | Chlorophyll a,b [25] | Dried Spinach | 70,000 @ 430 nm [26] | 0.12 |
| Xanthonoids (M) | α-Mangostin [27] | Mangosteen Pericarp dietary supplements | 51,800 @ 425 nm [28] | 0.14 |
| Phycobilins (P) | Phycobilin [29] | Blue Spirulina platensis powder | 98,000 @ 620 nm [29] | 0.02 |
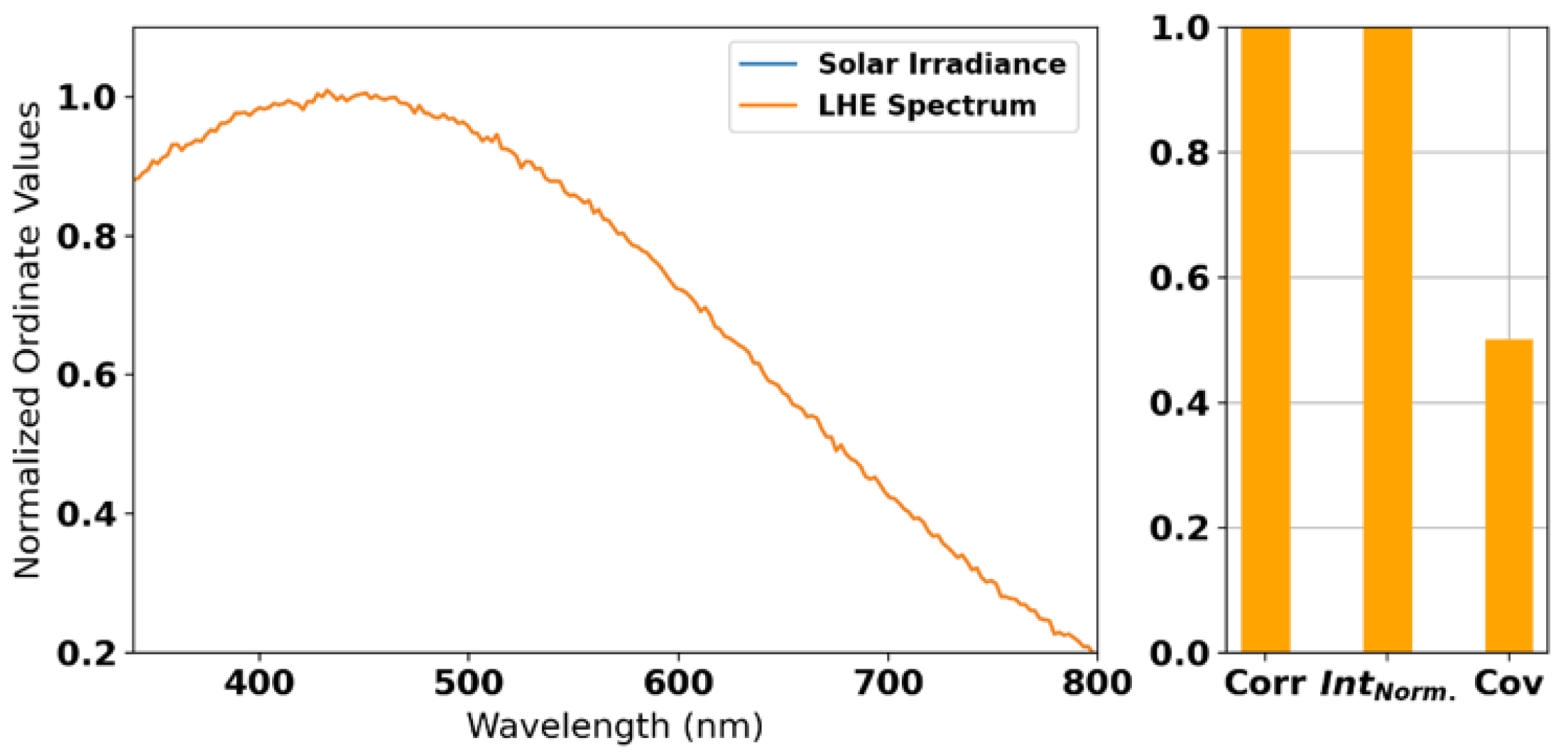
2.2. Radial Basis Function Interpolation and LHE Optimization
3. Results and Discussion
3.1. UV-Vis Absorbance Measurements
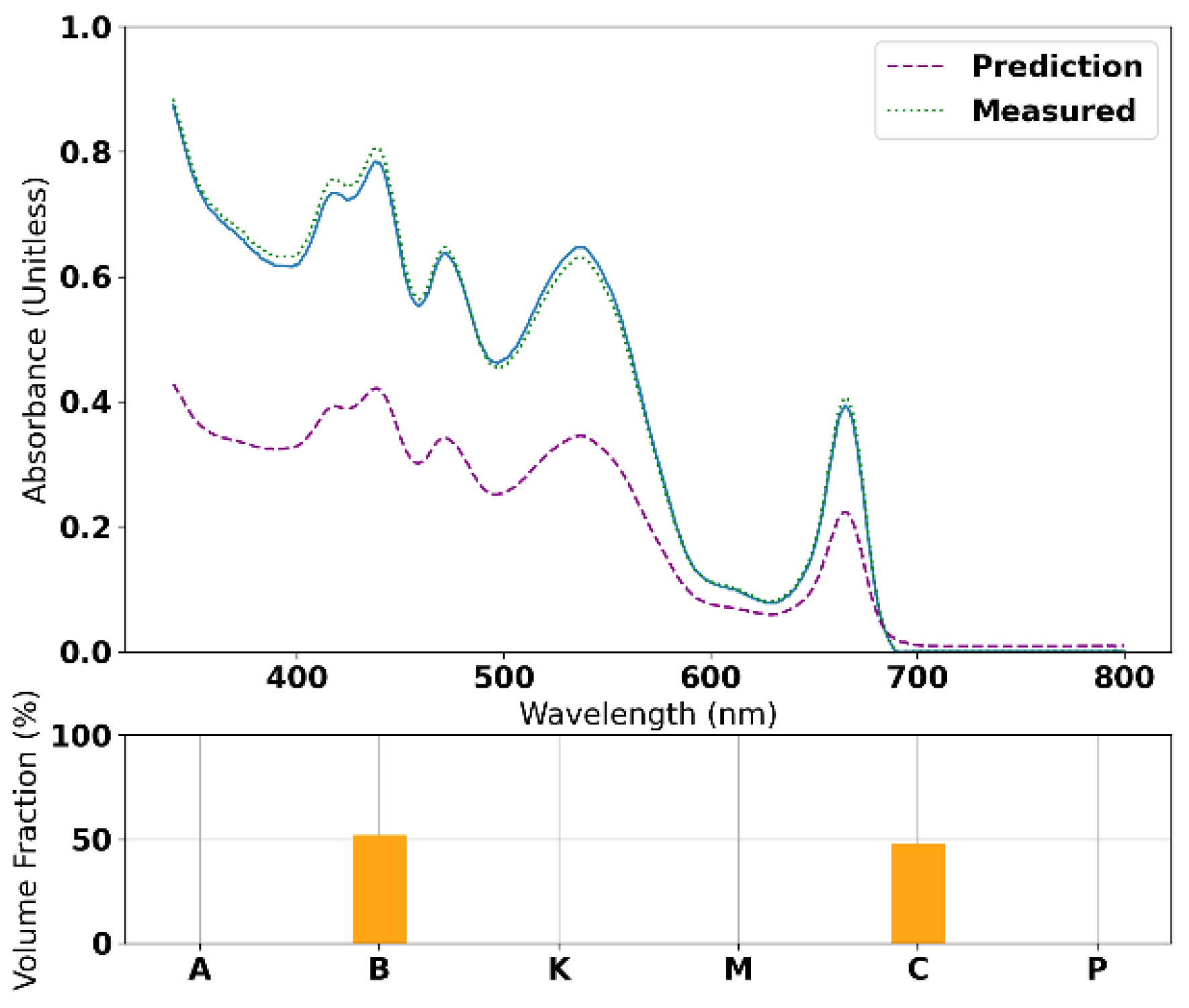
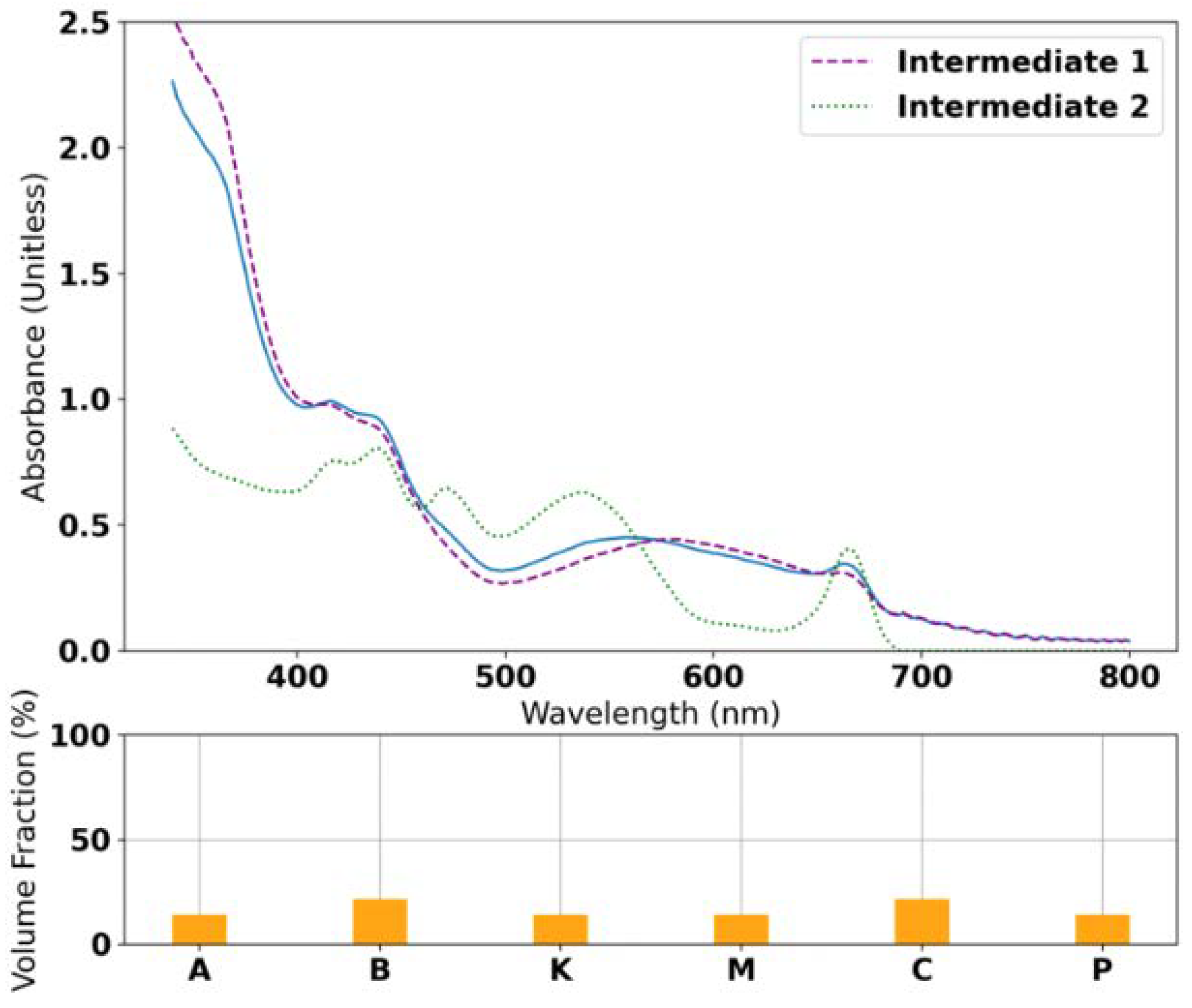
3.2. Radial Basis Function Interpolation and LHE Optimization Results
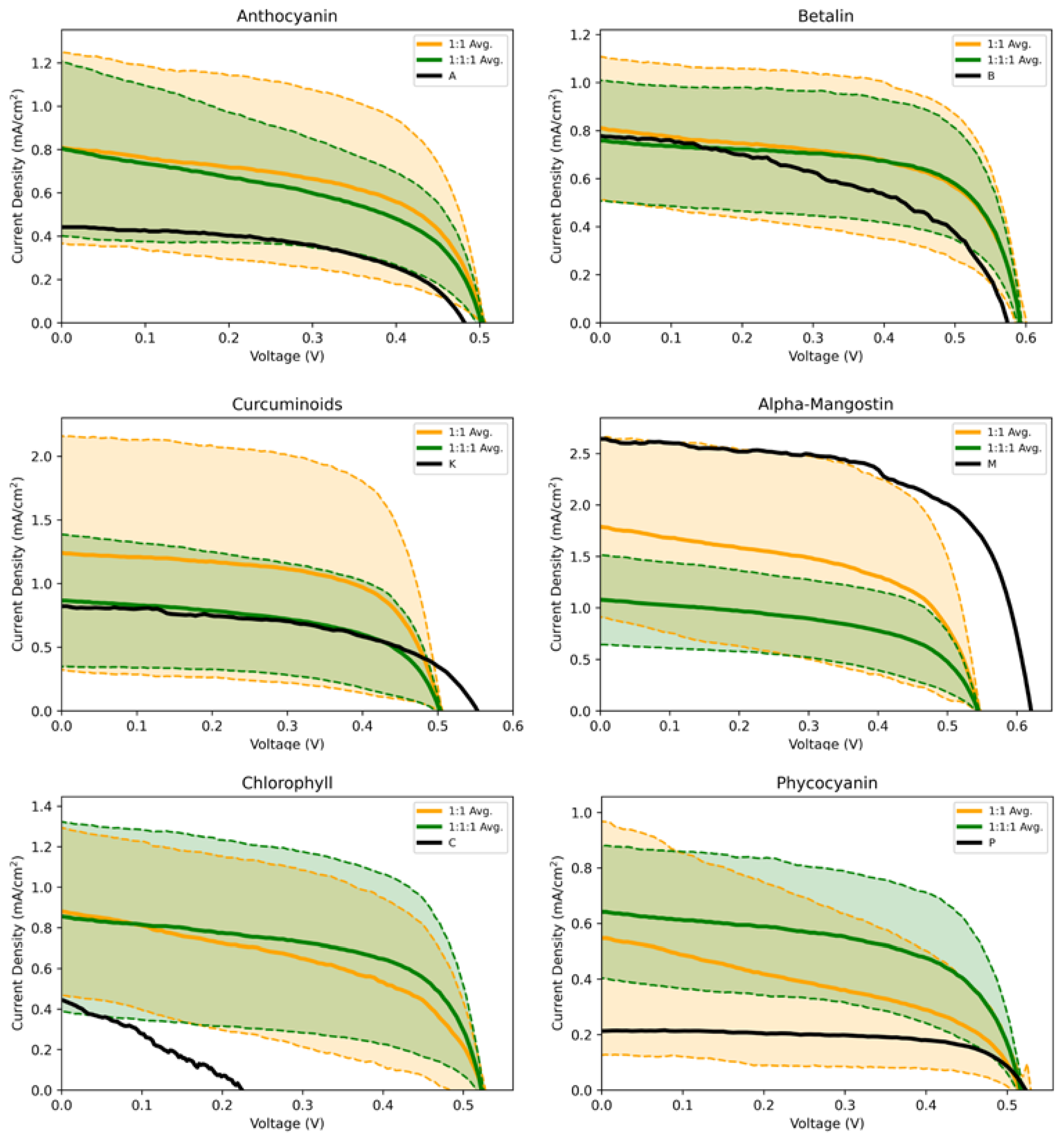
3.3. IV Measurements
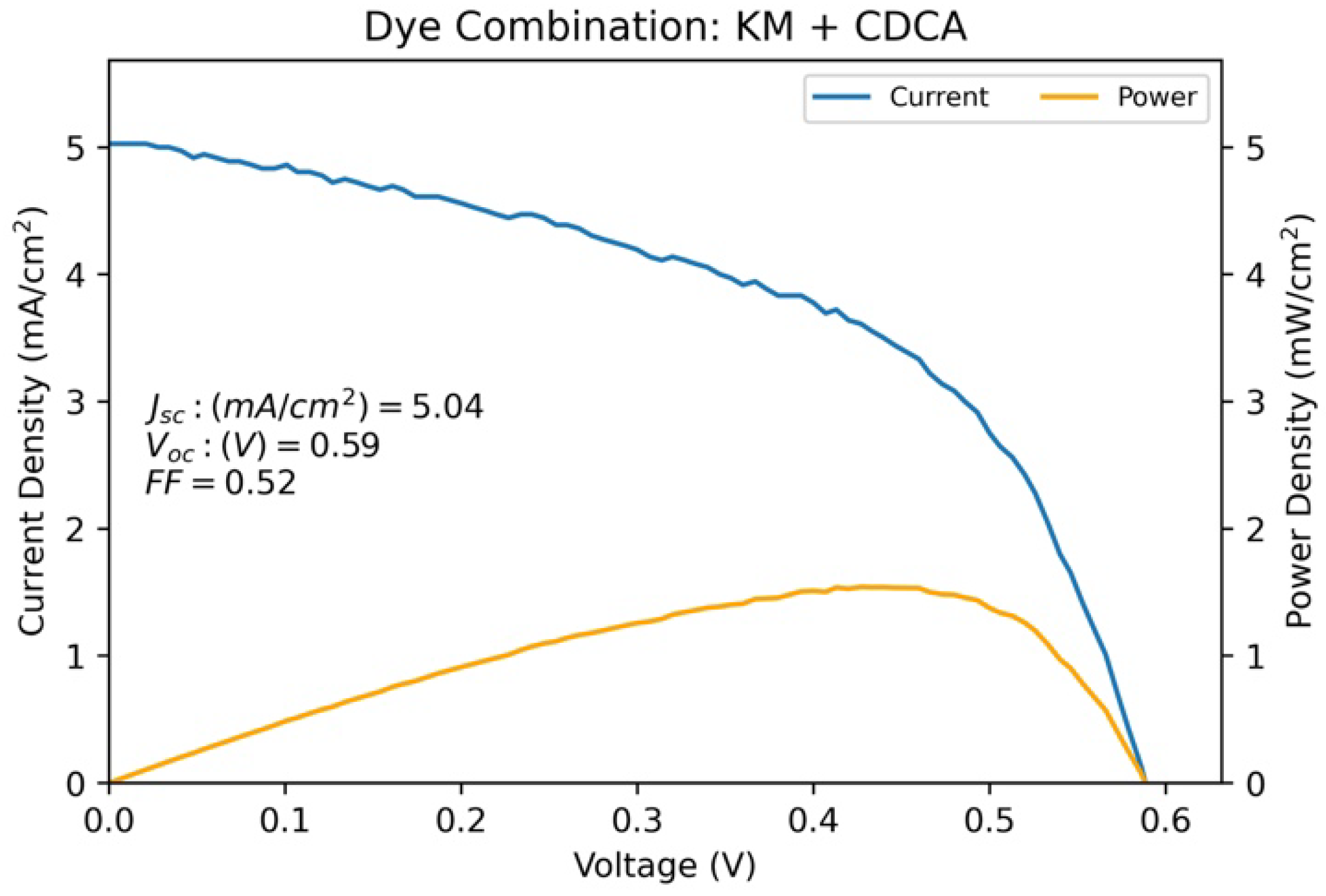
3.4. Co-Adsorption of KM with 1:1 CDCA
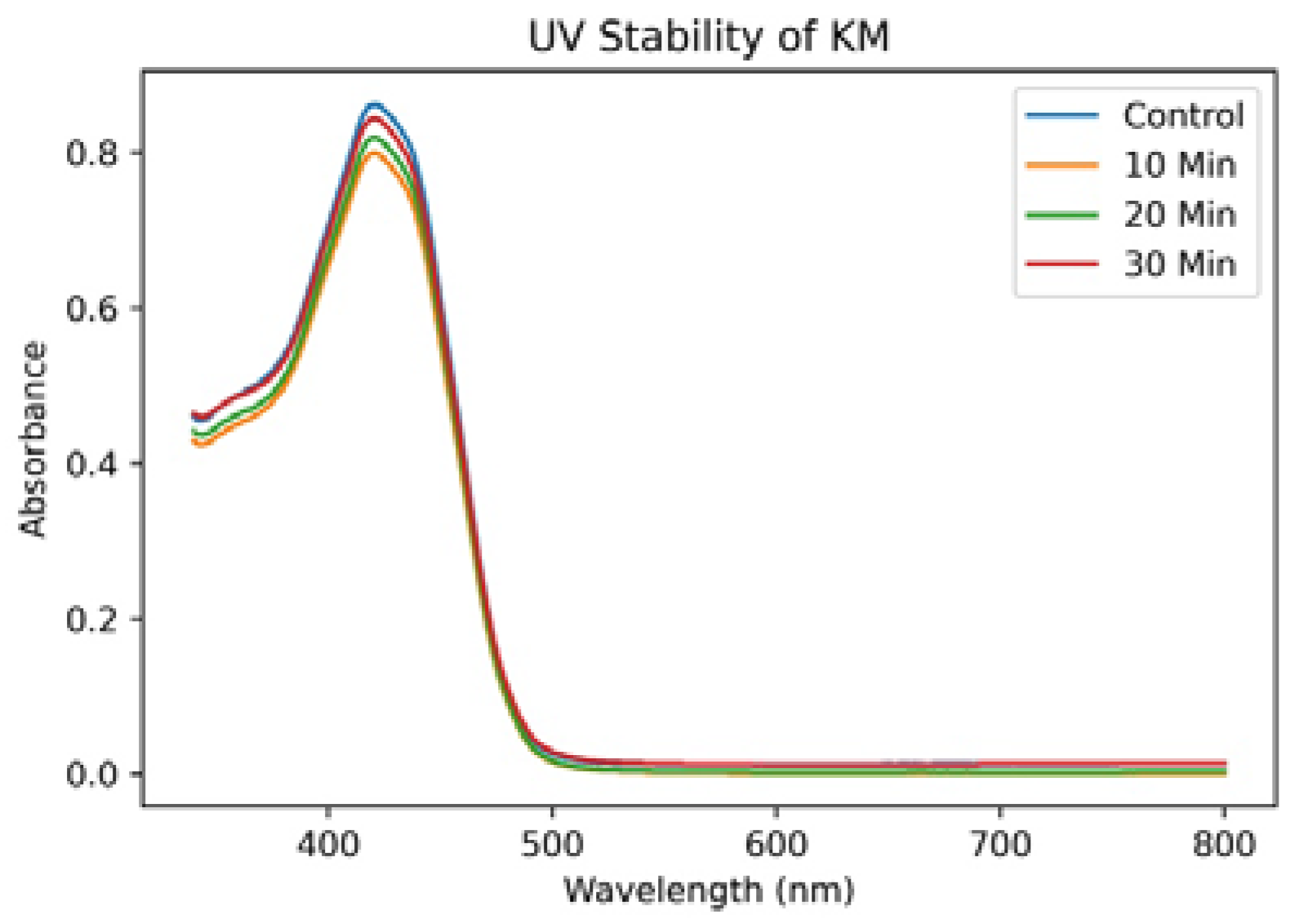
3.5. UV Stability of KM Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Dye Class (One Letter Abbreviation) | Major Chemical Species | Anode Anchoring Moiety | HOMO/LUMO (eV) |
|---|---|---|---|
| Anthocyanins (A) | Cyanidin-3-Glucoside [19] | OH | −5.89/−2.33 [47] |
| Betalins (B) | Betanin [20] | COOH | −4.75/−1.95 [48] |
| Curcuminoids (K) | Curcumin [21] | C=O, OH | −5.55/−1.97 [49] |
| Chlorophyll (C) | Chlorophyll a,b [22] | C=O | −5.93/−2.97 [50] |
| Xanthonoids (M) | α-Mangostin [23] | C=O, OH | −4.81/−2.27 [44] |
| Phycobilins (P) | Phycobilin [24] | COOH | −6.10/−3.85 [51] |
References
- Sharma, K.; Sharma, V.; Sharma, S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J. Photochem. Photobiol. 2004, 164, 3–14. [Google Scholar] [CrossRef]
- Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F. Vegetable-Based Dye-Sensitized Solar Cells. Chem. Soc. Rev. 2015, 44, 3244–3294. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.; Fahmid, K.; Manir, M.; Rahaman, M.; Hossian, M.; Barua, P.; Ghosh, B.; Mitsugi, F.; Ikegami, T.; Huque, S.; et al. Effect of Combination of Natural Dyes and the Blocking Layer on the Performance of DSSC. In Elseman, Solar Cells-Theory, Materials and Recent Advances; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Chang, H.; Kao, M.; Chen, T.; Chen, C.; Cho, K.; Lai, X. Characterization of Natural Dye Extracted from Wormwood and Purple Cabbage for Dye-Sensitized Solar Cells. Int. J. Photoenergy 2013, 2013, 159502. [Google Scholar] [CrossRef]
- Cho, K.; Chang, H.; Chen, C.; Kao, M.; Lai, X. A Study of Mixed Vegetable Dyes with Different Extraction Concentrations for Use as a Sensitizer for Dye-Sensitized Solar Cells. Int. J. Photoenergy 2014, 2014, 492747. [Google Scholar] [CrossRef]
- Golshan, M.; Osfouri, S.; Azin, R.; Jalali, T.; Moheimani, N. Co-sensitization of natural and low-cost dyes for efficient panchromatic light-harvesting using dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2021, 417, 113345. [Google Scholar] [CrossRef]
- Hosseinnezhad, M.; Gharanjig, K.; Moradian, S.; Reza-Saeb, M. In quest of power conversion efficiency in nature inspired dye-sensitized solar cells: Individual, co-sensitized or tandem configuration? Energy 2017, 134, 864–870. [Google Scholar] [CrossRef]
- Kabir, F.; Bhuiyan, M.; Hossian, M.; Bashar, H.; Rahaman, M.; Manir, M.; Ullah, S.; Uddin, S.; Mollah, M.; Kahn, R.; et al. Improvement of efficiency of Dye Sensitized Solar Cells by optimizing the combination ratio of Natural Red and Yellow dyes. Optik 2019, 179, 252–258. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Luo, W. The synergistic effect of two photosynthetic pigments in dye-sensitized mesoporous TiO2 solar cells. Dye. Pigment. 2008, 76, 327–331. [Google Scholar] [CrossRef]
- Lo, Y.; Chang, H. Pomegranate leaves and mulberry fruit as natural sensitizers for dye-sensitized solar cells. Sol. Energy 2010, 84, 1833–1837. [Google Scholar] [CrossRef]
- Obi, K.; Frolova, L.; Fuierer, P. Preparation and performance of prickly pear (Opuntia phaeacantha) and mulberry (Morus rubra) dye-sensitized solar cells. Sol. Energy 2020, 208, 312–320. [Google Scholar] [CrossRef]
- Orona-Navar, A.; Aguilar-Hernández, I.; López-Luke, T.; Zarazúa, I.; Romero-Arellano, V.; Guerrero, J.P.; Ornelas-Soto, N. Photoconversion efficiency of Titania solar cells co-sensitized with natural pigments from cochineal, papaya peel and microalga Scenedesmus obliquus. J. Photochem. Photobiol. A Chem. 2020, 388, 112216. [Google Scholar] [CrossRef]
- Patni, N.; Pillai, S.; Sharma, P. Effect of using betalain, anthocyanin and chlorophyll dyes together as a sensitizer on enhancing the efficiency of dye-sensitized solar cell. Int. J. Res. Energy 2020, 44, 10846–10859. [Google Scholar] [CrossRef]
- Prabavathy, N.; Shalini, S.; Balasundaraprabhu, R.; Velauthapillai, D.; Prasanna, S.; Balaji, G.; Muthukumarasamy, N. Algal buffer layers for enhancing the efficiency of anthocyanins extracted from rose petals for natural dye-sensitized solar cell (DSSC). Int. J. Energy Res. 2017, 42, 790–801. [Google Scholar] [CrossRef]
- Puspitasari, N.; Amalia, S.; Yudoyono, G. Effect of Mixing Dyes and Solvent in Electrolyte Toward Characterization of Dye Sensitized Solar Cell Using Natural Dyes as The Sensitizer. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 214, p. 012022. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Radha, N.; Maheswari, G.; Anandan, S.; Manoharan, S.; Williams, R. Betalain and anthocyanin dye-sensitized solar cells. J. Appl. Electrochem. 2016, 46, 929–941. [Google Scholar] [CrossRef]
- Manz, N. Optimizing the Combination of Natural Pigments for Co-Sensitization of Panchromatic TiO2 Dye Sensitized Solar Cells. Master’s Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, April 2022. [Google Scholar]
- Ahmadiani, N.; Robbins, R.; Collins, T.; Giusti, M. Anthocyanins Contents, Profiles, and Color Characteristics of Red Cabbage Extracts from Different Cultivars and Maturity Stages. Agric. Food Chem. 2014, 62, 7524–7531. [Google Scholar] [CrossRef]
- Gouvêa, A.; Santiago, M.; Schultz, D.; Pacheco, S.; Godoy, R.; Cabral, L. Anthocyanins standards (cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside) isolation from freeze-dried açaí (Euterpe oleraceae Mart.) by HPLC. Food Sci. Technol. 2012, 32, 43–46. [Google Scholar] [CrossRef]
- Khatabi, O.; Hanine, H.; Elothmani, D.; Hasib, A. Extraction and determination of polyphenols and betalain pigments in the Moroccan Prickly pear fruits (Opuntia ficus indica). Arab. J. Chem. 2016, 9, 278–281. [Google Scholar] [CrossRef]
- Tayyem, R.; Heath, D.; Al-Delaimy, W.; Rock, C. Curcumin Content of Turmeric and Curry Powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Bohn, T.; Walczyk, T.; Leisibach, S.; Hurrell, R. Chlorophyll-bound Magnesium in Commonly Consumed Vegetables and Fruits: Relevance to Magnesium Nutrition. J. Food Sci. 2006, 69, 347–350. [Google Scholar] [CrossRef]
- Li, Y.; Scales, N.; Blankenhip, R.; Willows, R.; Chen, M. Extinction coefficient for red-shifted chlorophylls: Chlorophyll d and chlorophyll f. Biochim. Biophys. Acta 2012, 1817, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Chung, P.; Owaga, E.; Tsai, I.; Wang, P.; Tsai, J.; Yeh, T.; Hsieh, R. Alpha-mangostin and mangsteen (Garcinia mangostana Linn.) pericarp extract reduces high fat-diet induced hepatic steatosis in rats by regulating mitochondria function and apoptosis. Nutr. Metab. 2016, 13, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Muchtaridi, M.; Suryani, D.; Qosim, W.; Saptarini, N. Quantitative analysis of a-Mangostin in Mangonsteen (Garcinia mangostana L.) pericarp extract from four district of west Java by HPLC method. Int. J. Pharm. Pharm. Sci. 2016, 8, 232–236. [Google Scholar]
- Khandual, S.; Sanchez, E.; Andrews, H.; De-la-Rosa, J. Phycocyanin content and nutritional profile of Arthrospira platensis from Mexico: Efficient extraction process and stability evaluation of phycocyanin. BMC Chem. 2021, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 197081, Cyanidin 3-O-Glucoside. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cyanidin-3-O-glucoside (accessed on 10 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6540685, Betanine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Betanine (accessed on 10 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 969516, Curcumin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Curcumin (accessed on 10 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 12085802, Chlorophyll a. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/12085802 (accessed on 10 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281650, Alpha-Mangostin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Mangostin (accessed on 10 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6438349, Phycobilin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phycobilin (accessed on 10 February 2023).
- Bett, J.; Radial Basis Function USRA. Github Repository. October 2016. Available online: https://github.com/jessebett/Radial-Basis-Function-USRA (accessed on 1 December 2022).
- Guo, M.; Xie, K.; Lin, J.; Yong, Z.; Tung-Yip, C.; Zhou, L.; Wang, Y.; Huang, H. Design and coupling of multifunctional TiO2 nanotube photonic crystal to nanocrystalline titania layer as semi-transparent photoanode for dye-sensitized solar cell: Supplementary information. Energy Environ. Sci. 2012, 12, 9881–9888. [Google Scholar] [CrossRef]
- Manz, N.; Bauman, A.; Brudos, E. Optimizing the Combination of Natural Pigments for Co-Sensitization of Panchromatic TiO2 Dye Sensitized Solar Cells [dataset]. Mendeley Data 2022, V3. [Google Scholar] [CrossRef]
- Pitigala, P.; Henary, M.; Perera, A. Effects of Physical Orientation of Dye Molecules and Molecular Orbitals on Performance of Solid-State Dye Sensitized Solar Cells. Mater. Today Proc. 2020, 23, 43–48. [Google Scholar] [CrossRef]
- Sewvandi, G.; Kakimoto, M.; Chen, C.; Hu, D.; Abeygunawardhana, P.; Feng, Q. Controlling Dye Coverage Instead of Addition of Organic Acid to Reduce Dye Aggregation in Dye-Sensitized Solar Cells. Sol. Energy 2020, 202, 507–513. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory (NREL). Reference Air Mass 1.5 Spectra. Available online: https://www.nrel.gov/grid/solar-resource/spectra-am1.5.html (accessed on 1 December 2022).
- Matsuzaki, H.; Murakami, T.; Masaki, N.; Furube, A.; Kimura, M.; Mori, S. Dye Aggregation Effect on Interfacial Electron-Transfer Dynamics in Zinc Phthalocyanine-Sensitized Solar Cells. J. Phys. Chem. 2014, 118, 17205–17212. [Google Scholar] [CrossRef]
- Cisneros, R.; Beley, M.; Lapicque, F. A study of the impact of co-adsorbents on DSSC electron transfer processes: Anti-pi-stacking vs. shield effect. Phys. Chem. Chem. Phys. 2016, 18, 9645–9651. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Ludin, N.; Hamid, N.; Ibrahim, M.; Sopain, K. The Effect of Chenodeoxycholic Acid (CDCA) in Mangosteen (Garcinia mangostana) Pericarps Sensitizer for Dye-Sensitized Solar Cell (DSSC). J. Phys. Conf. Ser. 2018, 1083, 012018. [Google Scholar] [CrossRef]
- Zaffino, C.; Russo, B.; Bruni, S. Surface-enhanced Raman scattering (SERS) study of Anthocyanidins. Mol. Biomol. Spectrosc. 2015, 149, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Sugimoto, N. The structural stability and catalytic activity of DNA and RNA oligonucleotides in the presence of organic solvents. Biophys. Rev. 2016, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yuliarto, B.; Prima, E.; Dipojono, H. Theoretical Investigation of Anthocyanidin Algycones as Photosensitizers for Dye-Sensitized TiO2 Solar Cells. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2015. [Google Scholar] [CrossRef]
- Ranjitha, S.; Aroulmoji, V.; Dhevi, R.L.; Rajarajan, G.; Gnanendra, S. Priminence of Using Betalin and Cubebin as Natural Dye Sensitizers for the Design of Eco-Friendly DSSC’s. Int. J. Adv. Sci. Eng. 2018, 4, 726. [Google Scholar] [CrossRef]
- Shahab, S.; Azarakhski, F.; Sheikhi, M. DFT Study of Physiorption Effect of the Curcumin on CNT(8,0-6) Nanotube for Biological Applications. Chin. J. Struct. Chem. 2019, 38, 42–57. [Google Scholar] [CrossRef]
- De Lile, J.R.; Kang, S.G.; Son, Y.A.; Lee, S.G. Do HOMO-LUMO Energy Levels and Band Gaps Provide Sufficient Understanding of Dye-Sensitizer Activity Trends for Water Purification? ACS Omega 2020, 5, 15052–15062. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Shoji, M.; Umena, Y.; Boero, M.; Shigeta, Y. Role of the Propinoic Acid Side Chain of C-Phycocyanin Chromophores in the Excited States of the Photosynthesis Process. Bull. Chem. Soc. Jpn. 2020, 93, 1509–1519. [Google Scholar] [CrossRef]



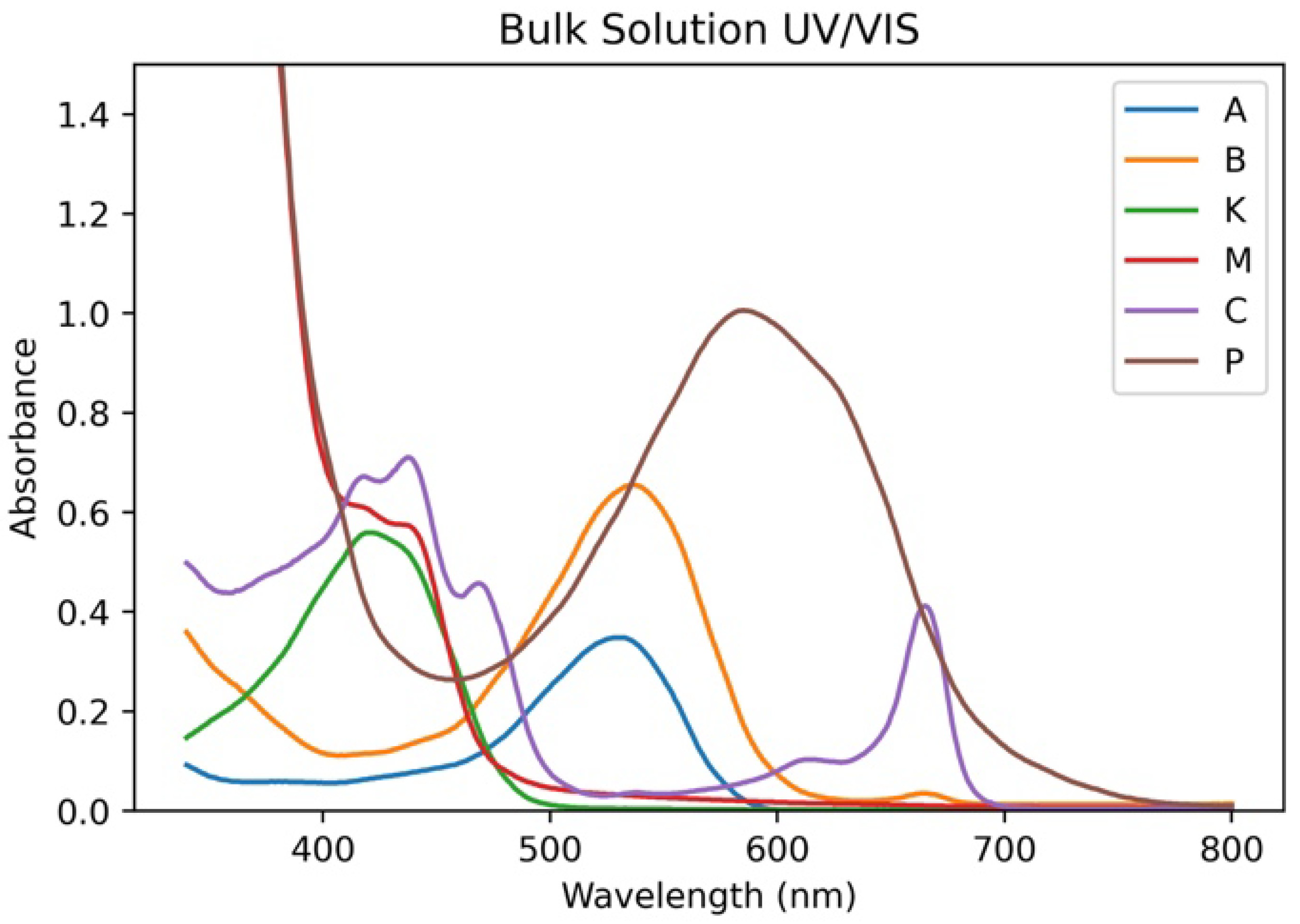
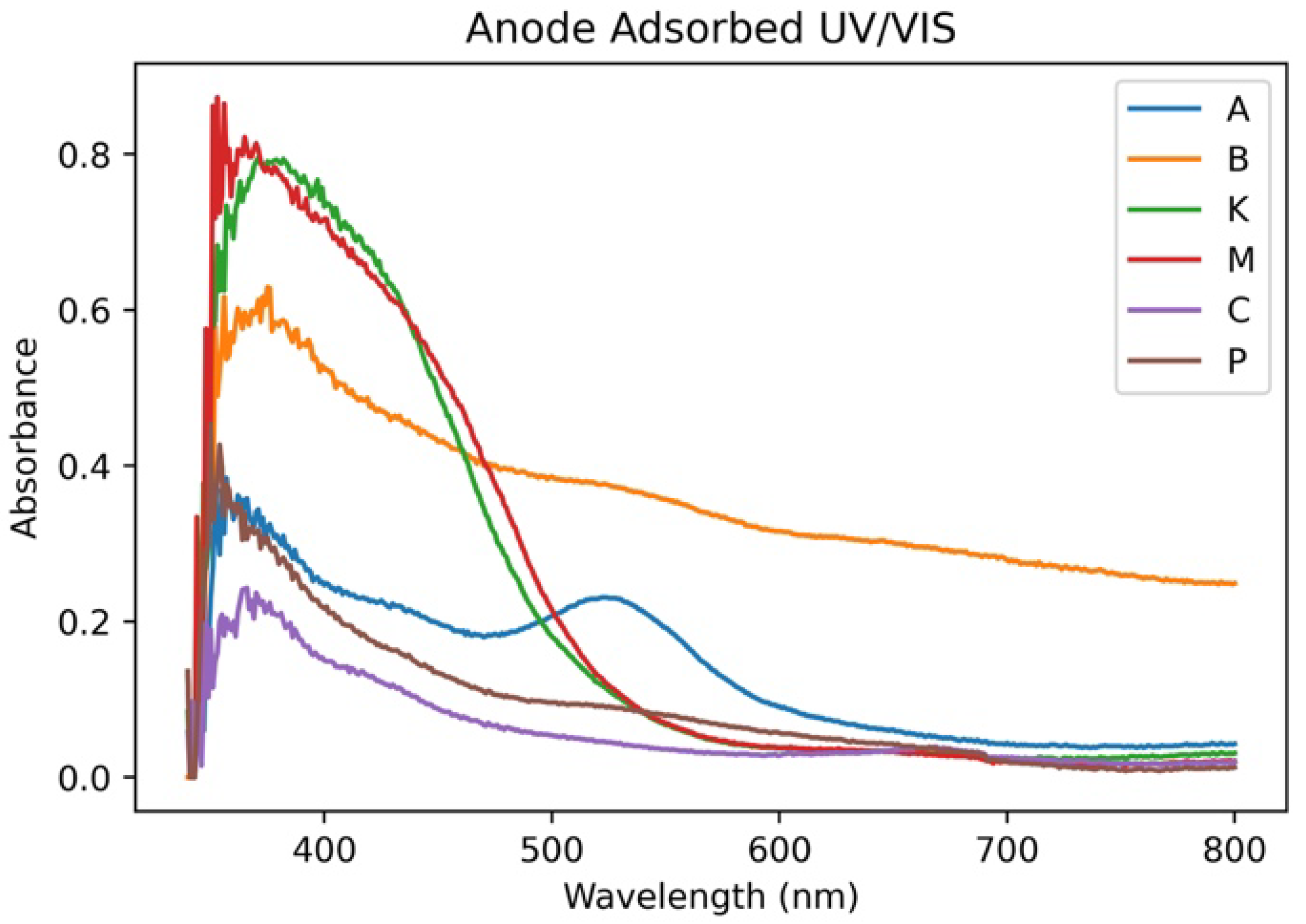
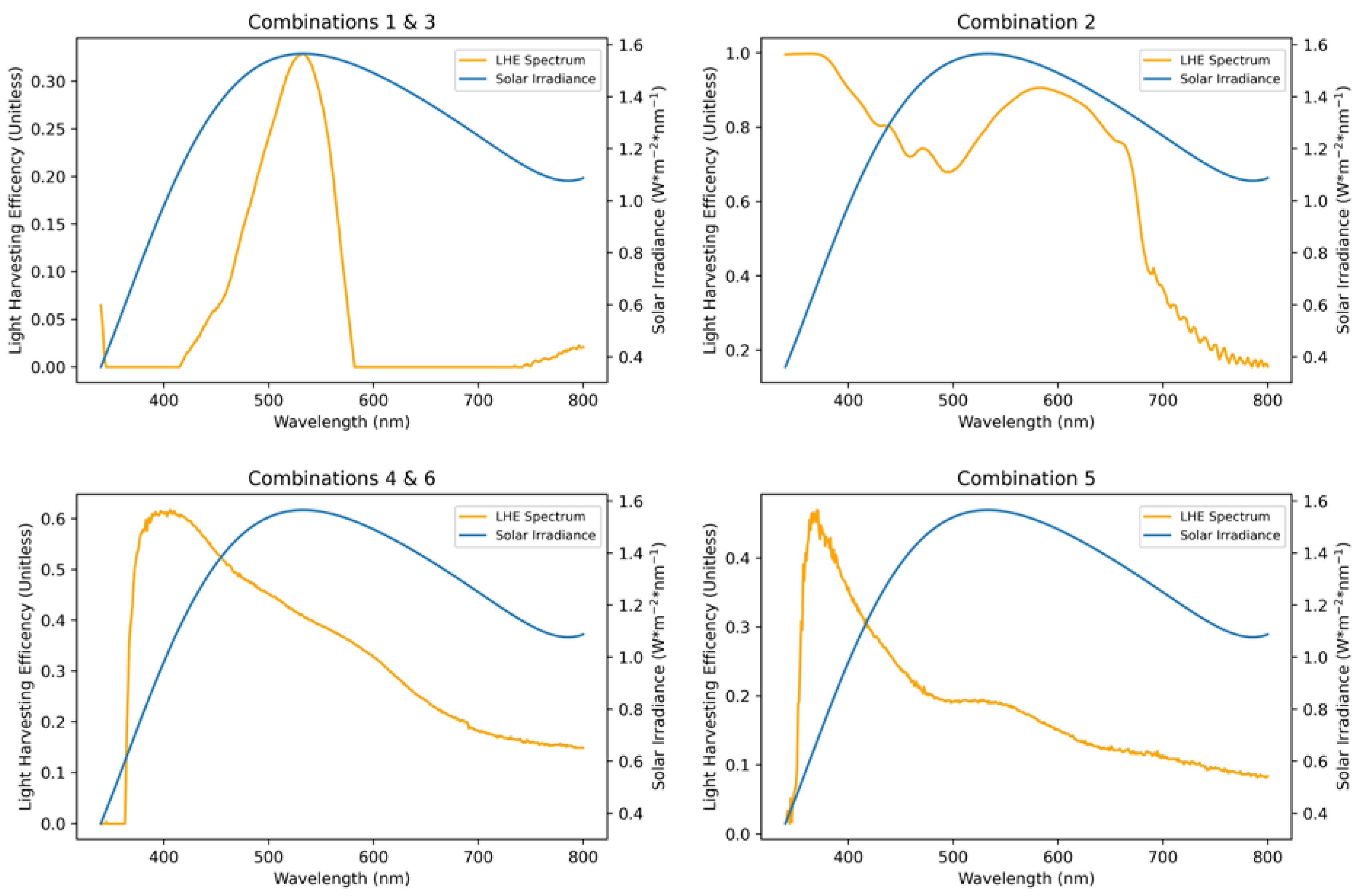
| Combination | Bulk Solution Peak Absorbance | Bulk Solution Integral | Anode-Adsorbed Peak Absorbance | Anode-Adsorbed Integral |
|---|---|---|---|---|
| A | 0.35 | 34.69 | 0.38 | 65.15 |
| B | 0.66 | 81.84 | 0.63 | 165.01 |
| K | 0.56 | 49.90 | 0.80 | 101.53 |
| M | 3.64 | 168.94 | 0.87 | 104.31 |
| C | 0.71 | 98.17 | 0.24 | 28.98 |
| P | 2.46 | 286.43 | 0.45 | 43.84 |
| AB | 0.49 | 63.15 | 0.45 | 66.22 |
| AK | 0.81 | 100.41 | 0.37 | 61.87 |
| AM | 3.41 | 170.20 | 0.67 | 123.40 |
| AC | 0.69 | 102.19 | 0.27 | 40.16 |
| AP | 2.57 | 293.47 | 0.33 | 46.38 |
| BK | 0.54 | 52.71 | 0.29 | 42.44 |
| BM | 3.39 | 208.88 | 0.68 | 69.36 |
| BC | 0.89 | 165.36 | 0.44 | 82.99 |
| BP | 2.46 | 308.67 | 0.37 | 26.29 |
| KM | 2.36 | 128.56 | 1.06 | 122.38 |
| KC | 1.20 | 145.31 | 0.41 | 43.00 |
| KP | 2.41 | 318.23 | 0.47 | 62.28 |
| MC | 3.74 | 235.69 | 0.91 | 102.10 |
| MP | 3.50 | 285.53 | 0.66 | 60.28 |
| CP | 2.45 | 301.86 | 0.51 | 59.30 |
| ABK | 0.50 | 70.19 | 0.45 | 51.18 |
| ABM | 3.84 | 232.12 | 0.47 | 57.55 |
| ABC | 0.83 | 157.65 | 0.58 | 62.85 |
| ABP | 0.32 | 41.38 | 0.25 | 40.08 |
| AKM | 3.81 | 217.62 | 0.64 | 92.77 |
| AKC | 1.14 | 146.07 | 0.46 | 55.76 |
| AKP | 2.45 | 300.71 | 0.59 | 99.61 |
| AMC | 3.67 | 230.30 | 0.57 | 100.88 |
| AMP | 3.40 | 286.19 | 0.44 | 51.72 |
| ACP | 2.43 | 323.68 | 0.28 | 42.68 |
| BKM | 3.38 | 248.50 | 0.52 | 67.45 |
| BKC | 0.69 | 111.51 | 0.46 | 36.96 |
| BKP | 2.44 | 331.19 | 0.51 | 51.07 |
| BMC | 3.00 | 267.46 | 0.36 | 44.26 |
| BMP | 3.01 | 266.14 | 0.32 | 32.19 |
| BCP | 2.40 | 341.48 | 0.40 | 43.52 |
| KMC | 3.54 | 263.29 | 0.83 | 90.33 |
| KMP | 2.76 | 241.07 | 0.49 | 97.11 |
| KCP | 1.27 | 200.71 | 0.36 | 36.05 |
| MCP | 2.69 | 257.95 | 0.63 | 49.99 |
| ABKMCP | 2.55 | 251.09 | 0.82 | 85.43 |
| Average | 2.13 | 199.68 | 0.52 | 66.83 |
| Combination | Fitment Condition | VA | VB | VK | VM | VC | VP |
|---|---|---|---|---|---|---|---|
| 1 | Correlation | 0.4 | 0.4 | 0 | 0 | 0 | 0.2 |
| 2 | Integral | 0.3 | 0 | 0 | 0 | 0.3 | 0.4 |
| 3 | Covariance | 0.4 | 0.4 | 0 | 0 | 0 | 0.2 |
| Combination | Fitment Condition | VA | VB | VK | VM | VC | VP |
|---|---|---|---|---|---|---|---|
| 4 | Correlation | 0 | 0 | 0.3 | 0.3 | 0 | 0.4 |
| 5 | Integral | 0 | 1.0 | 0 | 0 | 0 | 0 |
| 6 | Covariance | 0 | 0 | 0.3 | 0.3 | 0 | 0.4 |
| Combination | Jsc (mA/cm2) | Voc (V) | FF (Unitless) | Efficiency (%) |
|---|---|---|---|---|
| A | 0.442 | 0.482 | 0.517 | 0.110 |
| B | 0.775 | 0.574 | 0.489 | 0.217 |
| K | 0.822 | 0.553 | 0.522 | 0.237 |
| M | 2.642 | 0.620 | 0.612 | 1.003 |
| C | 0.447 | 0.225 | 0.284 | 0.028 |
| P | 0.214 | 0.523 | 0.660 | 0.073 |
| AB | 0.856 | 0.607 | 0.684 | 0.354 |
| AK | 1.042 | 0.499 | 0.528 | 0.275 |
| AM | 1.514 | 0.536 | 0.584 | 0.474 |
| AC | 0.506 | 0.502 | 0.557 | 0.141 |
| AP | 0.389 | 0.145 | 0.268 | 0.015 |
| BK | 0.825 | 0.617 | 0.686 | 0.349 |
| BM | 0.714 | 0.612 | 0.410 | 0.178 |
| BC | 1.247 | 0.632 | 0.691 | 0.544 |
| BP | 0.322 | 0.576 | 0.629 | 0.116 |
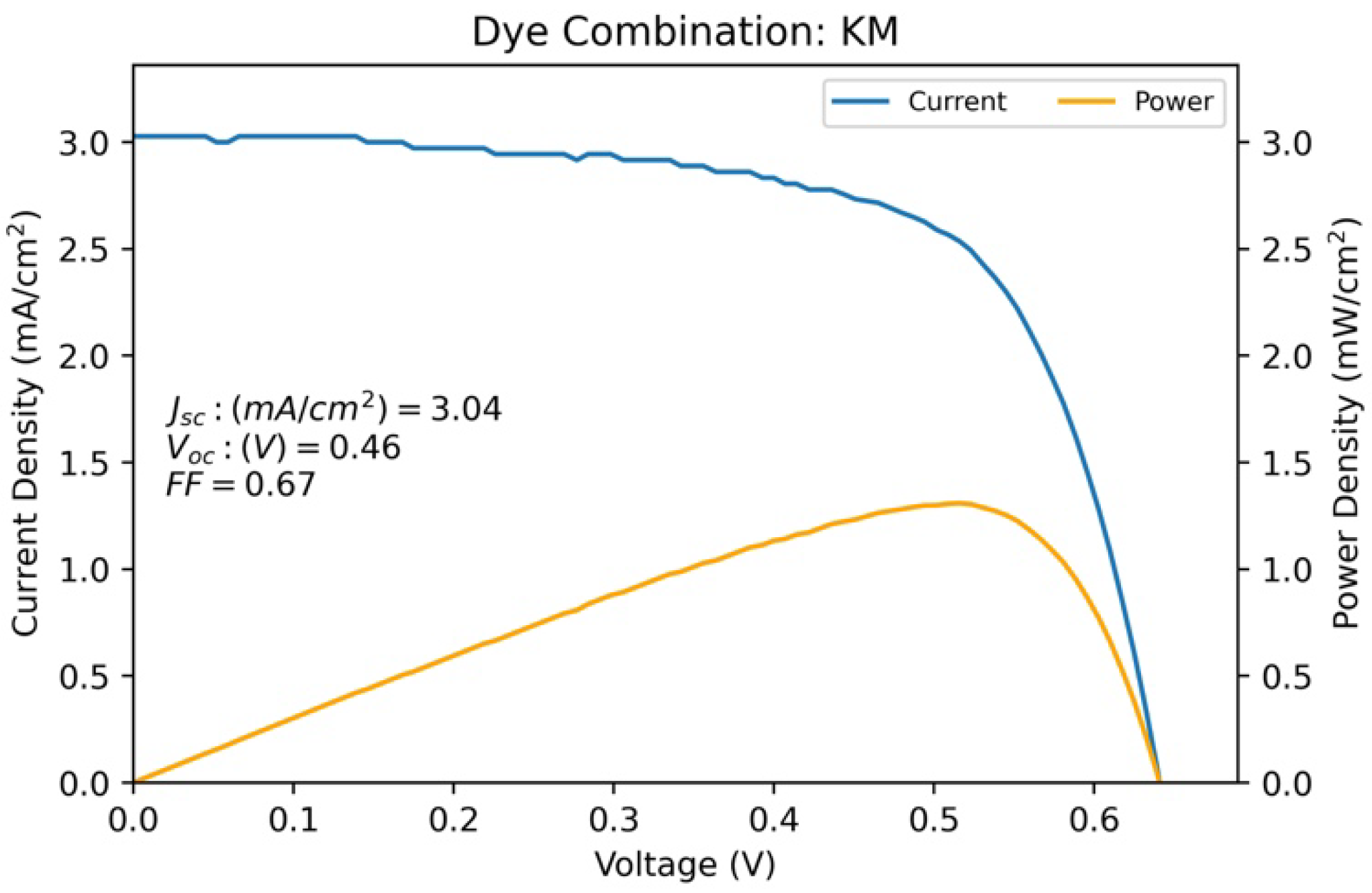
| KM | 3.039 | 0.461 | 0.672 | 1.308 |
| KC | 1.206 | 0.592 | 0.570 | 0.406 |
| KP | 0.417 | 0.538 | 0.647 | 0.145 |
| MC | 1.214 | 0.290 | 0.333 | 0.117 |
| MP | 1.325 | 0.562 | 0.394 | 0.293 |
| CP | 0.492 | 0.464 | 0.227 | 0.052 |
| ABK | 0.822 | 0.572 | 0.702 | 0.330 |
| ABM | 0.750 | 0.609 | 0.460 | 0.210 |
| ABC | 0.914 | 0.638 | 0.755 | 0.440 |
| ABP | 0.583 | 0.608 | 0.595 | 0.247 |
| AKM | 1.614 | 0.345 | 0.295 | 0.164 |
| AKC | 0.900 | 0.529 | 0.520 | 0.248 |
| AKP | 0.239 | 0.421 | 0.464 | 0.046 |
| AMC | 1.433 | 0.529 | 0.409 | 0.310 |
| AMP | 0.675 | 0.557 | 0.487 | 0.183 |
| ACP | 0.447 | 0.546 | 0.412 | 0.100 |
| BKM | 1.125 | 0.668 | 0.647 | 0.486 |
| BKC | 0.331 | 0.598 | 0.586 | 0.115 |
| BKP | 0.731 | 0.582 | 0.706 | 0.300 |
| BMC | 1.081 | 0.665 | 0.696 | 0.492 |
| BMP | 0.989 | 0.604 | 0.687 | 0.410 |
| BCP | 0.489 | 0.582 | 0.700 | 0.199 |
| KMC | 1.883 | 0.640 | 0.695 | 0.837 |
| KMP | 0.456 | 0.495 | 0.498 | 0.112 |
| KCP | 0.472 | 0.537 | 0.579 | 0.147 |
| MCP | 0.992 | 0.573 | 0.588 | 0.334 |
| ABKMCP | 0.564 | 0.605 | 0.534 | 0.182 |
| Combinations 1 and 3 | 0.567 | 0.555 | 0.585 | 0.184 |
| Combination 2 | 0.497 | 0.483 | 0.457 | 0.109 |
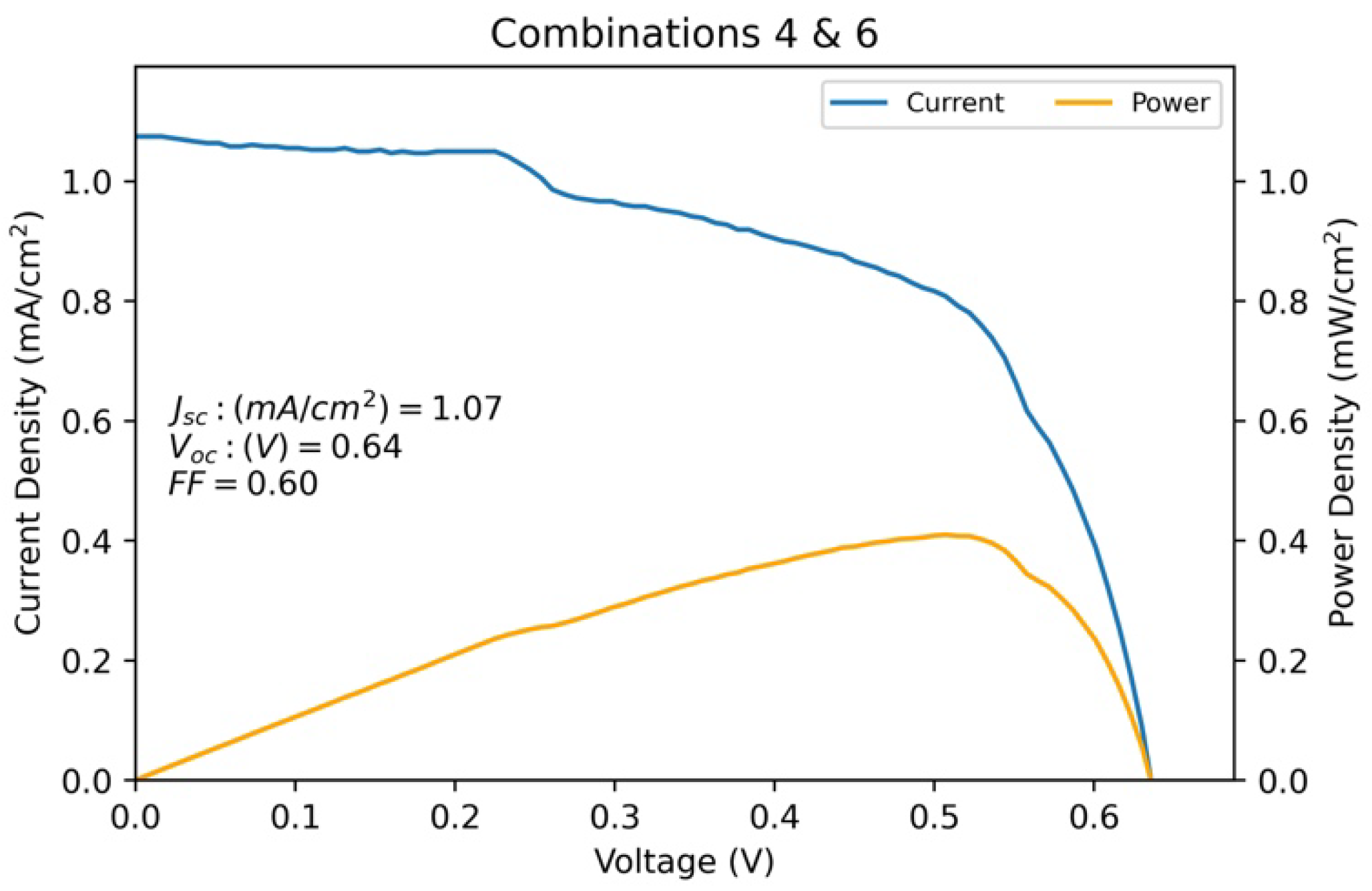
| Combinations 4 and 6 | 1.075 | 0.636 | 0.601 | 0.410 |
| Combination 5 | 0.775 | 0.574 | 0.489 | 0.217 |
| Average | 0.888 | 0.538 | 0.546 | 0.288 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manz, N.B.; Fuierer, P.A. Mathematical Approach to Optimizing the Panchromatic Absorption of Natural Dye Combinations for Dye-Sensitized Solar Cells. Colorants 2023, 2, 90-110. https://doi.org/10.3390/colorants2010007
Manz NB, Fuierer PA. Mathematical Approach to Optimizing the Panchromatic Absorption of Natural Dye Combinations for Dye-Sensitized Solar Cells. Colorants. 2023; 2(1):90-110. https://doi.org/10.3390/colorants2010007
Chicago/Turabian StyleManz, Noah B., and Paul A. Fuierer. 2023. "Mathematical Approach to Optimizing the Panchromatic Absorption of Natural Dye Combinations for Dye-Sensitized Solar Cells" Colorants 2, no. 1: 90-110. https://doi.org/10.3390/colorants2010007
APA StyleManz, N. B., & Fuierer, P. A. (2023). Mathematical Approach to Optimizing the Panchromatic Absorption of Natural Dye Combinations for Dye-Sensitized Solar Cells. Colorants, 2(1), 90-110. https://doi.org/10.3390/colorants2010007








