Abstract
Cyclohexanone is an important industrial intermediate in the synthesis of materials such as nylons, but preparing it efficiently through one-step hydrogenation of phenol is hindered by over-reduction to cyclohexanol. Using an efficient catalyst can enhance the selectivity of cyclohexanone at high phenol conversion. In this study, catalysts comprised of palladium nanoparticles supported on electrospun PVDF-HFP (polyvinylidene fluoride-co-hexafluoropropylene) nanofibers were prepared using the electrospinning technique. The catalysts were characterized using thermogravimetric analyzer (TGA), scanning electron microscopy (SEM), transmission electron microscope (TEM), and drop shape analyzer (DSA). The prepared catalysts were used to hydrogenate phenol into cyclohexanone in a batch reactor. The Pd/PVDF-HFP catalyst showed a very high product selectivity and high phenol conversion. The conversion of phenol achieved was 98% with 97% cyclohexanone selectivity in 7 h using 15 wt% of palladium (0.0021 moles) relative to phenol (0.0159 moles). The turnover number (TON) and turnover frequency (TOF) values calculated were 7.38 and 1.05 h−1, respectively. This paper presents original research in heterogeneous catalysis using novel electrospun nanofibers. Multiphase hydrogenation of phenol to cyclohexanone over electrospun Pd/PVDF-HFP catalyst has not been reported by any researcher in the literature. This work will also provide a research window for the application of electrospun polymeric nanofibers in multiphase reactions.
1. Introduction
Cyclohexanone is an important chemical and it is a raw material used to produce ε-caprolactam and adipic acid. Nylon6 and nylon66 are produced from ε-caprolactam and adipic acid respectively [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Oxidation of cyclohexane [7,8] and hydrogenation of phenol [1,2,3,4,5,6,9,10,11,12,13,14,15,16,18,19,20,21,22,23] are the main two reactions that produce cyclohexanone commercially. The first route generates undesirable byproducts due to high temperatures and pressures [15,18]. These byproducts lower the product selectivity and make the recovery/separation steps more difficult [15,18]. In the hydrogenation of phenol, cyclohexanone is produced either in a “two-step” or a “one-step” process. In the two-step process, first phenol undergoes hydrogenation to produce cyclohexanol, which further dehydrogenates to form cyclohexanone [1,10,14,15,17,18,23]. The one-step method is preferable since it eliminates the endothermic dehydrogenation step by directly and selectively hydrogenating phenol into cyclohexanone [18]. However, cyclohexanone can hydrogenate easily to cyclohexanol and other byproducts due to its high reactivity [1,10,14,15,17,18,23]. Generally, vapor phase phenol hydrogenation to cyclohexanone is carried out with palladium type catalysts such as Pd@Al2O3, Pd@MgO, and others [3,4,9,10,15,18,20,22]. However, deactivation by coke deposition of Al2O3-supported Pd catalysts can take place easily at high temperatures. Furthermore, the poor mechanical performance of MgO limits its use as a catalytic support in industrial application [5,14,15,18]. Liquid phase hydrogenation of phenol to cyclohexanone is in great interest since the reaction can be carried out at mild conditions. The capital cost can be improved effectively by having better product quality, reducing the catalyst deactivation, and operating at lower temperature and pressure. However, most of the catalysts suffered from the poising effect in the liquid phase, and a longer time is required to obtain high phenol conversion [1,2,5,9,12,13,14,16,20,23].
Selectivity and activity of a catalyst in the hydrogenation of phenol to cyclohexanone are influenced by the metal loading, the acidity and basicity of the support, the existence of the alkali and/or alkaline earth metal, the nature of the palladium catalyst precursor, the catalyst preparation method, and the reactant concentrations [5,6,9,11,13,15]. Many research efforts have been put forward looking for good phenol hydrogenation catalysts. Metals including palladium (Pd) [1,6,9,14,15,19,21], platinum (Pt) [11], and ruthenium (Ru) [4] supported on different materials such as aluminum oxide (Al2O3), silicon dioxide (SiO2), and carbon (C) have been prepared and examined for their catalytic properties. Huizhen Liu et al. showed that a combination of two common commercial materials: (1) palladium nanoparticles supported on carbon, alumina, or NaY zeolite, and (2) a Lewis acid such as AlCl3 synergistically promotes hydrogenation of phenol to cyclohexanone [1]. They achieved phenol conversion exceeding 99.9% with >99.9% selectivity within 7 h at 1.0 Mpa hydrogen pressure and 50 °C. It was demonstrated that the reaction could be carried out effectively at temperatures as low as 30 °C, and >99.9% conversion of phenol can be achieved with >99.9% selectivity to cyclohexanone. However, the hydrogen pressure was high (1 Mpa = 9.9 atm) which imposed safety concerns and rigid reactor design-requirements. They also used dichloromethane as a solvent for phenol and not water; it is not preferred based on green chemistry. Most importantly, the existence of Lewis acids as promoters inhibits the usage of these catalysts in industry. It is not practical to use these catalysts in hydrogenation applications in general because they add other chemical sensitivities restricting substrates, purities, and reaction conditions.
A mixture of Pd-Ce-B supported on Hydrotalcite was prepared by Jianliang and co-workers [20]. They found that the incorporation of Ce could effectively enhance both the reaction rate and the cyclohexanone selectivity. These researchers suggested that Ce improved Pd dispersion on the support leading to better catalyst performance. Also, the adsorption and activation of the reactants increased when adding Ce since Ce3+ species can act as Lewis basic sites, which lead to an increase in the intrinsic activity. Moreover, they believed that phenol adsorption on their catalyst was in a nonplanar form which favored the synthesis of the desired product. The maximum selectivity achieved in their study was 86% in 6 h, which is considered low. They also could not reach a complete phenol conversion. In addition, they used a high hydrogen pressure of 1 Mpa, and the solvents for phenol were ethanol and water. Most recently, Xu Yang and his group prepared Pt nanoparticles supported on mesoporous silica (MS), titanate nanotubes (TNT) [11]. They have also modified the TNT structure hydrophobically to entrap the Pt nanoparticles. Their modified entrapped Pt on TNT showed better performance compared to the other two prepared catalysts, Pt/MS and Pt/TNT. The reaction conditions were moderate (50 °C and 0.5 MPa). However, the reaction was done using dichloromethane as a solvent, and the highest selectivity achieved was 85%. Tieyong Xu et al. prepared palladium particles on carbon nanotubes [16]. They modified the surface of the carbon support with oxygen-containing groups (OCGs) to change the catalytic support properties. Even though the reaction time was very short compared to most other studies and water was used as a solvent, the phenol concentration was low (13 g/L) and the hydrogen pressure was high (1 Mpa). Most importantly, the best-achieved selectivity in this study was 87.3%. Yolanda Perez’s group studied the effect of the support on hydrogenation of phenol in the aqueous phase [24]. They used different supports, which were alumina (γ-Al2O3, acidic properties), carbon (C), alumina with high surface area (Al2O3-CWE) and hydroxyapatite (HA), and studied the effect of the nanocrystals size Pd on catalytic properties. The results were very encouraging in terms of conversion and selectivity. They found higher selectivity and activity to cyclohexanone by Pd supported on alumina than carbon supported catalysts. They concluded the support material is important to the activity and selectivity. The turn over frequency (TOF) of phenol hydrogenation increased with the Pd dispersion. The selectivity of the reaction to cyclohexanone increased from 78% to 98% using Pd/Al2O3 catalyst with Pd content from 18.5 to 63.7, respectively. The study proved that activity is a direct function of the metal content. Even though their phenol solvent was water, and their reaction time was short, Perez et al. used high hydrogen pressure of 5 bar. Most importantly, the support was a ceramic material that is brittle and can break into small parts in the liquid phase. This adds a filtration step to remove the catalyst from products. Also, ceramic supports have low mechanical flexibility, which makes it difficult to use in a multiphase flow reactor. Recent studies have shown that metal particles supported on polymer nanofiber mats produced by the electrospinning technique show good catalytic activities [25,26]. For example, Demir’s group utilized electrospun palladium particles with poly(acrylonitrile-co-acrylic acid) to produce a catalytic fiber mat. The mat was used to selectively hydrogenate dehydrolinalool. The electrospun Pd/polymer catalyst showed 4.5 times higher activity than Pd/Al2O3 supports. It was concluded that catalytic particles supported on electrospun polymer nanofibers performed better than using those supported on inorganic supports in organic reactions [26].
It is thus of great interest to prepare Pd supported on polymer support for multiphase hydrogenation of phenol. The electrospun fiber mat catalysts have the advantages of good mechanical strength, high porosity, high permeability, and other improved characteristics. In contrast to nanopowder or nanoparticles on the ceramic catalyst, the polymer catalytic membranes are very easy to separate from liquid phase products. Most importantly, the polymeric membrane catalysts can be used in different continuous flow reactors like membrane reactors, packed bed reactors or fluidized bed reactors.
In the present work, palladium nanoparticles were immobilized on PVDF-HFP (poly(vinylidene fluoride-co-hexafluoropropylene)) fiber mats using electrospinning. The fiber mat was characterized using different catalytic characterization techniques. PVDF-HFP was chosen because it is a semicrystalline polymer that has been used as a catalytic support. It is an inert, superhydrophobic polymer, and it has great chemical and mechanical characteristics [27,28,29,30,31,32,33,34,35,36].
2. Materials and Methods
2.1. Preparation of Pd/PVDF-HFP Catalyst
The PVDF-HFP with a molecular weight of 450,000 (Detonated by Arkema, Inc., King of Prussia, PA, USA) was dissolved in acetone (Sigma Aldrich, St. Louis, MO, USA) at room temperature to form electrospinning solutions having polymer concentrations of 10 wt%. Mild stirring for 24 h was done to make the electrospinning solutions. Then 0.3 g of palladium black (Sigma Aldrich) was added into 15 mL of the prepared PVDF-HFP solution and mixed for one day to disperse the catalytic metal particles uniformly in the polymer solution. Electrospinning of the PVDF-HFP solution containing Pd particles was executed at room temperature where the prepared solution was injected from a 5 mL syringe equipped with a 21-gauge needle through a syringe pump (SP220i, World Precision Instruments, Sarasota, FL, USA) at a feed rate of 15 mL/h [31]. The power supply was adjusted to 30 kV. A piece of aluminum foil was used as a collector for the produced fibers. The gap distance between the needle and the aluminum foil was 15 cm.
2.2. Catalytic Membrane Characterization
The thermal properties of the electrospun catalytic membrane were analyzed with a thermogravimetric analyzer (TGAQ500) (TA Instruments, New Castle, DE, USA). TGA analysis was performed in the temperature range of 30–800 °C with a 20 °C/min heating rate under N2. In addition, the electrospun catalytic membrane was placed in a furnace, Barnstead Thermolyne 1400 Furnace, at 120 °C under air conditioning for 12 h to measure the weight change. Scanning electron microscopy (SEM) was used to study the fiber morphology. SEM images of the catalytic membrane were acquired using FEI Quanta 200 at 15 kV and HITACHI TM3000 (Hitachi High Technologies America, Inc, Greenville, SC, USA). The images were taken with different magnifications at the voltage of 15 kV. The diameter of the nanofibers was measured directly from the SEM images using FibraQuant 1.3 software (nanoScaffold Technologies LLC, Chapel Hill, NC, USA) and was displayed as diameter histogram. The histogram was generated out of 100 measurements. Transmission Electron Microscope (TEM, JEM 1200XII, Jeol USA, Peabody, MA, USA) was used to acquire the catalytic particle size. To prepare the TEM sample, the fibers were directly dispersed on a TEM grid during electrospinning. An X-ray diffractometer (Bruker AXS Dimension D8 X-ray, BRUKER AXS, Inc. Madison, WI, USA) was used to determine the support and catalytic peaks. PVDF-HFP with and without catalytic particles was tested. Cu anode (Kα1 = 0.154056 nm) was used. The voltage was set to 40 kV and the current to 40 mA. Scans were collected with the step size of 0.1 degrees and scan speed of 0.5 degrees/min. The wettability of the membrane was characterized by the water contact angle. Water drops of 5 μL volumes were placed on the fiber mats and imaged using a drop shape analyzer (DSA20E, Krüss GmbH, Hamburg, Germany). To determine the contact angle, the contact angles were averaged from measurements of five independent drops. Frazier Air Permeability Tester (Frazier Precision Instrument Company, Inc., Hagerstown, MD, USA) was used to measure the permeability of the catalytic membrane. The permeability can be calculated using Darcy’s law. Darcy’s law provides a relationship between the pressure drop and the flow through a membrane with the permeability coefficient as given in Equation (1):
where is the permeability in m2, Po and Pl are the initial and final pressure in Pa, L is the thickness of the membrane in m, Q is the volumetric flow rate in m3/s, µ is the kinematic viscosity in NS/m2, and A is the area of the membrane in m. For thin media, as for the tested catalytic PVDF-HFP membrane, to obtain accurate permeability values, an accurate measure of the mat thickness is very important. Since, precise thickness measurements were not obtainable hence, the ratio of permeability to the thickness of the medium is reported for this work.
2.3. Catalytic Experiments
In a typical reaction, aqueous phenol (20 g/L) was first prepared by dissolving phenol in deionized water. Seventy-five mL of the phenol and different amounts of Pd/PVDF-HFP catalyst (5, 10, and 15 wt% of palladium relative to phenol), was placed into a three-necked flask in three different experiments. A balloon was connected to the flask to store the hydrogen. Prior to the reaction, the system was flushed with hydrogen three times to remove air. The mixing and heating of reactants were conducted using a magnetic stirrer with a hotplate. The temperature of the system was raised to 80 °C. After a period of time, a sample was taken and analyzed to monitor the progress of the reaction. The reaction products were analyzed using a gas chromatograph (Shimadzu GC-17A, Shimadzu America Inc., Columbia, MD, USA) equipped with a Flame Ionization Detector (FID) and Hewlett Packard free fatty acids phase (HP-FFAP) column. The GC conditions were as follows: Injection temperature, 220 °C; detector temperature, 240 °C; column temperature, 120 °C; and temperature ramping rate, 10 °C/min [20]. The conversion of cyclohexanone into products was calculated using
where CA0 and CA are initial and final cyclohexanone concentrations, respectively.
3. Results and Discussion
3.1. Pd/PVDF-HFP Characterization
Figure 1 shows photos of the PVDF-HFP fiber mat with (B) and without (A) spinning catalytic particles in the polymer solution. A clear color change was observed when adding the catalytic nanoparticles. This indicates that the nanoparticles are not agglomerated but homogenously dispersed on the surface of the membrane and expected to have catalytic active sites all over the membrane.

Figure 1.
Polyvinylidene fluoride-co-hexafluoropropylene (PVDF-HFP) electrospun fiber mat (A) without and (B) with catalytic nanoparticles.
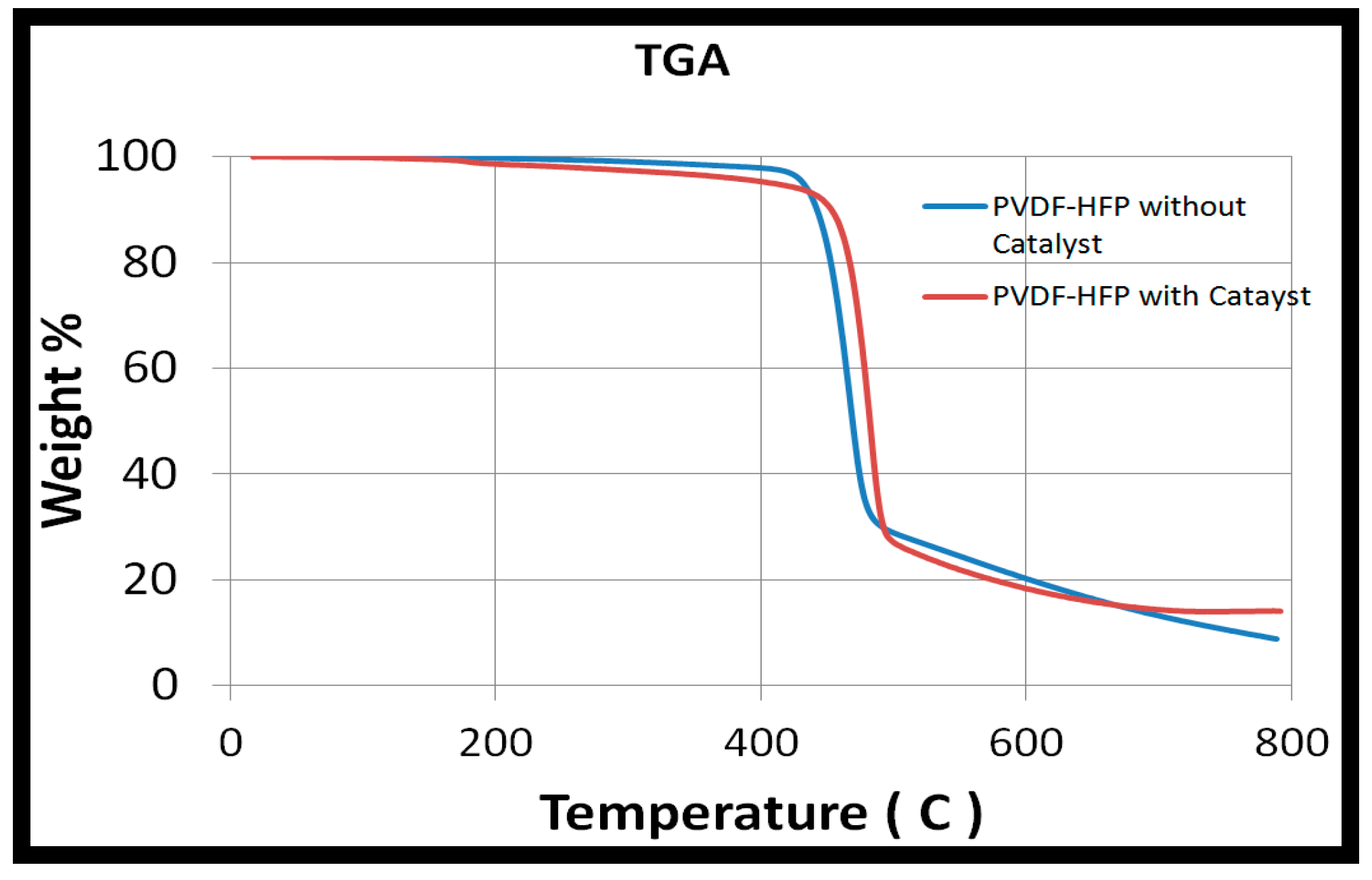
The TGA curve presented in Figure 2 shows that the PVDF-HFP catalytic membrane started degradation at around 420 °C. The PVDF-HFP membranes with and without catalyst have similar thermal properties. There was also no weight change for the sample that was placed in the furnace for 12 h. It was thus very safe to operate the reaction at 80 °C.
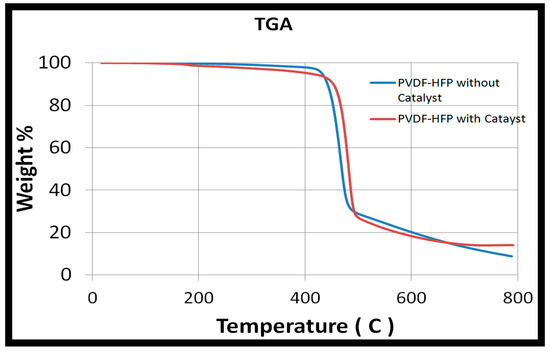
Figure 2.
Thermogravimetric analyzer (TGA) analysis of PVDF-HFP nanofibers.
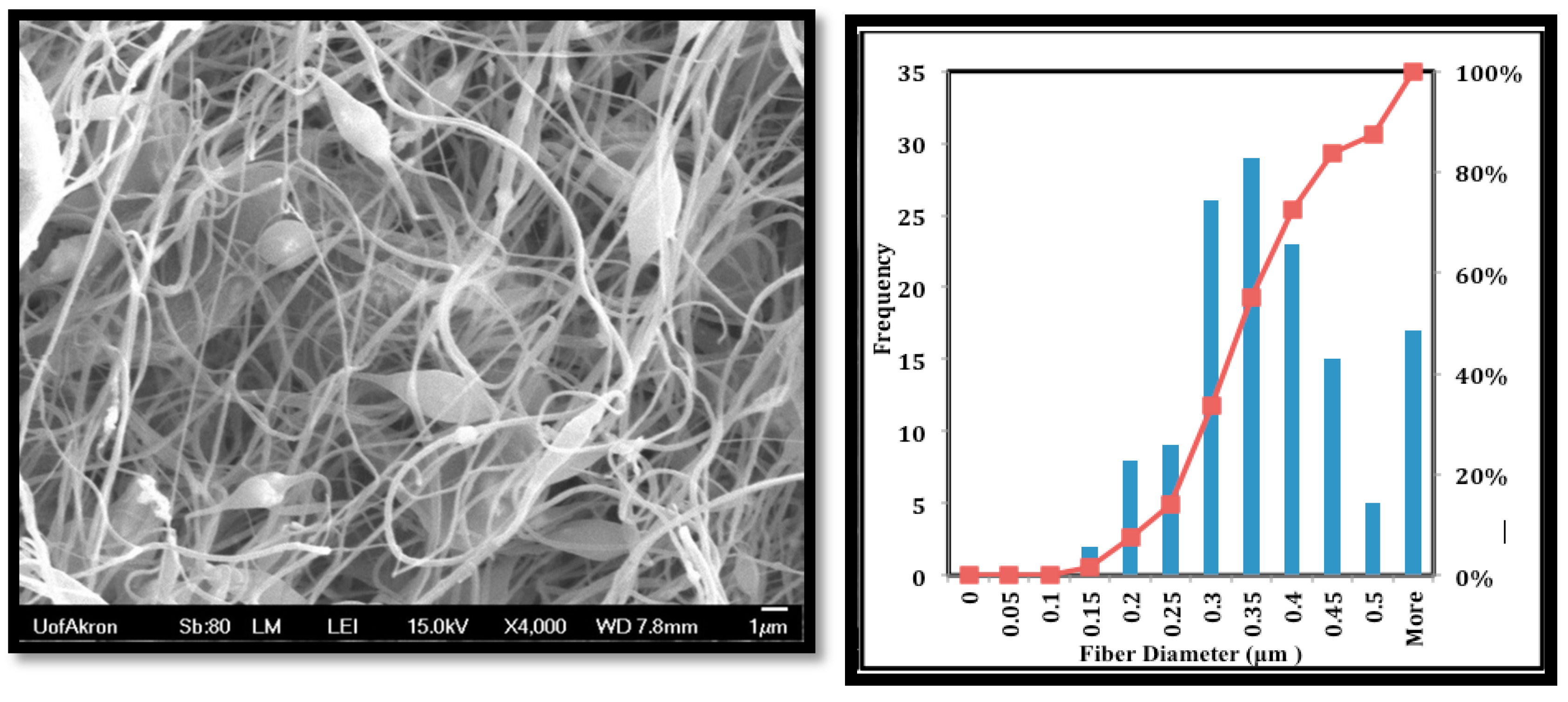
The fiber morphology was achieved using SEM as shown in Figure 3. The mean fiber diameter was around 357 ± 118 nm, and the mats have some beads.
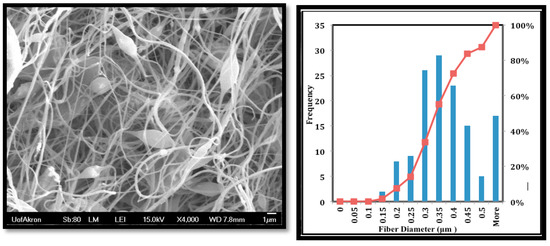
Figure 3.
Scanning electron microscopy (SEM) images of the PVDF-HFP nanofibers.
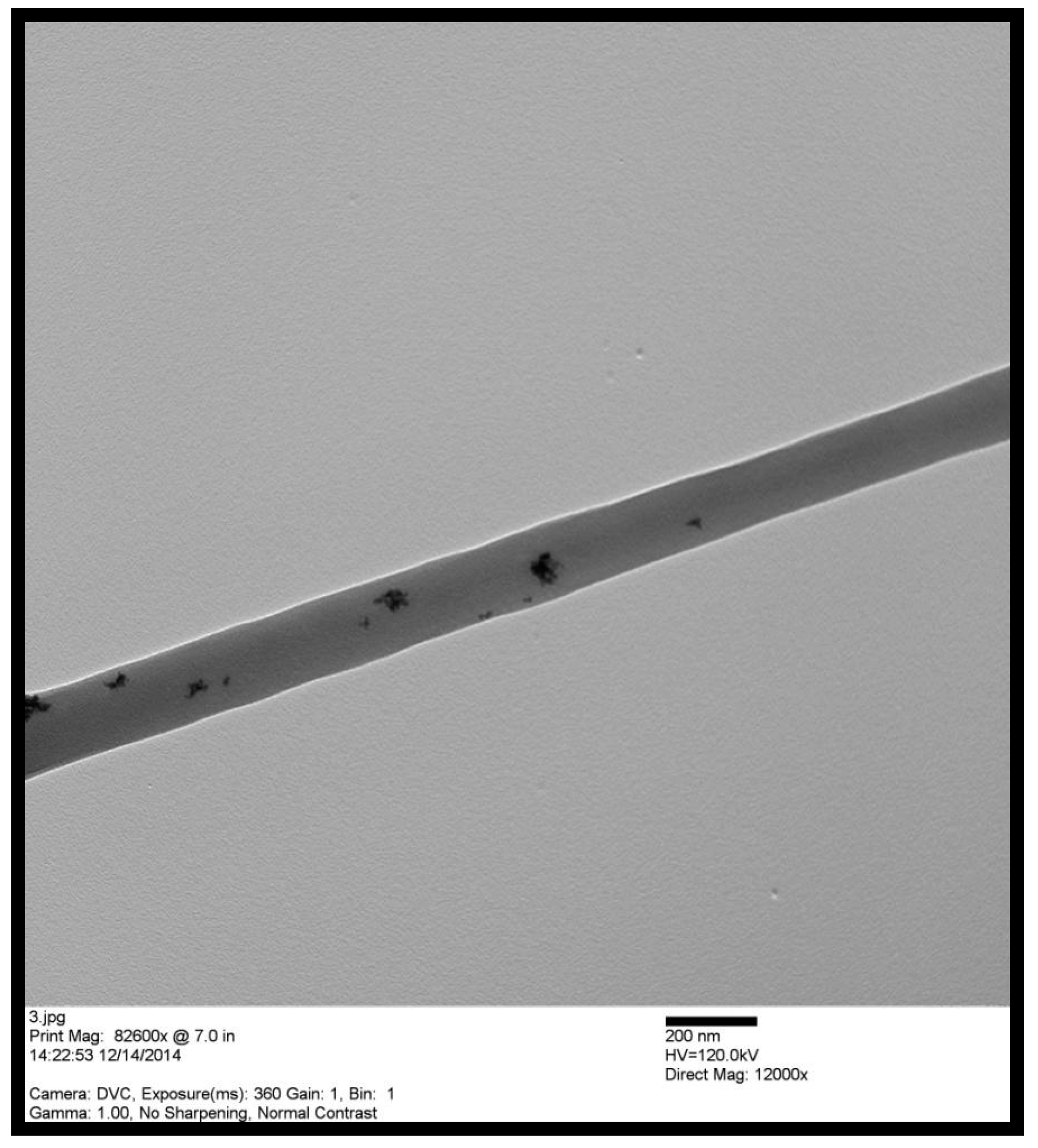
The TEM image shown in Figure 4 exhibits Pd particles dispersion on PVDF-HFP fibers. The average particle size was around 20 nm.

Figure 4.
Transmission electron microscope (TEM) image of Pd/PVDF-HFP nanofibers.
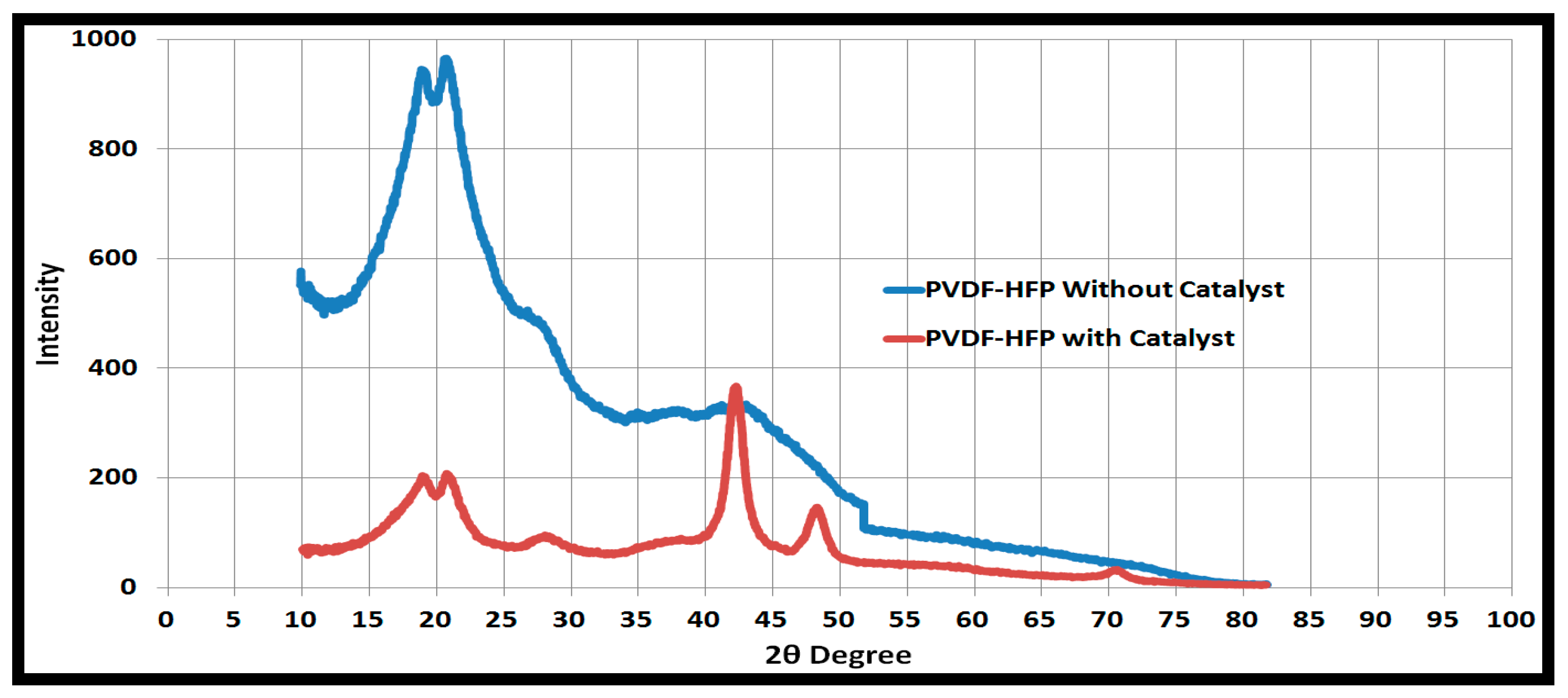
X-ray diffraction (XRD) peaks as shown in Figure 5 showed that peaks before 40 degrees are related to the polymer support, and peaks after 40 degrees are related to the catalyst. Peaks at 2θ = 41°, 47°, and 68° represent the indices of (111), (200), and (222) crystal planes of Pd [37,38,39], respectively.
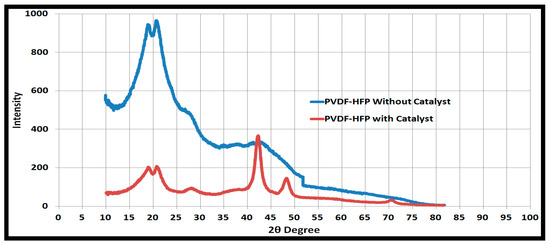
Figure 5.
X-Ray Defraction (XRD) plots of PVDF-HFP with and without catalytic nanoparticles.
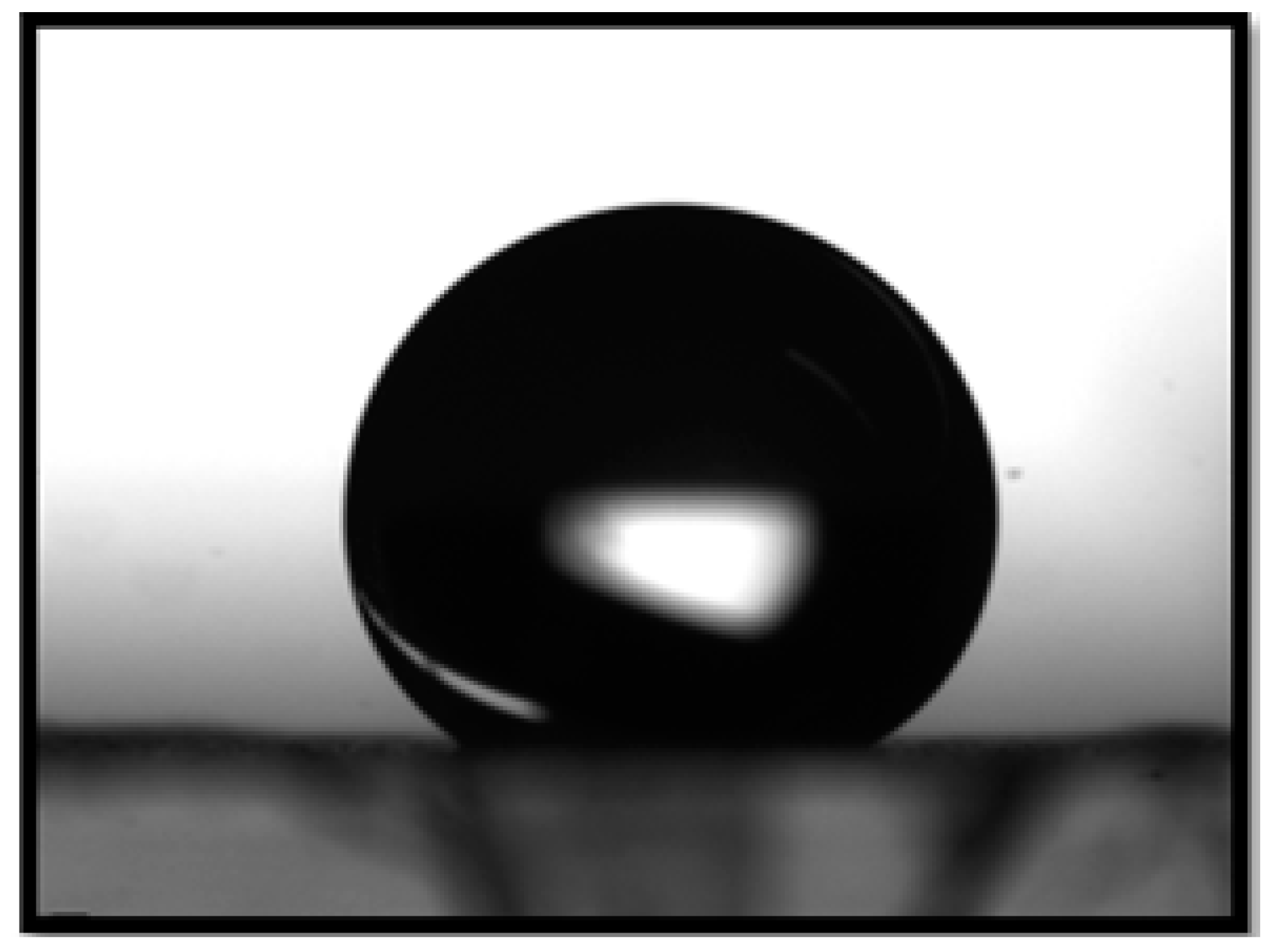
The contact angle of the catalytic media was 145 with respect to aqueous phenol as shown in Figure 6. The permeability of the catalyst calculated over the thickness of the fiber mat was m.
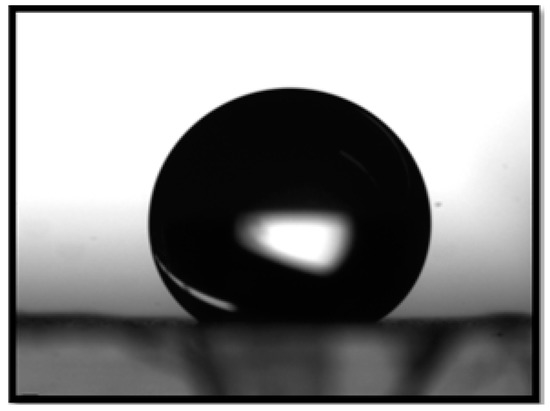
Figure 6.
Contact angle of aqueous phenol with respect to PVDF-HFP membrane.
3.2. Pd/PVDF-HFP Catalytic Performance Test
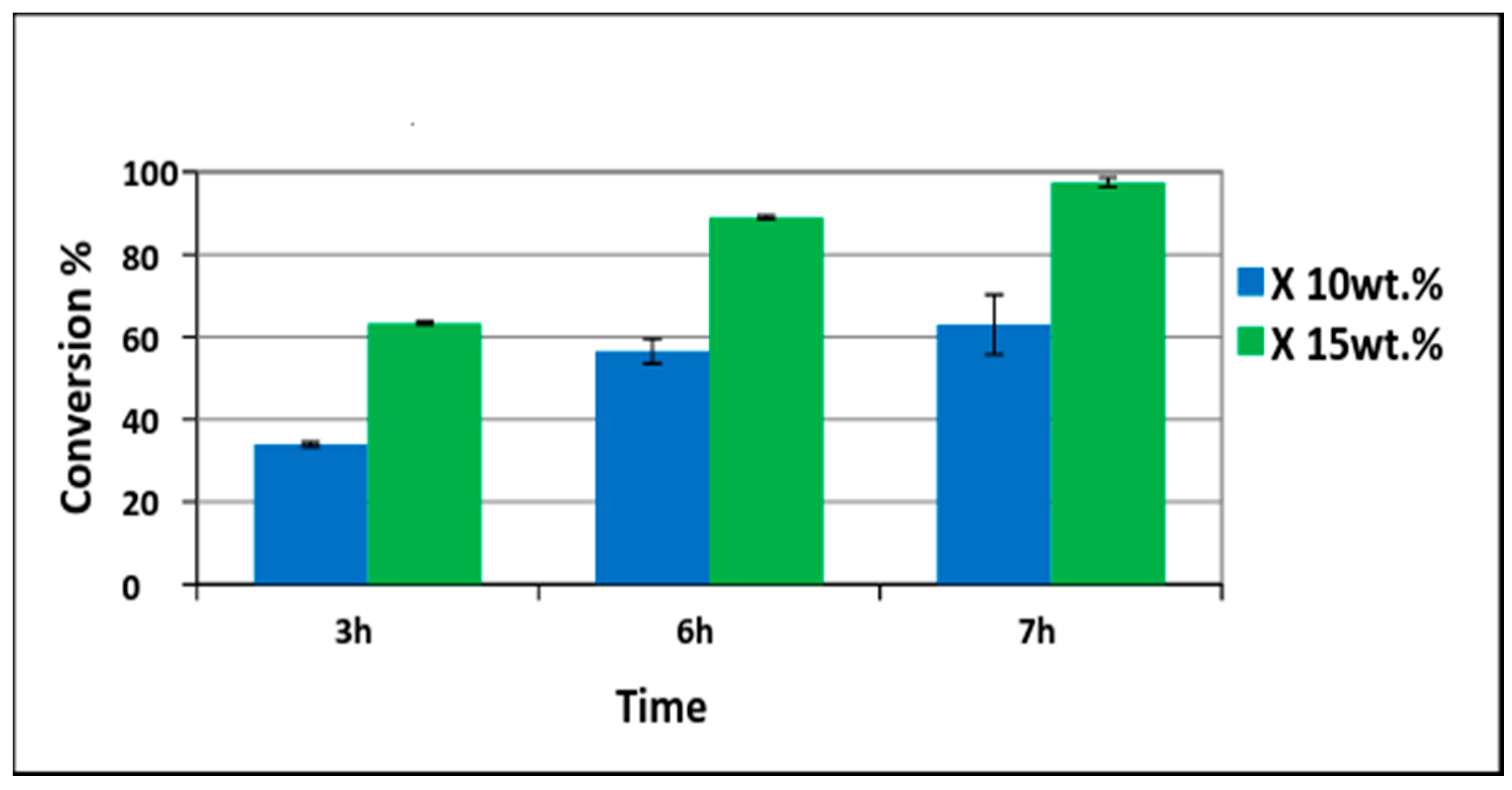
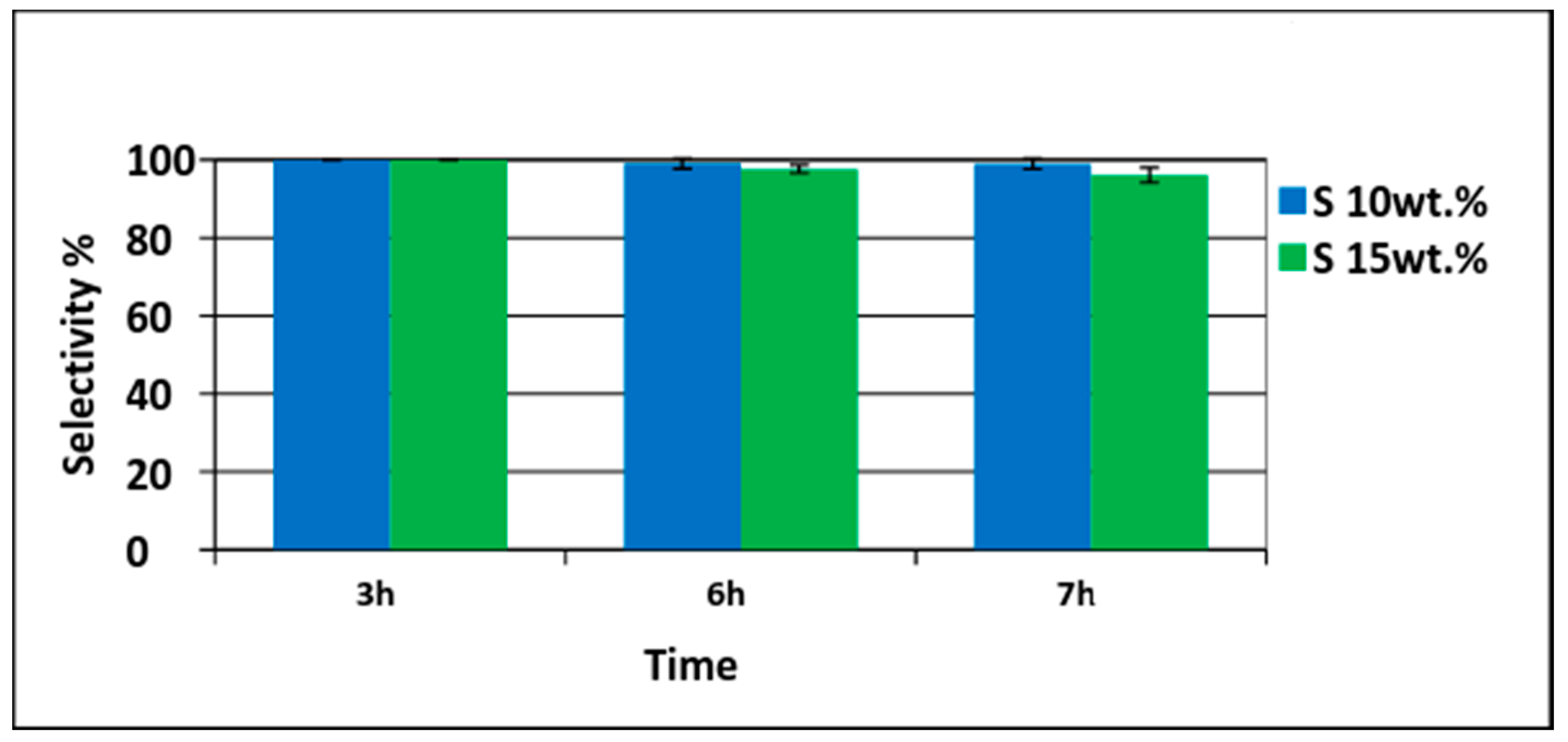
Different amounts of Pd/PVDF-HFP catalysts were tested. First, 10 wt% of palladium with respect to phenol (1.5 g phenol and 0.15 gPd/0.75 gPVDF-HFP) was tested. Conversion of phenol increased with time and was 37% in 3 h and 62% in 7 h as shown in Figure 7. Only cyclohexanone was detected at the first three hours indicating 100% selectivity. The cyclohexanone selectivity was decreased slightly to 99% at 6 h and to 98% at 7 h. To have a higher conversion, the amount of palladium was increased to 15 wt% with respect to phenol (1.5 g phenol and 0.225 gPd/1.125 g PVDF-HFP). The phenol conversion achieved was 60% in 3 h and 88% in 6 h. Almost complete phenol conversion was achieved at 7 h (98%) as shown in Figure 8. The TON (turnover number) and TOF (turnover frequency) values calculated were 7.38 and 1.05 h−1, respectively. The TON value was calculated based on the moles of phenol converted (0.0159 moles) with respect to moles of Pd utilized (0.0021) in the reaction while TON with respect to time of reaction was termed as TOF [40].
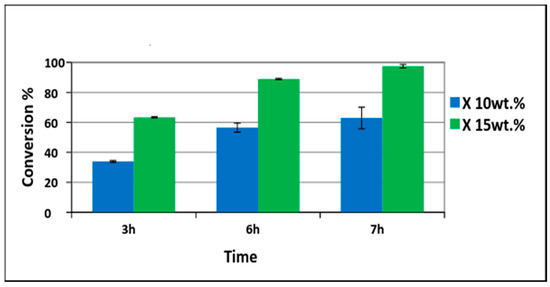
Figure 7.
Conversion of phenol using 10 wt% (phenol 1.5g (0.0159 moles) with palladium 0.15 g (0.0014 moles)) and 15 wt% (phenol 1.5 g (0.0159 moles) with palladium 0.225g (0.0021 moles)) of electrospun catalysts.
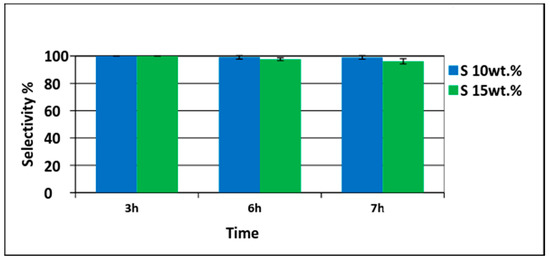
Figure 8.
Selectivity of cyclohexanone using 10 wt% (phenol 1.5 g (0.0159 moles) with palladium 0.15 g (0.0014 moles)) and 15 wt% (phenol 1.5 g (0.0159 moles) with palladium 0.225 g (0.0021 moles)) of electrospun catalysts.
The selectivity of cyclohexanone achieved was very high (>95%) even at high phenol conversion as shown in Figure 8. The result proved that phenol conversion could be increased by increasing the metal loading without a major decrease in the desired selectivity when using superhydrophobic nanofiber support. The high cyclohexanone selectivity is attributed to the superhydrophobicity of the PVDF-HFP support.
4. Conclusions
Hydrogenation of phenol to cyclohexanone was achieved actively and selectively using nanofiber supported catalysts. Palladium nanoparticles supported on PVDF-HFP nanofibers were synthesized using electrospinning. The superhydrophobic catalytic fiber mats directed the reaction toward the desired product leading to high cyclohexanone selectivity and high phenol conversion. The conversion of phenol achieved was 98% with 97% cyclohexanone selectivity within 7 h using 15 wt% of palladium relative to phenol. This paper presents novel research in heterogeneous catalysis using electrospun nanofibers. Multiphase hydrogenation of phenol to cyclohexanone over electrospun Pd/PVDF-HFP catalyst has not been reported earlier in the literature. This work can open a research window for applications of polymeric nanofibers in multiphase reactions. The polymeric structure provided the advantages of easy separation and high mechanical strength.
Author Contributions
Reaction experiment, original draft preparation, A.A. (Ahmed Abutaleb); characterization and analysis, D.L., A.A. (Abdulwahab Aljuhani) and H.U.S.; partial writing, review and editing, M.A.A.; writing and editing, A.A.Y.H. and I.M.H.M.; advising and supervision, G.G.C.
Funding
This research received external funding.
Acknowledgments
The author would like to thank SABIC Company and Jazan University for financially supporting this project (Grant No. SABIC 3/2018/1). Special thanks go to Arkema for denoting the PVDF-HFP polymer.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, H.; Jiang, T.; Han, B.; Liang, S.; Zhou, Y. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd-Lewis acid catalyst. Science 2009, 326, 1250–1252. [Google Scholar] [CrossRef]
- Xiang, Y.; Ma, L.; Lu, C.; Zhang, Q.; Li, X. Aqueous system for the improved hydrogenation of phenol and its derivatives. Green Chem. 2008, 10, 939–943. [Google Scholar] [CrossRef]
- Fujita, S.; Yamada, T.; Akiyama, Y.; Cheng, H.; Arai, M. Hydrogenation of phenol with supported Rh catalysts in the presence of compressed CO2: Its effects on reaction rate, product selectivity and catalyst life. J. Supercrit. Fluids 2010, 54, 190–201. [Google Scholar] [CrossRef]
- Hu, S.; Yang, G.; Jiang, H.; Liu, Y.; Chen, R. Selective hydrogenation of phenol to cyclohexanone over Pd@CN (N-doped porous carbon): Role of catalyst reduction method. Appl. Surf. Sci. 2018, 435, 649–655. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, R.; Wang, Q.; Wu, C.; Yu, Y.; Zhao, F. Selective reduction of phenol derivatives to cyclohexanones in water under microwave irradiation. New J. Chem. 2012, 36, 1085–1090. [Google Scholar] [CrossRef]
- Yang, X.; Du, L.; Liao, S.; Li, Y.; Song, H. High-performance gold-promoted palladium catalyst towards the hydrogenation of phenol with mesoporous hollow spheres as support. Catal. Commun. 2012, 17, 29–33. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. N2O-Free single-pot conversion of cyclohexane to adipic acid catalysed by an iron(II) scorpionate complex. Green Chem. 2017, 19, 1499–1501. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1-yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Sikhwivhilu, L.M.; Coville, N.J.; Naresh, D.; Chary, K.V.R.; Vishwanathan, V. Nanotubular titanate supported palladium catalysts: The influence of structure and morphology on phenol hydrogenation activity. Appl. Catal. A Gen. 2007, 324, 52–61. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, C.; Liu, Y.; Jiang, H.; Chen, R. Selective hydrogenation of phenol to cyclohexanone in water over Pd@N-doped carbons derived from ZIF-67: Role of dicyandiamide. Appl. Surf. Sci. 2017, 425, 484–491. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Long, L.; Wang, T.; Ma, L.; Wu, L.; Bai, Y.; Li, X.; Liao, S. Pt nanoparticles entrapped in titanate nanotubes (TNT) for phenol hydrogenation: the confinement effect of TNT. Chem. Commun. 2014, 50, 2794–2796. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Li, H. Liquid-phase selective hydrogenation of phenol to cyclohexanone over the Ce-doped Pd–B amorphous alloy catalyst. Mater. Lett. 2008, 62, 297–300. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, F.; Fujita, S.; Arai, M. Hydrogenation of phenol in scCO2 over carbon nanofiber supported Rh catalyst. Catal. Commun. 2008, 9, 362–368. [Google Scholar] [CrossRef]
- Chatterjee, M.; Kawanami, H.; Sato, M.; Chatterjee, A.; Yokoyama, T.; Suzuki, T. Hydrogenation of Phenol in Supercritical Carbon Dioxide Catalyzed by Palladium Supported on Al-MCM-41: A Facile Route for One-Pot Cyclohexanone Formation. Adv. Synth. Catal. 2009, 351, 1912–1924. [Google Scholar] [CrossRef]
- Velu, S.; Kapoor, M.P.; Inagaki, S.; Suzuki, K. Vapor phase hydrogenation of phenol over palladium supported on mesoporous CeO2 and ZrO2. Appl. Catal. A Gen. 2003, 245, 317–331. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Q.; Cen, J.; Xiang, Y.; Li, X. Selectivity tailoring of Pd/CNTs in phenol hydrogenation by surface modification: Role of CO oxygen species. Appl. Surface Sci. 2015, 324, 634–639. [Google Scholar] [CrossRef]
- Makowski, P.; Cakan, R.D.; Antonietti, M.; Goettmann, F.; Titirici, M.-M. Selective partial hydrogenation of hydroxy aromatic derivatives with palladium nanoparticles supported on hydrophilic carbon. Chem. Commun. 2008, 8, 999–1001. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Li, H.; Su, D.; Antonietti, M. Highly selective hydrogenation of phenol and derivatives over a Pd@carbon nitride catalyst in aqueous media. J. Am. Chem. Soc. 2011, 133, 2362–2365. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.U.; Lolla, D.; Nikolov, Z.; Chase, G.G. Pd–Au nanoparticles supported by TiO2 fibers for catalytic NO decomposition by CO. J. Ind. Eng. Chem. 2016, 33, 91–98. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Li, H. Liquid-Phase Selective Hydrogenation of Phenol to Cyclohexanone over Pd-Ce-B/Hydrotalcite Catalyst. Chin. J. Catal. 2007, 28, 312–316. [Google Scholar] [CrossRef]
- Mahata, N.; Vishwanathan, V. Influence of Palladium Precursors on Structural Properties and Phenol Hydrogenation Characteristics of Supported Palladium Catalysts. J. Catal. 2002, 196, 262–270. [Google Scholar] [CrossRef]
- Sulman, E.M.; Ivanov, A.A.; Chernyavsky, V.S.; Sulman, M.G.; Bykov, A.I.; Sidorov, А.I.; Doluda, V.Y.; Matveeva, V.G.; Bronstein, L.M.; Stein, B.D. Kinetics of phenol hydrogenation over Pd-containing hypercrosslinked polystyrene. Chem. Eng. J. 2011, 33, 176–177. [Google Scholar] [CrossRef]
- Matos, J.; Corma, A. Selective phenol hydrogenation in aqueous phase on Pd-based catalysts supported on hybrid TiO2-carbon materials. Appl. Catal. A Gen. 2011, 404, 103–112. [Google Scholar] [CrossRef]
- Pérez, Y.; Fajardo, M.; Corma, A. Highly selective palladium supported catalyst for hydrogenation of phenol in aqueous phase. Catal. Commun. 2011, 12, 1071–1074. [Google Scholar] [CrossRef]
- Demir, M.M.; Gulgun, M.A.; Menceloglu, Y.Z.; Erman, B.; Yolu, R.; Abramchuk, S.S.; Makhaeva, E.E.; Matveeva, A.R.; Sulman, M.G. Palladium Nanoparticles by Electrospinning from Poly(acrylonitrile-co-acrylic acid)−PdCl2 Solutions. Relations between Preparation Conditions, Particle Size, and Catalytic Activity. Macromolecules 2004, 37, 1787–1792. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, H.; Wang, S.; Shen, M.; Guo, R.; Cao, X.; Zhu, M.; Shi, X. Efficient catalytic reduction of hexavalent chromium using palladium nanoparticle-immobilized electrospun polymer nanofibers. ACS Appl. Mater. Interfaces 2012, 4, 3054–3061. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. PVDF−HFP Membrane Formation by Supercritical CO2 Processing: Elucidation of Formation Mechanisms. Ind. Eng. Chem. Res. 2006, 45, 8939–8945. [Google Scholar] [CrossRef]
- Cardea, S.; Reverchon, E. Nanostructured PVDF-HFP membranes loaded with catalyst obtained by supercritical CO2 assisted techniques. Chem. Eng. Process Process Intensif. 2011, 50, 630–636. [Google Scholar] [CrossRef]
- Priya, A.R.S.; Subramania, A.; Jung, Y.; Kim, K. High-performance quasi-solid-state dye-sensitized solar cell based on an electrospun PVdF−HFP membrane electrolyte. Langmuir 2008, 24, 9816–9819. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Huang, J.; Xu, J.J.; Khalfan, A.; Greenbaum, S.G. Li Ion Conducting Polymer Gel Electrolytes Based on Ionic Liquid/PVDF-HFP Blends. J. Electrochem. Soc. 2007, 154, A1048–A1057. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.U.; Patel, S.U.; Chase, G.G. Electrospun superhydrophobic poly(vinylidene fluoride-co-hexafluoropropylene) fibrous membranes for the separation of dispersed water from ultralow sulfur diesel. Energy Fuels 2013, 27, 2458–2464. [Google Scholar] [CrossRef]
- Carlin, R.T.; Fuller, J. Ionic liquid–polymer gel catalytic membrane. Chem. Commun. 1997, 1345–1346. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—A review. J. Mater. Process Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Frenot, A.; Chronakis, I.S. Polymer Nanofibers Assembled by Electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Park, S.J. Catalytic Decomposition of Nitric Oxide and Carbon Monoxide Gases Using Nanofiber Based Filter Media. Ph.D. Dissertation, The University of Akron, Akron, OH, USA, August 2008. [Google Scholar]
- Fan, L.; Zhang, L.; Shen, Y.; Liu, D.; Wahab, N.; Hasan, M.M. Liquid-phase Hydrogenation of Phenol to Cyclohexanone over Supported Palladium Catalysts. Bull. Chem. Reac. Eng. Catal. 2016, 11, 354–360. [Google Scholar] [CrossRef]
- Chen, A.; Li, Y.; Chen, J.; Zhao, G.; Ma, L.; Yu, Y. Selective Hydrogenation of Phenol and Derivatives over Polymer-Functionalized Carbon-Nanofiber-Supported Palladium Using Sodium Formate as the Hydrogen Source. ChemPlusChem 2013, 78, 1370–1378. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. “Turning Over” Definitions in Catalytic Cycles. ACS Catal. 2012, 2, 2787–2794. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).