Variation in Activation Parameters for the Preparation of Cellulose-Based Porous Carbon Fibers Used for Electrochemical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
2.3. Electrochemical Measurement
3. Results
3.1. Physicochemical Properties
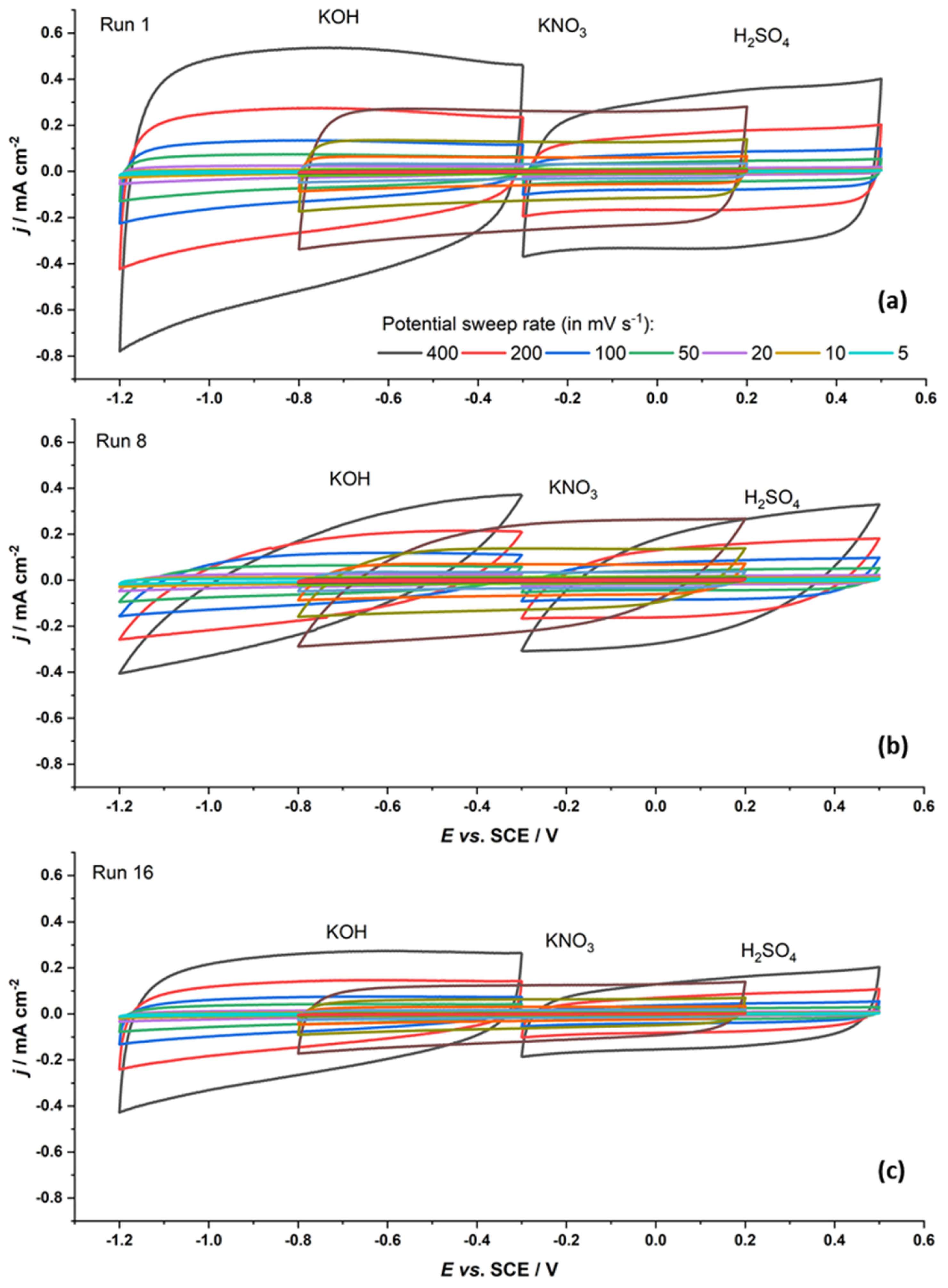
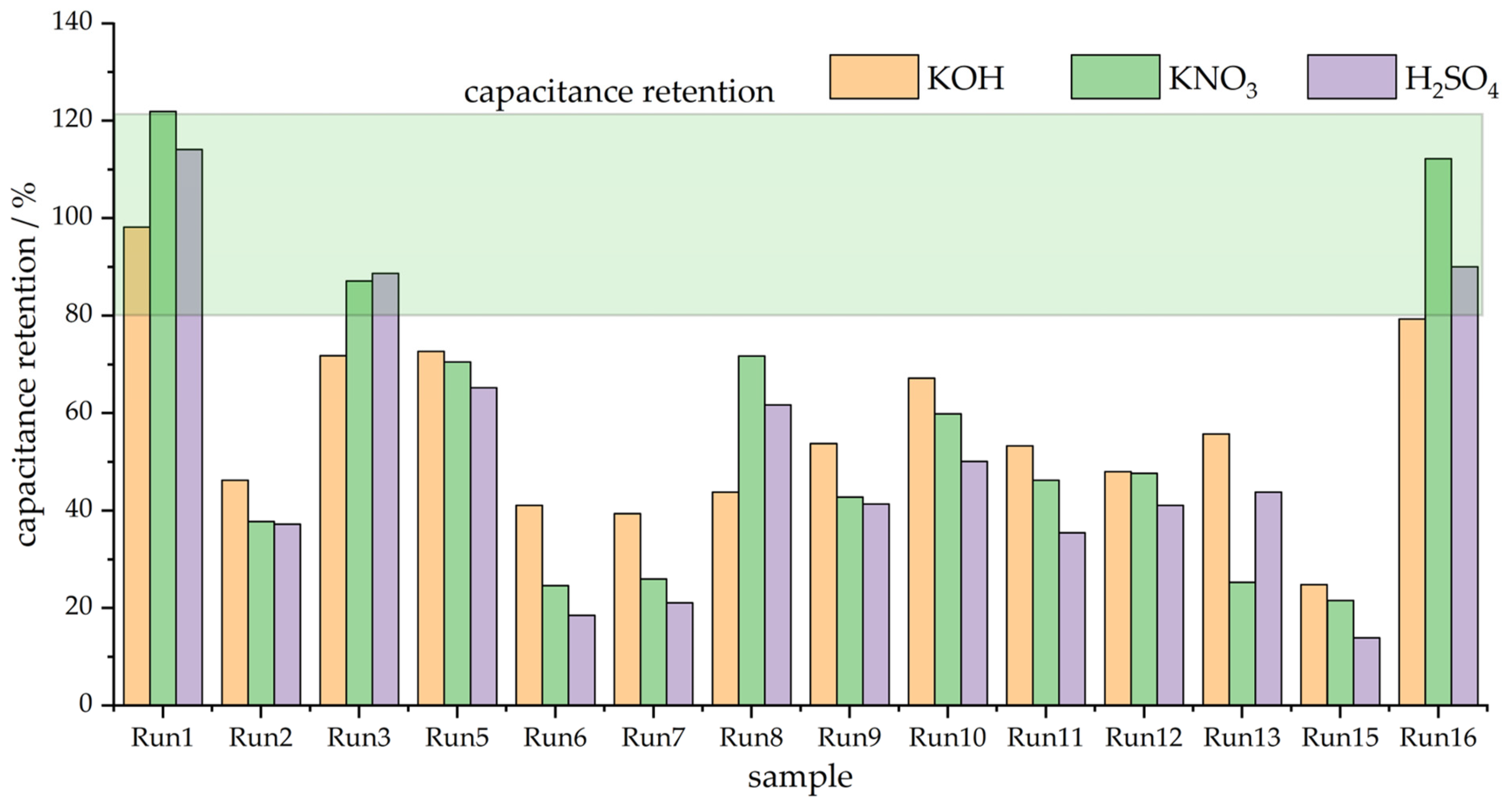
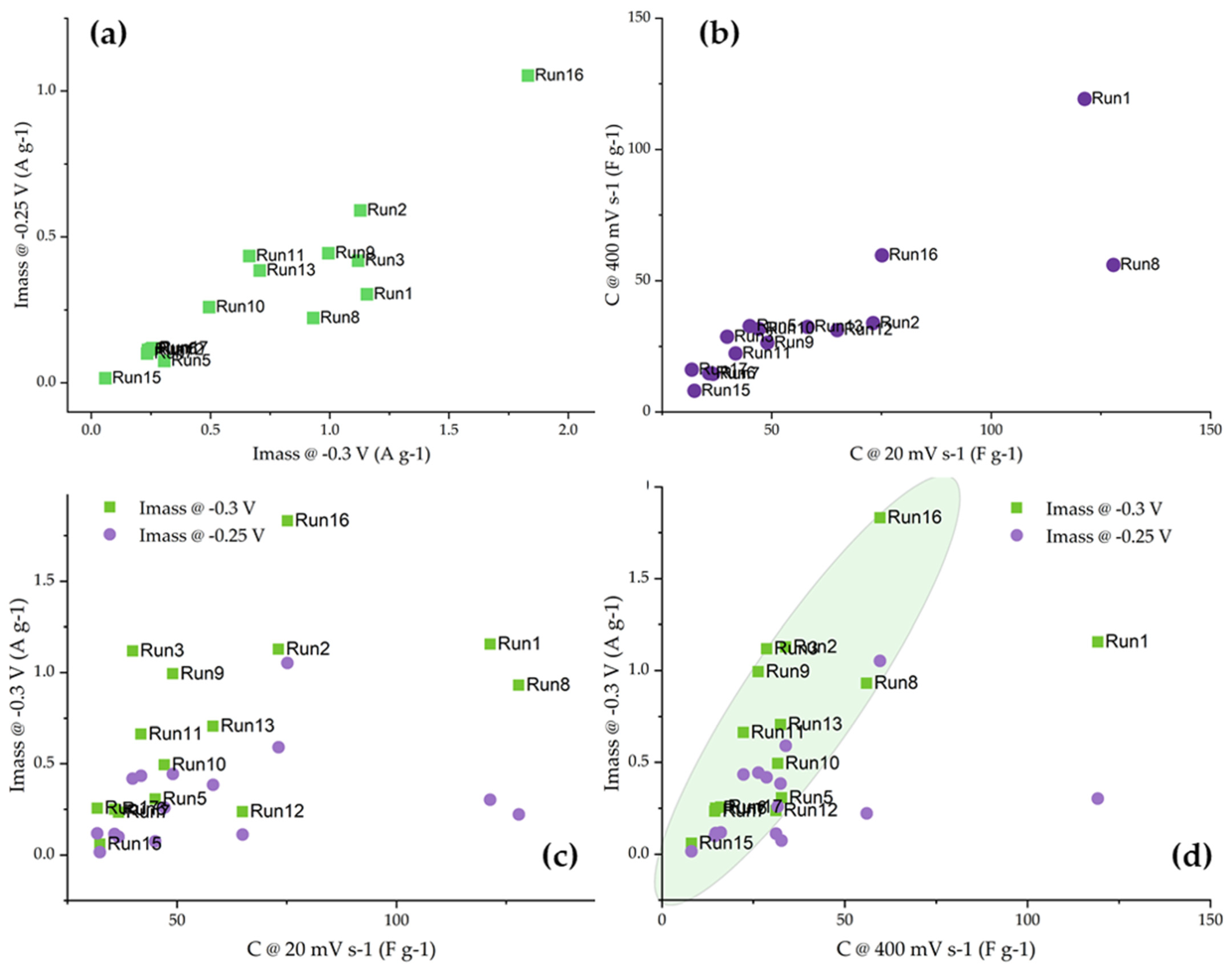
3.2. Capacitive Properties
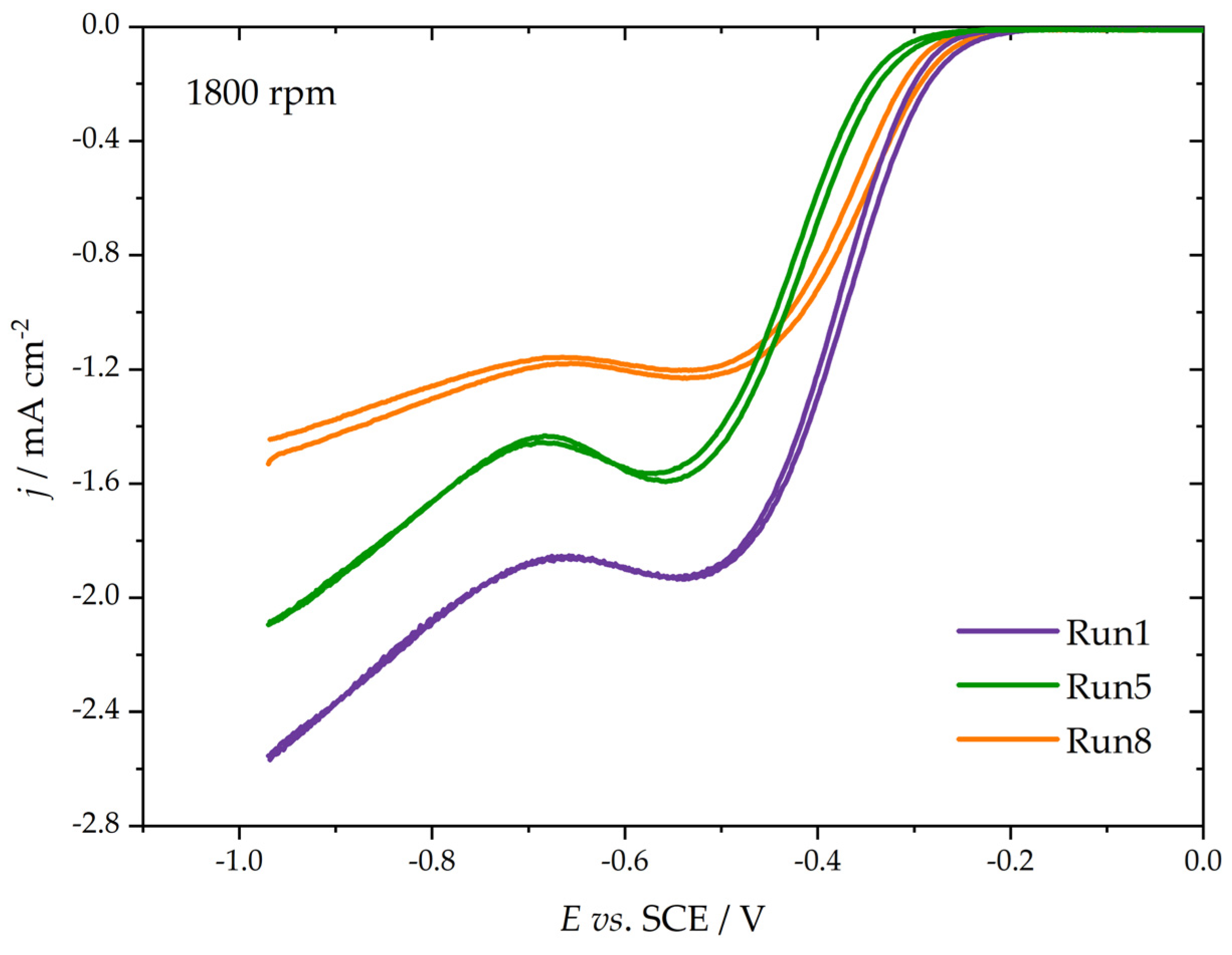
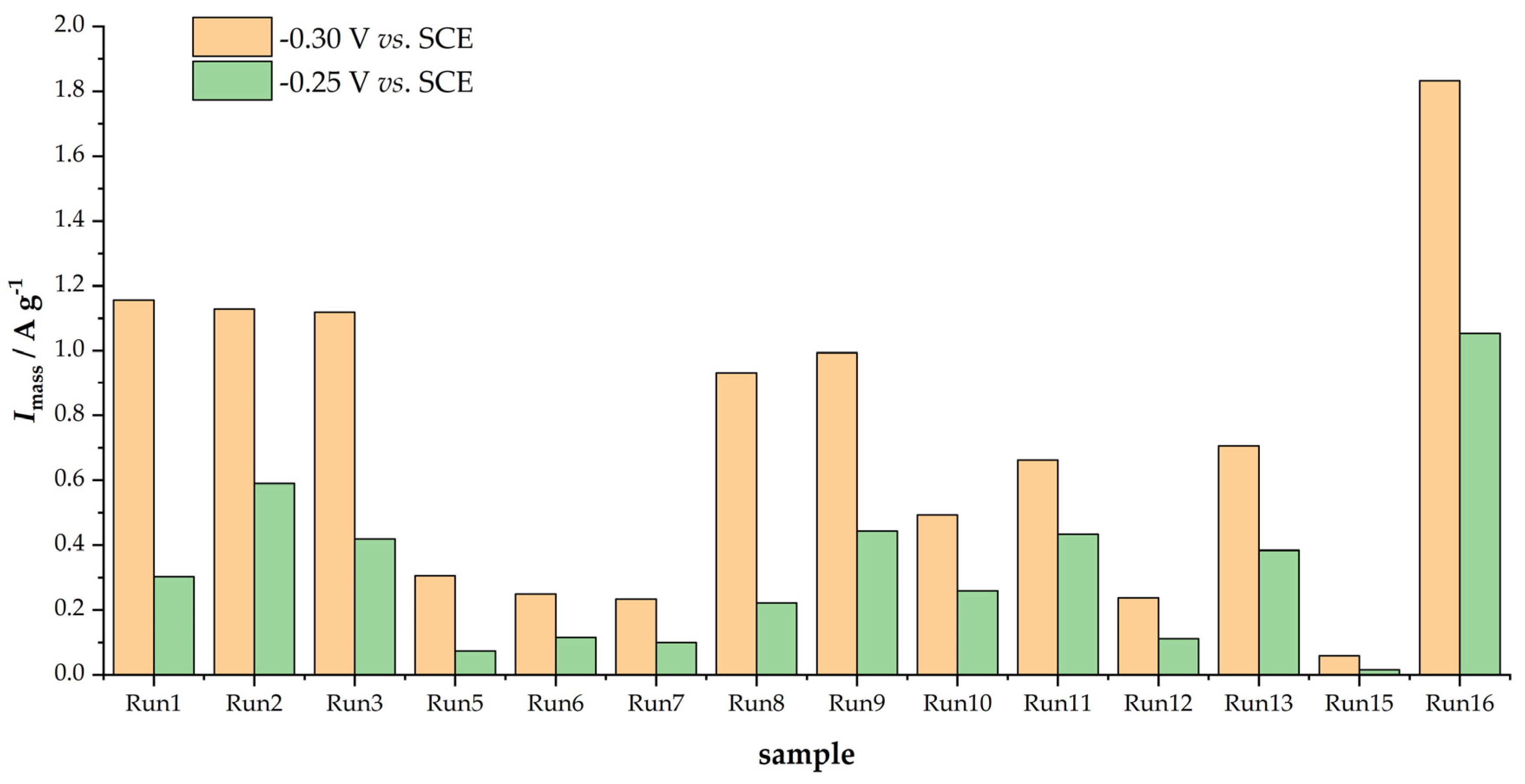
3.3. ORR Measurements
4. Discussion
4.1. Capacitive vs. Electrocatalytic Properties
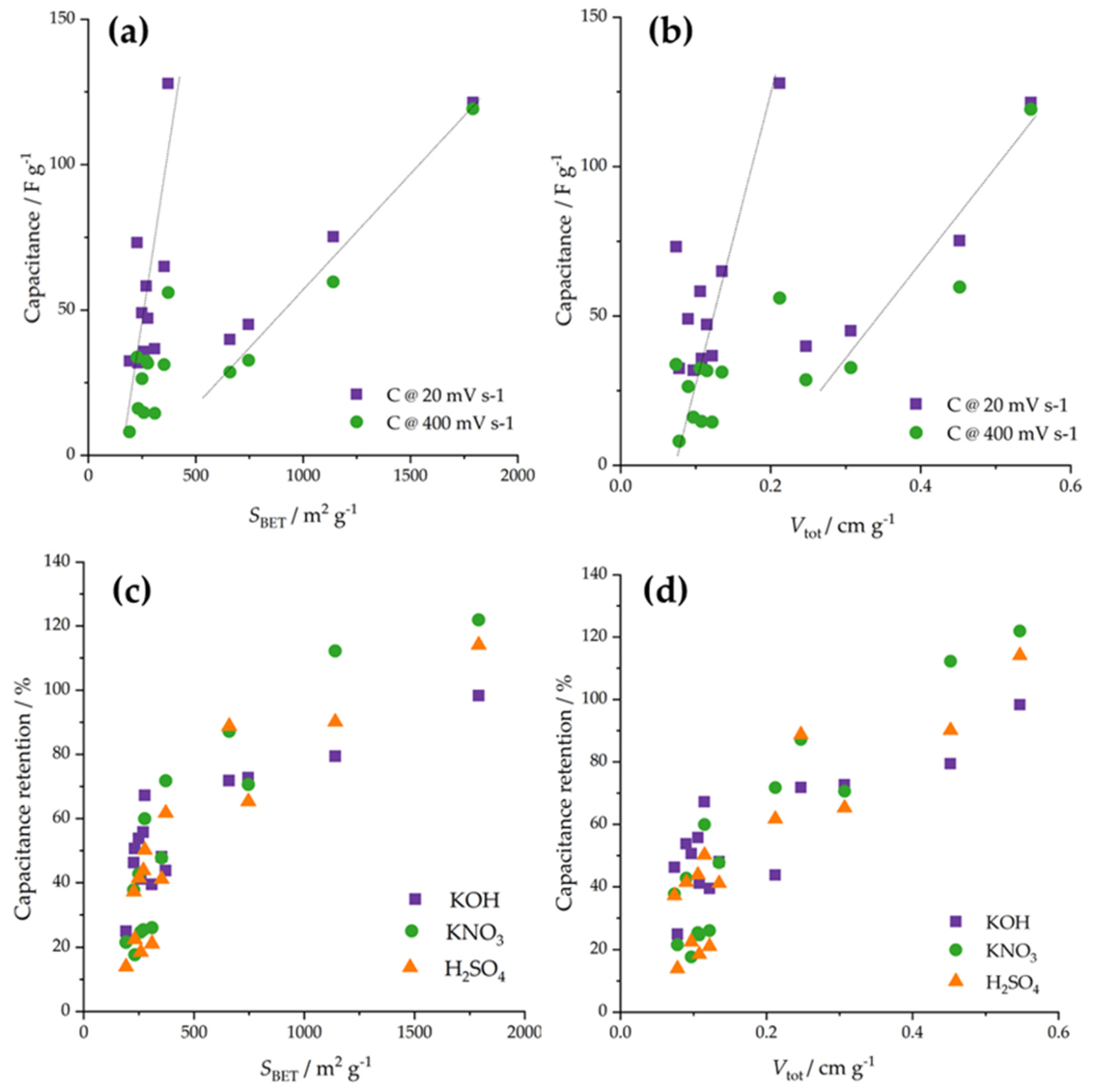
4.2. Linking to Materials Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Ma, C.; Wang, K.; Chen, J.-S. Recent advances in porous carbons for electrochemical energy storage. N. Carbon Mater. 2023, 38, 1–17. [Google Scholar] [CrossRef]
- Suzuki, M. Activated carbon fiber: Fundamentals and applications. Carbon 1994, 32, 577–586. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jung, S.-C.; Lee, H.-M.; Kim, B.-J. Comparison of Pore Structures of Cellulose-Based Activated Carbon Fibers and Their Applications for Electrode Materials. Int. J. Mol. Sci. 2022, 23, 3680. [Google Scholar] [CrossRef] [PubMed]
- Gindl-Altmutter, W.; Czabany, I.; Unterweger, C.; Gierlinger, N.; Xiao, N.; Bodner, S.C.; Keckes, J. Structure and electrical resistivity of individual carbonised natural and man-made cellulose fibres. J. Mater. Sci. 2020, 55, 10271–10280. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, C.; Huang, C.; Deng, Y.; Zou, X.; Ma, W.; Fang, G.; Ragauskas, A.J. Cellulose regulated lignin/cellulose-based carbon materials with hierarchical porous structure for energy storage. Adv. Compos. Hybrid Mater. 2024, 7, 600. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, S.; Strømme, M.; Xu, C. All-cellulose-based freestanding porous carbon nanocomposites and their versatile applications. Compos. Part B Eng. 2022, 232, 109602. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 443. [Google Scholar] [CrossRef]
- Ani, P.C.; Nzereogu, P.U.; Agbogu, A.C.; Ezema, F.I.; Nwanya, A.C. Cellulose from waste materials for electrochemical energy storage applications: A review. Appl. Surf. Sci. Adv. 2022, 11, 100298. [Google Scholar] [CrossRef]
- Breitenbach, S.; Duchoslav, J.; Mardare, A.I.; Unterweger, C.; Stifter, D.; Hassel, A.W.; Fürst, C. Comparative Behavior of Viscose-Based Supercapacitor Electrodes Activated by KOH, H2O, and CO2. Nanomaterials 2022, 12, 677. [Google Scholar] [CrossRef]
- Adam, D. Cellulose: A new bio-support for aqueous phase catalysts. Nature 2001, 1, 21. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Yop Rhee, K.; Park, S.-J. A rational design of cellulose-based heteroatom-doped porous carbons: Promising contenders for CO2 adsorption and separation. Chem. Eng. J. 2021, 420, 130421. [Google Scholar] [CrossRef]
- Bilgin Simsek, E.; Novak, I.; Sausa, O.; Berek, D. Microporous carbon fibers prepared from cellulose as efficient sorbents for removal of chlorinated phenols. Res. Chem. Intermed. 2017, 43, 503–522. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.H.; Rio, S.; Faur, C.; Le Coq, L.; Le Cloirec, P.; Nguyen, T.H. Production of fibrous activated carbons from natural cellulose (jute, coconut) fibers for water treatment applications. Carbon 2006, 44, 2569–2577. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Liu, J.; Sun, J.; Zhang, Z.; Zhu, Q. Sustainable cellulose nanomaterials for environmental remediation—Achieving clean air, water, and energy: A review. Carbohydr. Polym. 2022, 285, 119251. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Cui, J.; Lu, T.; Li, F.; Zhang, M.; Liu, K.; Zhang, Q.; He, S.; Huang, C. Design and fabrication of cellulose derived free-standing carbon nanofiber membranes for high performance supercapacitors. Carbohydr. Polym. Technol. Appl. 2021, 2, 100117. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Wang, Y. Recent progress in cellulose-based electrospun nanofibers as multifunctional materials. Nanoscale Adv. 2021, 3, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Anusiya, G.; Jaiganesh, R. A review on fabrication methods of nanofibers and a special focus on application of cellulose nanofibers. Carbohydr. Polym. Technol. Appl. 2022, 4, 100262. [Google Scholar] [CrossRef]
- Shi, J.; Huang, T.; Wu, R.; Wu, J.; Li, Y.; Kuang, Y.; Xing, H.; Zhang, W. Direct carbonization of cellulose toward hydroxyl-rich porous carbons for pseudocapacitive energy storage. Int. J. Biol. Macromol. 2024, 264, 130460. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, Y.; Li, H.; Li, X.; Zhang, C.; Guo, F.; Liu, X.; Wang, K.; Zhao, X.S. Waste-cellulose-derived porous carbon adsorbents for methyl orange removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Hina, K.; Zou, H.; Qian, W.; Zuo, D.; Yi, C. Preparation and performance comparison of cellulose-based activated carbon fibres. Cellulose 2018, 25, 607–617. [Google Scholar] [CrossRef]
- Hassan, M.F.; Sabri, M.A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent trends in activated carbon fibers production from various precursors and applications—A comparative review. J. Anal. Appl. Pyrolysis 2020, 145, 104715. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Lan, P.; Xu, H.; Lin, N. Hydrophobic and thermal-insulating aerogels based on rigid cellulose nanocrystal and elastic rubber. Carbohydr. Polym. 2022, 275, 118708. [Google Scholar] [CrossRef] [PubMed]
- Bandosz, T.J. Revealing the impact of small pores on oxygen reduction on carbon electrocatalysts: A journey through recent findings. Carbon 2022, 188, 289–304. [Google Scholar] [CrossRef]
- Breitenbach, S.; Gavrilov, N.; Pašti, I.; Unterweger, C.; Duchoslav, J.; Stifter, D.; Hassel, A.W.; Fürst, C. Biomass-Derived Carbons as Versatile Materials for Energy-Related Applications: Capacitive Properties vs. Oxygen Reduction Reaction Catalysis. J. Carbon Res. C 2021, 7, 55. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Kupgan, G.; Liyana-Arachchi, T.P.; Colina, C.M. NLDFT Pore Size Distribution in Amorphous Microporous Materials. Langmuir 2017, 33, 11138–11145. [Google Scholar] [CrossRef] [PubMed]
- Tasić, T.; Milanković, V.; Batalović, K.; Breitenbach, S.; Unterweger, C.; Fürst, C.; Pašti, I.A.; Lazarević-Pašti, T. Application of Viscose-Based Porous Carbon Fibers in Food Processing-Malathion and Chlorpyrifos Removal. Foods 2023, 12, 2362. [Google Scholar] [CrossRef]
- Hodnik, N.; Baldizzone, C.; Cherevko, S.; Zeradjanin, A.; Mayrhofer, K.J.J. The Effect of the Voltage Scan Rate on the Determination of the Oxygen Reduction Activity of Pt/C Fuel Cell Catalyst. Electrocatalysis 2015, 6, 237–241. [Google Scholar] [CrossRef]
- Wan, K.; Long, G.-F.; Liu, M.-Y.; Du, L.; Liang, Z.-X.; Tsiakaras, P. Nitrogen-doped ordered mesoporous carbon: Synthesis and active sites for electrocatalysis of oxygen reduction reaction. Appl. Catal. B Environ. 2015, 165, 566–571. [Google Scholar] [CrossRef]

| Sample | Carbon (at.%) | Oxygen (at.%) | Vtot/cm3 g−1 | dmean/nm | SBET/m2 g−1 |
|---|---|---|---|---|---|
| Run1 | 91.9 | 7.6 | 0.547 | 1.951 | 1791 |
| Run2 | 92.6 | 7.2 | 0.074 | 1.029 | 227 |
| Run3 | 92.1 | 7.6 | 0.247 | 0.479 | 659 |
| Run5 | 92.4 | 7.3 | 0.307 | 0.479 | 746 |
| Run6 | 93.4 | 6.3 | 0.108 | 0.718 | 259 |
| Run7 | 93.6 | 6.1 | 0.122 | 0.718 | 309 |
| Run8 | 92.2 | 7.4 | 0.212 | 0.718 | 372 |
| Run9 | 92.3 | 7.4 | 0.090 | 0.718 | 250 |
| Run10 | 93.1 | 6.6 | 0.115 | 1.077 | 277 |
| Run11 | 92.1 | 7.2 | 0.094 | 0.524 | 263 |
| Run12 | 91.8 | 7.9 | 0.135 | 0.524 | 353 |
| Run13 | 92.6 | 7.1 | 0.106 | 0.718 | 270 |
| Run15 | 94.0 | 5.8 | 0.078 | 1.029 | 192 |
| Run16 | 92.4 | 7.3 | 0.452 | 0.718 | 1141 |
| Run17 | 93.1 | 6.6 | 0.097 | 0.574 | 232 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unterweger, C.; Gavrilov, N.; Breitenbach, S.; Fürst, C.; Pašti, I.A. Variation in Activation Parameters for the Preparation of Cellulose-Based Porous Carbon Fibers Used for Electrochemical Applications. Fibers 2024, 12, 46. https://doi.org/10.3390/fib12060046
Unterweger C, Gavrilov N, Breitenbach S, Fürst C, Pašti IA. Variation in Activation Parameters for the Preparation of Cellulose-Based Porous Carbon Fibers Used for Electrochemical Applications. Fibers. 2024; 12(6):46. https://doi.org/10.3390/fib12060046
Chicago/Turabian StyleUnterweger, Christoph, Nemanja Gavrilov, Stefan Breitenbach, Christian Fürst, and Igor A. Pašti. 2024. "Variation in Activation Parameters for the Preparation of Cellulose-Based Porous Carbon Fibers Used for Electrochemical Applications" Fibers 12, no. 6: 46. https://doi.org/10.3390/fib12060046
APA StyleUnterweger, C., Gavrilov, N., Breitenbach, S., Fürst, C., & Pašti, I. A. (2024). Variation in Activation Parameters for the Preparation of Cellulose-Based Porous Carbon Fibers Used for Electrochemical Applications. Fibers, 12(6), 46. https://doi.org/10.3390/fib12060046







