Sustainable Approach to Development of Antimicrobial Textile Pads for Sweat Absorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Tested Textile Fabrics
2.3. Impregnating Liquid Formulations and Preparation
2.4. Determination of Composition of Impregnating Liquid Using GC-MS Qualitative Analysis
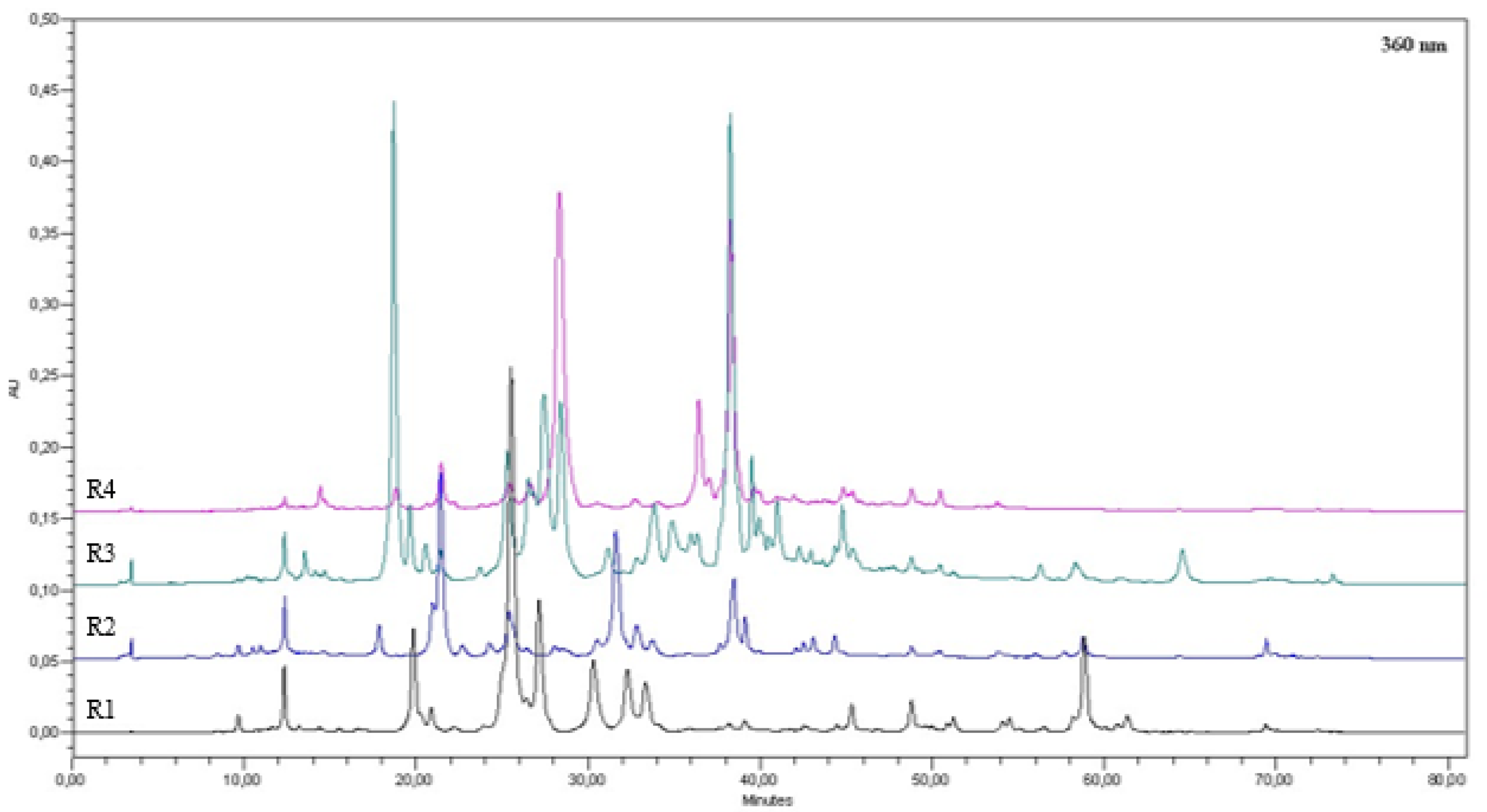
2.5. Determination of Total Amount of Phenolic Compounds in an Impregnating Liquid Using HPLC Analysis
2.6. Antimicrobial Treatment
2.7. Static Water Absorption
2.8. Dynamic Water Absorption
3. Results and Discussion
3.1. Selection of Impregnating Liquid Composition
3.2. Composition of Liquids
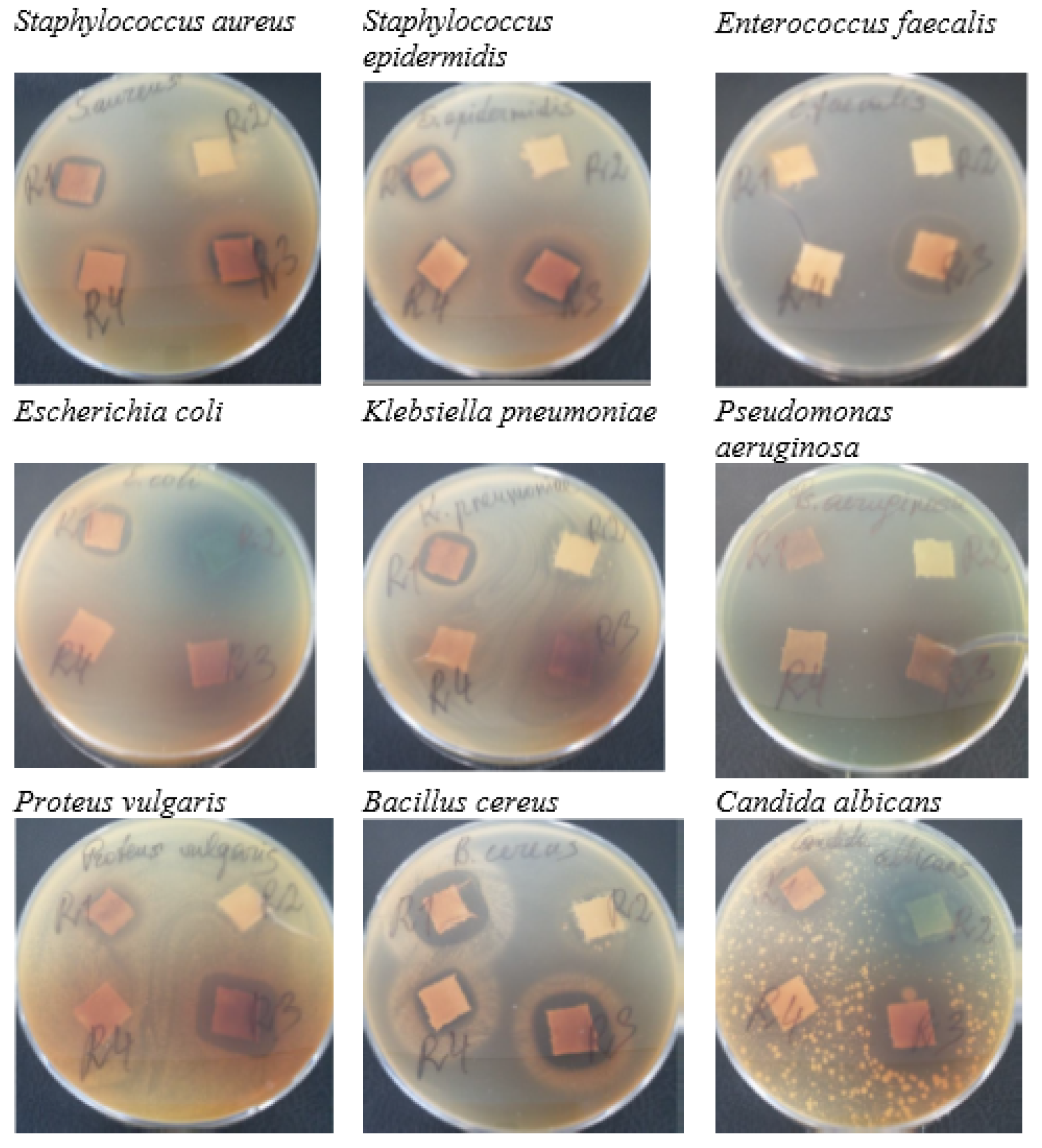
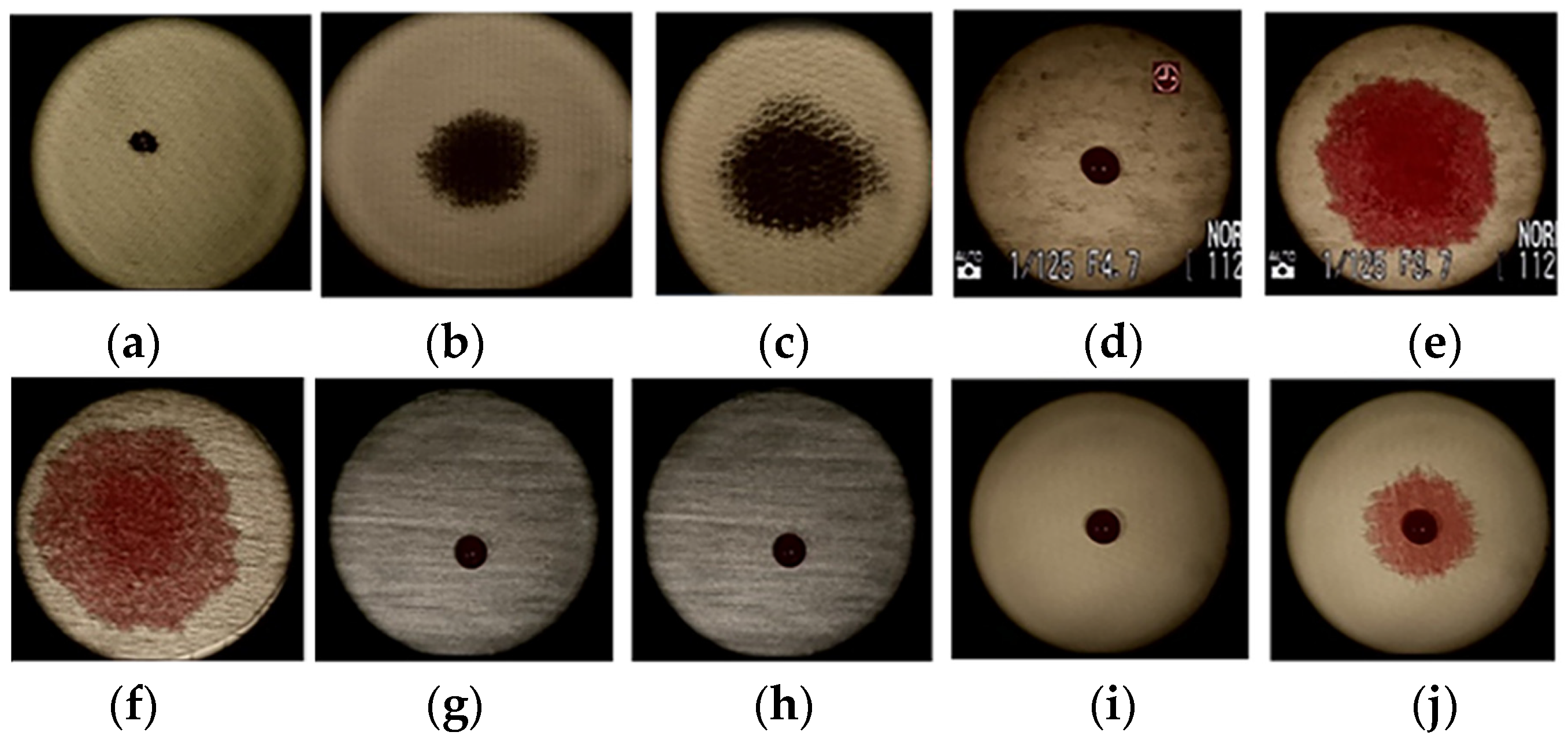
3.3. Antimicrobial Activity
3.4. Static Water Absorption
3.5. Dynamic Water Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansoor, T.; Hes, L.; Bajzik, V.; Noman, M.T. Novel Method on Thermal Resistance Prediction and Thermo-Physiological Comfort of Socks in a Wet State. Text. Res. J. 2020, 90, 1987–2006. [Google Scholar] [CrossRef]
- Raccuglia, M.; Hodder, S.; Havenith, G. Human Wetness Perception in Relation to Textile Water Absorption Parameters under Static Skin Contact. Text. Res. J. 2017, 87, 2449–2463. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, D.; Zheng, R.; Tang, K.P.M.; Fu, B.; Zhang, X.; Ma, P. Moisture and Thermal Transport Properties of Different Polyester Warp-Knitted Spacer Fabric for Protective Application. Autex Res. J. 2021, 21, 182–191. [Google Scholar] [CrossRef]
- Abramavičiute, J.; Mikučioniene, D.; Čiukas, R. Static Water Absorption of Knits from Natural and Textured Yarns. Fibres Text. East. Eur. 2011, 86, 60–63. [Google Scholar]
- Eryuruk, S.H. Analyzing Thermophysiological Comfort and Moisture Management Behavior of Cotton Denim Fabrics. Autex Res. J. 2020, 21, 248–254. [Google Scholar] [CrossRef]
- Bivainyte, A.; Mikučioniene, D. Influence of Shrinkage on Air and Water Vapour Permeability of Double-Layered Weft Knitted Fabrics. Medziagotyra 2012, 18, 271–274. [Google Scholar] [CrossRef]
- Marolleau, A.; Salaun, F.; Dupont, D.; Gidik, H.; Ducept, S. Influence of Textile Properties on Thermal Comfort. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 182007. [Google Scholar] [CrossRef]
- Bivainyte, A.; Mikučioniene, D.; Milašiene, D. Influence of the Knitting Structure of Double-Layered Fabrics on the Heat Transfer Process. Fibres Text. East. Eur. 2012, 91, 40–43. [Google Scholar]
- Yoo, S.; Kim, E. Effects of Multilayer Clothing System Array on Water Vapor Transfer and Condensation in Cold Weather Clothing Ensemble. Text. Res. J. 2008, 78, 189–197. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Borayek, R.; Qu, H.; Nandakumar, D.K.; Zhang, Q.; Ding, J.; Tan, S.C. Super-Hygroscopic Film for Wearables with Dual Functions of Expediting Sweat Evaporation and Energy Harvesting. Nano Energy 2020, 75, 104873. [Google Scholar] [CrossRef]
- Gerrett, N.; Griggs, K.; Redortier, B.; Voelcker, T.; Kondo, N.; Havenith, G. Sweat from Gland to Skin Surface: Production, Transport, and Skin Absorption. J. Appl. Physiol. 2019, 125, 459–469. [Google Scholar] [CrossRef]
- James, A.G.; Austin, C.J.; Cox, D.S.; Taylor, D.; Calvert, R. Microbiological and Biochemical Origins of Human Axillary Odour. FEMS Microbiol. Ecol. 2013, 83, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Fredrich, E.; Barzantny, H.; Brune, I.; Tauch, A. Daily Battle against Body Odor: Towards the Activity of the Axillary Microbiota. Trends Microbiol. 2013, 21, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla, L.). J. Essent. Oil-Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Miraj, S.; Alesaeidi, S. A Systematic Review Study of Therapeutic Effects of Matricaria recuitta Chamomile (Chamomile). Electron. Physician 2016, 8, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- El Joumaa, M.M.; Borjac, J.M. Matricaria Chamomilla: A Valuable Insight into Recent Advances in Medicinal Uses and Pharmacological Activities. Phytochem. Rev. 2022, 21, 1913–1940. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A Herbal Medicine of the Past with a Bright Future (Review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.L.; Cardoso, L.M.L.; Akisue, G.; Pereira, C.A.; Junqueira, J.C.; Jorge, A.O.C.; Santos, P.V., Jr. Adherence of Microorganisms to Sutures after Extraction of Unerupted Third Molars. In Vivo 2011, 19, 476–482. [Google Scholar]
- Efstratiou, E.; Hussain, A.I.; Nigam, P.S.; Moore, J.E.; Ayub, M.A.; Rao, J.R. Antimicrobial Activity of Calendula officinalis Petal Extracts against Fungi, as Well as Gram-Negative and Gram-Positive Clinical Pathogens. Complement. Ther. Clin. Pract. 2012, 18, 173–176. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, J. The Use of Botanical Extracts as Topical Skin-Lightening Agents for the Improvement of Skin Pigmentation Disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef]
- Tober, C.; Schoop, R. Modulation of Neurological Pathways by Salvia officinalis and Its Dependence on Manufacturing Process and Plant Parts Used. BMC Complement. Altern. Med. 2019, 19, 128. [Google Scholar] [CrossRef]
- Flinčec Grgac, S.; Tesla, T.; Čorak, I.; Žuvela Bošnjak, F. Hydrothermal Synthesis of Chitosan and Tea Tree Oil on Plain and Satin Weave Cotton Fabrics. Materials 2022, 15, 5034. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Chen, L.W.; Chen, L.G.; Chang, T.L.; Huang, C.W.; Huang, M.C.; Wang, C.C. Correlations of the Components of Tea Tree Oil with Its Antibacterial Effects and Skin Irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Biswas, N.N.; Saha, S.; Ali, M.K. Antioxidant, Antimicrobial, Cytotoxic and Analgesic Activities of Ethanolic Extract of Mentha arvensis L. Asian Pac. J. Trop. Biomed. 2014, 4, 792–797. [Google Scholar] [CrossRef]
- Grgac, S.F.; Jablan, J.; Inić, S.; Malinar, R.; Kovaček, I.; Čorak, I. The Effect of Ultrasonic Treatment on the Binding of the Inclusion Complex β-Cyclodextrin-Peppermint Oil with Cellulose Material. Materials 2022, 15, 470. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal Plants of the Genus Betula—Traditional Uses and a Phytochemical–Pharmacological Review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.A.; Jin, D.Q.; Hwang, Y.K.; Lee, I.S.; Hwang, J.K.; Ha, I.; Han, J.S. Macelignan Attenuates LPS-Induced Inflammation and Reduces LPS-Induced Spatial Learning Impairments in Rats. Neurosci. Lett. 2008, 448, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Dziedziński, M.; Kobus-cisowska, J.; Szymanowska-powałowska, D. Polyphenols Composition, Antioxidant and Antimicrobial Properties of Pinus sylvestris L. Shoots Extracts Depending on Different Drying Methods. Emir. J. Food Agric. 2020, 32, 229–237. [Google Scholar] [CrossRef]
- Macedo, L.M.; De Mendes, É.; Milit, L.; Tundisi, L.L.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651–663. [Google Scholar] [CrossRef]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef] [PubMed]
- Chahla, B.; Salih, B.M.; Adriana, B.; Viviana, M.; Guido, F.; Sergio, S.; Federica, C.; Rosaria, N.; Marina, P.; Abdelmounaim, K.; et al. Chemical Composition and Biological Activities of Oregano and Lavender Essential Oils. Appl. Sci. 2021, 11, 5688. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Denys, P.; Kowalczyk, E. Antibacterial and Immunostimulatory Effect of Essential Oils. Int. Rev. Allergol. Clin. Immunol. 2011, 17, 40–44. [Google Scholar]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological Activities of Lavender Essential Oil. Phyther. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Kaur, H.; Bhardwaj, U.; Kaur, R. Cymbopogon Nardus Essential Oil: A Comprehensive Review on Its Chemistry and Bioactivity. J. Essent. Oil Res. 2021, 33, 205–220. [Google Scholar] [CrossRef]
- Kenny, G.P.; Journeay, W.S. Human Thermoregulation: Separating Thermal and Nonthermal Effects on Heat Loss. Front. Biosci. 2010, 15, 259–290. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Nishiyasu, T.; Inoue, Y.; Koga, S. Non-Thermal Modification of Heat-Loss Responses during Exercise in Humans. Eur. J. Appl. Physiol. 2010, 110, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Mekjavic, I.B.; Eiken, O. Contribution of Thermal and Nonthermal Factors to the Regulation of Body Temperature in Humans. J. Appl. Physiol. 2006, 100, 2065–2072. [Google Scholar] [CrossRef]
- Shibasaki, M.; Wilson, T.E.; Crandall, C.G. Neural Control and Mechanisms of Eccrine Sweating during Heat Stress and Exercise. J. Appl. Physiol. 2006, 100, 1692–1701. [Google Scholar] [CrossRef]
- Kazlauskaite, J.A.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. The Effect of Traditional and Cyclodextrin-Assisted Extraction Methods on Trifolium pratense L. (Red Clover) Extracts Antioxidant Potential. Antioxidants 2022, 11, 435. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Liaudanskas, M.; Ivanauskas, L.; Ivaskiene, M.; Ramanauskiene, K. Extracts of Poplar Buds (Populus balsamifera L., Populus nigra L.) and Lithuanian Propolis: Comparison of Their Composition and Biological Activities. Plants 2021, 10, 828. [Google Scholar] [CrossRef]
- Kazlauskaite, J.A.; Matulyte, I.; Marksa, M.; Lelesius, R.; Pavilonis, A.; Bernatoniene, J. Application of Antiviral, Antioxidant and Antibacterial Glycyrrhiza glabra L., Trifolium pratense L. Extracts and Myristica fragrans Houtt. Essential Oil in Microcapsules. Pharmaceutics 2023, 15, 464. [Google Scholar] [CrossRef]
- LST EN ISO 139:2005; Textiles—Standard Atmospheres for Conditioning and Testing. ISO: London, UK, 2019.
- ISO 8655-6:2022; The High Art of Pippete Calibration. ISO: London, UK, 2022.
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral, and Cytotoxic Properties—An Overview. Complement. Med. Res. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, S.; Farahpour, M.R. Topical Application of Salvia officinalis Hydroethanolic Leaf Extract Improves Wound Healing Process. Indian J. Exp. Biol. 2017, 55, 98–106. [Google Scholar] [PubMed]
- Abdel-Fattah, A.; Aboelazab, Y.; Khallaf, M.; El-Kenany, Y. Antimicrobial Activity of Ethanolic Extracts of Clove and Thyme. Arab Univ. J. Agric. Sci. 2019, 27, 491–499. [Google Scholar] [CrossRef]
- Erin Chen, Y.; Fischbach, M.A.; Belkaid, Y. Skin Microbiota-Host Interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Older, C.E.; Diesel, A.; Patterson, A.P.; Meason-Smith, C.; Johnson, T.J.; Mansell, J.; Suchodolski, J.S.; Hoffmann, A.R. The Feline Skin Microbiota. PLoS ONE 2017, 12, e0178555. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Gousia, P.; Economou, V.; Sakkas, V.; Petsios, S.; Papadopoulou, C. In Vitro Antimicrobial Activity of Five Essential Oils on Multidrug Resistant Gram-Negative Clinical Isolates. J. Intercult. Ethnopharmacol. 2016, 5, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kunicka-Styczyńska, A.; Sikora, M.; Kalemba, D. Antimicrobial Activity of Lavender, Tea Tree and Lemon Oils in Cosmetic Preservative Systems. J. Appl. Microbiol. 2009, 107, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Duttke, C.; Hild, J.; Müller, H.J. In Vitro Activity of Essential Oils on Microorganisms Isolated from Vaginal Infections. Int. J. Aromather. 2006, 16, 169–174. [Google Scholar] [CrossRef]
- Delgado, A.J.M.; Velázquez, U.C.; González, J.G.B.; Montes, A.C.; Villarreal, S.M.L.; García, L.E.V.; Casas, R.M.S.; Luis, O.E.R. Evaluation of the Essential Oil of Citrus Paradisi; as an Alternative Treatment against Candida albicans. Open J. Stomatol. 2020, 10, 258–270. [Google Scholar] [CrossRef]
- Sepehri, Z.; Javadian, F.; Khammari, D.; Hassanshahian, M. Antifungal Effects of the Aqueous and Ethanolic Leaf Extracts of Echinophora Platyloba and Rosmarinus Officinalis. Curr. Med. Mycol. 2016, 2, 30–35. [Google Scholar] [CrossRef]
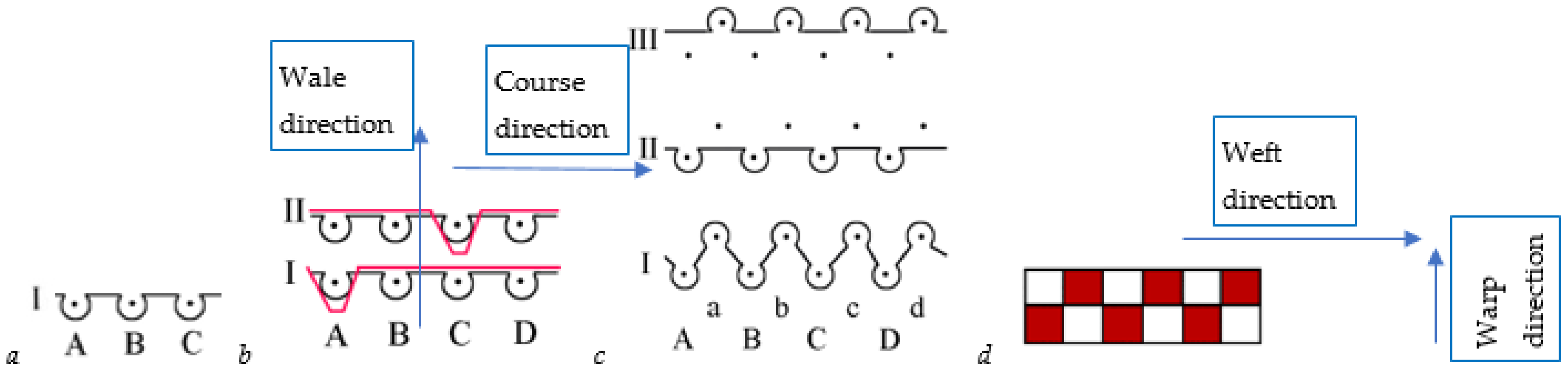
| Knitted Fabrics | ||||||
|---|---|---|---|---|---|---|
| Sample Code | Raw Material | Area Density, g/m2 | Course Density, cm−1 | Wale Density, cm−1 | Pattern | Linear Density of Yarns, Tex |
| 1M | 100% CO | 300 | 22.0 | 15.5 | Fleece | 24 (CO) |
| 2M | 70% MO + 25% CO + 5% EL | 230 | 20.0 | 14.0 | Fleece | 22.5 (MO) + 21 (CO) + 4 (EL) |
| 3M | 95% CO + 5% EL | 330 | 21.0 | 15.0 | Fleece | 20 (CO) + 4 (EL) |
| 4M | 95% CO + 5% EL | 310 | 19.0 | 12.0 | Milano rib | 24 (CO) + 2.2 (EL) |
| 5M | 94% VI + 6% EL | 220 | 21.0 | 15.0 | Single jersey | 16.5 (VI) + 4 (EL) |
| 6M | 100% CO | 190 | 18.0 | 13.0 | Single jersey | 25.5 (CO) |
| Woven Fabrics | ||||||
| Sample Code | Raw Material | Area Density, g/m2 | Weft Density, cm−1 | Warp Density, cm−1 | Pattern | Yarn Linear Density, Tex |
| 1A | 100% PA | 32 | 60 | 37 | Plain weave | 10 (PA) |
| 2A | 100% PES | 44 | 66 | 34 | Plain weave | 12 (PES) |
| Material | Function | Extract Concentration * | Amount (w/w%) |
|---|---|---|---|
| Nutmeg seeds (Myristica fragrans Houtt.) | Extract | 1:20 | 49 |
| Birch leaves (Betula pendula Roth.) | Extract | 1:5 | 49 |
| Tea tree (Melaleuca alternifolia) | Essential oil | - | 1 |
| Polysorbate 80 | Emulsifier | - | 1 |
| Material | Function | Extract Concentration * | Amount (w/w%) |
|---|---|---|---|
| Marigold flower (Calendula officinalis L.) | Extract | 1:5 | 49 |
| Rosemary leaves (Rosmarinus officinalis L.) | Extract | 1:5 | 49 |
| Pine needle (Pinus sylvestris L.) | Essential oil | - | 0.5 |
| Grapefruit peel (Citrus x paradisi L.) | Essential oil | - | 0.5 |
| Polysorbate 80 | Emulsifier | - | 1 |
| Material | Function | Extract Concentration * | Amount (w/w%) |
|---|---|---|---|
| Chamomile flower (Matricaria chamomilla L.) | Extract | 1:5 | 49 |
| Oregano herb (Origanum vulgare L.) | Extract | 1:5 | 49 |
| Lavender flower (Lavandula angustifolia L.) | Essential oil | - | 0.5 |
| Lemongrass (Cymbopogon nardus L.) | Essential oil | 0.5 | |
| Polysorbate 80 | Emulsifier | - | 1 |
| Material | Function | Extract Concentration * | Amount (w/w%) |
|---|---|---|---|
| Sage leaves (Salvia officinalis L.) | Extract | 1:5 | 49 |
| Thyme herb (Thymus vulgaris L.) | Extract | 1:5 | 49 |
| Mentha herb (Mentha arvensis L.) | Essential oil | - | 1 |
| Polysorbate 80 | Emulsifier | - | 1 |
| Formulation | Determined Compounds |
|---|---|
| R1 | 1,1-dicyclopropylethene; 6,6-dimethylspiro [3,4-diazabicyclo [3.1.0]hex-3-ene-2,1′-cyclopropane]; (E)-2,7-dimethyloct-3-en-5-yne; 1,4-methano-1H-cyclopenta[d]pyridazine, 4,4; 6-methyl-6-hepten-2-on; terpinen-4-ol; caryophyllene, humulene; 3,3,6,6,9,9-hexamethyl tetracyclo [6.1.0.02,4.05,7]nonane. |
| R2 | 1,1-dicyclopropylethene; 6,6-dimethylspiro [3,4-diazabicyclo [3.1.0]hex-3-ene-2,1′-cyclopropane]; 1,3,6-octatriene, 1-methyl-4-prop-1-en-2-ylcyclohexene (limonene) |
| R3 | 3-cyclohexylpent-4-en-2-one; 3-cyclohexyl-4-penten-2-one, (3R)-3,7-dimethylocta-1,6-dien-3-ol (linalool); 4-carvomenthenol; longipinenepoxide; linalyl acetate, trans-2-cis-6-nonadienal; [(2E,6Z)-nona-2,6-dienyl] acetate, caryophyllene. |
| R4 | 1-oxacyclopropyl-3,4-epoxycyclohexane; 3-(Allyloxy)-2-methyl-1-propene; tert-dodecylmercaptan; 2-Methylpent-2-en-1-ol; 1-undecyne; cis-1,7-octadien-3-yl acetate; caryophyllene |
| Microorganism | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| Diameter of Non-Growth Zones of Reference Microorganisms (mm) | ||||
| Staphylococcus aureus ATCC 25923 | 15.4 ± 0.4 | 10.4 ± 0.6 | 13.5 ± 0.5 | 11.0 ± 1.0 |
| Staphylococcus epidermidis ATCC 12228 | 17.6 ± 0.5 | 14.0 ± 0.1 | 15.7 ± 1.3 | 14.6 ± 1.1 |
| Enterococcus faecalis ATCC 29212 | 18.3 ± 0.6 | N | 22.0 ± 0.1 | 15.2 ± 0.4 |
| Escherichia coli ATCC 25922 | 16.4 ± 0.3 | N | N | N |
| Klebsiella pneumoniae ATCC 13883 | 17.1 ± 0.6 | 13.0 ± 0.1 | N | N |
| Pseudomonas aeruginosa ATCC 27853 | N | N | 16.1 ± 0.1 | N |
| Proteus vulgaris ATCC 8427 | 10.6 ± 0.5 | 14.1 ± 0.1 | 18.3 ± 0.5 | N |
| Bacillus cereus ATCC 11778 | 21.7 ± 0.3 | 12.7 ± 0.4 | 18.2 ± 0.1 | 14.1 ± 0.2 |
| Candida albicans ATCC 10231 | 13.4 ± 0.6 | 18.0 ± 0.1 | N | N |
| Sample Code | Mass of Dry Sample, md, g | Mass of Wet Sample, mw, g | Static Water Absorption, Sw, % |
|---|---|---|---|
| M1 | 2.01 ± 0.08 | 7.57 ± 0.15 | 276.6 |
| M2 | 1.93 ± 0.10 | 6.24 ± 0.11 | 223.3 |
| M3 | 2.41 ± 0.06 | 8.85 ± 0.12 | 267.2 |
| M4 | 2.11 ± 0.04 | 6.52 ± 0.08 | 209.0 |
| M5 | 1.90 ± 0.03 | 3.99 ± 0.10 | 110.0 |
| M6 | 1.82 ± 0.04 | 4.64 ± 0.12 | 154.9 |
| A1 | 0.27 ± 0.02 | 0.27 ± 0.01 | 0.00 |
| A2 | 0.26 ± 0.01 | 0.37 ± 0.01 | 42.31 |
| Sample Code | Liquid Spot Area, mm2 | ||||
|---|---|---|---|---|---|
| 1 s | 5 s | 30 s | 60 s | 180 s | |
| M1 | 20.8 ± 0.42 | 40.4 ± 0.47 | 61.6 ± 1.85 | 72.9 ± 2.32 | 74.0 ± 2.65 |
| M2 | 22.8 ± 0.51 | 39.6 ± 0.35 | 52.3 ± 1.79 | 68.6 ± 2.25 | 70.2 ± 2.42 |
| M3 | 24.2 ± 0.62 | 39.9 ± 0.38 | 59.1 ± 1.62 | 70.9 ± 2.42 | 72.7 ± 2.59 |
| M4 | 20.5 ± 0.39 | 34.2 ± 0.46 | 50.0 ± 1.63 | 62.6 ± 2.39 | 69.2 ± 2.40 |
| M5 | 28.0 ± 0.25 | 57.9 ± 0.86 | 98.1 ± 1.92 | 119.8 ± 2.59 | 120.2 ± 2.72 |
| M6 | 35.9 ± 0.34 | 65.2 ± 0.71 | 119.8 ± 1.64 | 130.6 ± 2.81 | 132.0 ± 2.12 |
| A1 | N | N | N | N | N |
| A2 | 21.4 ± 0.12 | 21.4 ± 0.18 | 22.9 ± 0.15 | 23.1 ± 0.06 S | 23.2 ± 0.04 S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikucioniene, D.; Kazlauskaite, J.A.; Matulyte, I.; Petkuviene, B.; Laureckiene, G.; Marksa, M.; Bernatoniene, J. Sustainable Approach to Development of Antimicrobial Textile Pads for Sweat Absorption. Fibers 2024, 12, 20. https://doi.org/10.3390/fib12030020
Mikucioniene D, Kazlauskaite JA, Matulyte I, Petkuviene B, Laureckiene G, Marksa M, Bernatoniene J. Sustainable Approach to Development of Antimicrobial Textile Pads for Sweat Absorption. Fibers. 2024; 12(3):20. https://doi.org/10.3390/fib12030020
Chicago/Turabian StyleMikucioniene, Daiva, Jurga Andreja Kazlauskaite, Inga Matulyte, Brigita Petkuviene, Ginta Laureckiene, Mindaugas Marksa, and Jurga Bernatoniene. 2024. "Sustainable Approach to Development of Antimicrobial Textile Pads for Sweat Absorption" Fibers 12, no. 3: 20. https://doi.org/10.3390/fib12030020
APA StyleMikucioniene, D., Kazlauskaite, J. A., Matulyte, I., Petkuviene, B., Laureckiene, G., Marksa, M., & Bernatoniene, J. (2024). Sustainable Approach to Development of Antimicrobial Textile Pads for Sweat Absorption. Fibers, 12(3), 20. https://doi.org/10.3390/fib12030020










