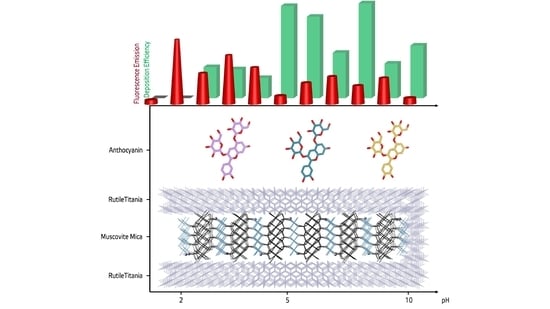
pH-Dependent Coloring of Combination Effect Pigments with Anthocyanins from Brassica oleracea var. capitata F. rubra
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mica/Titania (MT) Synthesis
2.2. Anthocyanin Extraction
2.3. Combination Pigment Synthesis
2.4. Characterization
3. Results
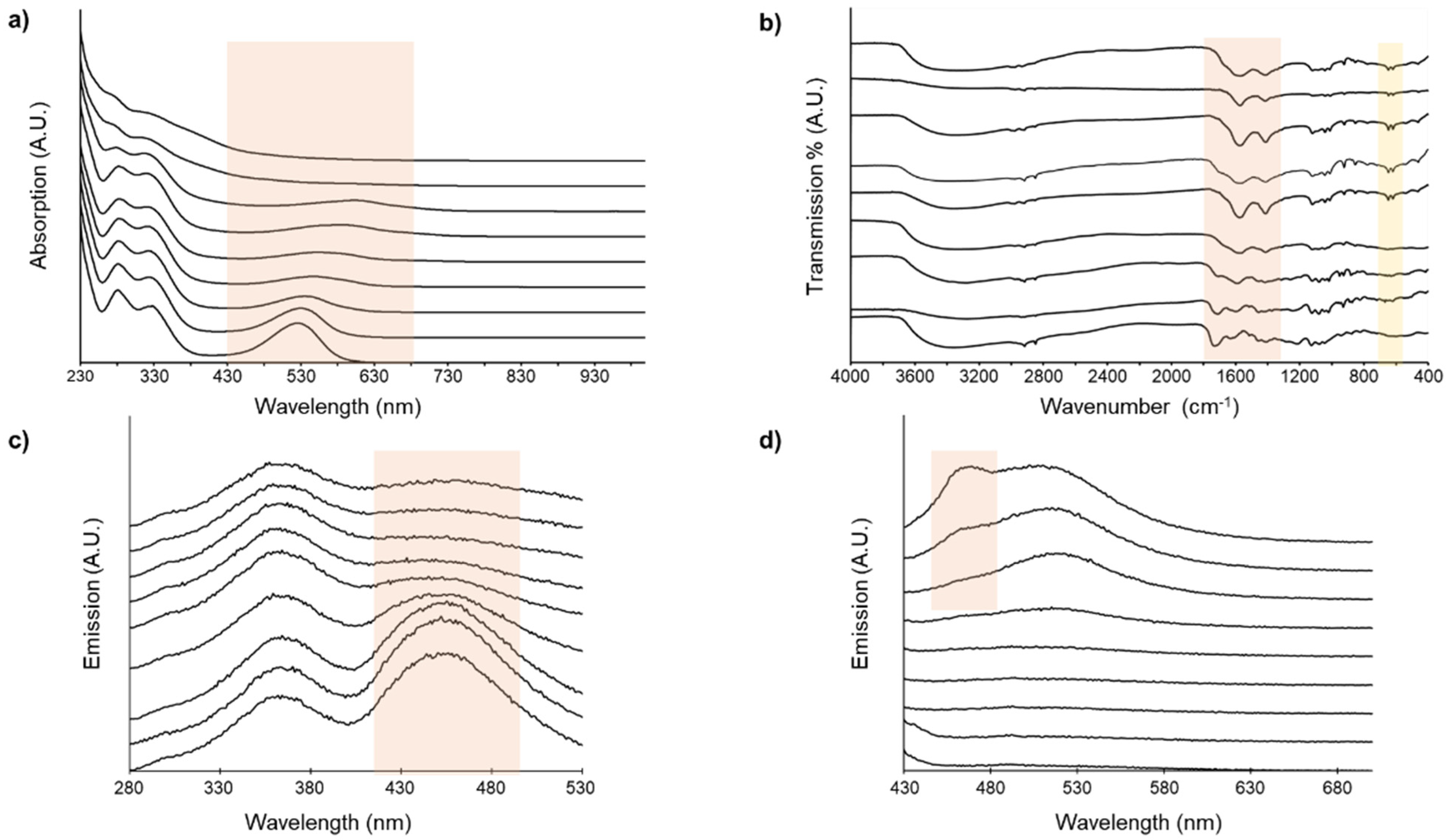
3.1. Anthocyanin Extraction and Spectral Analyses
3.2. Combination Pigments
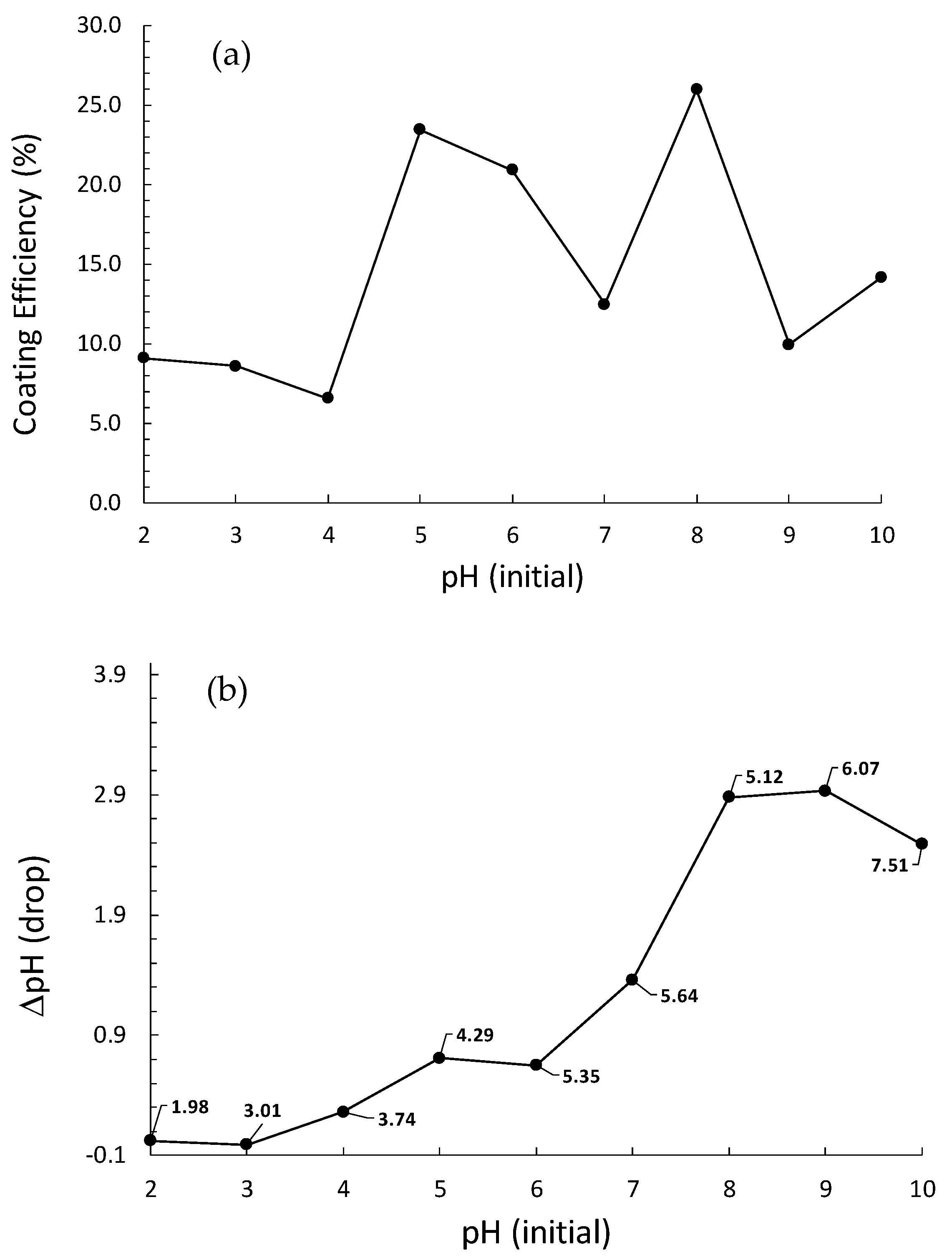
3.2.1. pH-Specific Depositions
3.2.2. Thermogravimetric Analyses
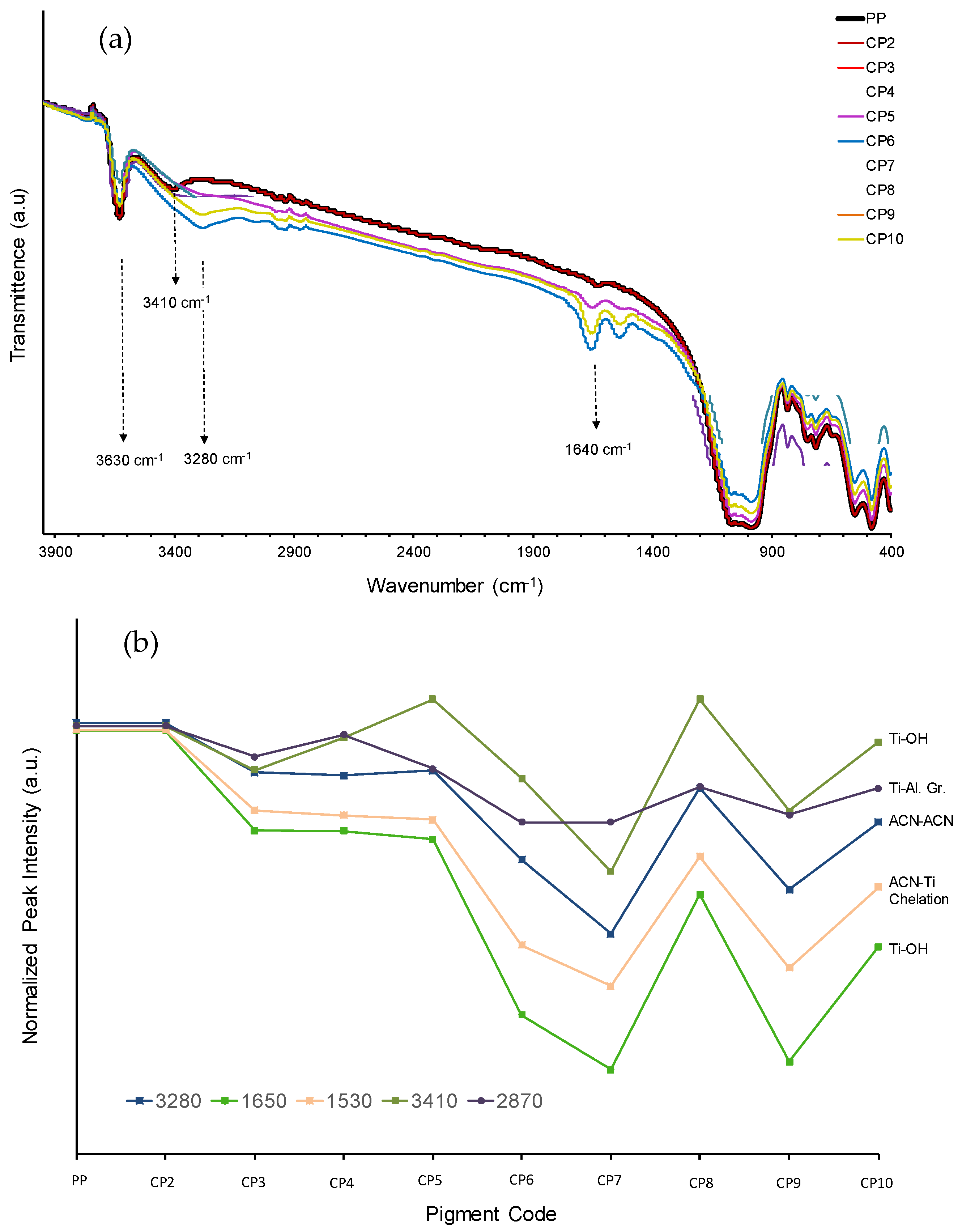
3.2.3. FTIR Spectra of Combination Pigments
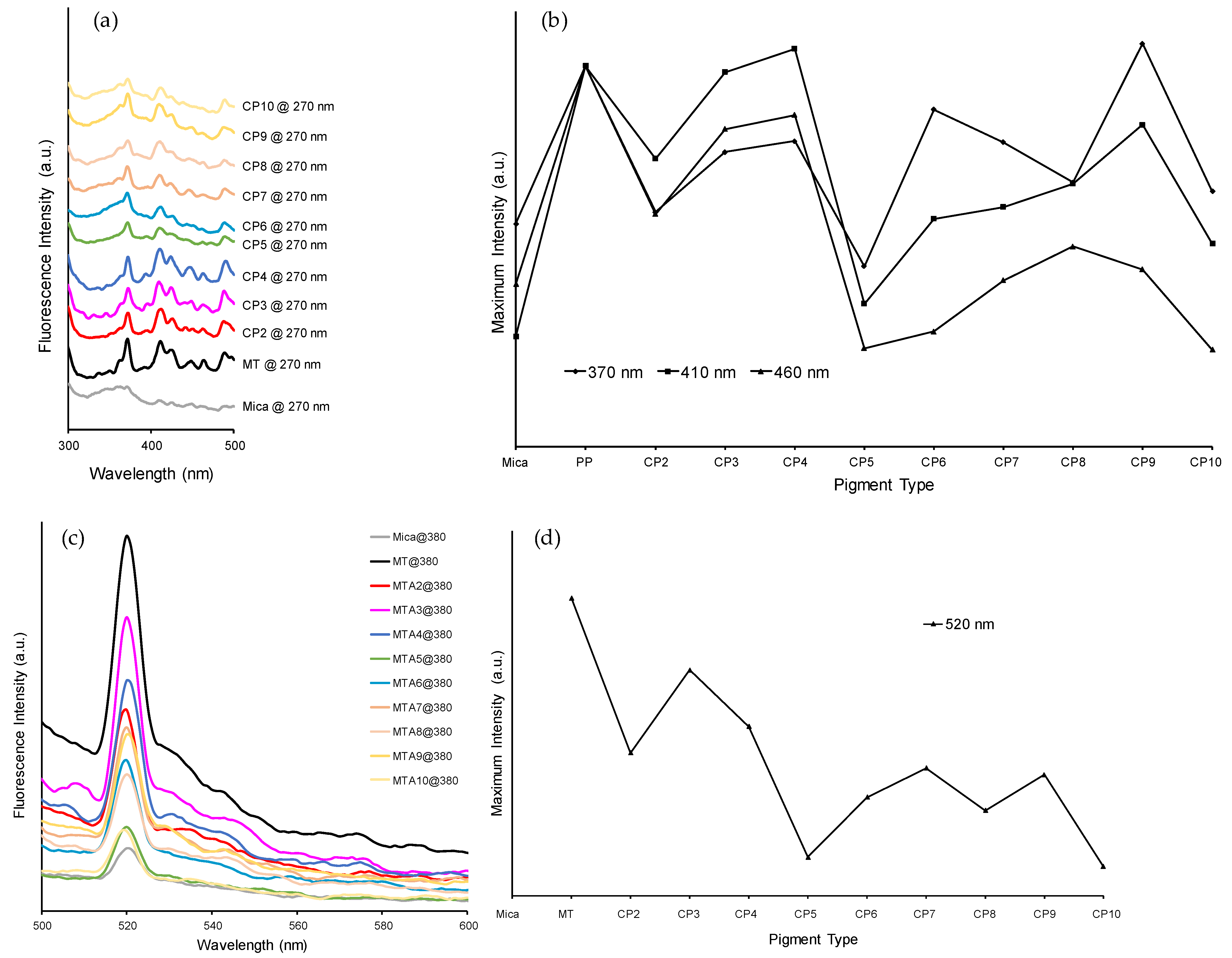
3.2.4. Fluorescence Spectra of Combination Pigments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Steyn, W.J. Prevalence and Functions of Anthocyanins in Fruits. In Anthocyanins: Biosynthesis, Functions, and Applications; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2009; pp. 86–105. [Google Scholar]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Brouillard, R.; Delaporte, B. Chemistry of anthocyanin pigments. 2. Kinetic and thermodynamic study of proton transfer, hydration, and tautomeric reactions of malvidin 3-glucoside. J. Am. Chem. Soc. 1977, 99, 8461–8468. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.-A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zyoud, A.H.; Saleh, F.; Helal, M.H.; Shawahna, R.; Hilal, H.S. Anthocyanin-Sensitized TiO2 Nanoparticles for Phenazopyridine Photodegradation under Solar Simulated Light. J. Nanomater. 2018, 2018, 2789616. [Google Scholar] [CrossRef] [Green Version]
- Gokilamani, N.; Muthukumarasamy, N.; Thambidurai, M.; Ranjitha, A.; Velauthapillai, D. Utilization of natural anthocyanin pigments as photosensitizers for dye-sensitized solar cells. J. Sol-Gel Sci. Technol. 2013, 66, 212–219. [Google Scholar] [CrossRef]
- Ezike, S.C.; Hyelnasinyi, C.N.; Salawu, M.A.; Wansah, J.F.; Ossai, A.N.; Agu, N.N. Synergestic effect of chlorophyll and anthocyanin Co-sensitizers in TiO2-based dye-sensitized solar cells. Surf. Interfaces 2021, 22, 100882. [Google Scholar] [CrossRef]
- Martinez-Pacheco, M.; Lozada-Ramirez, J.D.; Martinez-Huitle, C.A.; Cerro-Lopez, M. Hibiscus sabdariffa L. Anthocyanins Immobilization on TiO2 Nanotubes and Its Electrochemical Characterization as a Hydrogen Peroxide Sensing Electrode. J. Electrochem. Soc. 2019, 166, B1506–B1512. [Google Scholar] [CrossRef]
- Fang, J.-L.; Luo, Y.; Yuan, K.; Guo, Y.; Jin, S.-H. Preparation and evaluation of an encapsulated anthocyanin complex for enhancing the stability of anthocyanin. LWT 2020, 117, 108543. [Google Scholar] [CrossRef]
- Topuz, B.B.; Gündüz, G.; Mavis, B.; Çolak, Ü. The effect of tin dioxide (SnO2) on the anatase-rutile phase transformation of titania (TiO2) in mica-titania pigments and their use in paint. Dyes Pigment. 2011, 90, 123–128. [Google Scholar] [CrossRef]
- Pfaff, G. Special Effect Pigments. In High Performance Pigments; Wiley-VCH: Weinheim, Germany, 2001; pp. 75–101. [Google Scholar]
- Maile, F.J.; Pfaff, G.; Reynders, P. Effect pigments—past, present and future. Prog. Org. Coat. 2005, 54, 150–163. [Google Scholar] [CrossRef]
- Ren, M.; Yin, H.; Ge, C.; Huo, J.; Li, X.; Wang, A.; Yu, L.; Jiang, T.; Wu, Z. Preparation and characterization of inorganic colored coating layers on lamellar mica-titania substrate. Appl. Surf. Sci. 2012, 258, 2667–2673. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Fan, Y.; Du, C. Influence of the seed layer on photoactivity inhibition of mica–titania pigments. Ceram. Int. 2016, 42, 6595–6600. [Google Scholar] [CrossRef]
- Fang, X.; Lu, G.; Mahmood, A.; Wang, Y.; Wang, X.; Xie, X.; Tang, Z.; Sun, J. A novel ternary mica-titania@rGO composite pearlescent pigment for the photocatalytic degradation of gaseous acetaldehyde. Chem. Eng. J. 2020, 396, 125312. [Google Scholar] [CrossRef]
- Tan, J.; Shen, L.; Fu, X.; Hou, W.; Chen, X. Preparation and conductive mechanism of mica titania conductive pigment. Dyes Pigment. 2004, 62, 107–114. [Google Scholar] [CrossRef]
- Zuo, S.; Liu, Z.; Liu, W.; Li, X.; Li, Z.; Yao, C.; Chen, Q.; Fu, Y. TiO2 nanorod arrays on the conductive mica combine photoelectrochemical cathodic protection with barrier properties. J. Alloys Compd. 2019, 776, 529–535. [Google Scholar] [CrossRef]
- Topuz, B.B.; Gündüz, G.; Mavis, B.; Çolak, Ü. Synthesis and characterization of copper phthalocyanine and tetracarboxamide copper phthalocyanine deposited mica-titania pigments. Dyes Pigment. 2013, 96, 31–37. [Google Scholar] [CrossRef]
- Kahya, S.S.; Sönmez, Y.; Gündüz, G.; Mavis, B. Combination effect pigments with enhanced fluorescence. Pigment. Resin Technol. 2019, 48, 277–292. [Google Scholar] [CrossRef]
- Silva, G.T.M.; da Silva, K.M.; Silva, C.P.; Rodrigues, A.C.B.; Oake, J.; Gehlen, M.H.; Bohne, C.; Quina, F.H. Highly fluorescent hybrid pigments from anthocyanin- and red wine pyranoanthocyanin-analogs adsorbed on sepiolite clay. Photochem. Photobiol. Sci. 2019, 18, 1750–1760. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purification of Anthocyanins. Curr. Protoc. Food Anal. Chem. 2001, F1.1.1–F1.1.11. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Sarapulova, V.; Klevtsova, A.; Mikhaylin, S.; Bazinet, L. Adsorption of Anthocyanins by Cation and Anion Exchange Resins with Aromatic and Aliphatic Polymer Matrices. Int. J. Mol. Sci. 2020, 21, 7874. [Google Scholar] [CrossRef] [PubMed]
- Pina, F.; Oliveira, J.; de Freitas, V. Anthocyanins and derivatives are more than flavylium cations. Tetrahedron 2015, 71, 3107–3114. [Google Scholar] [CrossRef]
- Singh, M.; Singh, L. Vibrational spectroscopic study of muscovite and biotite layered phyllosilicates. Indian J. Pure Appl. Phys. 2016, 54, 116–122. [Google Scholar]
- Prima, E.C.; Nugroho, H.S.; Nugraha; Refantero, G.; Panatarani, C.; Yuliarto, B. Performance of the dye-sensitized quasi-solid state solar cell with combined anthocyanin-ruthenium photosensitizer. RSC Adv. 2020, 10, 36873–36886. [Google Scholar] [CrossRef]
- Gregorkiewitz, M.; Rausell-Colom, J.A. Characterization and properties of a new synthetic silicate with highly charged mica-type layers. Am. Mineral. 1987, 72, 515–527. [Google Scholar]
- Nie, B.; Stutzman, J.; Xie, A. A Vibrational Spectral Maker for Probing the Hydrogen-Bonding Status of Protonated Asp and Glu Residues. Biophys. J. 2005, 88, 2833–2847. [Google Scholar] [CrossRef] [Green Version]
- Dawy, M. Electrical Properties and Infrared Studies of Heated Mica Sheets. Egypt. J. Solids 2002, 25, 137–152. [Google Scholar] [CrossRef]
- Buchweitz, M.; Gudi, G.; Carle, R.; Kammerer, D.R.; Schulz, H. Systematic investigations of anthocyanin–metal interactions by Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 2001–2007. [Google Scholar] [CrossRef]
- Ribeiro, H.L.; Oliveira, A.V.d.; Brito, E.S.d.; Ribeiro, P.R.V.; Souza Filho, M.d.s.M.; Azeredo, H.M.C. Stabilizing effect of montmorillonite on acerola juice anthocyanins. Food Chem. 2018, 245, 966–973. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH-dependent surface charging and points of zero charge: V. Update. J. Colloid Interface Sci. 2011, 353, 1–15. [Google Scholar] [CrossRef]
- Andersen, Ø.M.M.K.R. Flavonoids: Chemistry, Biochemistry, and Applications; CRC, Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hawkins, T. Surface Charge Characterization of Anatase and Rutile Using Flow Adsorption Microcalorimetry. Master’s Thesis, Georgia State University, Atlanta, GA, USA, 2016. [Google Scholar]
- Alfarano, S.R.; Pezzotti, S.; Stein, C.J.; Lin, Z.; Sebastiani, F.; Funke, S.; Hoberg, C.; Kolling, I.; Ma, C.Y.; Mauelshagen, K.; et al. Stripping away ion hydration shells in electrical double-layer formation: Water networks matter. Proc. Natl. Acad. Sci. USA 2021, 118, e2108568118. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, C.S.K.; Wanninnayake, W.M.N.M.B.; Kumara, G.R.A.; Rajapakshe, R.M.G.; Sirimanne, P.M. An enhancement of efficiency of a solid-state dye-sensitized solar cell due to cocktail effect of N719 and black dye. Optik 2014, 125, 813–815. [Google Scholar] [CrossRef]
- Rajkumar, S.; Kumar, M.N.; Suguna, K.; Muthulakshmi, S.; Kumar, R.A. Enhanced performance of dye-sensitized solar cells using natural cocktail dye as sensitizer. Optik 2019, 178, 224–230. [Google Scholar] [CrossRef]
- Hao, S.; Wu, J.; Huang, Y.; Lin, J. Natural dyes as photosensitizers for dye-sensitized solar cell. Sol. Energy 2006, 80, 209–214. [Google Scholar] [CrossRef]
- Chien, C.-Y.; Hsu, B.-D. Optimization of the dye-sensitized solar cell with anthocyanin as photosensitizer. Sol. Energy 2013, 98, 203–211. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çoruh, O.; Gündüz, G.; Çolak, Ü.; Maviş, B. pH-Dependent Coloring of Combination Effect Pigments with Anthocyanins from Brassica oleracea var. capitata F. rubra. Colorants 2022, 1, 149-164. https://doi.org/10.3390/colorants1020010
Çoruh O, Gündüz G, Çolak Ü, Maviş B. pH-Dependent Coloring of Combination Effect Pigments with Anthocyanins from Brassica oleracea var. capitata F. rubra. Colorants. 2022; 1(2):149-164. https://doi.org/10.3390/colorants1020010
Chicago/Turabian StyleÇoruh, Orkun, Güngör Gündüz, Üner Çolak, and Bora Maviş. 2022. "pH-Dependent Coloring of Combination Effect Pigments with Anthocyanins from Brassica oleracea var. capitata F. rubra" Colorants 1, no. 2: 149-164. https://doi.org/10.3390/colorants1020010
APA StyleÇoruh, O., Gündüz, G., Çolak, Ü., & Maviş, B. (2022). pH-Dependent Coloring of Combination Effect Pigments with Anthocyanins from Brassica oleracea var. capitata F. rubra. Colorants, 1(2), 149-164. https://doi.org/10.3390/colorants1020010