Abstract
Pulpal pathology in young permanent teeth, caused by dental caries or trauma, can lead to disruption of root formation, leaving the tooth with an uncertain prognosis. Current therapies for such cases present a number of limitations; thus, the aim of this article is to provide an overview on the use of nanofibers in endodontics. The search was conducted on two databases and eight articles met the inclusion criteria for this systematic review. Data on nanofiber production and fiber characteristics were extracted and systematized in tables. Moreover, the ability of novel scaffolds to deliver either drugs or different therapeutic agents without interfering with the products’ characteristics is analyzed from the in vitro and in vivo data. The potential for nanofiber-based scaffolds to induce cellular differentiation and overcome the limitations of classic regenerative endodontic treatment is also discussed.
1. Introduction
Dental caries is a multifactorial infectious disease in which the oral microbiome’s shift into acidogenic and acid tolerant bacteria plays a decisive role. It can affect the tooth’s structure during the eruption phase, leading to pulpal pathology before the root has fully developed [1,2].
The bacterial biofilm on the dental surface contains salivary proteins and glycoproteins that compose the pellicle, the associated microorganisms and their products, components derived from gingival sulcus fluid and blood, and food debris [3].
The acquired pellicle enables the attachment of oral bacteria, thus promoting the formation of the dental biofilm, and also limits the acid diffusion, playing the role of a physical barrier. Furthermore, microorganisms can be maintained close to the surface by weak transient van der Waal forces, without covalent or ionic interactions between the bacterial wall and the components in the biofilm [3,4]. Bacterial attachment can be reinforced by permanent bonds between the adhesins expressed by the bacteria and the specialized receptors in the acquired biofilm [4,5].
During the acquired pellicle buildup, Streptococcus and Actinomyces species act as early colonizers due to the expression of surface receptors that enable the direct link to the dental biofilm glycoproteins. Subsequently, other species, such as the Veillonella genus, provide a favorable substrate for further attachment of later colonizers, thus leading to a culmination in microorganisms colonization [4,6].
Due to particular features such as a low external mineral content, high pulp–mineralized tissue ratio, deep occlusal pits, and fissures, young permanent teeth are prone to develop rampant caries. These rapidly-progressing caries frequently overcome the dentin deposition and infect the pulpal tissue, affecting the root development [7,8]. Consequently, the high fracture risk and improper crown-to-root ratio have a negative impact on the life span of affected teeth [9].
The endodontic management of pulp necrosis in young permanent teeth is challenging. The classic approach conforms with the principles of apexification; this method consists of the application of a calcium hydroxide dressing in the root canal in order to promote apical closure and to enable subsequent filling with gutta-percha [10,11]. This method has some limitations due to the need for repeated calcium hydroxide applications that come with multiple dental office visits and higher costs. A more recent therapeutic option consists of producing a quick apical barrier through the application of a bio-ceramic-based material (e.g., mineral trioxide aggregate—MTA) as an apical plug, thus shortening the treatment time [11,12].
The limitations of the aforementioned therapies can be overcome using the evoked bleeding (EB) strategy, which promotes dentinal wall thickening, as well as root lengthening [13,14]. The aim of this therapy is to obtain a microbial-free environment (a good disinfection) within the endodontic system and to produce apical bleeding, which enables stem cells’ recruitment and differentiation into odontoblasts [15,16]. The blood clot acts as a scaffold for the apical stem cells and the dentinal proteins induce their differentiation into odontoblasts [17,18].
The disinfection of the endodontic system is usually achieved through calcium hydroxide dressing and/or antibiotic paste dressing [19]. Calcium hydroxide is an excellent endodontic disinfectant when applied into the main root canal, but its effectiveness within the depth of thin dentinal tubules is poor, due to low solubility, on the one hand [20], and the buffer capacity, on the other hand [21].
Triple antibiotic paste (TAP) offers good disinfection, but it potentially inhibits the revascularization of the newly formed pulpal tissue. Improper vascularization could interfere with stem cells’ differentiations and could also result in dentin discoloration [22,23,24]. TAP, which was based on metronidazole (MET), ciprofloxacin (CIP), and minocycline (MINO) has been replaced by a double-antibiotic paste (DAP); in order to avoid dentin discoloration, MINO was excluded [25,26]. Moreover, antibiotic pastes are difficult to remove from the endodontic system [27], and, due to the acid pH, TAP could promote dentin decalcification after prolonged use [28].
In the past, regenerative endodontics took advantage of tissue engineering principles for managing non-vital young permanent teeth. The key to success in tissue engineering therapies is the use of three elements: stem cells, bioactive molecules such as growth factors, and scaffolds [9,14].
The use of nanofiber-based scaffolds with simple compositions or loaded with antibiotics and/or cellular inducing factors has been suggested as an alternative to TAP due to better dosage of the delivered antibiotic [29,30].
The aim of this paper is to provide a comprehensive review on current data regarding the endodontic therapy of young permanent teeth diagnosed with pulpal necrosis, in relation to nanofiber technology.
2. Materials and Methods
The current article is based on the search of two databases: PubMed and Mesh.
The search was conducted using the following Boolean search terms:
- #1—“Nanofibers”[Mesh] OR “Nanofibers”[tw] OR “Tissue Scaffolds”[Mesh];
- #2—“Endodontics”[Mesh] OR “Regenerative Endodontics”[Mesh] OR “Root Canal Preparation”[Mesh];
- #3—(“Nanofibers”[Mesh] OR “Nanofibers”[tw] OR “Tissue Scaffolds”[Mesh]) AND (“Endodontics”[Mesh] OR “Regenerative Endodontics”[Mesh] OR “Root Canal Preparation”[Mesh]).
The articles eligible for inclusion in this review were assessed by two independent researchers.
The including criteria required the studies to be conducted using nanofibers as a scaffold for either disinfection purposes or cellular inductivity, or both. The use of hard dental tissue, dental stem cells, or oral bacteria was mandatory either in vivo or in vitro. The time period searched was the last ten years and articles had to be written in English. Additionally, review articles were excluded.
The data assessment focused on the type of nanofibers used, their manufacturing process, antimicrobial activity, cellular-inductive potential, and the most relevant data offered by the paper.
3. Results
The used search terms provided a total of 76 articles that were further processed by the title and abstract. After the article’s selection, a number of eight scientific papers met the inclusion criteria and were included in this review.
The overall statistics are as follows: reports sought for retrieval (n = 56); reports not retrieved (n = 1); reports assessed for eligibility (n = 55); reports excluded: Did not present nanofiber containing scaffolds (n = 47); and studies included in review (n = 8).

Table 1.
Data systematization showing the types of scaffolds used.

Table 2.
Data systematization showing scaffold obtaining parameters and characteristics.

Table 3.
Data systematization showing antimicrobials and pathogens used.

Table 4.
Data systematization showing cellular effect assessment and the main results.
The PRISMA flow chart reveals the selection stage (Figure 1).
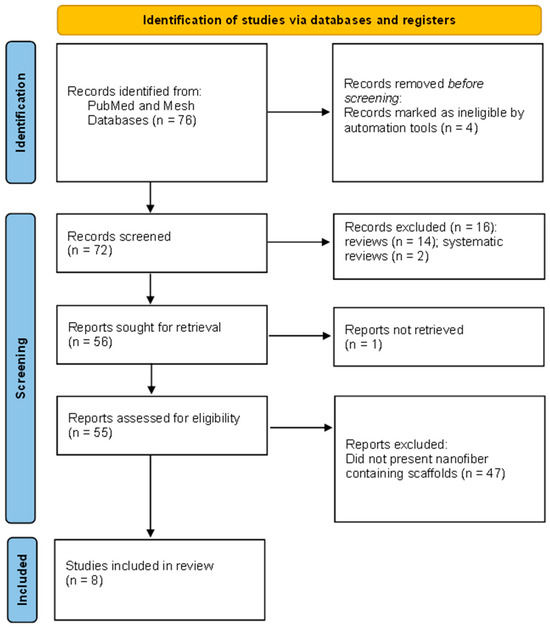
Figure 1.
Identification of studies via databases.
4. Discussion
The included articles were published between 2013 and 2022 and were based on studies mainly conducted in vitro, with only one in vivo research-oriented article on a canine model [24]. The use of nanofiber-based scaffolds for endodontic purposes was mentioned earlier in the literature, e.g., the use of poly(epsilon-caprolactone) (PCL)/gelatin scaffolds mentioned by Yang et al. [36]. They reported promising results regarding hard tissue formation when the matrix loaded with human-derived pulp cells (hDPCs) was subcutaneously inserted in mice.
Polydioxanone is a polyester that resembles the PCL and polylactic acid (PLA) and presents excellent biodegradability as well as biocompatibility due to ester bonds. Moreover, the ester bonds provided great flexibility and relatively low mechanical strength [37]. This product disintegrated much faster than the other mentioned polymers and released less acidic products [38].
4.1. Nanofiber Obtaining Process, Characteristics, and Dynamics
Our data revealed that the most frequently used polymer was PDS by electrospinning and with the electrospun parameters set between 1 and 2 mL/h rate of injection 18–20 cm distance from the injection point to the collector device, and 12–19 KV power supply.
Electrospinning is a high-voltage-dependent method of producing nanofibers from a natural or synthetic polymer-based solution; these nanofibers closely resemble the tissue extracellular matrix [31,39]. This technique is based on inducing a strong potential difference between the polymer solution flowing through a narrow tip and a metallic collector. By this method, an uneven arrangement of the fibers in the scaffold as well as scaffolds with aligned fibers were obtained [40].
Polydioxanone used in the studies was mainly derived from the suture material that contained PDS in a monofilament form [24,29,31]. The PDS was loaded with either antimicrobials, metronidazole (MET), ciprofloxacin (CIP), and minocycline (MINO) or with halloysite aluminosilicate clay nanotubes (HNTs) for scaffold production [31], and all combinations produced homogenous 3D scaffolds evaluated under scanning electron microscopy (SEM). The HNTs belong to the kaolinite family and contain aluminum and silica particles, used for drug or growth factor delivery. Cationic and anionic drugs can be loaded on HNT-containing scaffolds due to negatively charged silica present on the outer surface of the nanotubes and positively charged aluminum present in the lumen of the tubes [41,42].
The scaffold-containing triple antibiotics offer a more homogenous aspect than simple PDS [24]. An explanation could rely on the more hydrophilic nature of the antimicrobial-loaded solution, thus making it less viscous, altering the resultant electrospinning fibers [43,44]. If the solution presented a low viscosity, the jet turned into droplets; on the other hand, if the solution had a too high viscosity, it was difficult to pomp through the capillary and dried on the tip [40].
The same idea could be applied to nanofibers loaded with HNTs, which offered significant increases in the fiber diameter and a broader distribution for 10 wt% HNTs but higher tensile strength for 0.5 wt% [31]. Once the amount of HNTs was higher, the viscosity of the solution changed, consequently reducing the scaffold’s strength [42,44]. Besides viscosity, the conglomeration of HNTs in stress points along the nanofibers could be responsible for the loss of strength [45].
Bottino et al. [29] and Palasuk et al. [30] obtained a decrease in fiber diameter when two antibiotics, MET and CIP, were added to the scaffold. The fiber diameter is an important parameter of the scaffold, playing essential roles in the mechanical resistance as well as in the drug-release process. Addition of CIP to the fiber could result in a decrease in the material’s strength and a reduction in drug release, explained by the difference in molecular weight (Mw) between antimicrobials, since CIP’s Mw is almost double MET’s Mw [46]. Moreover, according to the literature, a smaller diameter fiber offered a larger surface area for cellular adhesion and enabled a more gradual release of drugs loaded in the scaffold [47,48].
Moonesi Rad et al. [34] used the thermally induced phase separation method to obtain a three-dimensional scaffold. They obtained boron (B)-modified bioactive glass nanoparticles (BG-NPs) containing a cellulose acetate/oxidized pullulan/gelatin (CA/ox-PULL/GEL) scaffold. This method was based on the fact that the polymer containing solution was thermodynamically unstable. Thus, when the solution’s temperature was lowered, it separated into a polymer-rich phase and a solvent-rich phase [49,50]. The solvent was then extracted using another solvent that could be sublimated, and the porogen material (KCl) was leached out; subsequently, the remaining nanofibers’ porous structure was freeze-dried [34,51].
The above-mentioned authors reported obtaining a scaffold with an even distribution of BG-NPs and with lower weight loss when compared with BG-NP free scaffolds at the solubility assessment. This aspect could have derived from the release of alkali ions from the bio-glass particles that were capable of neutralizing the acidic product derived from the dissolution of the polymeric scaffold, as the authors explained; therefore, a decrease in solubility could be obtained by increasing the bio-glass in the scaffold [34]. Additionally, the water absorbance (WA) of the scaffold was enhanced by the bio-glass addition due to the hydrophilic properties of the material and the weak bonds between the fiber and the glass [52,53].
It is worth mentioning the deposition of hard minerals on the scaffold when submersed into simulated body fluid (SBF). The deposition of calcium and phosphate was uniformly distributed. The explanation resides in the bio-glass negative charge and the silica ions, offering a nucleation point for the mineral deposition. The replacement of silica oxide with boron oxide enhanced the deposition of apatite crystals due to the opening of the scaffold’s structure [54,55].
The addition of BG-NPs enhanced the mechanical properties of the scaffold but decreased its porosity. The balance between the mechanical resistance and the porosity of the scaffold had to be maintained, since larger pores promoted the development of mineralized tissue in the bio-material, but it impaired the strength of the scaffold, making it difficult to manipulate [56].
4.2. Antimicrobial Assessment
Five of the eight articles included in this review investigated the antimicrobial loading on the nanofiber scaffold and four studies assessed the antimicrobial activity of the scaffolds. The antibacterial substances (i.e., MET, CIP, and MINO) were integrated in the scaffold in the range of 5 wt% to 35 wt%. An initial drug release burst was observed in the first 24 to 48 h, with MET presenting a steeper release: up to 50% during the first two days [29]. The antibiotic-containing fibers became hydrophilic, being easier to degrade progressively through hydrolysis, and thus sustaining the therapeutic drug release [24,57]. Another important parameter to be taken in consideration is the pH. MET’s dissolution could influence CIP release from the fibers. MET’s solubility was indirectly proportional to the pH, with a minimum solubility at pH = 8, whereas CIP had optimal solubility at neutral pH. Thus, the dosage ratio of the two drugs should be taken in consideration [58,59].
Notably, the electrospinning method used for obtaining the scaffold did not affect the properties of the antimicrobials [29]. This aspect was in accordance with the literature regarding other antimicrobials (example: diclofenac sodium or indomethacin) or growth factors [60,61,62].
The microbial species investigated were the main endodontic pathogens found in cases of dental pulp gangrene and are often responsible for failure of endodontic treatment (e.g., Ef) [8,63,64].
According to the data, MET-only scaffolds failed to be effective against the pathogens, whereas TAP-containing scaffolds (35 wt%) presented efficiency comparable to TAP paste against Actinomyces naeslundii (An) and the mix of MET and CIP also showed efficiency against the studied pathogens [24,29,30,33].
4.3. Cell Viability, Differentiation, Proliferation, and Toxicity Assessment
All studies evaluated the performance of nanofiber scaffolds in relation to stem cells (hDPSCs) for in vitro studies, or cells harvested through the EB method for in vivo studies [24,29,31,39].
Antibiotic-containing scaffolds demonstrated good biocompatibility, similar to simple scaffolds, without associated antimicrobials. When antibiotics were administered into the root canal using nanofiber-based scaffolds, drug release was better controlled and in lower concentrations (5 wt% MET = 386 μg, 25 wt% MET = 1.38 mg, 5 wt% CIP = 280 μg, and 25 wt% CIP = 1 mg) [29], compared to administration as TAP paste (1 g/mL). The literature cites toxic cellular effects of antibiotic concentrations exceeding 1 g/mL; the survival rate of cells derived from the apical papillae increased only below this concentration [26,65].
Remarkably, MET presented less cytotoxicity than CIP. Even though MET has been shown to have cytotoxic effects directly related to the concentration used [66,67], it seemed to enhance the viability of hDPSCs, as observed by Kamoki et al. [32]. The explanation could be that MET promoted cell proliferation through a cytokine-mediated inflammatory response [68]. Fluoroquinolones, group of antimicrobials that include CIP, are notorious cytotoxic substances [69]; thus, the negative impact of CIT on cell proliferation and viability is easily comprehended. On the other hand, Bottino et al. [29] evidenced that CIP-containing PDS scaffolds had equally effective antibacterial properties in concentrations of both 25 wt% and 5 wt%; thus, loading the nanofibers with a reduced quantity of CIP, but at an effective dosage, could be the answer for cell viability.
Similar to TAP, the in vivo use of PDS scaffolds loaded with antimicrobials promoted apical closure through an osseous dentin-like tissue, although an inflammatory infiltrate was reported in the apical area [24]. The dental pulp stem cells had a lesser potential of differentiation into odontoblasts than cells derived from the apical papillae, which were more likely to survive the disinfection protocol [70]. Growth factors and differentiation determining growth factors are known to be found in the dentinal tissue [28,71] and antimicrobial therapy had a negative impact on the release of these factors; thus, formation of osteoblast like cells was more likely to occur [18,24].
The use of HNTs in the scaffold revealed enhanced cellular proliferation of the hDPSCs. HNT scaffolds proved good biocompatibility toward other cellular lines, such as osteoblasts and fibroblasts [72]. Halloysite nanotubes presented the capacity to encapsulate antimicrobials, being able to prevent the initial drug burst release from the fibers [73].
Even with no disinfection potential, PCL-based nanofiber-containing scaffolds associated with fibronectin (FN)-coated collagen hydrogel had higher cellular proliferation, chemotaxis, and gene expression of pulp regeneration markers than PCL nanofibers [35]. The biocompatibility, wound healing, and enhanced protein secretion of collagen and fibronectin was evidenced in the literature toward dental pulp cells, as well as other cell types (e.g.,hepatocytes) [74,75,76].
Another promoter of cellular differentiation proved to be the scaffold loaded with bio-glass nanoparticles and boron [34]. Alkaline phosphatase (ALP)’s increased activity demonstrated that hDPSCs differentiated into odontoblasts [77]. Moreover, confocal laser scanning microscopy analysis (CSLM) revealed the presence of cellular processes in the newly differentiated cells, a characteristic of odontoblasts [78].
Also, the differentiation markers investigated by Moonesi Rad et al. [34] through immune-histochemical methods revealed greater expression in the bio-glass boron-enriched-containing scaffolds. Collagen type I, which represents the main protein in the dentinal matrix [79], as well as dentin phosphoprotein (DPP)—the main non-collagenous protein—and osteopontin (OPN) [80], proved the odontoblastic differentiation [81].
4.4. Challenges and Future Research
Future research should focus on developing three-dimensional scaffolds based on materials with high biocompatibility, which enables stem cell cultivation or recruitment, also promoting cell growth and controlling cell differentiation. Such three-dimensional matrices should facilitate the influx of nutrients deep into the scaffold, as well as efflux of metabolites. Additionally, the synthetic matrices should be biodegradable with no toxic effect on human cells, so that the newly formed tissue could produce its own matrix.
Advanced endodontic therapies should focus on fully restoring the dental pulp, with all the morphological and functional characteristics. One possibility for achieving this ideal is to develop a scaffold that already contains various types of pre-differentiated stem cells in its structure. Maybe the way to truly regenerate the pulpal–dentin complex throughout the endodontic system is to create a microenvironment suitable for cell survival and not necessarily for cell differentiation, since the latter is more environmentally sensitive.
In line with the aforementioned ideas, cell insemination should be achieved by adding the cells in layers to obtain constructs with a characteristic architecture that mimics the natural tissue (e.g., blood and lymphatic vascular circulation, nerve fibers, etc.). The idea of loading the scaffolds with antimicrobials in non-toxic doses should also be considered, since the sterilization of the endodontic root canal system is difficult to obtain. Another aspect to be taken in consideration is the possibility of performing the endodontic therapy in such a manner that the tooth structure is not weakened, which could increase the risk of dental fracture, leading to therapeutic failure.
In summary, the articles cited in this review reveal that the clinical scenario of dental pulp necrosis can be mimicked by introducing biofilm-derived bacteria into the root canals and demonstrated that the use of nanofiber scaffolds loaded with antibiotics in a much lower concentration could be as effective as the use of TAP [24]. Bottino et al. also reported that the use of antibiotic-loaded scaffolds proved as efficient as DAP [30]. Nanofiber scaffolds composed of PDS as a carrier also ensured a drug-efficient dosage against common endodontic pathogens [29,33]. All studies demonstrated the antibacterial efficiency of scaffolds against the biofilm, in balance with the capability of modulating cell differentiation. Through the support provided to the non-differentiated cells, scaffolds could act as an inducer of cell differentiation. Moreover, the inductive properties of scaffolds could be improved by the addition of cell growth factors such as fibronectin and collagen [35]. Leite et al. used a PCL scaffold associated with fibronectin and collagen hydrogel to stimulate the migration and proliferation of hDPSCs, and cell differentiation was highlighted through expression of pulp regeneration markers [35]. Promising results regarding modulation of stem cells’ function was obtained by the addition of B and BGNPs to a cellulose-based scaffold obtained through phase separation. The authors reported superior biological properties of the scaffold for controlling cell differentiation, demonstrated by the expression of marker proteins and the deposition of hard tissue on the scaffold [34]. The ability of delivering bioactive agents to the endodontic system could be optimized through the addition of HNTs to a PDS scaffold. The hollow tube can be used as a carrier for a wide variety of substances for cell modulation and bacterial control [31].
5. Conclusions
Several in vitro and in vivo studies reported promising results regarding the use of scaffolds for endodontic treatment. The nanofiber-based scaffolds offer a tremendous advantage through possible loading with different materials and drugs compared with the classic TAP revitalization method. The fiber properties, antimicrobial potential, and cellular impact of the scaffold reveal the start of a new era in endodontic therapy in young permanent teeth. In conclusion, nanofiber-based scaffolds could represent an alternative to current endodontic therapies, but further in vivo studies should be conducted for a better understanding of the scaffolds’ advantages and limitations.
Author Contributions
Conceptualization: S.C., A.M. and A.I.; methodology, I.R.B., A.B.B. and A.I.; investigation, S.C., A.-M.B. and A.B.; writing—original draft preparation, S.C., A.-M.B. and C.N.F.; project administration, A.M.; writing—review and editing, A.M., A.B.B. and A.I.; data curation, A.B. and A.B.B.; resources, C.N.F., I.R.B. and A.B.B.; supervision, I.R.B. and A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Research, Innovation and Digitization, CNCS—UEFISCDI, project number PN-III-P4-PCE-2021-1140, within PNCDI III, a financial support for which the authors are thankful.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Abbreviation | Meaning |
| MTA | Mineral trioxide aggregate |
| EB | Evoked bleeding |
| TAP | Triple antibiotic paste |
| MET | Metronidazole |
| CIP | Ciprofloxacin |
| MINO | Minocycline |
| DAP | Double antibiotic paste |
| PDS | Polydioxanone |
| HNTs | Halloysite aluminosilicate clay nanotubes |
| TB-SC | Tubular scaffold poly(caprolactone) |
| FN | Fibronectin |
| H | Collagen hydrogel |
| CA/ox-PULL/GEL | Cellulose acetate/oxidized pullulan/gelatin |
| BGNPs | Bioactive glass nanoparticles |
| B-BGNPs | Boron-modified bioactive glass nanoparticles |
| B | Boron |
| SEM | Scanning electron microscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| TEM | Transmission electron microscopy |
| HPLC | High-performance liquid chromatography |
| Pg | Porphyromonas gingivalis |
| Ef | Enterococcus faecalis |
| An | Actinomyces naeslundii |
| Fn | Fusobacterium nucleatum |
| CLSM | Confocal laser scanning microscopy |
| PBS | Phosphate-buffered solution |
| H&E | Hematoxylin–eosin |
| TA-3DC | Triple antibiotic-eluting constructs |
| qPCR | Quantitative polymerase chain reaction |
| EthD-1 | Ethidium homodimer |
| WA | Water absorption |
| ALP | Alkaline phosphatase |
| DPP | Dentin phosphoprotein |
| DSPP | Dentin sialophosphoprotein |
| OPN | Osteopontin |
| ITGA5, ITGAV | Genes that encode the protein integrin α 5 and α V |
| COL1A1, COL3A1 | Genes that encode collagen type I and III |
| hDPSCs | Human-derived pulp cells |
| PCL | Poly(epsilon caprolactone) |
| PLA | Polylactic acid |
| PGA | Polyglycolic acid |
| Mw | Molecular weight |
| SBF | Simulated body fluid |
References
- Tanner, A.C.R.; Kressirer, C.A.; Faller, L.L. Understanding Caries from the Oral Microbiome Perspective. J. Calif. Dent. Assoc. 2016, 44, 437–446. [Google Scholar] [CrossRef]
- Tong, H.J.; Seremidi, K.; Stratigaki, E.; Kloukos, D.; Duggal, M.; Gizani, S. Deep Dentine Caries Management of Immature Permanent Posterior Teeth with Vital Pulp: A Systematic Review and Meta-Analysis. J. Dent. 2022, 124, 104214. [Google Scholar] [CrossRef]
- Chawhuaveang, D.D.; Yu, O.Y.; Yin, I.X.; Lam, W.Y.-H.; Mei, M.L.; Chu, C.-H. Acquired Salivary Pellicle and Oral Diseases: A Literature Review. J. Dent. Sci. 2021, 16, 523–529. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial Interactions in Dental Biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. In Microbiota of the Human Body; Schwiertz, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 902, pp. 45–60. ISBN 978-3-319-31246-0. [Google Scholar]
- Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Streptococcus Adherence and Colonization. Microbiol. Mol. Biol. Rev. MMBR 2009, 73, 407–450. [Google Scholar] [CrossRef]
- Carvalho, J.C. Caries Process on Occlusal Surfaces: Evolving Evidence and Understanding. Caries Res. 2014, 48, 339–346. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Soares, A.J.; Souza-Filho, F.J.; Zaia, A.A.; Ferraz, C.C.R.; Almeida, J.F.A.; Gomes, B.P.F.A. Microbial Evaluation of Traumatized Teeth Treated with Triple Antibiotic Paste or Calcium Hydroxide with 2% Chlorhexidine Gel in Pulp Revascularization. J. Endod. 2014, 40, 778–783. [Google Scholar] [CrossRef]
- Albuquerque, M.T.P.; Valera, M.C.; Nakashima, M.; Nör, J.E.; Bottino, M.C. Tissue-Engineering-Based Strategies for Regenerative Endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef]
- Bottino, M.C.; Pankajakshan, D.; Nör, J.E. Advanced Scaffolds for Dental Pulp and Periodontal Regeneration. Dent. Clin. N. Am. 2017, 61, 689–711. [Google Scholar] [CrossRef]
- Trope, M. Treatment of the Immature Tooth with a Non-Vital Pulp and Apical Periodontitis. Dent. Clin. N. Am. 2010, 54, 313–324. [Google Scholar] [CrossRef]
- Diogenes, A.; Ruparel, N.B. Regenerative Endodontic Procedures: Clinical Outcomes. Dent. Clin. N. Am. 2017, 61, 111–125. [Google Scholar] [CrossRef]
- Galler, K.M. Clinical Procedures for Revitalization: Current Knowledge and Considerations. Int. Endod. J. 2016, 49, 926–936. [Google Scholar] [CrossRef]
- Diogenes, A.; Henry, M.A.; Teixeira, F.B.; Hargreaves, K.M. An Update on Clinical Regenerative Endodontics. Endod. Top. 2013, 28, 2–23. [Google Scholar] [CrossRef]
- Namour, M.; Theys, S. Pulp Revascularization of Immature Permanent Teeth: A Review of the Literature and a Proposal of a New Clinical Protocol. Sci. World J. 2014, 2014, 737503. [Google Scholar] [CrossRef]
- Torabinejad, M.; Nosrat, A.; Verma, P.; Udochukwu, O. Regenerative Endodontic Treatment or Mineral Trioxide Aggregate Apical Plug in Teeth with Necrotic Pulps and Open Apices: A Systematic Review and Meta-Analysis. J. Endod. 2017, 43, 1806–1820. [Google Scholar] [CrossRef]
- Ostby, B.N. The Role of the Blood Clot in Endodontic Therapy. An Experimental Histologic Study. Acta Odontol. Scand. 1961, 19, 324–353. [Google Scholar] [CrossRef]
- Galler, K.M.; Buchalla, W.; Hiller, K.-A.; Federlin, M.; Eidt, A.; Schiefersteiner, M.; Schmalz, G. Influence of Root Canal Disinfectants on Growth Factor Release from Dentin. J. Endod. 2015, 41, 363–368. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Münchow, E.A.; Ferreira Bordini, E.A.; de Oliveira da Rosa, W.L.; Bottino, M.C. Antimicrobial Therapeutics in Regenerative Endodontics: A Scoping Review. J. Endod. 2020, 46, S115–S127. [Google Scholar] [CrossRef]
- Ercan, E.; Dalli, M.; Dülgergil, C.T. In Vitro Assessment of the Effectiveness of Chlorhexidine Gel and Calcium Hydroxide Paste with Chlorhexidine against Enterococcus Faecalis and Candida Albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, e27–e31. [Google Scholar] [CrossRef]
- Portenier, I.; Haapasalo, H.; Rye, A.; Waltimo, T.; Ørstavik, D.; Haapasalo, M. Inactivation of Root Canal Medicaments by Dentine, Hydroxylapatite and Bovine Serum Albumin. Int. Endod. J. 2001, 34, 184–188. [Google Scholar] [CrossRef]
- Dubey, N.; Xu, J.; Zhang, Z.; Nör, J.E.; Bottino, M.C. Comparative Evaluation of the Cytotoxic and Angiogenic Effects of Minocycline and Clindamycin: An In Vitro Study. J. Endod. 2019, 45, 882–889. [Google Scholar] [CrossRef]
- Porter, M.L.A.; Münchow, E.A.; Albuquerque, M.T.P.; Spolnik, K.J.; Hara, A.T.; Bottino, M.C. Effects of Novel 3-Dimensional Antibiotic-Containing Electrospun Scaffolds on Dentin Discoloration. J. Endod. 2016, 42, 106–112. [Google Scholar] [CrossRef]
- Bottino, M.C.; Albuquerque, M.T.P.; Azabi, A.; Münchow, E.A.; Spolnik, K.J.; Nör, J.E.; Edwards, P.C. A Novel Patient-Specific Three-Dimensional Drug Delivery Construct for Regenerative Endodontics. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1576–1586. [Google Scholar] [CrossRef]
- Reynolds, K.; Johnson, J.D.; Cohenca, N. Pulp Revascularization of Necrotic Bilateral Bicuspids Using a Modified Novel Technique to Eliminate Potential Coronal Discolouration: A Case Report. Int. Endod. J. 2009, 42, 84–92. [Google Scholar] [CrossRef]
- Ruparel, N.B.; Teixeira, F.B.; Ferraz, C.C.R.; Diogenes, A. Direct Effect of Intracanal Medicaments on Survival of Stem Cells of the Apical Papilla. J. Endod. 2012, 38, 1372–1375. [Google Scholar] [CrossRef]
- Berkhoff, J.A.; Chen, P.B.; Teixeira, F.B.; Diogenes, A. Evaluation of Triple Antibiotic Paste Removal by Different Irrigation Procedures. J. Endod. 2014, 40, 1172–1177. [Google Scholar] [CrossRef]
- Yassen, G.H.; Eckert, G.J.; Platt, J.A. Effect of Intracanal Medicaments Used in Endodontic Regeneration Procedures on Microhardness and Chemical Structure of Dentin. Restor. Dent. Endod. 2015, 40, 104–112. [Google Scholar] [CrossRef]
- Bottino, M.C.; Kamocki, K.; Yassen, G.H.; Platt, J.A.; Vail, M.M.; Ehrlich, Y.; Spolnik, K.J.; Gregory, R.L. Bioactive Nanofibrous Scaffolds for Regenerative Endodontics. J. Dent. Res. 2013, 92, 963–969. [Google Scholar] [CrossRef]
- Palasuk, J.; Kamocki, K.; Hippenmeyer, L.; Platt, J.A.; Spolnik, K.J.; Gregory, R.L.; Bottino, M.C. Bimix Antimicrobial Scaffolds for Regenerative Endodontics. J. Endod. 2014, 40, 1879–1884. [Google Scholar] [CrossRef]
- Bottino, M.C.; Yassen, G.H.; Platt, J.A.; Labban, N.; Windsor, L.J.; Spolnik, K.J.; Bressiani, A.H.A. A Novel Three-Dimensional Scaffold for Regenerative Endodontics: Materials and Biological Characterizations. J. Tissue Eng. Regen. Med. 2015, 9, E116–E123. [Google Scholar] [CrossRef]
- Kamocki, K.; Nör, J.E.; Bottino, M.C. Dental Pulp Stem Cell Responses to Novel Antibiotic-Containing Scaffolds for Regenerative Endodontics. Int. Endod. J. 2015, 48, 1147–1156. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Albuquerque, M.T.P.; Evans, J.D.; Kamocka, M.M.; Gregory, R.L.; Bottino, M.C. Triple Antibiotic Polymer Nanofibers for Intracanal Drug Delivery: Effects on Dual Species Biofilm and Cell Function. J. Endod. 2016, 42, 1490–1495. [Google Scholar] [CrossRef]
- Moonesi Rad, R.; Atila, D.; Akgün, E.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Evaluation of Human Dental Pulp Stem Cells Behavior on a Novel Nanobiocomposite Scaffold Prepared for Regenerative Endodontics. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 928–948. [Google Scholar] [CrossRef]
- Leite, M.L.; de Oliveira Ribeiro, R.A.; Soares, D.G.; Hebling, J.; de Souza Costa, C.A. Poly(Caprolactone)-Aligned Nanofibers Associated with Fibronectin-Loaded Collagen Hydrogel as a Potent Bioactive Scaffold for Cell-Free Regenerative Endodontics. Int. Endod. J. 2022, 55, 1359–1371. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. The Performance of Dental Pulp Stem Cells on Nanofibrous PCL/Gelatin/nHA Scaffolds. J. Biomed. Mater. Res. A 2010, 93, 247–257. [Google Scholar] [CrossRef]
- Liu, X.; Feng, S.; Wang, X.; Qi, J.; Lei, D.; Li, Y.; Bai, W. Tuning the Mechanical Properties and Degradation Properties of Polydioxanone Isothermal Annealing. Turk. J. Chem. 2020, 44, 1430–1444. [Google Scholar] [CrossRef]
- Panchal, S.S.; Vasava, D.V. Biodegradable Polymeric Materials: Synthetic Approach. ACS Omega 2020, 5, 4370–4379. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Janowski, G.M. A Novel Spatially Designed and Functionally Graded Electrospun Membrane for Periodontal Regeneration. Acta Biomater. 2011, 7, 216–224. [Google Scholar] [CrossRef]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef]
- Qi, R.; Cao, X.; Shen, M.; Guo, R.; Yu, J.; Shi, X. Biocompatibility of Electrospun Halloysite Nanotube-Doped Poly(Lactic-Co-Glycolic Acid) Composite Nanofibers. J. Biomater. Sci. Polym. Ed. 2012, 23, 299–313. [Google Scholar] [CrossRef]
- Dargaville, B.L.; Vaquette, C.; Rasoul, F.; Cooper-White, J.J.; Campbell, J.H.; Whittaker, A.K. Electrospinning and Crosslinking of Low-Molecular-Weight Poly(Trimethylene Carbonate-Co-(L)-Lactide) as an Elastomeric Scaffold for Vascular Engineering. Acta Biomater. 2013, 9, 6885–6897. [Google Scholar] [CrossRef] [PubMed]
- Haroosh, H.J.; Chaudhary, D.; Dong, Y. Electrospun PLA/PCL Fibers with Tubular Nanoclay: Morphological and Structural Analysis. J. Appl. Polym. Sci. 2012, 124, 3930–3939. [Google Scholar] [CrossRef]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an Electrospun Nano-Apatite/PCL Composite Membrane for GTR/GBR Application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef]
- Tungprapa, S.; Jangchud, I.; Supaphol, P. Release Characteristics of Four Model Drugs from Drug-Loaded Electrospun Cellulose Acetate Fiber Mats. Polymer 2007, 48, 5030–5041. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Newehy, M.H.; Wnek, G.E. Controlled Release of Ketoprofen from Electrospun Poly(Vinyl Alcohol) Nanofibers. Mater. Sci. Eng. A 2007, 1–2, 390–396. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Newehy, M.H.; Wnek, G.E. Processing of Polymer Nanofibers through Electrospinning as Drug Delivery Systems. Mater. Chem. Phys. 2009, 113, 296–302. [Google Scholar] [CrossRef]
- Gupte, M.J.; Ma, P.X. Nanofibrous Scaffolds for Dental and Craniofacial Applications. J. Dent. Res. 2012, 91, 227–234. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Macroporous and Nanofibrous Polymer Scaffolds and Polymer/Bone-like Apatite Composite Scaffolds Generated by Sugar Spheres. J. Biomed. Mater. Res. A 2006, 78, 306–315. [Google Scholar] [CrossRef]
- Lei, B.; Shin, K.-H.; Noh, D.-Y.; Jo, I.-H.; Koh, Y.-H.; Kim, H.-E.; Kim, S.E. Sol-Gel Derived Nanoscale Bioactive Glass (NBG) Particles Reinforced Poly(ε-Caprolactone) Composites for Bone Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1102–1108. [Google Scholar] [CrossRef]
- Pourhaghgouy, M.; Zamanian, A. Ice-Templated Scaffolds of Bioglass Nanoparticles Reinforced-Chitosan. In Proceedings of the 2014 21th Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 26–28 November 2014; pp. 48–52. [Google Scholar] [CrossRef]
- Tainio, J.; Paakinaho, K.; Ahola, N.; Hannula, M.; Hyttinen, J.; Kellomäki, M.; Massera, J. In Vitro Degradation of Borosilicate Bioactive Glass and Poly(l-Lactide-Co-ε-Caprolactone) Composite Scaffolds. Materials 2017, 10, 1274. [Google Scholar] [CrossRef] [PubMed]
- Araujo, H.C.; Nakamune, A.C.M.S.; Garcia, W.G.; Pessan, J.P.; Antoniali, C. Carious Lesion Severity Induces Higher Antioxidant System Activity and Consequently Reduces Oxidative Damage in Children’s Saliva. Oxid. Med. Cell. Longev. 2020, 2020, 3695683. [Google Scholar] [CrossRef]
- Mour, M.; Das, D.; Winkler, T.; Hoenig, E.; Mielke, G.; Morlock, M.M.; Schilling, A.F. Advances in Porous Biomaterials for Dental and Orthopaedic Applications. Materials 2010, 3, 2947–2974. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and Hydrolytic Effects in Dental Polymer Networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Breda, S.A.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Olivera, M.E. Solubility Behavior and Biopharmaceutical Classification of Novel High-Solubility Ciprofloxacin and Norfloxacin Pharmaceutical Derivatives. Int. J. Pharm. 2009, 371, 106–113. [Google Scholar] [CrossRef]
- Rediguieri, C.F.; Porta, V.; Nunes, D.S.G.; Nunes, T.M.; Junginger, H.E.; Kopp, S.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; et al. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Metronidazole. J. Pharm. Sci. 2011, 100, 1618–1627. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Ruktanonchai, U.; Supaphol, P. Drug-Loaded Electrospun Mats of Poly(Vinyl Alcohol) Fibres and Their Release Characteristics of Four Model Drugs. Nanotechnology 2006, 17, 2317–2329. [Google Scholar] [CrossRef]
- Sahoo, S.; Ang, L.T.; Goh, J.C.-H.; Toh, S.-L. Growth Factor Delivery through Electrospun Nanofibers in Scaffolds for Tissue Engineering Applications. J. Biomed. Mater. Res. A 2010, 93, 1539–1550. [Google Scholar] [CrossRef]
- Waeiss, R.A.; Negrini, T.C.; Arthur, R.A.; Bottino, M.C. Antimicrobial Effects of Drug-Containing Electrospun Matrices on Osteomyelitis-Associated Pathogens. J. Oral Maxillofac. Surg. 2014, 72, 1310–1319. [Google Scholar] [CrossRef]
- Madhubala, M.M.; Srinivasan, N.; Ahamed, S. Comparative Evaluation of Propolis and Triantibiotic Mixture as an Intracanal Medicament against Enterococcus Faecalis. J. Endod. 2011, 37, 1287–1289. [Google Scholar] [CrossRef]
- Pallotta, R.C.; Ribeiro, M.S.; de Lima Machado, M.E. Determination of the Minimum Inhibitory Concentration of Four Medicaments Used as Intracanal Medication. Aust. Endod. J. 2007, 33, 107–111. [Google Scholar] [CrossRef]
- Chuensombat, S.; Khemaleelakul, S.; Chattipakorn, S.; Srisuwan, T. Cytotoxic Effects and Antibacterial Efficacy of a 3-Antibiotic Combination: An In Vitro Study. J. Endod. 2013, 39, 813–819. [Google Scholar] [CrossRef]
- Chatzkel, J.A.; Vossough, A. Metronidazole-Induced Cerebellar Toxicity. Pediatr. Radiol. 2010, 40, 1453. [Google Scholar] [CrossRef]
- Kuriyama, A.; Jackson, J.L.; Doi, A.; Kamiya, T. Metronidazole-Induced Central Nervous System Toxicity: A Systematic Review. Clin. Neuropharmacol. 2011, 34, 241–247. [Google Scholar] [CrossRef]
- Rizzo, A.; Paolillo, R.; Guida, L.; Annunziata, M.; Bevilacqua, N.; Tufano, M.A. Effect of Metronidazole and Modulation of Cytokine Production on Human Periodontal Ligament Cells. Int. Immunopharmacol. 2010, 10, 744–750. [Google Scholar] [CrossRef]
- Sobolewska, B.; Hofmann, J.; Spitzer, M.S.; Bartz-Schmidt, K.U.; Szurman, P.; Yoeruek, E. Antiproliferative and Cytotoxic Properties of Moxifloxacin on Rat Retinal Ganglion Cells. Curr. Eye Res. 2013, 38, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Tziafas, D.; Kodonas, K. Differentiation Potential of Dental Papilla, Dental Pulp, and Apical Papilla Progenitor Cells. J. Endod. 2010, 36, 781–789. [Google Scholar] [CrossRef]
- Cassidy, N.; Fahey, M.; Prime, S.S.; Smith, A.J. Comparative Analysis of Transforming Growth Factor-Beta Isoforms 1-3 in Human and Rabbit Dentine Matrices. Arch. Oral Biol. 1997, 42, 219–223. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Guo, B.; Liu, M.; Liao, R.; Rabie, A.B.M.; Jia, D. Poly(Vinyl Alcohol)/Halloysite Nanotubes Bionanocomposite Films: Properties and in Vitro Osteoblasts and Fibroblasts Response. J. Biomed. Mater. Res. A 2010, 93, 1574–1587. [Google Scholar] [CrossRef]
- Qi, R.; Shen, M.; Cao, X.; Zhang, L.; Xu, J.; Yu, J.; Shi, X. Electrospun Poly(Lactic-Co-Glycolic Acid)/Halloysite Nanotube Composite Nanofibers for Drug Encapsulation and Sustained Release. J. Mater. Chem. 2010, 20, 10622–10629. [Google Scholar] [CrossRef]
- Kaufman, G.; Whitescarver, R.A.; Nunes, L.; Palmer, X.-L.; Skrtic, D.; Tutak, W. Effects of Protein-Coated Nanofibers on Conformation of Gingival Fibroblast Spheroids: Potential Utility for Connective Tissue Regeneration. Biomed. Mater. 2018, 13, 025006. [Google Scholar] [CrossRef]
- Das, P.; DiVito, M.D.; Wertheim, J.A.; Tan, L.P. Collagen-I and Fibronectin Modified Three-Dimensional Electrospun PLGA Scaffolds for Long-Term In Vitro Maintenance of Functional Hepatocytes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110723. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.L.; Soares, D.G.; Anovazzi, G.; Anselmi, C.; Hebling, J.; de Souza Costa, C.A. Fibronectin-Loaded Collagen/Gelatin Hydrogel Is a Potent Signaling Biomaterial for Dental Pulp Regeneration. J. Endod. 2021, 47, 1110–1117. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kang, M.-S.; Mahapatra, C.; Kim, H.-W. Effect of Aminated Mesoporous Bioactive Glass Nanoparticles on the Differentiation of Dental Pulp Stem Cells. PLoS ONE 2016, 11, e0150727. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, B.N.; Zeitlin, B.D.; Nör, J.E. A Hydrogel Scaffold That Maintains Viability and Supports Differentiation of Dental Pulp Stem Cells. Dent. Mater. 2013, 29, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, I.J.; Martins, M.D.; Katayama, E.; Antoniazzi, J.H.; Segmentilli, A.; Marques, M.M. Collagen Analysis in Human Tooth Germ Papillae. Braz. Dent. J. 2006, 17, 208–212. [Google Scholar] [CrossRef][Green Version]
- Saito, K.; Nakatomi, M.; Ida-Yonemochi, H.; Ohshima, H. Osteopontin Is Essential for Type I Collagen Secretion in Reparative Dentin. J. Dent. Res. 2016, 95, 1034–1041. [Google Scholar] [CrossRef]
- Deshpande, A.S.; Fang, P.-A.; Zhang, X.; Jayaraman, T.; Sfeir, C.; Beniash, E. Primary Structure and Phosphorylation of Dentin Matrix Protein 1 (DMP1) and Dentin Phosphophoryn (DPP) Uniquely Determine Their Role in Biomineralization. Biomacromolecules 2011, 12, 2933–2945. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).