Production of Decolorized Mushroom Pulp for Nonwoven Cotton Composite
Abstract
Highlights
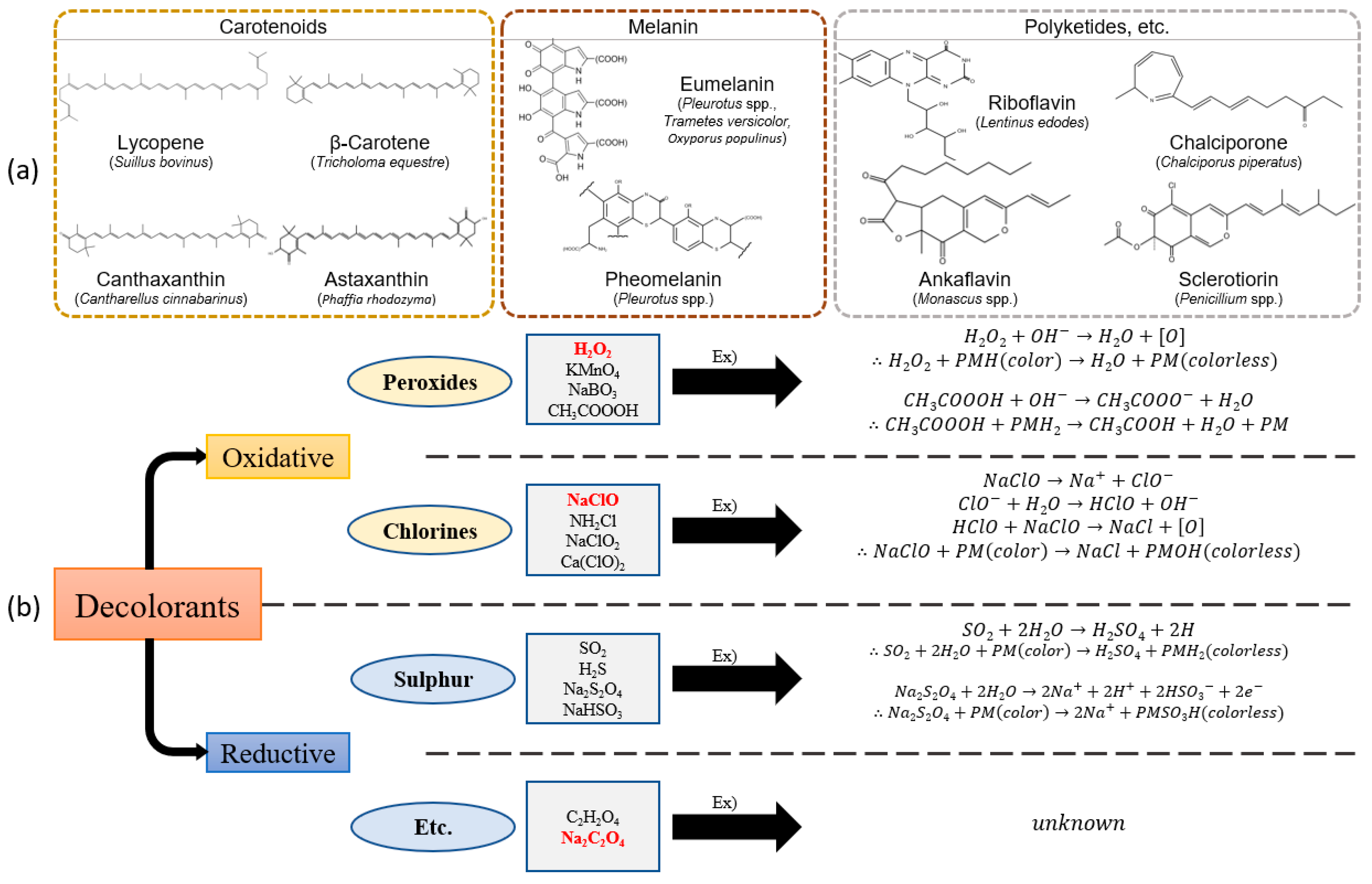
- Sustainable Cotton Alternative: Decolorized mushroom pulp (DMP) was developed as an eco-friendly substitute for cotton, with hydrogen peroxide (H2O2) being the most effective decolorization agent for fruiting bodies.
- Non-woven Textile Fabrication: DMP was successfully combined with cotton fibers to create non-woven textiles, highlighting its potential for sustainable fabric production.
- Environmental Impact: Using DMP as a cotton alternative can reduce water consumption, pesticide use, and soil degradation in the textile industry.
- Industrial Application: The successful integration of DMP into non-woven fabrics suggests its potential for use in the commercial-scale production of sustainable textiles.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strain
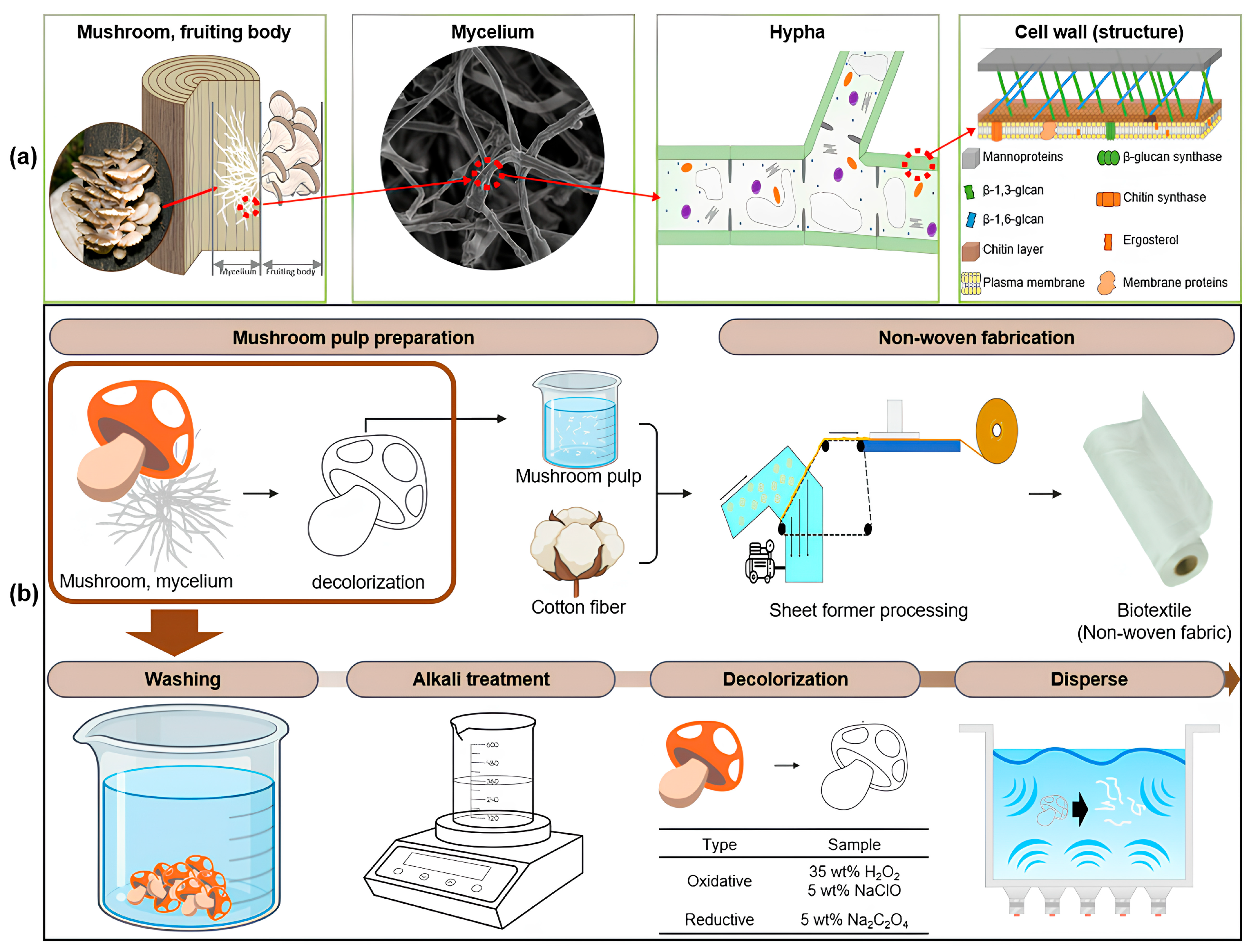
2.3. DMP Manufacturing
2.4. Nonwoven Composite Fabrication
2.5. Analysis
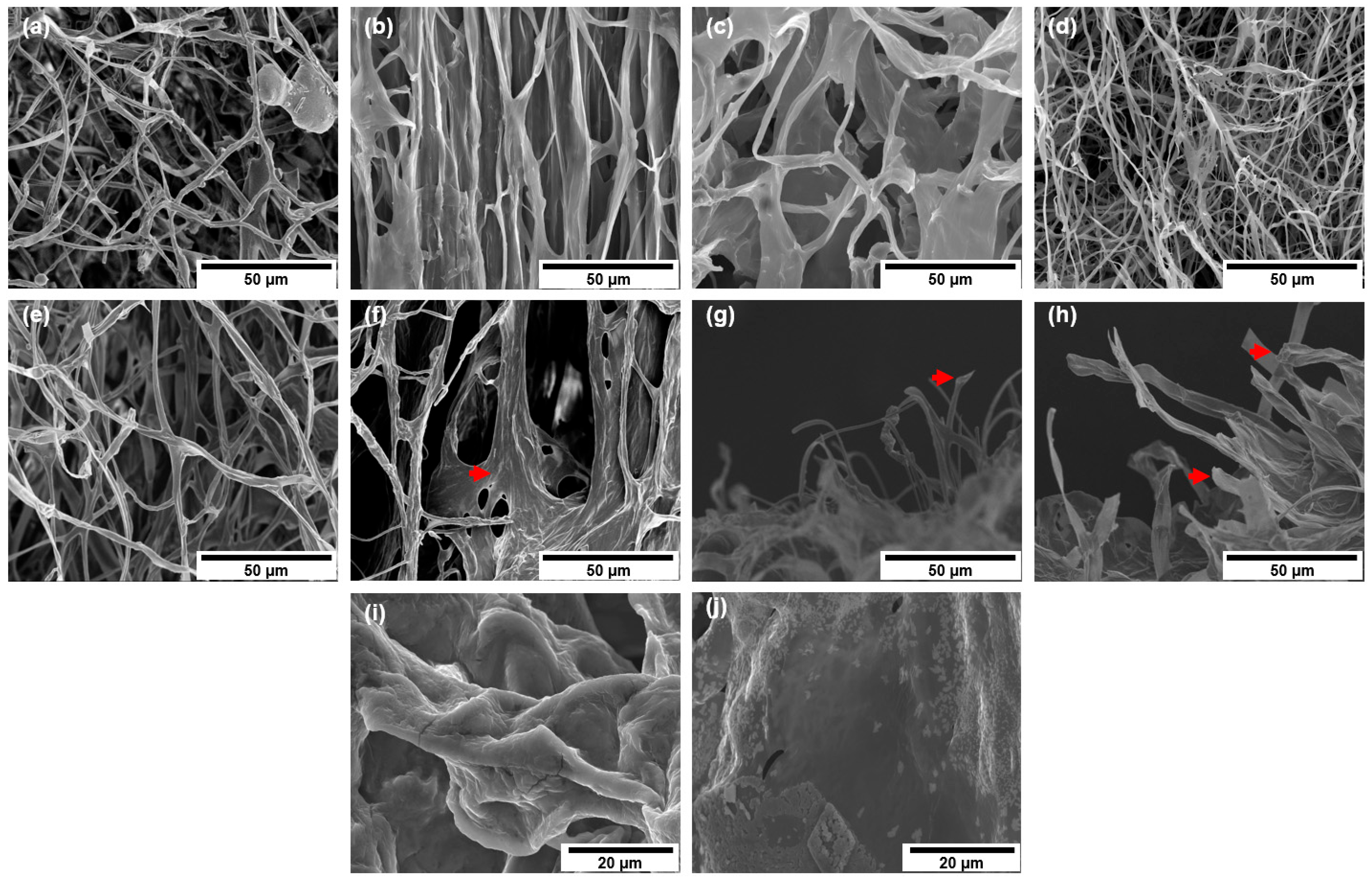
2.5.1. Morphology
2.5.2. Confocal Laser Scanning Microscopy (CLSM)
2.5.3. Fourier-Transform Infrared (FT-IR)
2.5.4. X-Ray Diffractometer (XRD)
2.5.5. Color Parameter
3. Results and Discussion
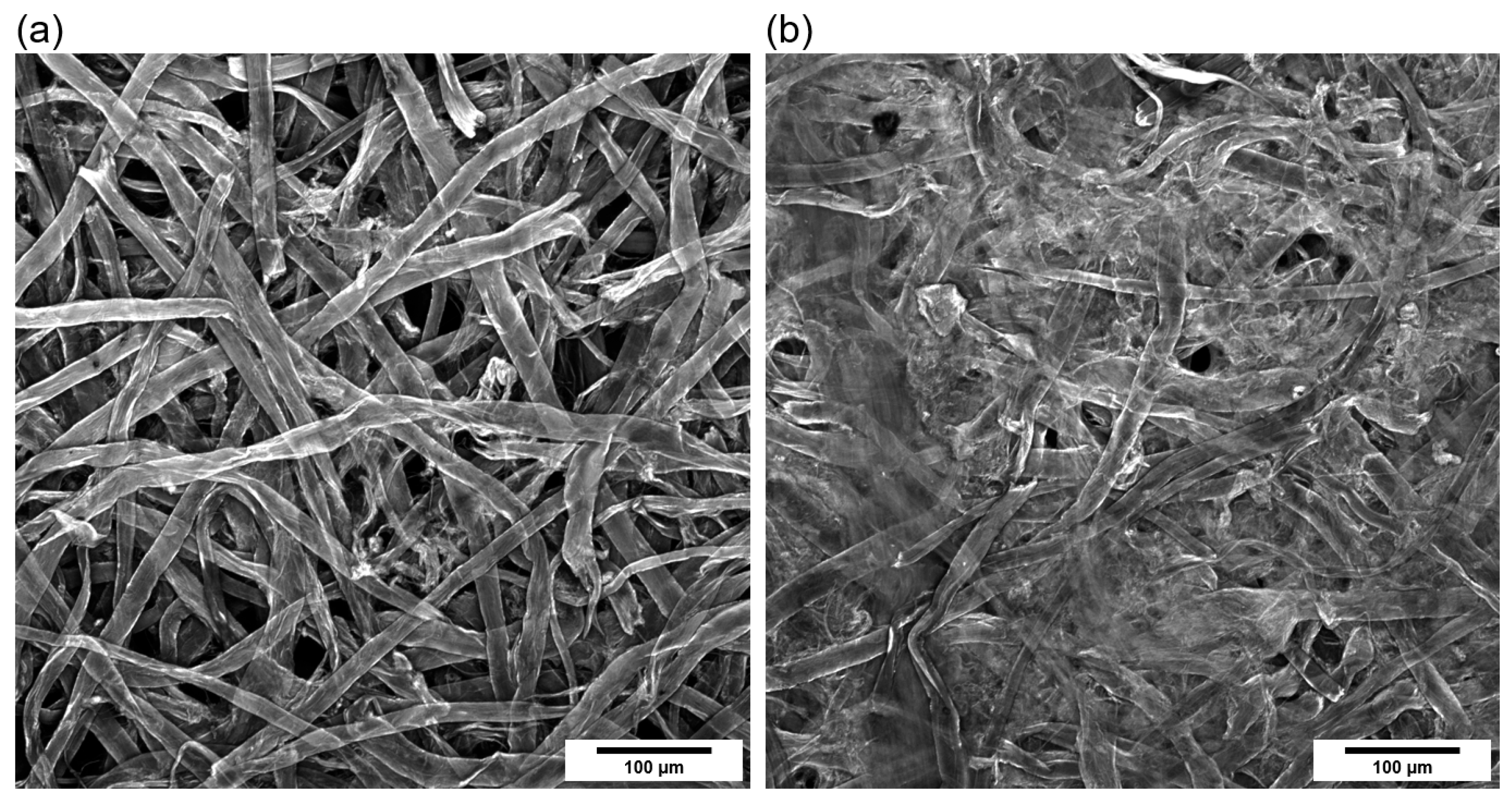
3.1. DMP Morphology and Elemental Contents
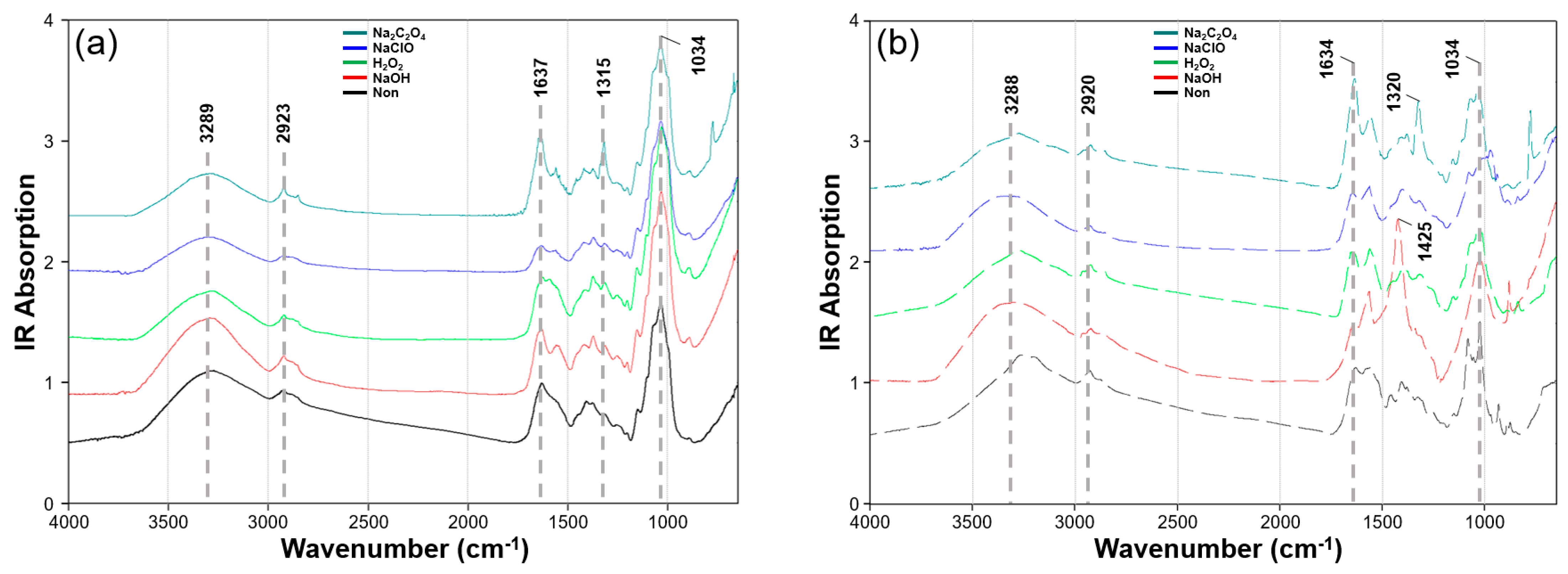
3.2. FT-IR
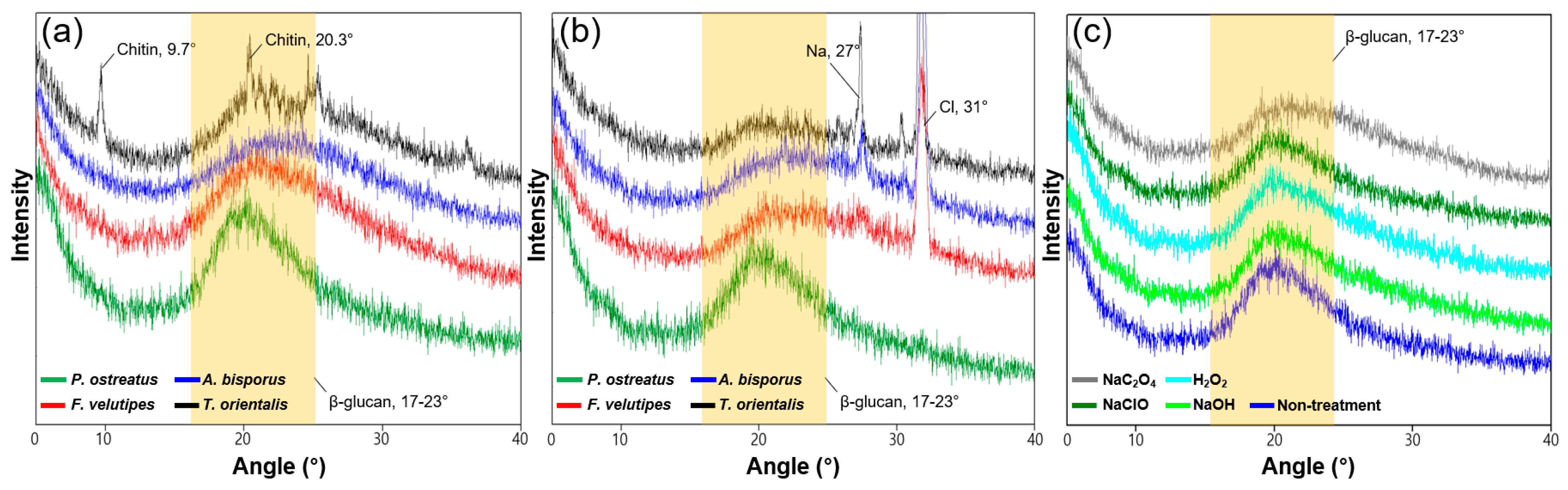
3.3. Crystalline Structure
3.4. Color
3.5. Nonwoven Fabrication
3.6. Nonwoven Textile Produced from a Composite of Cotton and DMP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Meteorological Organization (WMO). Available online: https://wmo.int/publication-series/state-of-global-climate-2023 (accessed on 1 September 2024).
- Chang, A.K.; Hoekstra, A.Y.; Savenije, H.H.G.; Gautam, R. The water footprint of cotton consumption: An assessment of the impact of worldwide consumption of cotton products on the water resources in the cotton producing countries. Ecol. Econ. 2006, 60, 186–203. [Google Scholar]
- Jans, Y.; Bloh, W.V.; Schaphoff, S.; Müller, C. Global cotton production under climate change implications for yield and water consumption. Hydrol. Earth Syst. Sci. 2021, 25, 2027–2044. [Google Scholar] [CrossRef]
- Altenbuchner, C.; Vogel, S.; Larcher, M. Social, economic and environmental impacts of organic cotton production on the livelihood of smallholder farmers in Odisha, India. Renew. Agric. Food Syst. 2018, 33, 373–385. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Biswas, A.; Wang, J.; Dong, H.; Chen, J.; Liu, C.; Fan, X. Impact of climate change and crop management on cotton phenology based on statistical analysis in the main-cotton-planting areas of China. J. Clean. Prod. 2021, 298, 126750. [Google Scholar] [CrossRef]
- Gao, Y.; Serrenho, A.C. Greenhouse gas emissions from nitrogen fertilizers could be reduced by up to one-fifth of current levels by 2050 with combined interventions. Nat. Food 2023, 4, 170–178. [Google Scholar] [CrossRef]
- Akromah, S.; Chandarana, N.; Eichhorn, S.J. Mycelium composites for sustainable development in developing countries: The case of Africa. Adv. Sustain. Syst. 2024, 8, 2300305. [Google Scholar] [CrossRef]
- Raman, J.; Kim, D.S.; Kim, H.S.; Oh, D.S.; Shin, H.J. Mycofabrication of mycelium-based leather from brown-rot fungi. J. Fungi 2022, 8, 317. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Sukchot, S.; Phonphimai, P.; Ketnawa, S.; Chaijan, M.; Grossmann, L.; Rawdkuen, S. Mushroom-legume-based minced meat: Physico-chemical and sensory properties. Foods 2023, 12, 2094. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Fan, X.; Yu, X. Naturally grown mycelium-composite as sustainable building insulation materials. J. Clean. Prod. 2022, 342, 130784. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of cultivation substrate and microbial community on improving mushroom productivity: A review. Biology 2022, 11, 569. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Kapri, M.; Tiwari, A.; Sharma, S. Medicinal importance of mushroom mycelium: Mechanisms and applications. J. Funct. Foods 2019, 56, 182–193. [Google Scholar] [CrossRef]
- Porter, D.L.; Naleway, S.E. Hyphal systems and their effect on the mechanical properties of fungal systems. Acta Biomater. 2022, 145, 272–282. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as potential sources of active metabolites and medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Huang, C.; Zhang, Z.; Gao, W. Isolation and identification of pigments from oyster mushrooms with black, yellow and pink caps. Food Chem. 2022, 372, 131171. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, J. Fungal pigments and their roles associated with human health. J. Fungi 2020, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Rimbach, G. Canthaxanthin: From molecule to function. Mol. Nutr. Food Res. 2017, 61, 1600469. [Google Scholar] [CrossRef]
- Alaux, N.; Vašatko, H.; Malerhofer, D.; Saade, M.R.M.; Stavrlc, M.; Passer, A. Environmental potential of fungal insulation: A prospective life cycle assessment of mycelium-based composites. Int. J. Life Cycle Assess. 2024, 29, 255–272. [Google Scholar] [CrossRef]
- Enarevba, D.R.; Haapala, K.R. A comparative life cycle assessment of expanded polystyrene and mycelium packaging box inserts. Procedia CIRP 2023, 116, 654–659. [Google Scholar] [CrossRef]
- Akromah, S.; Chandarana, N.; Rowlandson, J.L.; Eichhorn, S.J. Potential environmental impact of mycelium composites on African communities. Sci. Rep. 2024, 14, 11867. [Google Scholar] [CrossRef]
- National Institute of Horticultural and Herbal Science. Available online: https://www.nihhs.go.kr/farmer/statistics/statistics.do?t_cd=0206 (accessed on 10 January 2025).
- Jeong, Y.H.; Kim, D.S.; Kim, H.S.; Oh, D.S.; Shin, H.J. Effect of culture and method and medium components on Trametes orientalis mycelium mat formation (Yasuda) Imazeki. J. Mushrooms 2022, 20, 69–77. [Google Scholar]
- Da Silva, C.J.G.; de Medeiros, A.D.L.M.; de Amorim, J.D.P.; do Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Bacterial cellulose biotextiles for the future of sustainable fashion: A review. Environ. Chem. Lett. 2021, 19, 2967–2980. [Google Scholar] [CrossRef]
- Somogyi Škoc, M.; Stevelić, N.; Rezić, I. Development and characterization of sustainable coatings on cellulose fabric and nonwoven for medical applications. Sustainability 2024, 16, 857. [Google Scholar] [CrossRef]
- Rizwan, M.; Gilani, S.R.; Durrani, A.I.; Naseem, S. Cellulose extraction of Alstonia scholaris: A comprarative study on efficiency of different bleaching reagents for its isolation and characterization. Int. J. Biol. Macromol. 2021, 191, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, H.; Amano, Y.; Tagawa, S. Preparation of mycelium pulp from mushroom fruiting bodies. ACS Sustain. Chem. Eng. 2023, 11, 15789–15794. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, D.S.; Shin, H.J. Trametes orientalis mycelium mat, can be used as an alternative to elastomers? Biotechnol. Bioprocess Eng. 2023, 28, 602–611. [Google Scholar] [CrossRef]
- Yoon, M.J.; Doh, S.J.; Im, J.N. Preparation and characterization of carboxymethyl cellulose nonwovens by a wet-laid process. Fibers Polym. 2011, 12, 247–251. [Google Scholar] [CrossRef]
- Fischer, M.W.F.; Money, N.P. Why mushrooms form gills: Efficiency of the lamellate morphology. Fungal Biol. 2010, 114, 57–63. [Google Scholar] [CrossRef][Green Version]
- Wang, C.S.; Virgilio, N.; Carreau, P.J.; Heuzey, M.C. Understanding the effect of conformational rigidity on rheological behavior and formation of polysaccharide-based hybrid hydrogels. Biomacromolecules 2021, 22, 4016–4026. [Google Scholar] [CrossRef]
- Nawaz, R.; Naqvi, S.T.R.; Fatima, B.; Zulfiqar, N.; Farooq, M.U.; Haq, M.N.; Hussain, D.; Javeed, A.; Rasul, A.; Jafri, L.; et al. Cost-effective fabrication, antibacterial application and cell viability studies of modified nonwoven cotton fabric. Sci. Rep. 2022, 12, 2493. [Google Scholar] [CrossRef]
- Kurita, K. Controlled functionalization of polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Gómez-Salazar, J.A.; Galván-Navarro, A.; Lorenzo, J.M.; Sosa-Morales, M.E. Ultrasound effect on salt reduction in meat products: A review. Curr. Opin. Food Sci. 2021, 38, 71–78. [Google Scholar] [CrossRef]
- Baeva, E.; Bleha, R.; Sedliaková, M.; Sushytsktyi, L.; Švec, I.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Evaluation of the cultivated mushroom Pleurotus ostreatus basidiocarps using vibration spectroscopy and chemometrics. Appl. Sci. 2020, 10, 8156. [Google Scholar] [CrossRef]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus mushrooms content in glucans and ergosterol assessed by ATR-FTIR spectroscopy and multivariate analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Sills, D.L.; Gossett, J.M. Using FTIR to predict saccharification from enzymatic hydrolysis of alkali-pretreated biomasses. Biotechnol. Bioeng. 2012, 109, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Bekiaris, G.; Koutrotsios, G.; Tarantilis, P.A.; Pappas, C.S.; Zervakis, G.I. FTIR assessment of compositional changes in lignocellulosic wastes during cultivation of Cyclocybe cylindracea mushrooms and use of chemometric models to predict production performance. J. Mater. Cycles Waste Manag. 2020, 22, 1027–1035. [Google Scholar] [CrossRef]
- Bikmurzin, R.; Bandezevičiūtė, R.; Maršalka, A.; Maneikis, A.; Kalėdienė, L. FT-IR method limitations for β-glucan analysis. Molecules 2022, 27, 4616. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra: A practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Pylkkänen, R.; Werner, D.; Bishoyl, A.; Well, D.; Scoppola, E.; Wagermaler, W.; Safeer, A.; Bahrl, S.; Baldus, M.; Paananen, A.; et al. The complex structure of Fomes fomentarius represents an architectural design for high-performance ultralightweight materials. Sci. Adv. 2023, 9, eade5417. [Google Scholar] [CrossRef]
- Shams, R.; Singh, J.; Dash, K.K.; Dar, A.H. Comparative study of freeze drying and cabinet drying of button mushroom. Appl. Food Res. 2022, 2, 100084. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Zhang, X.; Zhang, H. Characteristics and rheological properties of polysaccharide nanoparticles from edible mushrooms (Flammulina velutipes). J. Food Sci. 2017, 82, 687–693. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Ospina Álvares, S.P.; Ramírez Cadavid, D.A.; Escobar Sierra, D.M.; Ossa Orozco, C.P.; Rojas Vahos, D.F.; Zapata Ocampo, P.; Atehortúa, L. Comparison of extraction methods of chitin from Ganoderma lucidum mushroom obtained in submerges culture. BioMed Res. Int. 2014, 2014, 169071. [Google Scholar]
- Xu, L.; Sun, J.; Qaria, M.A.; Gao, L.; Zhu, D. Dye decoloring peroxidase structure, catalytic properties and applications: Current advancement and futurity. Catalysts 2021, 11, 955. [Google Scholar] [CrossRef]
- Jeon, G.C.; Kim, C.H.; Cho, U.M.; Hwang, E.T.; Hwang, H.S.; Min, J.H. Melanin-decoloring activity of antioxidant enzymes, glutathione peroxidase, thiol peroxidase, and catalase. Mol. Biotechnol. 2021, 63, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.A.; Rahman, M.H.; Rahman, M.S.; Arif, M.; Nazir, K.H.M.N.; Dufossé, L. Fungal pigments: Carotenoids, riboflavin, and polyketides with diverse applications. J. Fungi 2023, 9, 454. [Google Scholar] [CrossRef]
- Molelekoa, T.; da Silva, L.S.; Regnier, T.; Augustyn, W. Application and stability of fungal pigments using jellysweets as a food model system. Food Sci Technol. 2023, 58, 6761–6774. [Google Scholar]
- Bayram, S.; Aygün, B.; Karadayi, M.; Alaylar, B.; Güllüce, M.; Karabulut, A. Determination of toxicity and radioprotective properties of bacterial and fungal eumelanin pigments. Int. J. Radiat. Biol. 2023, 99, 1785–1793. [Google Scholar] [CrossRef]
- Luo, H.J.; Zhan, Y.K.; Wang, S.Z.; Lin, S.Q.; Wang, L.F.; Lin, Z.X.; Lu, G.D.; Lin, D.M. Structural characterization and anti-oxidative activity for a glycopeptide from Ganoderma lucidum fruiting body. Int. J. Biol. Macromol. 2024, 261, 129793. [Google Scholar] [CrossRef]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of chitin nanofibers from mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Lehto, J.; Heiningen, A.V.; Patil, R.; Louhelainen, J.; Alén, R. Effect of sodium borohydride and hydrogen peroxide pretreatments on soda pulping of sugar maple (Acer saccharum). JWCT 2021, 41, 128–136. [Google Scholar] [CrossRef]
- Farr, J.P.; Smith, W.L.; Steichen, D.S. Bleaching agents. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 43–81. [Google Scholar]
- Yi, H.; Qin, X.; Zhai, L.; Duan, H.; Chen, H.; Zuo, Y.; Lian, X.; Tian, K.; Zhang, J.; Liu, Z.; et al. Positional isomeric thiophene-based π-conjugated chromophores: Synthesis, structure, and optical properties. Precis. Chem. 2023, 1, 548–554. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.; Zhang, G.; Zhu, P. Decolorization of dark-colored waste cotton fabric using redox decoloring agents. RSC Adv. 2022, 12, 17689–17700. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, D.; Cartabia, M.; Scalet, G.; Buratti, S.; Landro, L.D.; Benedetti, A.; Auricchio, F.; Babbini, S.; Savino, E.; Dondi, D. Innovative chitin-glucan based material obtained from mycelium of wood decay fungal strains. Heliyon 2024, 10, e28709. [Google Scholar] [CrossRef] [PubMed]
- Venu, L.B.S.; Shim, E.; Anantharamaiah, N.; Pourdeyhimi, B. Three-dimensional structural characterization of nonwoven fabrics. Microsc. Microanal. 2012, 18, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, A.; Nabiyeva, G.; Rasmussen, C.J.; Alkhoury, K.; Assem, N.; Bauer, J.; Chester, S.A.; Khalizov, A.F.; Gor, G.Y. Effects of humidity on mycelium-based leather. ACS Appl. Bio Mater. 2024, 7, 6441–6450. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A.; Koukoulas, A.A. Wet-laid nonwovens manufacture-Chemical approaches using synthetic and cellulosic fibers. BioResources 2016, 11, 5500–5552. [Google Scholar] [CrossRef]
- Kang, Y.J.; Song, S.H. Effects of surfactants on dispersion behavior of Vectran® in water (II)-Study on the manufacture and properties of wet-laid fabrics-. Text. Color. Finish. 2015, 27, 327–333. [Google Scholar] [CrossRef]
- Seidel, S.; Mozaffari, F.; Maschke, R.W.; Kraume, M.; Eibl-Schindler, R.; Eibl, D. Automated shape and process parameter optimization for scaling up geometrically non-similar bioreactors. Processes 2023, 11, 2703. [Google Scholar] [CrossRef]
- Xia, J.Y.; Wang, G.; Lin, J.H.; Wang, Y.H.; Chu, J.; Zhuang, Y.P.; Zhang, S.L. Advances and Practices of Bioprocess Scale-up. In Bioreactor Engineering Research and Industrial Applications II; Bao, J., Ye, Q., Zhong, J.J., Eds.; Advances in Biochemical Engineering-Biotechnology; Springer: Berlin/Heidelberg, Germany, 2016; Volume 152, pp. 137–151. [Google Scholar]
- Hildebrandt, J.; Thrän, D.; Bezama, A. The circularity of potential bio-textile production routes: Comparing life cycle impacts of bio-based materials used within the manufacturing of selected leather substitutes. J. Clean. Prod. 2021, 287, 125470. [Google Scholar] [CrossRef]
- Jung, D.H.; Son, J.E. Carbon dioxide emission modeling of king oyster mushroom before and after thinning processes according to temperature and growth stage. J. Bio-Environ. Control 2021, 30, 140–148. [Google Scholar] [CrossRef]
- Chen, F.; Ji, X.; Chu, J.; Xu, P.; Wang, L. A Review: Life Cycle Assessment of Cotton Textiles. Ind. Textila 2021, 72, 19–29. [Google Scholar] [CrossRef]
- Williams, E.; Cenian, K.; Golsteijn, L.; Morris, B.; Scullin, M.L. Life Cycle Assessment of MycoWorks’ ReishiTM: The First Low-Carbon and Biodegradable Alternative Leather. Environ. Sci. Eur. 2022, 34, 120. [Google Scholar] [CrossRef]
- Rong, H.; Bhat, G.S. Binder fiber distribution and tensile properties of thermally point bonded cotton-based nonwovens. J. Appl. Polym. Sci. 2004, 91, 3148–3155. [Google Scholar] [CrossRef]
- Maino, A.; Janszen, G.; Di Landro, L. Glass/Epoxy and Hemp/Bio Based Epoxy Composites: Manufacturing and Structural Performances. Polym. Compos. 2019, 40, E723–E731. [Google Scholar] [CrossRef]
- Woo, Y.E.; Oh, K.W. Fabrication of polyester fabrics with tungsten bronze nanorods and a silane coupling agent for improved thermal storage and washing durability. Fash. Text. 2023, 10, 1. [Google Scholar] [CrossRef]
- Flores, A.L.L.; Kairytė, A.; Šeputytė-Jucikė, J.; Makowska, S.; Lavoratti, A.; de Avila Delucis, R.; Amico, S.C. Effect of chemical treatments on the mechanical properties of jute/polyester composites. Materials 2024, 17, 2320. [Google Scholar] [CrossRef]



| Fungal Species | Abbreviation | Type | Structure |
|---|---|---|---|
| Agaricus bisporus Hongo | CSU *-AB | Fruiting body | Monomitic |
| Pleurotus ostreatus (Jacq.) P. Kumm. | CSU-FV | ||
| Flammulina velutipes (Curtis) Singer | CSU-PO | Dimitic | |
| Trametes orientalis (Yasuda) Imazeki | CSU-ToM | Mycelial mat | Trimitic |
| No. | Component | Wavenumber (cm−1) | Ref. |
|---|---|---|---|
| 1 | Polysaccharides (Chitin, glucans) | 1372, 1322, 1250, 1200, 1150, 1076–1071, 1040–1033, 1020, 910–880 | [34,35,36,37,38,39,40] |
| 2 | Proteins | 1650–1632, 1554–1535 | [36] |
| 3 | Lipids | 2930, 2868 | [40] |
| 4 | Phosphates | 1407–1150 | [24,41] |
| 5 | Water and Hydrogen bond | 3280 (broad) | [40] |
| Pleurotus ostreatus | Flammulina velutipes | |||||||
|---|---|---|---|---|---|---|---|---|
| a Non | NaOH | H2O2 | NaClO | Non | NaOH | H2O2 | NaClO | |
| b NaOH | L* < 0.001 a* < 0.001 b* < 0.01 | L* < 0.001 a* < 0.001 b* < 0.001 | ||||||
| c H2O2 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | ||||
| d NaClO | L* < 0.001 a* < 0.001 b* < 0.001 | L*: 0.198 a* < 0.001 b*: 0.790 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a*: 0.990 b*: 0.773 | L* < 0.05 a* < 0.001 b* < 0.001 | ||
| e Na2C2O4 | L* < 0.001 a* < 0.001 b* < 0.001 | L*:0.758 a*: 0.054 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | L*: 0.848 a*: 0.959 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a*: 0.955 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a*: 0.766 b* < 0.001 |
| Agaricus bisporus | Trametes orientalis | |||||||
| Non | NaOH | H2O2 | NaClO | Non | NaOH | H2O2 | NaClO | |
| NaOH | L* < 0.001 a* < 0.001 b* < 0.001 | L*: 0.336 a* < 0.001 b* < 0.001 | ||||||
| H2O2 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | L*: 0.933 a* < 0.001 b* < 0.001 | L*: 0.802 a* < 0.001 b* < 0.001 | ||||
| NaClO | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a*: 0.051 b*: 0.114 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a*: 0.519 b* < 0.01 | L* < 0.001 a* < 0.001 b*: 0.163 | ||
| Na2C2O4 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a*: 0.311 b*: 0.447 | L* < 0.001 a* < 0.001 b* < 0.001 | L* < 0.001 a* < 0.001 b* < 0.001 | L*: 0.820 a* < 0.05 b*: 0.654 | L*: 0.923 a*: 0.706 b* < 0.001 | L*: 0.998 a* < 0.001 b* < 0.001 | L* < 0.001 a* < 0.05 b* < 0.001 |
| Specimen | Mix Ratio (a CF:b M) | Area Density (g/m2) | Tensile Strength (MPa) | p-Value |
|---|---|---|---|---|
| CF | 10:0 | 136.59 ± 13.761 | 1.097 ± 0.069 ns | - |
| CF + c PoD | 8:2 | 134.56 ± 16.207 | 1.086 ± 0.078 ns | 0.9763 |
| CF + d FvD | 130.40 ± 9.251 | 1.037 ± 0.057 ns | 0.5589 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, H.-S.; Tagawa, S.; Jeong, J.-S.; Shin, H.-J. Production of Decolorized Mushroom Pulp for Nonwoven Cotton Composite. Fibers 2025, 13, 30. https://doi.org/10.3390/fib13030030
Im H-S, Tagawa S, Jeong J-S, Shin H-J. Production of Decolorized Mushroom Pulp for Nonwoven Cotton Composite. Fibers. 2025; 13(3):30. https://doi.org/10.3390/fib13030030
Chicago/Turabian StyleIm, Ho-Seong, Satomi Tagawa, Jae-Seok Jeong, and Hyun-Jae Shin. 2025. "Production of Decolorized Mushroom Pulp for Nonwoven Cotton Composite" Fibers 13, no. 3: 30. https://doi.org/10.3390/fib13030030
APA StyleIm, H.-S., Tagawa, S., Jeong, J.-S., & Shin, H.-J. (2025). Production of Decolorized Mushroom Pulp for Nonwoven Cotton Composite. Fibers, 13(3), 30. https://doi.org/10.3390/fib13030030







