Abstract
The innovation of drug delivery vehicles with controlled properties for cancer therapy is the aim of most pharmaceutical research. This study aims to fabricate a new type of smart biocompatible stealth-nanoliposome to deliver curcumin for cancer treatment. Herein, four different types of liposomes (with/without pH-responsive polymeric coating) were synthesized via the Mozafari method and then characterized with several tests, including dynamic light scattering (DLS), Fourier-transform infrared spectroscopy (FTIR), Zeta potential, and field emission scanning electron microscopes (FE-S EM). The loading and release profile of curcumin were evaluated in two pH of 7.4 and 6.6. Finally, the MTT assay was used to assess the cytotoxicity of the samples. FE-SEM results revealed a mean size of about 40 and 50 nm for smart stealth-liposome and liposome, respectively. The results of drug entrapment revealed that non-coated liposome had about 74% entrapment efficiency, while it was about 84% for PEGylated liposomes. Furthermore, the drug released pattern of the nanocarriers showed more controllable release in stealth-liposome in comparison to non-coated one. The results of the cytotoxicity test demonstrated the toxicity of drug-loaded carriers on cancer cells. Based on the results of this study, the as-prepared smart stealth pH-responsive nanoliposome could be considered as a potential candidate for cancer therapy.
1. Introduction
One of the most promising approaches for cancer treatment is the use of nanomaterials as drug delivery vehicles. The tiny size of these materials leads to the facility of their moving through the thinnest blood vessel and also the ability of their penetrating via the vessel to the tissues in specific sites, especially cancerous tissues [1,2,3]. Moreover, they can protect the drug cargo molecules from elimination by the immune system and release them at the targeted site, which not only prevents side effect of drugs on the healthy tissues but also improves the effectiveness of the drugs and thus causes a reduction in the dose [4,5,6].
Among the most applicable types of nanoparticles are liposomes, which are bilayer nano-drug delivery systems that were innovated by Bangham and his coworkers in 1965 for the first time. Liposomes are self-assembled spherical vesicles that consist of different types of phospholipids and cholesterol that have the most structural similarity with the cellular membrane [1,2]. Different types of phospholipid were considered for the preparation of liposomes, which are categorized into two main classes: Natural phospholipids like egg phosphatidylcholine and soy phosphatidylcholine (lecithin), and synthetic phospholipid, which contains a wide range of saturated and unsaturated phospholipids among the most important of them is 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) which can donate the most stability to the liposomal structure and also improve drug loading and the released pattern [3,4].
The specific structure of liposomes enables them to load both hydrophobic and hydrophilic drugs. In other words, the hydrophobic drugs could be placed inside the bilayer structure, and hydrophilic ones could be encapsulated inside the central hollow part or be loaded on the surface of the liposome [5,6,7,8]. Despite their different benefits, they have a defect in their drug released pattern so that drug molecules show leakage from the liposomal structure. Moreover, their rapid interaction with plasma proteins leads to their elimination from the body circulation system. This has led to the design of new types of the liposome, known as stealth liposome, in which the phospholipid bilayer is covered by a polymeric layer [9,10,11]. Utilizing the polymeric shell could increase the circulation time of liposomes, enhance their fluidity and stability, and reduce the uncontrollable drug release from them [12].
One of the most prevalent polymeric shells used for the liposome covering is Polyethylene glycol (PEG). This is an FDA-approved amphiphilic polymer with a hydrophilic surface that can improve the hydrophilic property of liposomes. The PEGylated liposomes could also show a protein resistance property that leads to improving blood circulation and prevents their clearance by the mononuclear phagocyte system (MPS). Doxil™ and Caelyx are two types of FDA-approved PEGylated liposomes containing doxorubicin as the drug component [13,14,15].
To improve the drug molecules’ performance, eliminate their side effect, and decrease the drug dosage, it is necessary to selectively accumulate the drug components in their targeted sites. Indeed, there exist three main strategies for delivering the nanoparticles to their targeted cancer tissue: Passive targeting, active targeting, and smart drug delivery. Passive targeting is based on the physiological property of cancerous tissue, the enhanced permeability and retention (EPR) effect, which increases the penetration of nanoparticles through the cancerous vessel due to the increase in their intercellular distance [16]. Active targeting is based on the functionalizing nanocarriers with targeting ligands that have an affinity to a receptor on the surface of cancer cells or a specific intercellular organelle. This targeting strategy works more effectively than an inactive one for cancer treatment [17]. The last strategy is based on the drug release in a specific site due to the structural change in response to the intrinsic (pH, and temperature) or extrinsic (light and magnetic) stimulation [18].
It is confirmed in different literature that the pH of cancer tissues is a little lower (6.5~7) than the pH of normal tissues (7.4), which results from a difference in the glucose metabolic pathway [19,20]. This intrinsic feature of cancer tissue could be used for the fabrication of smart pH-responsive drug delivery systems, which could release the drug component due to the cleavage that occurs in their structure in response to the pH-change. Based on these features, this study aimed to design and fabricate a new type of smart pH-responsive stealth liposome for the delivery of curcumin as an anticancer drug. Curcumin is a type of herbal drug extracted from the rhizome of Curcuma longa and shows several biological activities, from anti-inflammatory, anti-oxidant, anti-diabetic, and anti-atherosclerotic to anti-cancer, anti-angiogenic, and anti-metastatic [21]. Consequently, four types of liposomes were fabricated (with/without stealth cover) by the Mozafari method and characterized by different physicochemical and biological tests. Herein, citraconic anhydride was chosen as a pH-responsive cross-linker between PEG and phospholipid in the structure of nanoliposomes. During these tests, we also checked the effect of the amount of cholesterol on the properties of nanoliposomes.
2. Materials and Methods
2.1. Materials
Citraconic anhydride (CA) 98%, 1,2-distearoyl-sn-glycero-3-phosphocholine ≥ 99% (DSPC), 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine ≥ 99% (DSPE), cholesterol ≥ 99%, pyridine 99.8%, dichloromethane ≥ 99.9%, diethyl ether ≥ 99.9%, and glycerol ≥ 99.0% were purchased from Sigma, USA. 4-Dimethylaminopyridine and dicyclohexylcarbodiimide were prepared from Merck, Germany. Polyethylene glycol (PEG-2000) was received from Roth, Germany. Roswell Park Memorial Institute (RPMI) 1640 medium, fetal bovine serum (FBS), phosphate buffer saline (PBS), penicillin-streptomycin, and trypsin-EDTA were also received from BioIdea, Iran. The MCF7 and L929 cell lines were also purchased from the Pasture Institute of Iran.
2.2. Methods
2.2.1. Preparation of pH-Responsive Agents
In this research, CA was used as a pH-responsive polymer to donate the smart feature to the nanoliposome. For this purpose, CA was attached to the PEG and DSPE via two different chemical reactions: Amidation and esterification. In detail, 34 mg of DSPE and 40 μL of CA were dissolved in 3 mL of pyridine and then added to 5 mL of dichloromethane. After vigorously stirring the mixture for 24 h at room temperature, the products (DSPE- -CIT-COOH) were precipitated in cold diethyl ether.
In the second step, the as-prepared DSPE- -CIT-COOH was dissolved in 5 mL of dichloromethane, and then 50 mg of PEG was added to it. Certain amounts of DMAP and DDC as the coupling agents were also added to the above mixture, and the final solution was stirred for about 48 h under N2 atmosphere at room temperature. Then, it was precipitated in cold diethyl ether and dialyzed against water for 72 h to remove the solvent and all the non-reacted agents and final product (DSPE-citraconic-PEG (DSPE-CIT-PEG)) were dried with a freeze-drier (VaCo5, Zirbus, Bad Grund, Germany) [22].
2.2.2. Preparation of Liposome by the Mozafari Method
In this research, four different types of nanoliposomes were prepared with the aim of the Mozafari method. For this purpose, different amounts of DSPC, DSPE, DSPE-CIT-PEG, and cholesterol were weighted according to Table 1. Cholesterol was first dispersed in water, and then it was warmed to 120 °C on a heater-stirrer for about 2 h until all cholesterols were dissolved in the water.

Table 1.
The amounts of different components of the nano-liposomes. DSPE: Distearoyl-sn-glycero-3-phosphorylethanolamine; DSPC: Distearoyl-sn-glycero-3-phosphocholine; PEG: Polyethylene glycol.
In a separate three naked balloon, DSPC and DSPE-CIT-PEG/DSPE were added to 5 mL of water with 3% v/w glycerol, and the mixture was heated to be above the transition temperature of DSPC (about 70 °C) under N2 atmosphere. When the phospholipid reagents were completely dissolved in water, curcumin (with a concentration of 50 μg/mL of the final solution) was added to the solution. After about 10 min, the cholesterol solution was also added drop-wisely, and the final solution was vigorously stirred for about 1 h at 70 °C. Then, the temperature and the speed of the heater-stirrer were slowly decreased to allow the components to get in touch with each other and the nanoliposome was prepared. To decrease the size of the liposome, the solution was exposed to ultrasound waves for 30 min and then kept at the above critical temperature (Tc) without stirring for 1 h to let the liposomes be stable [23].
2.2.3. Characterization
To confirm the correct preparation of the DSPE-CIT-PEG and liposomes, different analytical tests were used. The size and morphology of the nanoliposomes were determined by FE-SEM (MIRA3 TESCAN, Brno, Czech Republic). The powder samples were coated with Au at first, and then their size and morphology were checked by the FE-SEM. FTIR spectroscopy (400–4000 cm−1) was chosen to evaluate the surface characteristics of liposomes with and without drug components and the correct preparation of DSPE-CIT-PEG (JASCO 6300 spectroscope, Tokyo, Japan, transmission mode). To this end, the samples’ powder was mixed by KBr and after preparing the tablet, their absorbance was collected by the FTIR instrument. Zeta sizer (HORIBA, scientific SZ100, Tokyo, Japan) was also applied to determine the surface charge and hydrodynamic diameter of the samples. In this test, samples were dispersed in deionized (DI) water via sonicating for at least 15 min, and then their hydrodynamic size and surface charge were conducted by the zeta sizer.
2.2.4. Determining the Drug Loading and Released Profile
- Drug loading determining
Curcumin is a type of herbal drug with several excellent properties like anti-oxidant, anti-bacterial, UV protective, and anticancer, but due to its low water solubility, it eliminates from the circulation system rapidly. So, a water-soluble biocompatible carrier could enhance the bioavailability of this drug. Based on this, a new formulation of liposome was selected in this research for the delivery of this drug. The drug loading process was conducted during the synthesis of liposomes since it might be absorbed in the interlayer space. To determine the amount of non-loaded drugs, liposomes were centrifuged after the preparation process to precipitate the liposomes. The supernatant, which contained non-loaded drugs, was mixed with a certain amount of ethanol (99.9%) to dissolve the drug. Then the absorbance of the solution was determined by UV-Visible spectroscopy (V670, Japan). The entrapment efficiency of the loaded drug was calculated by the following equation [24]:
- pH-responsive drug release
The drug release behavior of the nano-liposomes in response to the pH-change was determined at 25 °C and 37 °C over 5 days. For this purpose, a batch of 5 mg of liposomes was dissolved in 3 mL of deionized water and put in the dialyzes bag with molecular cut off 3.5 kD and placed in PBS solution with two different pH (7.4 and 6.6). After a certain period (24, 48, 72, and 96 h), the PBS solution was replaced with fresh PBS. Then, the drug containing PBS was centrifuged at 9000 RPM, the supernatant was discarded, and the precipitate which contained the drug molecules was dissolved in alcohol, and the absorbance of it was calculated to determine the amounts of the released drug.
2.2.5. Stability Assessment
The stability of nano-liposomes was determined by evaluating changes in the size (by FE-SEM) and drug release ability for 3 months.
2.2.6. Cell Viability Assessment
MTT colorimetric assay was chosen to assess the cytotoxicity effect of drug-loaded nano-liposomes. MTT assay is the most usual test used for this purpose, which is based on the conversion of yellow MTT components to the purple formazan by an enzymatic reaction inside the viable cells [25]. In this research, MCF-7 as the cancerous cell line, and L929 as the Healthy cells were chosen, and the concentration of 8000 cells in 100 μL of medium were cultured in each well of 96 well plates, and then cells were incubated for 24 h at 37 °C and 5% CO2. After 24 h, the medium culture of each well was replaced by fresh media containing different concentrations of drug-loaded nano-liposomes (25, 50, and 100 μg/mL). Moreover, the concentration of 100 μg/mL of liposome without a drug and two concentrations of free curcumin were also tested. After 24, 48, and 72 h of cells incubation at 37 °C, the medium of each well was discarded, cells were washed with 100 μL of PBS twice, and then 100 μL of pure media and 10 μL of MTT solution (5 mg/mL in PBS) were added to each well and incubated for 3.5 h. After that, the media was replaced by 100 μL of DMSO and incubated for another 1 h, and then the absorbance of each well was read at 493 nm by ELISA reader (Bio-Rad, Hercules, CA, USA) [26].
3. Results
3.1. Preparation of DSPE-CIT-PEG
In this research, the DSPE-CIT-PEG component was used as the pH-responsive agent that degraded in acidic pH and turned to release the drugs in the acidic environment of the cancerous tissues. On the other hand, the presence of this component in the structure of liposomes led to the enhance in biocompatibility and water solubility of nano-liposomes. Moreover, it could manage the drug released profile of the nano-liposomes in a more controllable manner. This component was prepared based on a two-step reaction in which amid and ester bonds were created between citraconic anhydride and DSPE and PEG, respectively (Scheme 1).
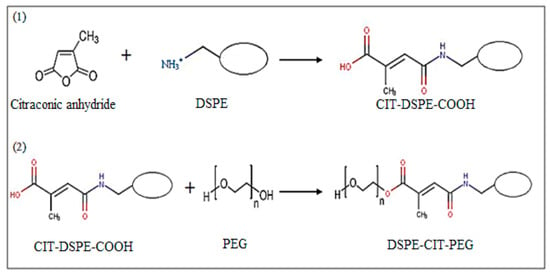
Scheme 1.
Two-step preparation of DSPE-CIT-PEG.
The correct preparation of this component was assessed by FTIR analysis (Figure 1). In other words, the emergence of peaks at around 1570, 1690, 3300, and 3400 cm−1 in the DSPE-CIT-PEG spectrum, which were attributed to the alkene group, amide bond, NH, and OH groups, respectively, in response to the spectrum of PEG and DSPE, confirmed the DSPE-CIT-PEG fabrication [22]. It is worth mentioning that the peaks of NH2 and OH groups were clear in the spectrum of DSPE and PEG, respectively. Other peaks shown in the figure are as follow: The alkane peaks at a wavenumber of about 2800–2900 cm−1, respectively, that were presented in the spectrum of all three components, the ester bond at around 1730 cm−1 in the spectrum of DSPE and DSPE-CIT-PEG [27], and the COC peak at around 1100 cm−1 in the spectrum of PEG and DSPE-CIT-PEG [28,29].
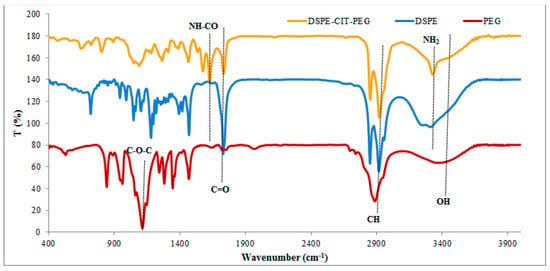
Figure 1.
FTIR results of PEG, DSPE, and DSPE-CIT-PEG.
3.2. Fabrication of Nanoliposomes
In this research, the effect of the concentration of cholesterol and the polymeric shell on the liposomal nanocarrier was evaluated. For this, four different types of nanoliposomes (with/without drug molecules) were prepared based on the Mozafari method with two different concentrations of cholesterol (5 and 10 M) and with/without PEG coating. The main advantage of this method is that it leads to the preparation of safe liposomes since the products of this method are prepared in the aquatic environment. Thus, they have no toxic agents which may be present in the structure of liposomes derived from other methods [30].
Different analytical analyses were used for the evaluation of the as-prepared nanoliposomes. In the first step, FTIR was selected to evaluated differences between liposomes and PEG-coated liposomes (stealth liposomes) with/without curcumin (Figure 2). Figure 2a shows the differences between the FTIR of liposome and stealth liposome. The presence of NH, CONH, and C=C groups is apparent at wavenumbers of 3300, 1690, and 1550 cm−1, respectively, in the spectrum of stealth liposome in response to the spectrum of the liposome. In Figure 2b, the differences between curcumin, liposome, and stealth liposomes (with/without curcumin) were also shown. The absence of curcumin at about 1277, 1428, 1509, 1623, and 3508 cm−1 which are referred to the aromatic C-O, olefinic C-H, C=C, and C=O, C=C vibrations of the benzene ring, and phenolic O-H groups [31] peaks in the spectrum of drug-loaded nanocarrier is a good reason which confirmed the encapsulation of drugs in the interlayer spaces of the liposomes.
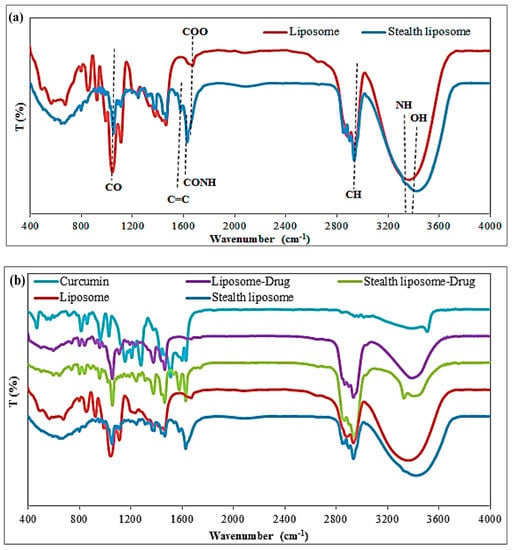
Figure 2.
FTIR differences of (a) liposome and stealth liposome, and (b) liposome, stealth liposome, liposome-drug, stealth liposome-drug, and curcumin.
In the following section, the size and surface charge of the four types of nanoliposomes were determined via the Zeta-sizer to evaluate the effect of cholesterol concentration on the size of the nano-system. Table 2 shows the differences between the size and charge of the nano-liposomes. Based on the results, the high negative zeta potential of all types of nanoliposomes revealed their potential stability in the liquid media however, this charge in the nanosystems with lower amounts of cholesterol was more negative in response to other types. This negative charge is related to the presence of DSPE components in the structure of liposome. Results also revealed that the negative charge of stealth liposomes was lower than common liposomes in both types, which could be due to the interaction of DSPE with CIT-PEG that could modulate its negative charge [32]. We also hypothesize that this decrease may be due to the presence of OH groups on the surface of stealth liposomes that interact with the H atoms of the surrounded aquatic environment temporary via week hydrogen bonds and thus the emergence a local temporal positive charge on the surface which reduces the negative charge.

Table 2.
Size and zeta potential results of different types of nano-liposomes.
On the other hand, the size of these nanoliposomes was changed with cholesterol alteration so that, by increasing the concentrations of cholesterol, the size of liposomes was decreased which is the same result of other researches. Based on the literature, the concentration of cholesterol has a direct effect on the size, rigidity, and drug loading ability of the liposomes, in such a way that it could decrease the size of liposomes in the low molar ratios by affecting the structural properties and also increase the rigidity of liposomes at high molar ratios, and thus a concentration rage was determined for this component to prepared liposomes with an appropriate size and fluency [33,34].
Based on the results of this section, the samples (liposome and stealth liposome) with the higher molar ratio of cholesterol were selected for the further sections. The size and morphology of the nanoliposomes with and without drug molecules were determined by the FE-SEM analysis, and the results are shown in Figure 3. As it is clear in the figure, using PEG as a cover for the liposomes turned to the production of nanosystems with more distinctive morphology. In other words, in the absence of PEG, coating liposomes were aggregated and had no recognizable spherical shape in the FE-SEM figure. The size of the nanoliposomes was also determined, which was about 50 and 40 nm for the liposome and stealth liposome, respectively.
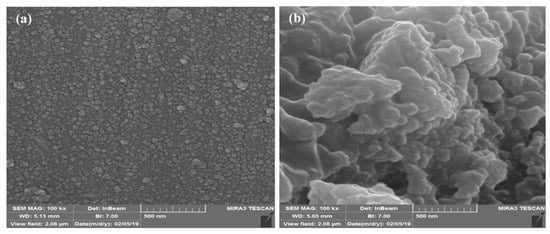
Figure 3.
FE-SEM results of drug-loaded: (a) Stealth liposome and (b) liposome.
3.3. Determination of Entrapment Efficiency
The amounts of drug-loaded in the nano-liposomes were determined by the measurements of the optical absorption of curcumin at 430 nm via UV-Visible spectroscopy. Table 3 shows the results of the entrapment efficiency of the nanoliposomes:

Table 3.
Percentage of curcumin entrapment efficiency.
Results demonstrated that there was no significant difference between nanosystems with different amounts of cholesterol. However, nanosystems with higher amounts of cholesterol showed a little lower entrapment efficiency, which could be due to the enhancement that occurred in the structural strength of these liposomal systems that prevent more drug loading. Moreover, the presence of the polymeric shell turned to improve drug loading. We suggested that it may be due to the presence of drug molecules near the hydrophobic chain of the polymers. It is revealed in other studies that cholesterol could affect the amount of loading drug especially in the case of a hydrophobic one. In other words, cholesterol competes with the drug components that are incorporated in the bilayer space and decrease the loading amount of hydrophobic drugs [35].
3.4. pH-Responsive Drug Release
The drug released profile of nanoliposomes was determined in response to the environmental pH at 37 °C for 5 days (Figure 4). The results of this section confirmed the effectiveness of the PEGylation on controlling the drug released profile so that the common form of liposome released approximately all 100% of its drug cargo at 37 °C in pH = 7.4 after 5 days, while this was about 61% for the stealth liposome with no drug released on the first day. The pH-responsive behavior of the stealth liposomes was also confirmed based on the results of the drug released. As it is clear in Figure 4b, the release of the drug component at pH = 6.6 showed two part, a burst released part during the first 24 h and a more controllable release in the following hours. At the first stage, the pH-responsive bond of citraconic components was disrupted, which lead to the separation of CIT-PEG layer from the liposomes [22]. This structural change turned to the release of drug components, which were entrapped in the polymeric shell as well as part of drug components inside the liposome. In the second stage, the drugs entrapped inside the liposome were released in a controllable manner in response to the pH and due to the presence of DSPE components that themselves played a pH-responsive role [36]. It is necessary to mention that the stealth liposome had a better drug release profile in response to the common liposome, and it could release nearly all of its components for 5 days [37].
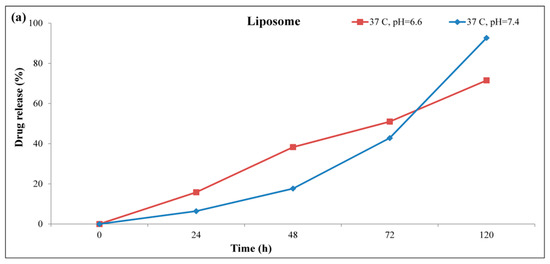
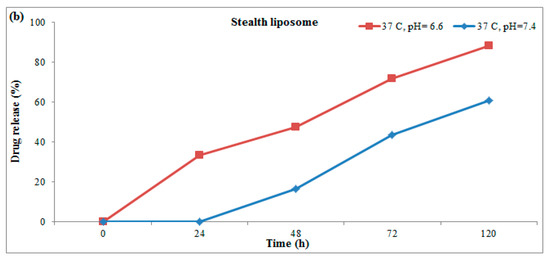
Figure 4.
pH-responsive drug released pattern of (a) liposome and (b) stealth liposome at 37 °C.
3.5. Stability Evaluation
The stability of the as-prepared liposomes was determined after 3 months by monitoring the changes that occurred in size and the drug released properties of them. Figure 5 shows the FE-SEM results of liposomes and stealth liposomes after 3 months. The results of electron microscopy revealed no changes in the size of both types of liposomes however, aggregation occurred for both of them.
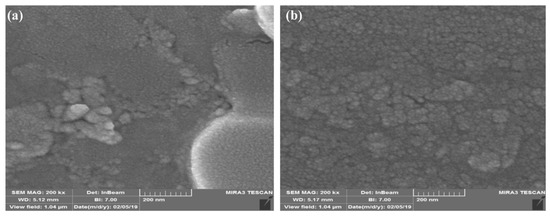
Figure 5.
FE-SEM results of (a) stealth liposome and (b) liposome.
The drug release ability of liposomes and stealth liposomes was also investigated after 3 months (Figure 6). As it clear in the figure, the drug release profile of liposomes had affected by the time, so that the capability of the drug released in normal pH was decreased, and in acid pH, it showed a burst release profile during just 3 days, which may represent the instability of liposomes. In contrast to common liposomes, stealth liposomes could preserve their released profile and released the drugs in a controllable manner at acidic pH however, they showed some reduction in the drug released property, too.
Figure 6.
pH-responsive drug released profile of (a) liposome, and (b) stealth liposome, after 3 months at 37 °C.
3.6. MTT Assay
The biocompatibility of different concentrations of liposomes and stealth liposomes was evaluated by MTT assay. In this test, drug-loaded nanoliposomes were exposed to normal and cancerous cells, and their cytotoxicity effect was assessed during 24–72 h. Figure 7a–c shows the cytotoxicity results of nanosystem on L929 cell line. As it is clear in this figure, not drug-loaded nanoliposomes nor nanocarrier alone showed a cytotoxicity effect against the normal cell line, while free drugs (in both concentrations) showed a significant cytotoxicity effect. Moreover, these nanosystems turn to an increase in the viability of the normal cells after 48 h, which could confirm the biocompatibility of them.
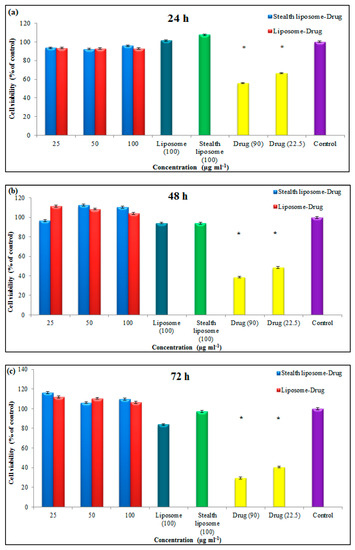
Figure 7.
Results of MTT assay of liposome and stealth liposome after (a) 24 h, (b) 48 h, and (c) 7 h on the L929 cell line (* p ≤ 0.05).
The effect of nanoliposomes on the cell viability of the MCF7 cell line was also evaluated during 24–72 h, and their results are shown in Figure 8a–c. Based on the results of this test, both types of nanoliposome showed a cytotoxicity effect against the cancer cells after 48 and 72 h moreover, the stealth liposomes had more cytotoxicity in comparison to the common liposomes so that they showed 31% and 29% cytotoxicity after 72 h at the concentration of 100 μg mL−1, respectively.
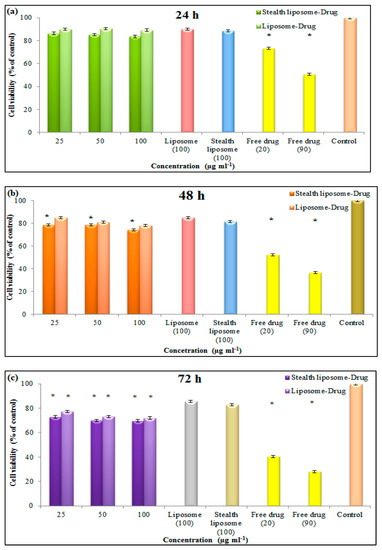
Figure 8.
Results of MTT assay of liposome and stealth liposome after (a) 24 h, (b) 48 h, and (c) 72 h on the MCF7 cell line (* p ≤ 0.05).
4. Discussion
In this paper, a new type of pH-responsive liposome was introduced to deliver curcumin to breast cancer tissue. In detail, four types of liposomes were fabricated via conjugation of DSPE and DSPC, as the phospholipid components, with cholesterol. To determine the effect of cholesterol on the stability and structure of liposomes, two concentrations of cholesterol were used for the fabrication of nanoliposomes. The results of different tests revealed that the lower amount of cholesterol could affect the structural features of nanoliposomes and lead to the production of unstable nanoparticles, which had similarities with other researches. Indeed, it is confirmed that the presence of cholesterol could affect the rigidity, fluidity, thickness, and stability of liposomes [34,38,39].
The nanoliposomes were prepared via the Mozafari method which is known as one of the best methods for the production of nanoliposomes since no hazard solvent is used for the preparation of the liposome by this method. The main aim of this study was to introduce a novel smart stealth liposome for the delivery of hydrophobic drugs. Utilizing the polymeric shell not only could enhance the stability and biocompatibility of the nanoliposomes, but also improve their drug loading capacity [40]. Here, we fabricated a type of pH-responsive nanoliposome using citraconic anhydride, which could be broken in response to acidic pH. Indeed, it was the first work in which pH-responsive nanoliposomes were fabricated by the Mozafari method for delivering curcumin to breast cancer tissues.
We test the effect of coating nanoliposome by CYT-PEG on its different properties, and the results revealed that it could improve different features of the nanosystem. On the other hand, it could reduce the size of nanoparticles, improve its drug loading, regulate its drug release pattern, and enhance the stability of the particles however, the potential charge of the nanoparticles was increased. The pH-responsibility of the nanoparticles was also tested in two different pH: pH = 7.4, which is the normal pH of the body, and pH = 6.6, which is the pH of cancerous tissues. It is worth mentioning that the presence of DSPE in the structure of the uncoated nanoliposomes could donate a pH-responsible ability [38], but they were non-stable liposomes with a burst released pattern at pH = 7.4 and unstable released pattern during the storage. While coated nanoparticles had better stability during the time, and could prevent drug release in the first 24 h at pH = 7.4 (which is the usual time needed for delivery of drug components to the targeted site).
The synthesis of pH-responsive stealth nanoliposomes is a new research topic that has received a lot of attention and several kinds of research have been done based on it [41,42]. For instance, in 2016, Zhao et al. prepared a pH-responsive liposome as a carrier of doxorubicin (DOX) for the treatment of glioblastoma. In this research, a type of pH-responsive peptide (H7K (R2)2) was used to functionalized stealth liposomes. They assessed the drug released pattern during 24 h in four different pH (5.5, 6, 6.5, and 7.4), and their results revealed that most of the drug components were released from the liposome during the first 8 h in the acidic environment while just 20% of them were released at a normal pH [43]. In comparison to this study, the current work has a more controllable drug release pattern and could release its drug components over a longer time, which could improve the effectiveness of the drug in cancer treatment.
In 2018, Lee et al. fabricated a pH-responsive liposome via functionalizing hydrogenated soy phosphatidylcholine (HSPC) by hyaluronic acid (HA) grafted with functional 3-diethylaminopropyl (DEAP) for the delivery of docetaxel (DTX) (a type of hydrophilic drug). They tested the release process for 24 h and saw that their system showed burst release during the first 4 h (for both normal and acidic pH), and after that, the release rate decreased for both however, the release rate was faster in acidic pH [44]. In comparison to this research, the liposome prepared in the current work had no release during the first 24 h at pH = 7.4, which makes it suitable for drug delivery application.
Curcumin is a type of hydrophobic herbal drugs with high potential application in the field of pharmacy which is resulted from its various therapeutic activities. It was widely used in different research as the anti-cancer drug for the treatment of different types of cancer, but the hydrophobic nature of this component has limited its effectiveness. To overcome this limitation several types of nanocarrier were used till now, among them are liposomes which could be prepared via different strategies [45]. In the current research, we hypothesized to increase the bioavailability of curcumin in the microenvironment of cancerous tissue via encapsulating it in pH-responsive nanoliposome.
In most of the studies, the active targeting method was used for the liposomal delivery of curcumin to its targeted site [46,47]. The main difference between these studies and the current work is that they just verified the release pattern in normal pH (7.40) or they had the same release pattern in different pH, while this work showed a different release rate. For instance, Mohammadi et al. in 2020 also fabricated a targeted nanoliposome for delivery of curcumin to MCF7 cancer cells. The lack of polymeric shell and pH-responsive agent in their nanosystem led to producing a similar curcumin releasing pattern in different pH (5.5, 6.5, and 7.4). Moreover, most of the drug components were released during 15 h which may cause a side effect on normal tissues [48]. While in the current work we did not see any release during the first day in pH 7.4, which is the normal pH of the body.
In the field of pH-responsivity, the route of administration plays an important role and could affect the type of material used. According to our knowledge, most of the pH-responsive curcumin loaded liposomes were fabricated for oral administration of this drug, which should show resistance against to acidic conditions of the stomach and release their components in a basic condition. For instance, Leo et al. in 2020 prepared a type of pH-responsive curcumin-loaded nanoliposomes for the aim of delivering the drug to the colon cells through oral administration. In this study, the nonmetric liposomes were prepared and coated by Eudragit S100 which is a pH-responsive shell that protects the liposomes from the acidic pH of the stomach and dissolves at pH > 7.0 [49]. While the liposomes prepared in the current work were suitable to be applied via blood administration. Indeed, they were responsive to the weakly acidic condition of the tumor tissue and enhanced the bio-availability of the curcumin in the tumor, while could limit the releasing of the drug in normal pH (at least for the first 24 h).
The cytotoxicity results of coated and non-coated liposomes confirmed the effectiveness of pH-responsive coating against cancerous tissues however, in vivo tests are needed for more assurance. In other words, the presence of bicarbonate in the formulation of media used for in vitro tests prevents the pH change during the test and could affect the results of these tests.
5. Conclusions
In summary, four different types of liposomes (with and without the pH-responsive agent) were fabricated via the Mozafari method via encapsulating curcumin between the bilayer of nanoliposomes. Nanoliposomes were characterized by different physicochemical methods. The FTIR results confirmed the preparation of DSPE-CIT-PEG component and curcumin-loaded liposomes. The results of DLS tests revealed that nanoliposomes with a lower amount of cholesterol had micron size and thus were not suitable for continuous tests. The nanometric size liposomes drug released in response to pH changes was also assessed, and its results showed coated liposomes had a controllable drug release pattern even after 3 months which represents their stability during storage time. Moreover, these pH-responsive stealth liposomes had better cytotoxicity against cancer cells. According to these results, it could be concluded that this new type of smart liposome could be considered as a good candidate for cancer therapy.
Author Contributions
Conceptualization, A.Z. (Ali Zarrabi) and A.Z. (Atefeh Zarepour); Methodology, A.Z. (Atefeh Zarepour) and A.K.; Data curation, A.K. and Z.A.; Writing—original draft preparation, A.Z. (Atefeh Zarepour) and A.K. and Z.A.; Writing—review and editing, A.Z. (Ali Zarrabi) and V.K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Medicinal Plants Research Center, Shahed University] grant number [15/2489].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We acknowledge the funding by “Medicinal Plants Research Center, Shahed University” under Grant Number 15/2489. No other benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Zangabad, P.S.; Mirkiani, S.; Shahsavari, S.; Masoudi, B.; Masroor, M.; Hamed, H.; Jafari, Z.; Taghipour, Y.D.; Hashemi, H.; Karimi, M.; et al. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnol. Rev. 2018, 7, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, P.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and Theranostics. Biomedicine 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Omri, A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004, 11, 33–39. [Google Scholar] [CrossRef]
- Li, T.; Cipolla, D.; Rades, T.; Boyd, B.J. Drug nanocrystallisation within liposomes. J. Control Release 2018, 288, 96–110. [Google Scholar] [CrossRef]
- Olusanya, T.O.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, M.; Mohamadi, N.; Zarrabi, A.; Abasi, S.; Dehghannoudeh, G.; Tamaddondoust, R.N.; Khanbabaei, H.; Mohammadinejad, R.; Thakur, V.K. Chitosan-based advanced materials for docetaxel and paclitaxel delivery: Recent advances and future directions in cancer Theranostics. Int. J. Biol. Macromol. 2020, 145, 282–300. [Google Scholar] [CrossRef]
- Yu, L.; Dong, A.; Guo, R.; Yang, M.; Deng, L.; Zhang, J. DOX/ICG coencapsulated liposome-coated thermosensitive nanogels for NIR-triggered simultaneous drug release and photothermal effect. ACS Biomater. Sci. Eng. 2018, 4, 2424–2434. [Google Scholar] [CrossRef]
- Kushwah, V.; Jain, D.K.; Agrawal, A.K.; Jain, S. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Colloids Surf. B Biointerfaces 2018, 172, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Jani, R.K.; Gohil, K.M. Liposomal Formulations in Cancer Therapy: Basic Concepts to Advanced Strategies. Int. J. Pharm. Sci. Drug Res. 2018, 10, 386–393. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Su, Y.; Tian, Q.; Li, B.; Quan, J.; Deng, Y. Are PEGylated liposomes better than conventional liposomes? A special case for vincristine. Drug Deliv. 2016, 23, 1092–1100. [Google Scholar] [CrossRef]
- Mastrotto, F.; Brazzale, C.; Bellato, F.; de Martin, S.; Grange, G.; Mahmoudzadeh, M.; Magarkar, A.; Bunker, A.; Salmaso, S.; Caliceti, P. In vitro and in vivo behavior of liposomes decorated with pegs with different chemical features. Mol. Pharm. 2019, 17, 472–487. [Google Scholar]
- Shen, Z.; Fisher, A.; Liu, W.K.; Li, Y. PEGylated “stealth” nanoparticles and liposomes. In Engineering of Biomaterials for Drug Delivery Systems, 1st ed.; Parambath, A., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–26. [Google Scholar]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef]
- Cho, H.Y.; Lee, C.K.; Lee, Y.B. Preparation and evaluation of PEGylated and folate-PEGylated liposomes containing paclitaxel for lymphatic delivery. J. Nanomater. 2015, 16, 36. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control Release. 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Zarepour, A.; Zarrabi, A.; Larsen, K.L. Fabricating Β-cyclodextrin based pH-responsive nanotheranostics as a programmable polymeric nanocapsule for simultaneous diagnosis and therapy. Int. J. Nanomed. 2019, 14, 7017–7038. [Google Scholar] [CrossRef] [PubMed]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Bejandi, A.K.; Hushmandi, K.; Ang, H.L.; et al. Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics 2020, 12, 1084. [Google Scholar] [CrossRef]
- Cao, J.; Su, T.; Zhang, L.; Liu, R.; Wang, G.; He, B.; Gu, Z. Polymeric micelles with citraconic amide as pH-sensitive bond in backbone for anticancer drug delivery. Int. J. Pharm. 2014, 471, 28–36. [Google Scholar] [CrossRef]
- Rasti, B.; Jinap, S.; Mozafari, M.R.; Abd-Manap, M.Y. Optimization on preparation condition of polyunsaturated fatty acids nanoliposome prepared by Mozafari method. J. Liposome Res. 2014, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, J.; Li, N.; Garamus, V.M.; Zou, A. Hyaluronic acid-coated liposome for active targeting on CD44 expressing tumors. J. Nanoparticle Res. 2018, 20, 235. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, 095505. [Google Scholar] [CrossRef] [PubMed]
- Ahani, E.; Toliyat, T.; Rad, M.M. Comparing size particle, release study and cytotoxicity activity of PHMB encapsulated in different liposomal formulations: Neutral and cationic liposomes. J. Bioeng. Res. 2019, 1. [Google Scholar] [CrossRef]
- Marasini, R.; Nguyen, T.D.T.; Rayamajhi, S.; Aryal, S. Synthesis and characterization of a tumor-seeking LyP-1 peptide integrated lipid–polymer composite nanoparticle. Mater. Adv. 2020, 1, 469–480. [Google Scholar] [CrossRef]
- Castillo, P.M.; de la Mata, M.; Casula, M.F.; Sánchez-Alcázar, J.A.; Zaderenko, A.P. PEGylated versus non-PEGylated magnetic nanoparticles as camptothecin delivery system. Beilstein J. Nanotechnol. 2014, 5, 1312–1319. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Liu, Y.; Ji, B.; Wang, Q.; Wang, M.; Dai, K.; Gao, D. On-demand drug release of ICG-liposomal wedelolactone combined photothermal therapy for tumor. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2019–2029. [Google Scholar] [CrossRef]
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J. Microencapsul. 2019, 36, 715–727. [Google Scholar] [CrossRef]
- Abe, K.; Higashi, K.; Watabe, K.; Kobayashi, A.; Limwikrant, W.; Yamamoto, K.; Moribe, K. Effects of the PEG molecular weight of a PEG-lipid and cholesterol on PEG chain flexibility on liposome surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2015, 474, 63–70. [Google Scholar] [CrossRef]
- Deniz, A.; Sade, A.; Severcan, F.; Keskin, D.; Tezcaner, A.; Banerjee, S. Celecoxib-loaded liposomes: Effect of cholesterol on encapsulation and in vitro release characteristics. Biosci. Rep. 2010, 30, 365–373. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2020, 592, 120051. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.; Moreira, J.N.; Fonseca, C.; Düzgüneş, N.; de Lima, M.C.P. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004, 56, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, X.; Zhang, Y.; Xu, J.; Xu, J.; Li, Y.; Min, H.; Shi, J.; Zhao, Y.; Wei, J.; et al. Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv. Mater. 2019, 31, 1900795. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, M.; Miao, Y.; He, S.; Dai, Z.; Song, W.; Liu, Y.; Song, S.; Ahmad, E.; Wang, D.; et al. Cholesterol-tuned liposomal membrane rigidity directs tumor penetration and anti-tumor effect. Acta Pharm. Sin. B 2019, 9, 858–870. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Chen, H.; Hou, X.; He, Y.; Shen, J.; Shi, J.; Feng, N. Advances in next-generation lipid-polymer hybrid nanocarriers with emphasis on polymer-modified functional liposomes and cell-based-biomimetic nanocarriers for active ingredients and fractions from Chinese medicine delivery. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102237. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D. Stimuli-responsive liposomes for drug delivery. Adv. Rev. 2017, 9, e1450. [Google Scholar] [CrossRef] [PubMed]
- de Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.Q.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control Release 2016, 222, 56–66. [Google Scholar] [CrossRef]
- Lee, J.M.; Park, H.; Oh, K.T.; Lee, E.S. pH-Responsive hyaluronated liposomes for docetaxel delivery. Int. J. Pharm. 2018, 547, 377–384. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Z.P.; Tian, G.X.; Pan, R.Y.; Xu, C.M.; Zhang, B.; Wu, J.L. Liver-targeted liposomes for codelivery of curcumin and combretastatin A4 phosphate: Preparation, characterization, and antitumor effects. Int. J. Nanomed. 2019, 14, 1789–1804. [Google Scholar] [CrossRef]
- Wang, W.Y.; Cao, Y.X.; Zhou, X.; Wei, B. Delivery of folic acid-modified liposomal curcumin for targeted cervical carcinoma therapy. Drug Des. Dev. Ther. 2019, 13, 2205–2213. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Mirahmadi-Babaheidri, S.A.; Delaviz, H.; Fouani, M.H.; Alipour, M.; Barmak, M.J.; Christiansen, G.; Bardania, H. RGD peptide-mediated liposomal curcumin targeted delivery to breast cancer cells. J. Biomater. Appl. 2020, 35, 743–753. [Google Scholar] [CrossRef] [PubMed]
- de Leo, V.; Di Gioia, S.; Milano, F.; Fini, P.; Comparelli, R.; Mancini, E.; Agostiano, A.; Conese, M.; Catucci, L. Eudragit s100 entrapped liposome for curcumin delivery: Anti-oxidative effect in Caco-2 cells. Coatings 2020, 10, 114. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).