Multilayer Electrospun Nanofibrous Membranes for Enhanced Heavy Metal Remediation
Highlights
- What are the main findings?
- Multilayer electrospun nanofibrous membranes based on polyacrylonitrile (PAN), chitosan (CS), and Nylon 6 (N6) were engineered and mechanically optimized for the adsorption of hexavalent chromium and cadmium from water.
- The optimized CS–N6–PAN architecture achieved removal efficiencies above 80% for and approximately 79% for in synthetic solutions, while maintaining high performance in real river water.
- What are the implications of these findings?
- These multilayer electrospun membranes, particularly with chitosan as the outer functional layer, offer a robust and environmentally friendly platform for heavy metal remediation in contaminated waters.
- The combination of high adsorption efficiency, mechanical stability, and effectiveness in real water matrices underscores their potential for scalable and sustainable water treatment applications.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Solution Preparation
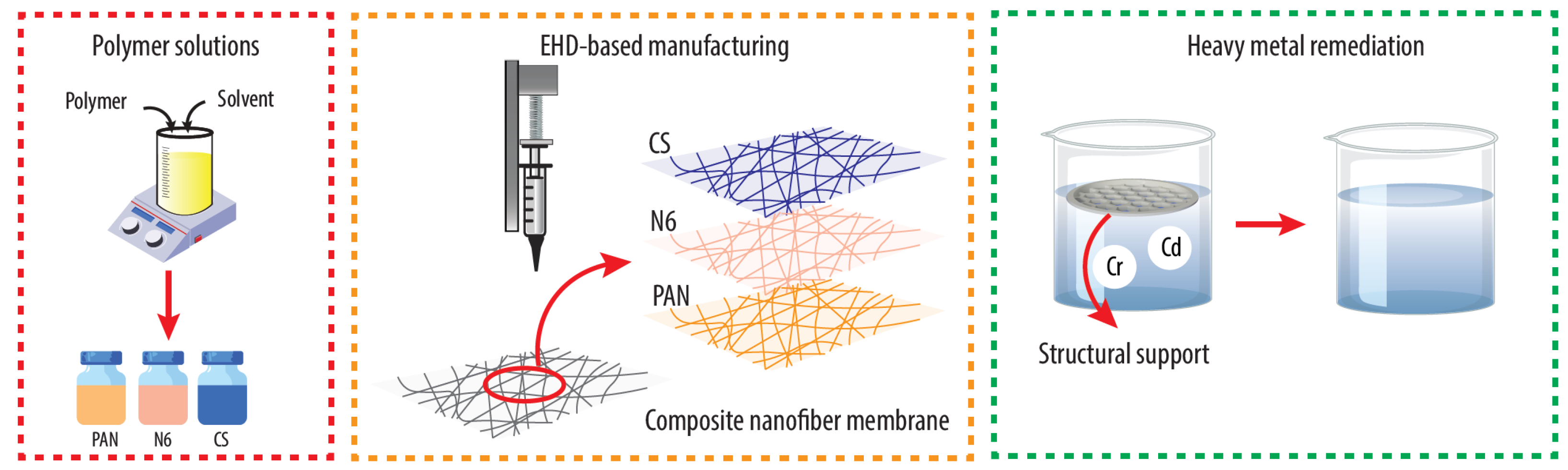
2.3. Membrane Fabrication
2.4. Membrane Layer Distribution
2.5. Metal Removal Tests
3. Results and Discussion
3.1. Preliminary Electrospinning Trials
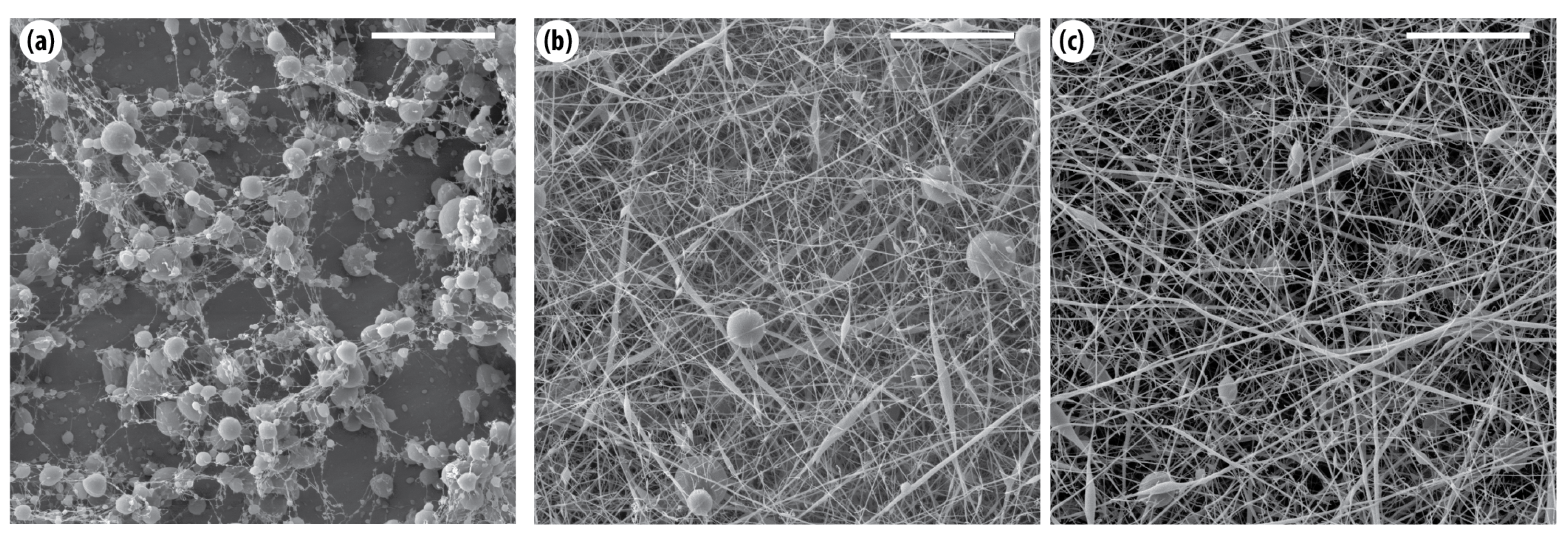
- Chitosan in acetic acid: When dissolved in 70% acetic acid at 2–3 wt%, CS generally failed to produce continuous fibers, resulting instead in either no structures or beaded fibers (see Figure 1a,b). Increasing the acetic acid concentration to 90% enabled the formation of stable fibers with fewer bead defects at 3 wt% CS, as shown in Figure 1c. In comparison, PVA in water typically required higher concentrations (around 7 wt%) together with optimized electrospinning parameters to produce uniform membranes; otherwise, the resulting structures were weak or of poor quality.
- PAN in DMF: As demonstrated in our previous research [34,35], fiber quality improved with increasing concentration. Low concentrations (5 wt%) failed to form membranes, while 12 wt% at lower voltages (10 kV) produced uniform, defect-free fibers, indicating that higher polymer content enhances stability and film integrity.
- Nylon 6 in formic acid: Continuous membranes were obtained only at high concentrations (≥18 wt%) and low flow rates ( L/h) with moderate voltages (14–16 kV). Lower concentrations resulted in beaded fibers, highlighting the importance of sufficient viscosity and solution conductivity for stable film formation [36].
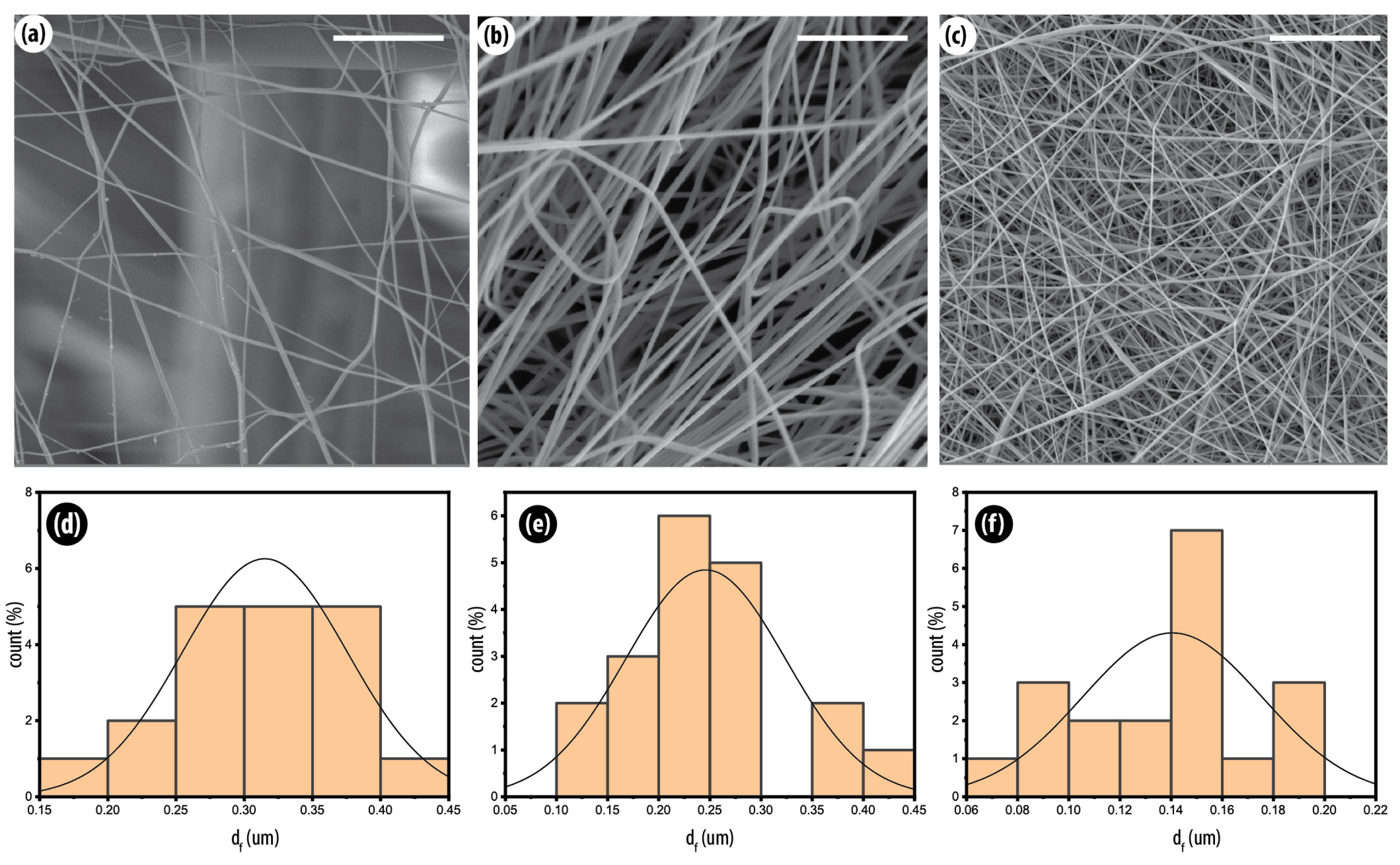
3.2. Fiber Morphology
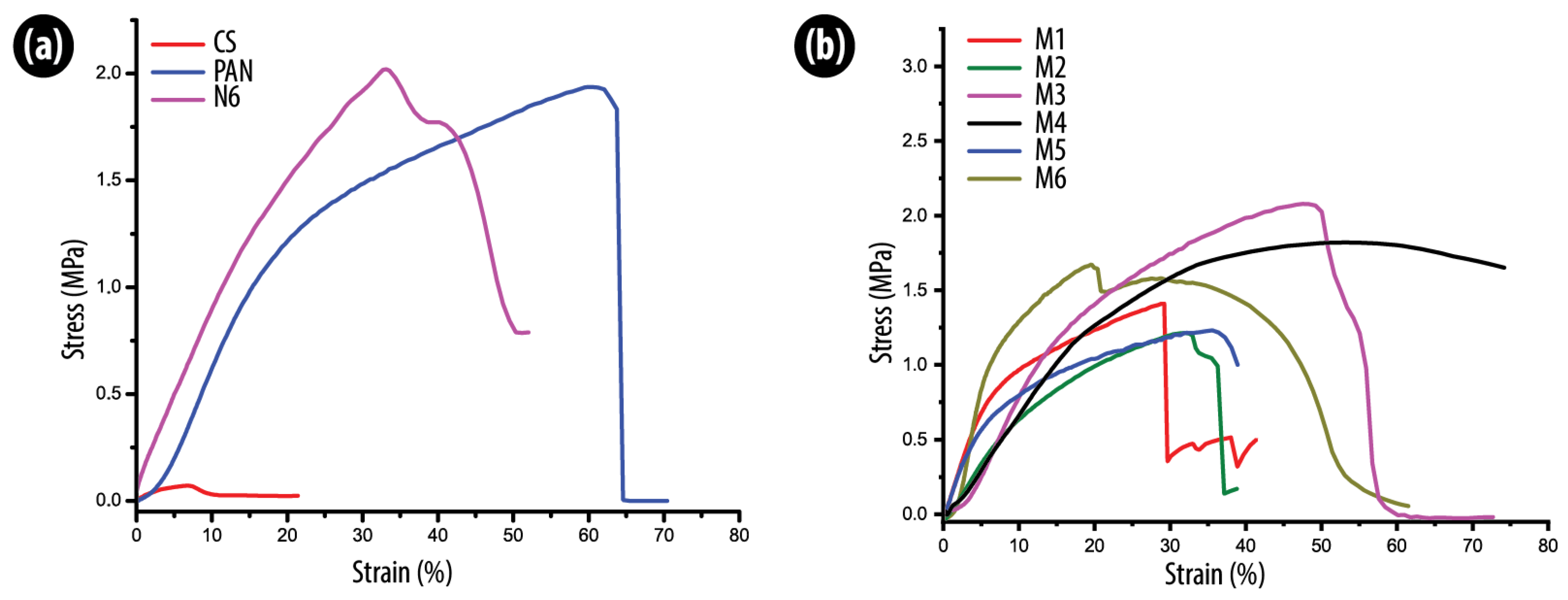
3.3. Mechanical Properties
3.4. Adsorption Performance of Electrospun Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, R.; Sharma, S.; Kaur, H. Heavy metals toxicity and the environment. J. Pharmacogn. Phytochem. 2019, 1, 247–249. [Google Scholar]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Mendonça, N.E.; Leão, C.A.; Alexis, F.; Ochoa-Herrera, V.; Zambrano-Romero, A.; Nobuyasu, R.S., Jr.; Miguez, F.B.; De Sousa, F.B. Exploiting Spiropyran Solvatochromism for Heavy Metal Ion Detection in Aqueous Solutions. ACS Omega 2025, 10, 36412–36420. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association and Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for environmental remediation: Materials and applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Guizard, C.; Bac, A.; Barboiu, M.; Hovnanian, N. Hybrid organic-inorganic membranes with specific transport properties: Applications in separation and sensors technologies. Sep. Purif. Technol. 2001, 25, 167–180. [Google Scholar] [CrossRef]
- Wang, W.; Hao, X.; Yan, Y.; Sun, R.; Petit, E.; Moderne, M.; Li, J.; Liu, J.; Wu, H.; Qi, K.; et al. 2D vermiculite nanolaminated membranes for efficient organic solvent nanofiltration. Adv. Funct. Mater. 2025, 35, 2410635. [Google Scholar] [CrossRef]
- Mohamad, S.H.; Idris, M.I.; Abdullah, H.Z.; Ismail, A.F. Short review of ultrafiltration of polymer membrane as a self-cleaning and antifouling in the wastewater system. Adv. Mater. Res. 2013, 795, 318–323. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Zamora-Ledezma, C.; Carrión-Matamoros, L.M.; Guerrero, I.E.; Debut, A.; Vizuete, K.; Haro, E.E.; López López, A.; Zamora-Ledezma, E. Influence of ultrasonication on the properties of hybrid electrospun polyacrylonitrile and silver nanoparticles fibers and their potential use in water decontamination. In Applied Technologies, Proceedings of the Third International Conference, ICAT 2021, Quito, Ecuador, 27–29 October 2021; Springer: Berlin/Heidelberg, Germany; pp. 176–188.
- Wu, S.; Li, K.; Shi, W.; Cai, J. Preparation and performance evaluation of chitosan/polyvinylpyrrolidone/polyvinyl alcohol electrospun nanofiber membrane for heavy metal ions and organic pollutants removal. Int. J. Biol. Macromol. 2022, 210, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Wang, L.; Nie, J.; Ma, G. Multilayer electrospun nanofibrous membranes with antibacterial property for air filtration. Appl. Surf. Sci. 2020, 515, 145962. [Google Scholar] [CrossRef]
- Borban, B.; Gohain, M.B.; Yadav, D.; Karki, S.; Ingole, P.G. Nano-electrospun membranes: Green solutions for diverse industrial needs. J. Hazard. Mater. Adv. 2023, 12, 100373. [Google Scholar] [CrossRef]
- Netto, J.F.Z.; Miguez, F.B.; Bahia, S.B.; Bolais-Ramos, L.G.; Verano-Braga, T.; Trigueiro, J.P.; Nobuyasu, R.S.; Brandao, T.A.; De Sousa, F.B. Core-sheath organic-inorganic hybrid electrospun fibers for organophosphorus heterogeneous catalysis. J. Environ. Chem. Eng. 2024, 12, 113267. [Google Scholar] [CrossRef]
- Wu, L.; Song, Y.; Xing, S.; Li, Y.; Xu, H.; Yang, Q.; Li, Y. Advances in electrospun nanofibrous membrane sensors for ion detection. RSC Adv. 2022, 12, 34866–34891. [Google Scholar] [CrossRef]
- Miguez, F.B.; Trigueiro, J.P.; Lula, I.; Moraes, E.S.; Atvars, T.D.; de Oliveira, L.F.; Alexis, F.; Nobuyasu, R.S.; De Sousa, F.B. Photochromic sensing of La3+ and Lu3+ ions using poly (caprolactone) fibers doped with spiropyran dyes. J. Photochem. Photobiol. A Chem. 2024, 452, 115568. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Guo, X.; Pang, H. Electrospun metal–organic framework nanofiber membranes for energy storage and environmental protection. Adv. Fiber Mater. 2022, 4, 1463–1485. [Google Scholar] [CrossRef]
- Sudário, A.C.; dos Santos, L.P.; Morais, D.C.; Tonon, G.J.; Ferreira, I.D.; Moreira, R.P.; Alves, J.d.F.; de Oliveira, L.F.; dos Santos Junior, G.A.; de Sousa, F.B.; et al. Electrospun PAN-derived carbon nanofibers enabling superior supercapacitor performance with Quinone redox electrolytes. J. Electroanal. Chem. 2025, 996, 119356. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef]
- Xiao, S.; Shen, M.; Ma, H.; Guo, R.; Zhu, M.; Wang, S.; Shi, X. Fabrication of water-stable electrospun polyacrylic acid-based nanofibrous mats for removal of copper (II) ions in aqueous solution. J. Appl. Polym. Sci. 2010, 116, 2409–2417. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Q.; Christodoulatos, C.; Meng, X. Lead and cadmium adsorption by electrospun PVA/PAA nanofibers: Batch, spectroscopic, and modeling study. Chemosphere 2019, 233, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Shen, L.; Hong, G.; Zhu, M.; Zhang, Y.; Wang, X.; Chen, Y.; Hsiao, B.S. Micro-nano structure poly (ether sulfones)/poly (ethyleneimine) nanofibrous affinity membranes for adsorption of anionic dyes and heavy metal ions in aqueous solution. Chem. Eng. J. 2012, 197, 88–100. [Google Scholar] [CrossRef]
- Almasian, A.; Giahi, M.; Fard, G.C.; Dehdast, S.; Maleknia, L. Removal of heavy metal ions by modified PAN/PANI-nylon core-shell nanofibers membrane: Filtration performance, antifouling and regeneration behavior. Chem. Eng. J. 2018, 351, 1166–1178. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Mallik, A.K.; Kabir, S.F.; Rahman, F.B.A.; Sakib, M.N.; Efty, S.S.; Rahman, M.M. Cu (II) removal from wastewater using chitosan-based adsorbents: A review. J. Environ. Chem. Eng. 2022, 10, 108048. [Google Scholar] [CrossRef]
- Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Mohd Yusoff, A.R.; Narkkun, T.; Faungnawakij, K. Electrospun nylon 6, 6/ZIF-8 nanofiber membrane for produced water filtration. Water 2019, 11, 2111. [Google Scholar] [CrossRef]
- Guevara, M.A.R.; Cepeda, Y.V.M.; Jiménez, B.D.C.; Sangucho, B.J.C.; Lugmaña, A.Y.J. El río Cutuchi, contaminante que fluye en nuestra sociedad y la afecta. RECIMUNDO 2024, 8, 51–60. [Google Scholar] [CrossRef]
- Esfahani, A.R.; Zhang, Z.; Sip, Y.Y.L.; Zhai, L.; Sadmani, A.A. Removal of heavy metals from water using electrospun polyelectrolyte complex fiber mats. J. Water Process Eng. 2020, 37, 101438. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Zamora-Ledezma, C.; Ryzhakov, P.; Pons-Prats, J.; Elango, J.; Mena, C.; Navarrete, F.; Morales-Flórez, V.; Cano-Crespo, R.; Segura, L.J. Improving glass-fiber epoxy composites via interlayer toughening with polyacrylonitrile/multiwalled carbon nanotubes electrospun fibers. J. Appl. Polym. Sci. 2023, 140, e53400. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Ponce, S.; Durán, C.; Aguayo, C.; Portero, C.; Guamán, J.; Debut, A.; Granda, M.; Alexis, F.; Zamora-Ledezma, E.; et al. Polyacrylonitrile/Silver Nanoparticles Composite for Catalytic Dye Reduction and Real-Time Monitoring. Polymers 2025, 17, 1762. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.R.; Bajgai, M.P.; Yi, C.; Nirmala, R.; Nam, K.T.; Baek, W.i.; Kim, H.Y. Effect of successive electrospinning and the strength of hydrogen bond on the morphology of electrospun nylon-6 nanofibers. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 370, 87–94. [Google Scholar] [CrossRef]
- Eda, G.; Shivkumar, S. Bead-to-fiber transition in electrospun polystyrene. J. Appl. Polym. Sci. 2007, 106, 475–487. [Google Scholar] [CrossRef]
- Vold, I.M.; Vårum, K.M.; Guibal, E.; Smidsrød, O. Binding of ions to chitosan—Selectivity studies. Carbohydr. Polym. 2003, 54, 471–477. [Google Scholar] [CrossRef]
- Caicho-Caranqui, J.; Vivanco, G.; Egas, D.A.; Chuya-Sumba, C.; Guerrero, V.H.; Ramirez-Cando, L.; Reinoso, C.; De Sousa, F.B.; Leon, M.; Ochoa-Herrera, V.; et al. Non-modified cellulose fibers for toxic heavy metal adsorption from water. Adsorption 2025, 31, 18. [Google Scholar] [CrossRef]


| Membrane | Components |
|---|---|
| M1 | PAN/CS/N6 |
| M2 | N6/CS/PAN |
| M3 | N6/PAN/CS |
| M4 | CS/PAN/N6 |
| M5 | CS/N6/PAN |
| M6 | PAN/N6/CS |
| Membrane | Removal (%) | Removal (%) | ||||
|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 | |
| M1 (N6/CS/PAN) | 0.00 | 64.20 | 61.15 | 0.00 | 45.13 | 55.23 |
| M2 (N6/PAN/CS) | 0.00 | 71.43 | 71.43 | 0.00 | 57.67 | 67.57 |
| M3 (PAN/N6/CS) | 0.00 | 71.55 | 71.55 | 0.00 | 46.89 | 49.81 |
| M4 (PAN/CS/N6) | 0.00 | 74.22 | 74.22 | 0.00 | 60.07 | 65.17 |
| M5 (CS/PAN/N6) | 0.00 | 75.33 | 75.43 | 0.00 | 56.89 | 65.93 |
| M6 (CS/N6/PAN) | 5.06 | 77.64 | 78.98 | 3.65 | 75.72 | 80.81 |
| Membrane | (mg/L) | (mg/L) | Removal (%) | Removal (%) |
|---|---|---|---|---|
| M5 | 2.330 | 1.570 | 74.3 | 75.2 |
| M6 | 2.251 | 1.551 | 77.1 | 82.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granda, M.; Zamora-Ledezma, E.; Macías Pro, M.; Guamán, J.; Debut, A.; Alexis, F.; De Sousa, F.B.; Narváez-Muñoz, C. Multilayer Electrospun Nanofibrous Membranes for Enhanced Heavy Metal Remediation. Fibers 2025, 13, 161. https://doi.org/10.3390/fib13120161
Granda M, Zamora-Ledezma E, Macías Pro M, Guamán J, Debut A, Alexis F, De Sousa FB, Narváez-Muñoz C. Multilayer Electrospun Nanofibrous Membranes for Enhanced Heavy Metal Remediation. Fibers. 2025; 13(12):161. https://doi.org/10.3390/fib13120161
Chicago/Turabian StyleGranda, Magaly, Ezequiel Zamora-Ledezma, Michael Macías Pro, Joseph Guamán, Alexis Debut, Frank Alexis, Frederico B. De Sousa, and Christian Narváez-Muñoz. 2025. "Multilayer Electrospun Nanofibrous Membranes for Enhanced Heavy Metal Remediation" Fibers 13, no. 12: 161. https://doi.org/10.3390/fib13120161
APA StyleGranda, M., Zamora-Ledezma, E., Macías Pro, M., Guamán, J., Debut, A., Alexis, F., De Sousa, F. B., & Narváez-Muñoz, C. (2025). Multilayer Electrospun Nanofibrous Membranes for Enhanced Heavy Metal Remediation. Fibers, 13(12), 161. https://doi.org/10.3390/fib13120161









