Abstract
This study successfully synthesized functionalized silver nanoparticle/TEMPO-oxidized cellulose nanofiber/chitosan (AgNP/TOCN/CS) composite fibers. First, the TOCN/CS composite fibers were prepared through the wet-spinning technique, yielding Ag/TOCN/CS composite fibers after immersion in a 5 mM AgNO3 aqueous solution for 3 h, followed by washing with 100 mL of deionized water five times. Second, upon heat treatment without adding other reducing agents, TOCN reduced the Ag+ in the Ag/TOCN/CS composite fibers to AgNP/TOCN/CS composite fibers on the surface of the CS fibers. The fiber color changed from white to yellow-orange when the temperature changed from 100 to 170 °C. In addition, the results suggest that the heat treatment at 130 °C for 20 min was the optimal heat treatment condition. Meanwhile, soaking the fibers in 50 mM ascorbic acid for 1 min is the best condition for ascorbic acid reduction. The antibacterial test results showed that the AgNP/TOCN/CS composite fibers formed via ascorbic acid reduction exhibited better antibacterial activity against both Escherichia coli and Bacillus subtilis than those produced via heat treatment. In summary, AgNPs formed on the fiber surface of AgNP/TOCN/CS composite fibers and showed antibacterial activity, confirming the successful addition of antibacterial properties to TOCN/CS composite fibers.
1. Introduction
Chitosan (CS) is a biopolymer obtained through the deacetylation of chitin. Although CS has antimicrobial activity, biocompatibility, biodegradability, and nontoxicity [1,2,3], it still has several limitations. For example, it has weaker antimicrobial activity against Escherichia coli (E. coli) than Staphylococcus aureus (S. aureus) [4]. Several methods involving adding antimicrobial functional groups to CS, such as silver nanoparticles (AgNPs), have been reported to improve its antimicrobial activity [5,6,7,8,9,10,11,12]. For instance, the antibacterial activity of hydrolyzed chitosan-silver nanoparticles/polyvinylpyrrolidone/polyvinyl alcohol nanofibers (HCS-AgNPs/PVP/PVA) against E. coli was increased from 74.4 to 99.9% compared with the HCS/PVP/PVA mats without AgNPs [6].
Recently, TEMPO-oxidized cellulose (TOC), which is prepared from cellulose using 2,2,6,6-tetramethylpiperidine-1-oxy radical (TEMPO)-mediated oxidation, has become an attractive material, particularly in the field of antimicrobial therapy. They can be synthesized using several methods and different chemicals [13,14,15,16,17,18,19]. TEMPO-oxidized cellulose nanofiber (TOCN) synthesized using Saito’s method is among the most popular [16,17,18,19]. Moreover, adding TOCN enhanced the mechanical and barrier properties of other polymers [20,21]. To promote the antimicrobial activity of TOC, silver is incorporated into TOC via chemisorption from an aqueous silver nitrate solution, followed by the reduction of silver salt on the surface of the TOC [13,14,15,22,23,24].
The use of TOCN remains limited because of their high price. Wet spinning is a suitable method for producing TOCN-coated fibers on a one-step process due to the electrostatic attraction between CS and TOCN. Using the wet spinning technique only requires a small amount of TOCN; hence, TOCN can be efficiently utilized. Recently, the consumption of bluefin tuna has dramatically increased. Hence, production of bluefin tuna using fully closed-cycle farming methods has been developed. Contaminants such as proteins, phytic acid, and ammonia in the water during culture affect the growth and survival rates of fish. Therefore, our group tried to solve this problem by preparing CS fibers coated with oxidized cellulose nanofibers (TOCN/CS fiber). TOCN/CS fibers have been shown to adsorb ammonia, protein, and phytic acid contamination from water [25]. Woven TOCN/CS fibers can be used as a filtration membrane to purify contaminated water from closed-cycle fish farming. Additionally, it would be useful if the filtration membrane had antimicrobial activity. Darder et al. found that the biohybrid polymer-coated copper-cystine high-aspect and TOC foam can protect E. coli and Staphylococcus epidermidis [26]. El-Sakhaway also found that TOCN/ZnO nanocomposites showed strong antibacterial activity against E. coli and Staphylococcus aureus [27]. In this study, we focused on adding antimicrobial functional groups to TOCN/CS composite fibers. TOCN/CS fibers were first prepared using a wet-spinning machine, then soaked in a silver nitrate solution, followed by reduction. The antimicrobial properties of the prepared AgNP/TOCN/CS composite fibers were then investigated using various assays.
2. Materials and Methods
2.1. Materials
Chitosan (CS; FM-80, DAC 88.3, Lot No. 0923-25) was supplied from Koyo Chemical Co., Ltd., Osaka, Japan TEMPO-oxidized cellulose nanofiber (TOCN; Reocrysta I-2SX; solid content, 2.3%; pH 7.1, viscosity, 64,000 mPa·s; Lot No. 65T443) was obtained from Dai-ichi Kogyo Seiyaku Co., Ltd. (DKS Co., Ltd., Tokyo, Japan). Silver nitrate (AgNO3), sodium hydroxide (NaOH), acetic acid, and ascorbic acid were purchased from Wako Pure Chemical Industries Ltd., Osaka, Japan. Luria–Bertani (LB) agar and LB broth were purchased from Becton, Dickinson and Company, New York, NY, USA. All chemicals were used without any treatment.
2.2. Preparation of TOCN/CS Composite Fibers
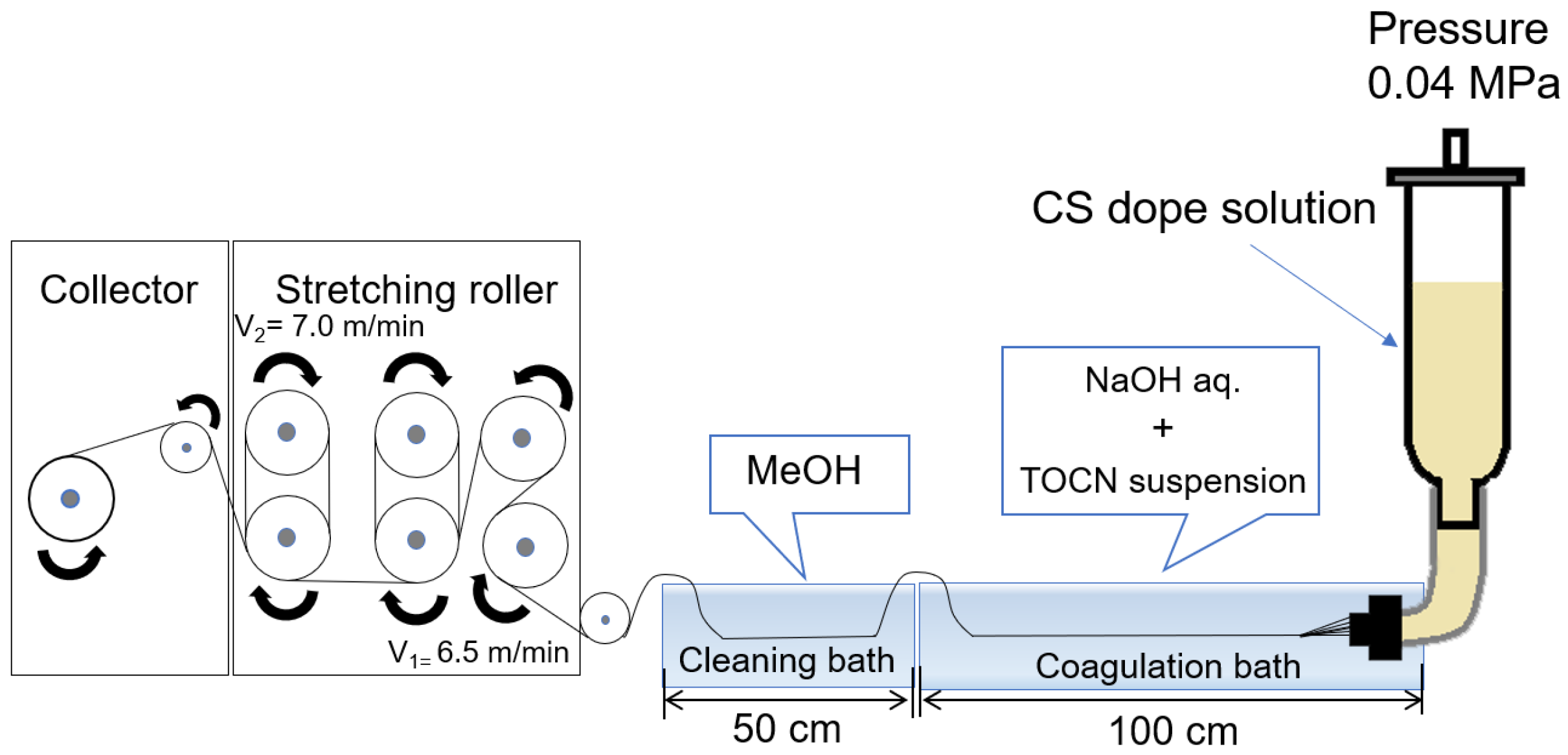
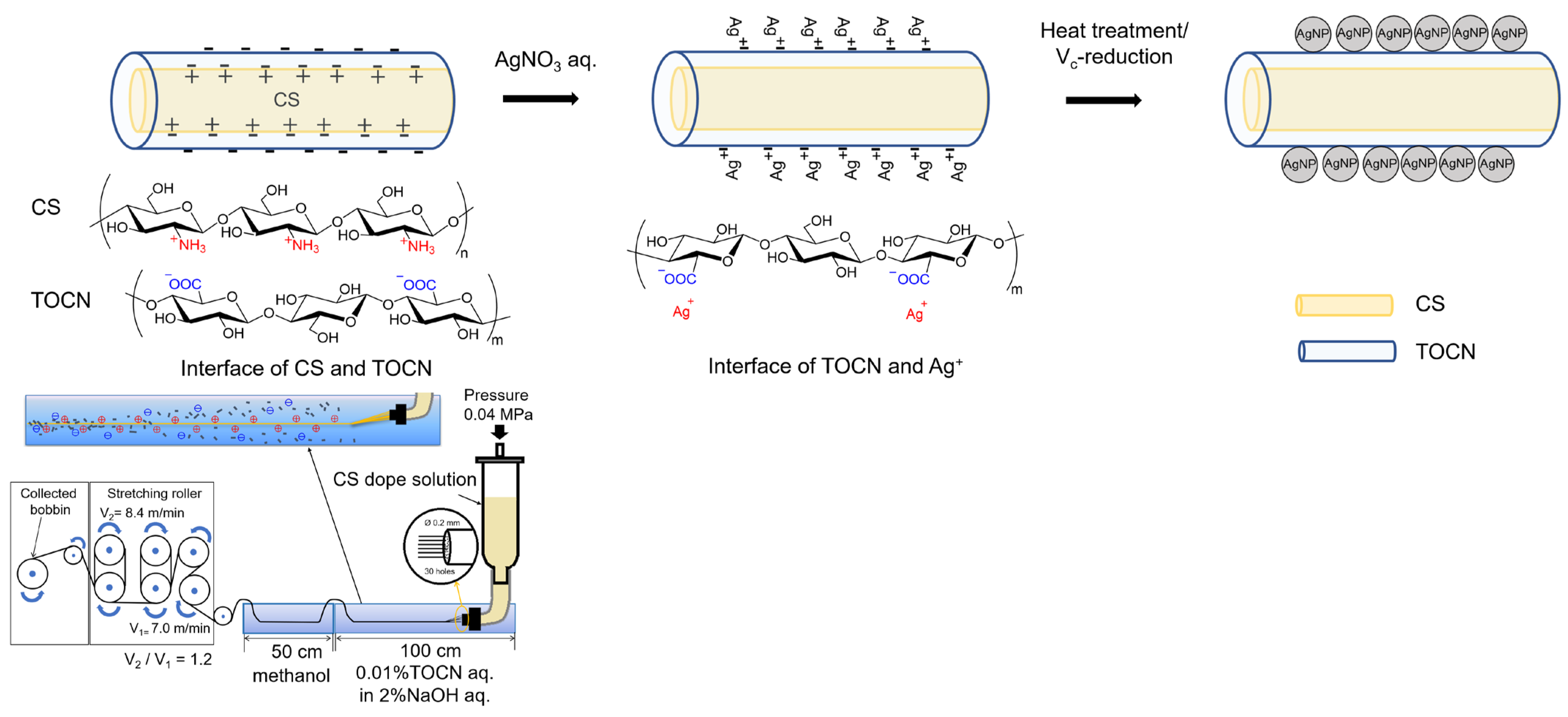
TOCN/CS composite fibers were prepared according to the method described by [25]. CS (10 g) was added to 240 g of a 2% w/v acetic acid solution to prepare a 4% w/w CS solution. The solution was stirred using a Hyper Stirrer System DxII (AS ONE Corporation, Tokyo, Japan) at a stirring speed of 200 rpm until the solution became homogenous. The solution was then filtered through a cotton cloth, packed in a column, and allowed to stand for 1 d. The defoamed solution was then used as the dope solution. Figure 1 shows a schematic of the wet-spinning equipment. Spinning was performed using a coagulation solution (2% w/w NaOH and 0.1% w/w TOCN) in the first bath and methanol as a cleaning agent in the second bath. After spinning, the fibers were washed with fresh methanol every 24 h for 5 d until the pH reached 7. Finally, the fibers were air-dried for 1 d to obtain TOCN/CS composite fibers.
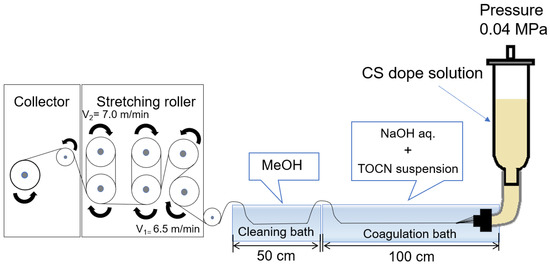
Figure 1.
Schematic diagram of the wet-spinning equipment used in this study.
2.3. Preparation of Ag/TOCN/CS Composite Fibers
2.3.1. Optimizing the Number of the Fiber Washing Rounds Using DI Water
Here, 16.98 mg of AgNO3 was dissolved in 10 mL of deionized (DI) water to prepare a 10 mM AgNO3 aqueous solution. Next, 10 mg of the obtained TOCN/CS composite fibers was soaked in an AgNO3 aqueous solution for 6 h. The Ag/TOCN/CS composite fibers were then washed with 100 mL of DI water under stirring for 24 h. The DI water was replaced ten times. The Ag+ content in each washing solution was examined using inductively coupled plasma atomic emission spectroscopy (ICP-AES; ICPS-7510, Shimadzu, Kyoto, Japan) at a wavelength of 328.068 nm.
2.3.2. Optimizing the Initial Concentration of the AgNO3 Aqueous Solution
Briefly, 10 mg of TOCN/CS composite fibers was soaked for 6 h in 10 mL of an AgNO3 aqueous solution with concentrations ranging from 1 to 10 mM. Each sample was then washed with 100 mL of DI water with stirring for 24 h. The washing process was repeated four times. The samples were then left to dry at room temperature in the dark. Each fiber sample was then placed in 5.0 mL of concentrated nitric acid and evaporated at 300 °C. The organic matter was entirely oxidized, and the remaining solid was dissolved in 25 mL of DI water before detecting the Ag content in the sample via ICP-AES.
2.3.3. Optimizing the Soaking Time of TOCN/CS Composite Fibers in the AgNO3 Aqueous Solution
Here, 10 mg of TOCN/CS composite fibers was soaked in 10 mL of an AgNO3 aqueous solution, with concentrations in the 5 mM range, for 1–6 h. Second, each sample was washed five times by stirring with 100 mL of DI water for 24 h. The samples were then left to dry at room temperature in the dark. The Ag+ content in each fiber sample was determined using ICP-AES.
2.4. Preparation of AgNP/TOCN/CS Composite Fibers
The obtained Ag/TOCN/CS composite fibers were converted into AgNP/TOCN/CS composite fibers via either heat treatment or ascorbic acid reduction. The optimal heat treatment temperature was first determined by heating the samples in an oven at temperatures ranging from 100 to 170 °C for 20 min. Second, the heat treatment period was optimized by performing heat treatment at 130 °C for 10–30 min. Next, ascorbic acid reduction was performed by immersing 10 mg of the AgNP/TOCN/CS composite fibers in an ascorbic acid solution at concentrations ranging from 1 to 100 mM for 1 min. The fibers were then washed with DI water and dried at 25 °C for 24 h.
2.5. Characterization of the AgNP/TOCN/CS Composite Fibers
The morphologies of the composites were observed via field-emission scanning electron microscopy (FE-SEM; JSM-6700F, JEOL, Tokyo, Japan). Before the measurements, the fibers were vacuum-dried for 24 h and coated with platinum. The presence of AgNPs on the fiber surface was confirmed using FE-SEM (S-4800, Hitachi High-Tech Corporation, Tokyo, Japan).
Fourier-transform infrared (FT-IR) spectroscopy was performed on a JASCO FTIR-4200 instrument (Tokyo, Japan) using the KBr method to qualitatively analyze the laminated membranes. The swollen membranes were freeze-dried before testing. A measurement range of 4000–400 cm−1, an accumulation count of 32, and a resolution of 2.0 cm−1 were applied in all measurements.
Thermogravimetric analysis (TGA) was performed using an EXSTAR6000 instrument (Hitachi High-Tech Corporation, Tokyo, Japan) to analyze the degradation of the fibers. The nitrogen flow rate was 200 mL min−1, the heating rate was 20 °C min−1, and the measurement temperature range was 30–900 °C in all analyses.
Elemental analysis of each composite fiber was performed using X-ray photoelectron spectroscopy (XPS; ESCA-3400, Shimadzu, Kyoto, Japan). Before the measurements, the fibers were vacuum-dried for 24 h. The measurement conditions were as follows: a radioactive source of MgKα X-rays (HV = 1253.6 eV, FWHM = 0.85 eV), an acceleration voltage of 10 kV, an emission current of 15 mA, an accumulation number of Ag 3d and C 1s of 128 times, and operation for eight times without etching.
2.6. Swelling and Weight Loss Measurements of the AgNP/TOCN/CS Composite Fibers
A swelling test was conducted to examine the water resistance of each composite fiber. Briefly, 0.02 g of the composite fibers was vacuum-dried at 35 °C overnight and weighed; the obtained weight was later considered as the dry weight. The fibers were then soaked in 20 mL of DI water for 24 h and then weighed; the obtained weight was later considered as the swelling weight. The swelling percentage (%) was calculated using Equation (1) as follows:
where Ws and Wd are the swelling weight (g) and dry weight (g) of the fibers, respectively.
Swelling percentage (%) = ((Ws − Wd)/Wd) × 100,
After the swelling test, the fibers were vacuum-dried again at 35 °C overnight, and their weights were then measured and considered as the dry weight after swelling. The remaining weight (%) after the swelling test was calculated using Equation (2) as follows:
where Wd1 and Wd2 indicate the dry weights (g) of the fiber before and after the swelling test, respectively.
Remaining weight (%) = (Wd2/Wd1) × 100,
2.7. Tensile Test
The tensile properties of each sample were determined using a universal testing machine (STA-1150; A&D Co., Ltd., Tokyo, Japan), and the diameter of the fiber was measured using an optical microscope. A fiber sample with a length of 10 mm was attached to the tensile test paper using an adhesive. The tensile test was performed at room temperature, with a humidity of 20–35% and a tensile rate of 10 mm/min. The reported values are the averages of 30 samples.
2.8. Antibacterial Test of the AgNP/TOCN/CS Composite Fibers
The disc diffusion method was used to analyze the antibacterial activity of the CS, TOCN/CS composite, heat-treated, and Vc-reduced fibers compared with a blank (no fiber) according to the JIS L 1920 guidelines [28]. E. coli, a representative Gram-negative bacterium, and B. subtilis, a representative Gram-positive bacterium, were used to determine the antibacterial performance of each sample. Firstly, E. coli or B. subtilis was added to an LB broth solution and cultured overnight at 37 °C with a shaking speed of 180 rpm. Then, the bacterial suspension was diluted to 1.0 × 106 CFU/mL. LB agar (0.8 g) was added to 20 mL of DI water and sterilized in an autoclave at 121 °C for 15 min. The agar medium was then transferred to a sterile petri dish. After solidification, 0.1 mL of the bacterial suspension was spread on the agar surface and incubated overnight at 37 °C. The zone of inhibition for each sample was recorded using a digital camera and analyzed. The average value and the standard deviation were calculated from triplicate tests and reported.
3. Results and Discussion
3.1. Effects of Washing and the Initial Concentration of the AgNO3 Aqueous Solution
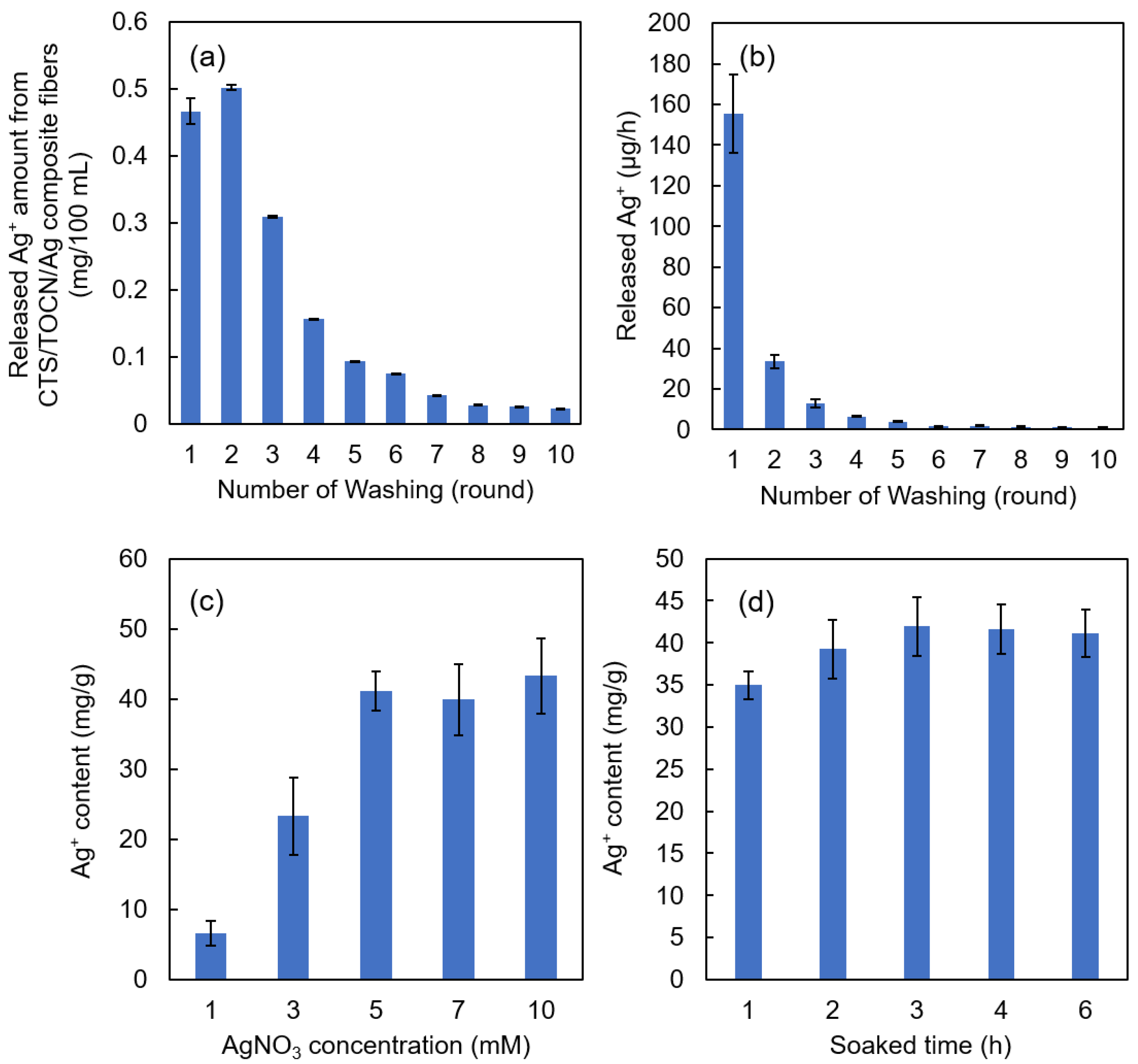
The amount and release rate of Ag+ from the Ag/TOCN/CS composite fibers versus the number of rounds of washing are shown in Figure 2a,b, respectively. Figure 2a shows that the released amount of Ag+ decreased after the second round of washing. As shown in Figure 2b, the Ag+ release rates from the fibers were significantly reduced after the second round of washing, from 155.4 µg·h−1 to 33.4 µg·h−1. The Ag+ release rate then gradually decreased until it reached a constant value of 0.9 µg·h−1 after ten rounds of washing. After six rounds of washing, the release rate tended to be constant and close to zero, indicating that the released amount of Ag+ from the TOCN fibers at that point was already very small.
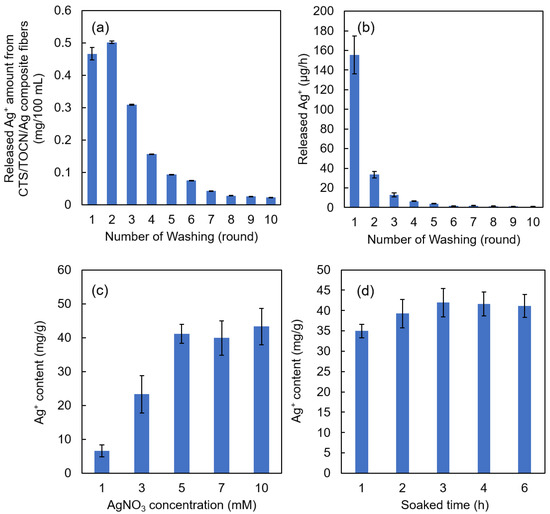
Figure 2.
(a) The amount of Ag+ released from the Ag/TOCN/CS composite fibers in relation to the number of washes. (b) The release rate of Ag+ from the Ag/TOCN/CS composite fibers in relation to the number of washes. (c) The relationship between the Ag+ content in the Ag/TOCN/CS composite fibers and the initial concentration of the AgNO3 used for soaking. (d) The relationship between the Ag+ content in the Ag/TOCN/CS composite fibers and the soaking time.
Figure 2c shows the relationship between the Ag+ content in the Ag/TOCN/CS composite fibers and the initial concentration of AgNO3 used in the soaking method. Figure 2d shows the relationship between the Ag+ content in the Ag/TOCN/CS composite fibers and the soaking time in the AgNO3 solution. These results show that the amount of Ag+ increased from 6.57 to 43.32 mg·per gram of fiber with the AgNO3 concentration, but no significant change was observed beyond 5 mM AgNO3. This means that Ag+ reached the fiber adsorption limit at a concentration of 5 mM AgNO3. In addition, no significant changes were observed after 3 h, implying that Ag+ had reached its adsorption limit at that time point. These results suggest that immersing the TOCN/CS composite fibers in a 5 mM AgNO3 aqueous solution for 3 h and washing with DI water five times are the optimum preparation conditions.
3.2. The Appearance of the Ag/TOCN/CS Composite Fibers
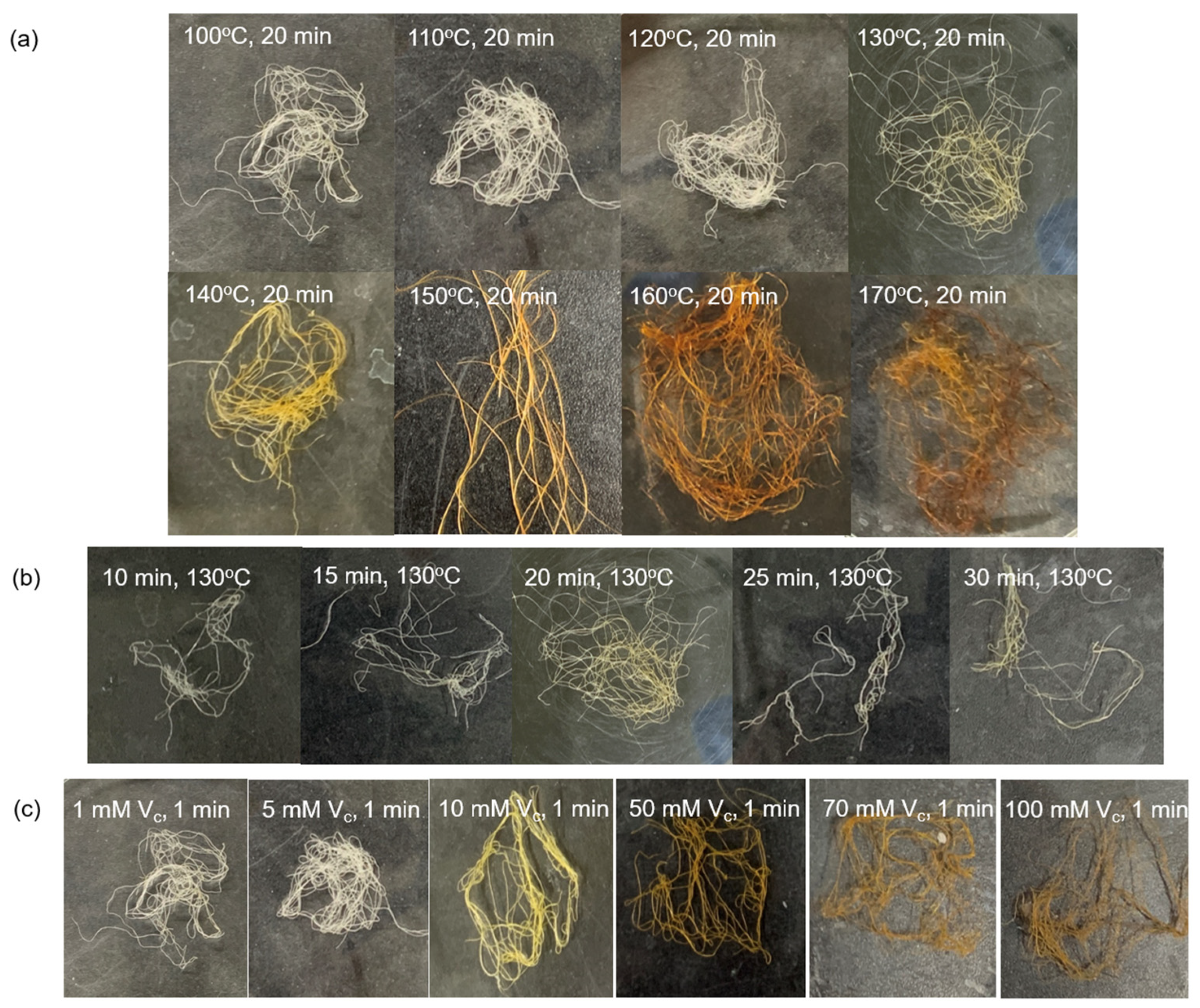
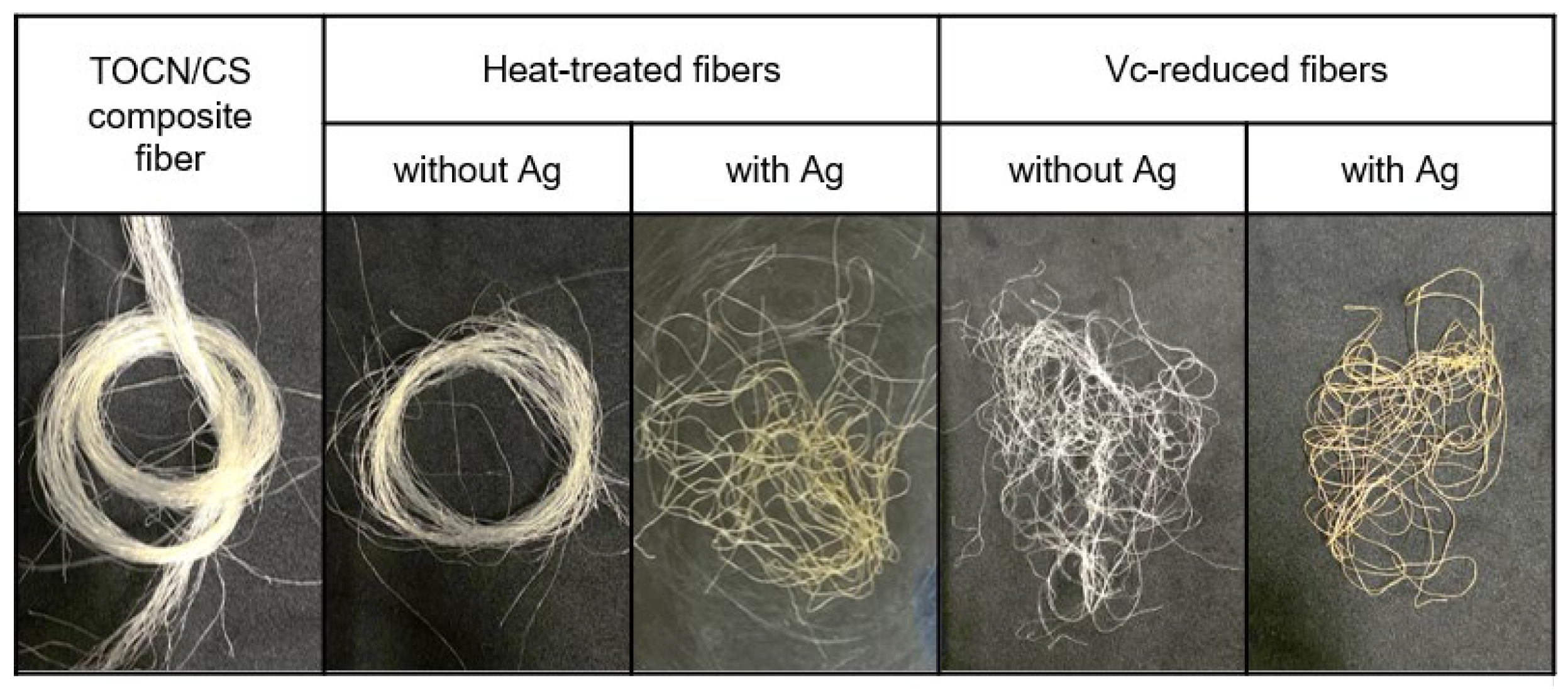
Figure 3 shows the color change of each Ag/TOCN/CS composite fiber after heat treatment. The results showed that the color of the fibers changed from off-white to ivory when the heating temperature changed from 100 to 120 °C. Meanwhile, the fiber color changed from off-white to yellow-orange when the heating temperature changed from 100 to 130–170 °C. When AgNPs are formed on the fiber surface, they turn yellow, owing to surface plasmon resonance. The optical properties of the composite material were induced by the interaction of the conduction electrons of the material with incident light. The uniform color distribution on the surface of the fiber indicates the uniform formation of nanoparticles on a macroscopic scale [29]. In addition, the color of the fiber changed to ivory at 10 and 15 min and yellow at 20–30 min of heat treatment. Moreover, there was a slight change in the yellow color of the fiber after treatment with low concentrations of ascorbic acid (1 and 5 mM); the fiber color changed to yellow after treatment with 10 mM ascorbic acid. After treatment with 50–100 mM ascorbic acid, the fiber color changed to orange. For the heat-treated fibers, the fiber color changed from off-white to yellow and orange because of the presence of AgNPs and the degradation of cellulose from heat treatment in air. In contrast, for ascorbic-acid-reduced fibers, the color change came from the AgNPs, not from ascorbic acid reduction (Figure 4).

Figure 3.
Appearance and color changes of the AgNP/TOCN/CS composite fibers obtained from (a) heat treatment at different temperatures, (b) heat treatment at different durations, and (c) reduction at different ascorbic acid concentrations.
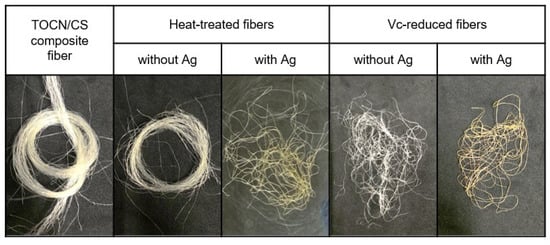
Figure 4.
The color change observed in TOCN/CS composite fibers after treatment with and without Ag.
3.3. Characterization of the Ag/TOCN/CS Composite Fibers
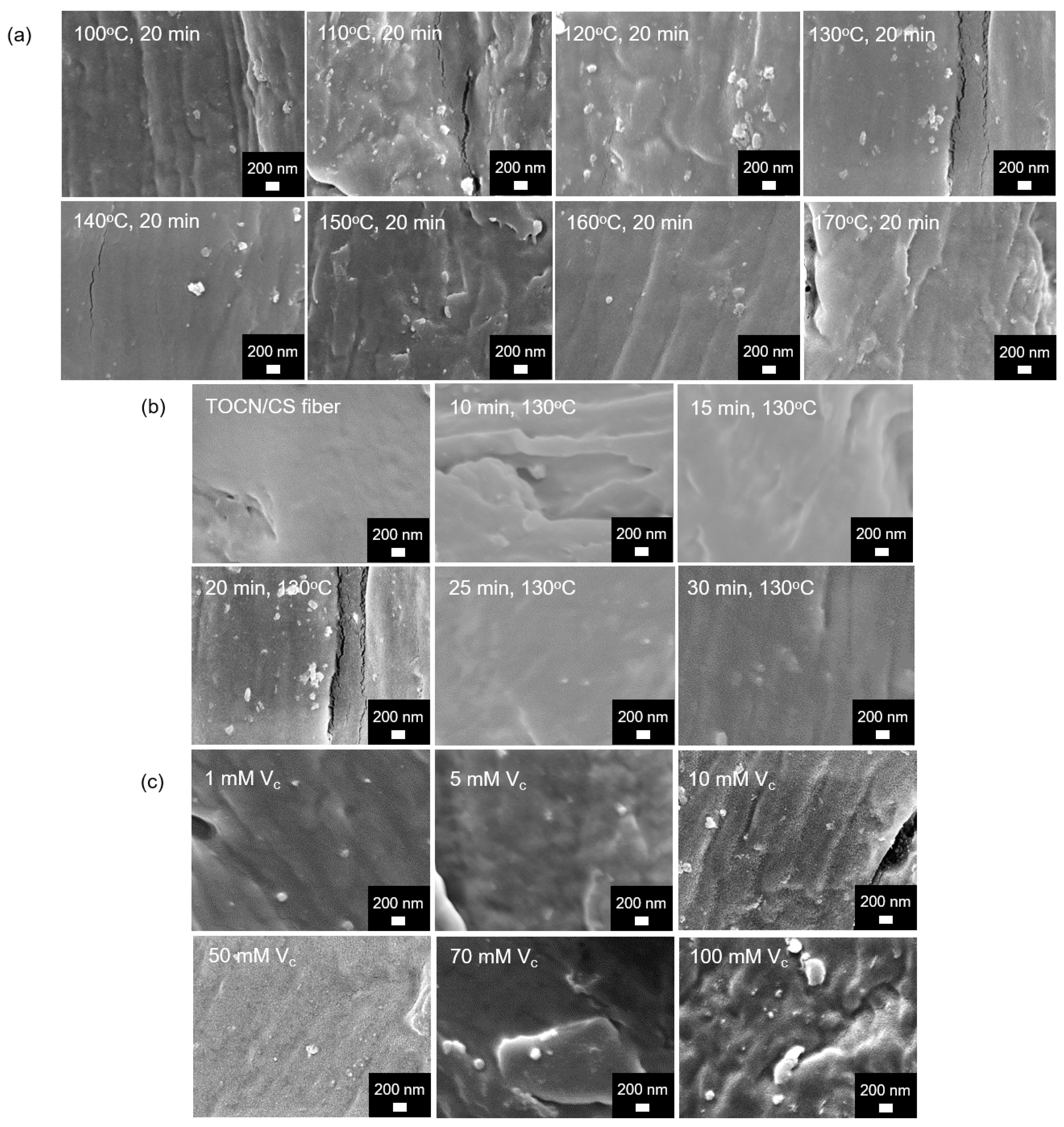
SEM images of the Ag/TOCN/CS composite fibers are shown in Figure 5. Small particles were observed on the fiber surfaces of all the samples except for the TOCN/CS fibers; these tiny particles were expected to be AgNPs. The presence of AgNPs on the fiber was confirmed via scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM-EDS).
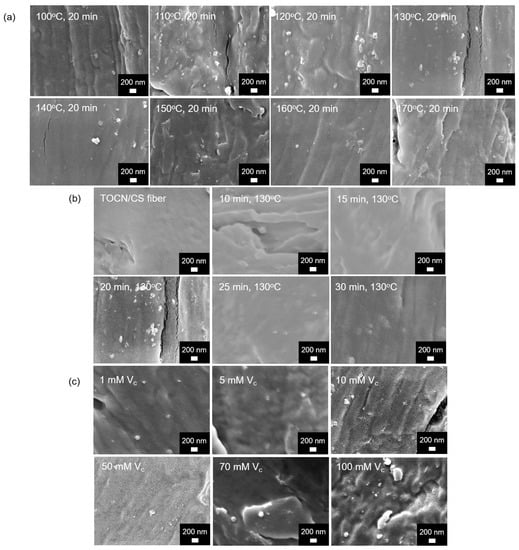
Figure 5.
FE-SEM micrographs of the AgNP/TOCN/CS composite fibers obtained from (a) heat treatment at different temperatures, (b) heat treatment at different durations, and (c) reduction at different ascorbic acid concentrations.
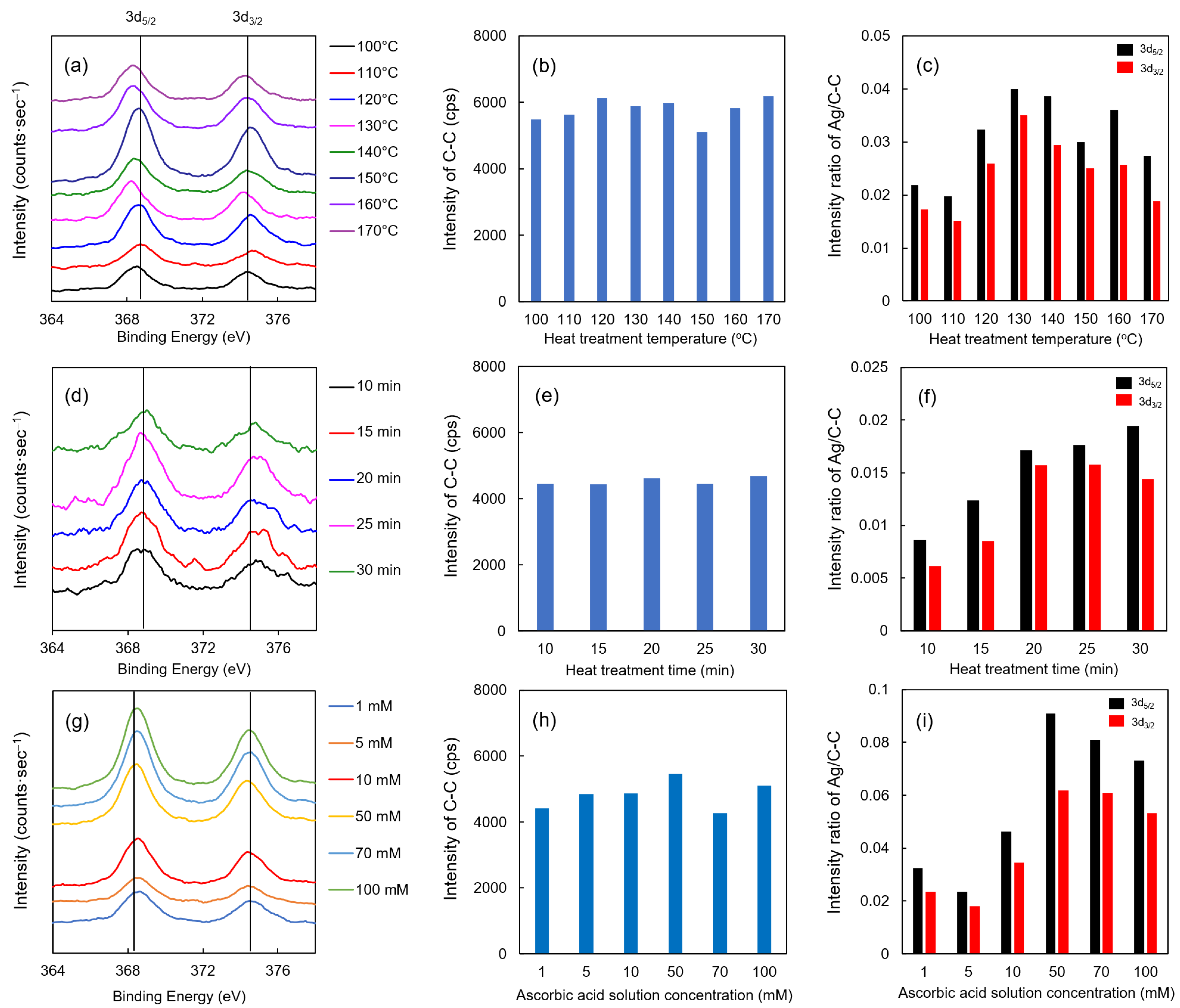
The XPS results of each Ag/TOCN/CS composite fiber are shown in Figure 6. Upon evaluating the effect of heat treatment temperature on the fibers, the binding energies of the Ag 3d5/2 and 3d3/2 orbital peaks were observed at all temperatures (Figure 6a). These binding energies had the same peaks as a standard Ag particle, implying that Ag was present on the fiber surface. However, no significant changes were observed between the different heat treatment temperatures. The intensity of carbon (single bond) can be stably detected at the time of measurement (Figure 6b,e,h); therefore, the intensity ratio of Ag to C–C was also demonstrated in this study. As shown in Figure 6c, the intensity ratio of Ag to C–C was the highest at 130 °C then gradually decreased beyond that temperature. This result suggests that the optimum temperature for heat reduction is 130 °C, where the binding energy is closest to the standard substance Ag. When the heat treatment was extended, higher intensities of the Ag 3d5/2 and 3d3/2 orbital peaks were observed (Figure 6d,f). However, no significant change was observed when heat treatment was applied for more than 20 min. Moreover, no relationship between the concentration and binding energy was found for ascorbic acid reduction. In contrast, when evaluating the relationship of the Ag to C–C intensity ratio to the ascorbic acid concentration, the highest ratio was observed when the concentration of the ascorbic acid solution was 50 mM (Figure 6g,i). Collectively, these results suggested that the optimum treatment condition, which was the highest intensity ratio of Ag to C–C, for heat treatment was 20 min at 130 °C, and the optimal treatment condition for ascorbic acid reduction was 1 min with 50 mM of ascorbic acid.
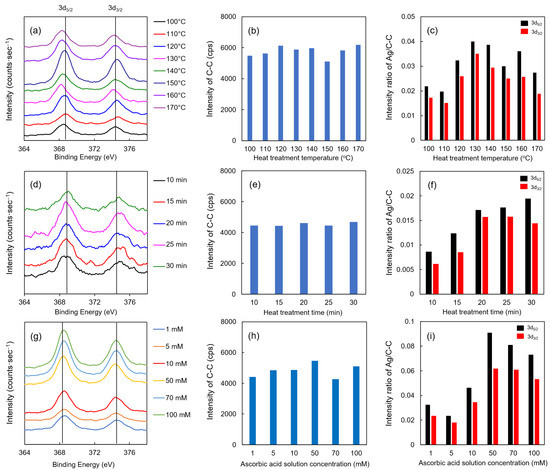
Figure 6.
XPS results of AgNP/TOCN/CS composite fibers obtained from (a–c) heat treatment at different temperatures, (d–f) heat treatment at different durations, and (g–i) reduction at different ascorbic acid concentrations.
Based on the above results, AgNPs were probably formed on the fiber surface. A schematic illustration of the preparation route for the AgNP/TOCN/CS composite fibers is shown in Figure 7. First, the CS dope solution was pressurized through a 30-hole nozzle and coagulated with an aqueous solution comprising 0.01% TOCN and 2% NaOH. This step yielded the CS fibers coated with TOCN when the CS cation reacts with the TOCN anion via an electrostatic reaction. Second, the TOCN/CS fibers were soaked in an AgNO3 aqueous solution. In this step, the anions on the surface of the TOCN/CS fiber reacted with Ag+, and excess Ag+ was removed from the Ag/TOCN/CS fiber surface. Finally, the Ag+ in the Ag/TOCN/CS fibers was reduced to Ag0 via the ascorbic acid reduction, yielding the AgNP/TOCN/CS fibers. During heat treatment, the reducing agent was probably TOCN, whereas, during ascorbic acid reduction, TOCN and ascorbic acid were presumed to be the reducing agents [30,31,32]. The involvement of the carboxylic groups of TOCN molecules in the direct reduction of Ag+ has been reported [24].
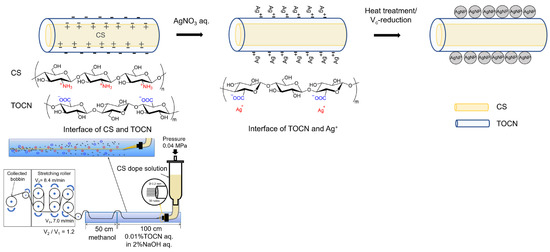
Figure 7.
A schematic illustration of the preparation of AgNP/TOCN/CS composite fibers.
Measurements were then performed using three types of fibers: TOCN/CS composite fibers, Ag/TOCN/CS composite fibers subjected to heat treatment at 130 °C for 20 min (named heat-treated fibers), and Ag/TOCN/CS composite fibers reduced with 50 mM ascorbic acid for 1 min (named Vc-reduced fibers).
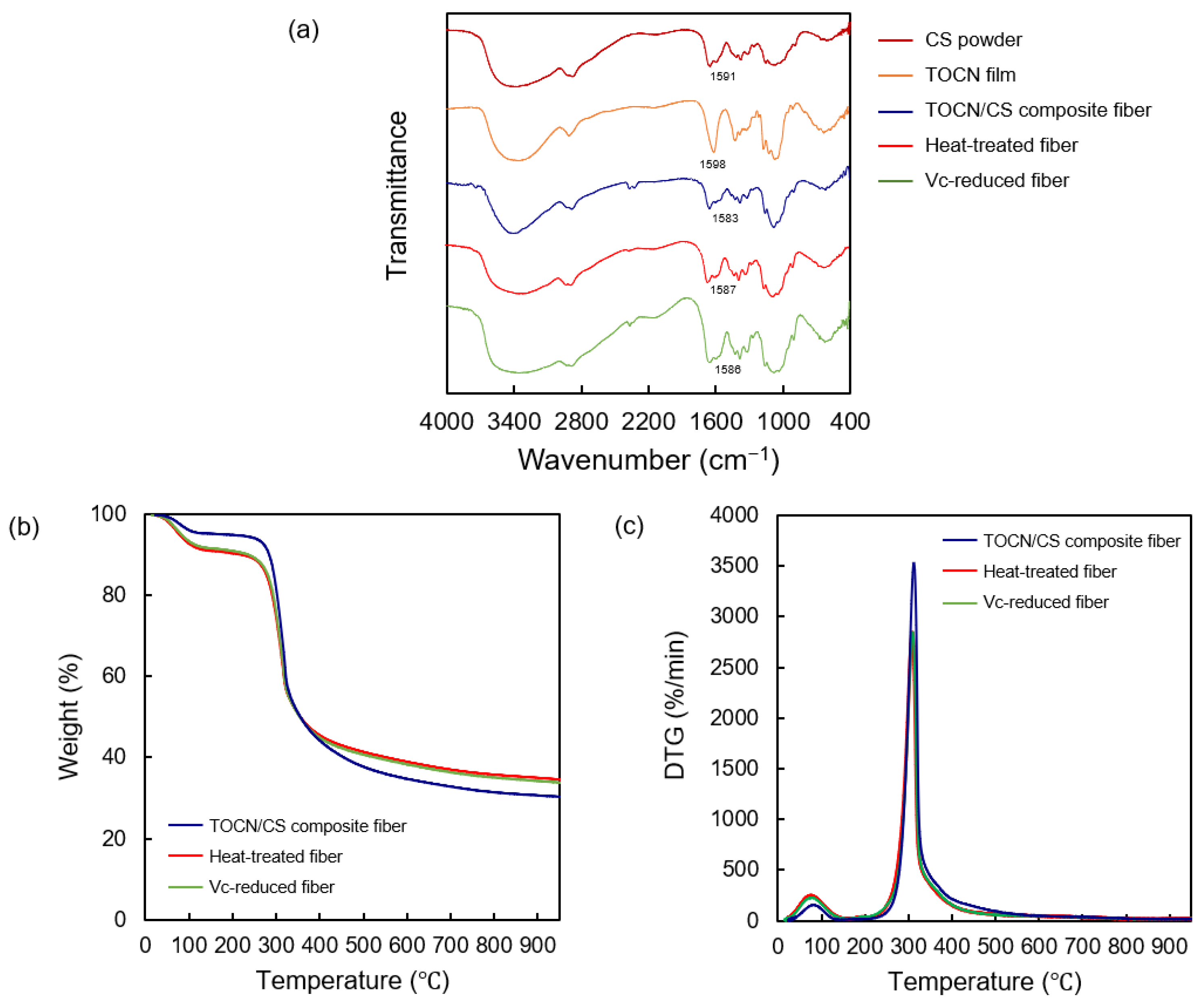
The FT-IR spectra of the CS powder, TOCN film, TOCN/CS composite fiber, heat-treated fiber, and Vc-reduced fiber are shown in Figure 8. An absorption band at 1598 and 1583 cm−1 was observed in the TOCN/CS composite fibers. Similarly, absorption bands at 1587 cm−1 and 1586 cm−1 were detected in the heat-treated and Vc-reduced fibers, respectively. The interaction between TOCN and AgNO3 was previously confirmed using FTIR-attenuated total reflection (FTIR-ATR) [24]. In that study, an absorption band at 1601 cm−1 derived from the carbonyl groups corresponded to the TEMPO-mediated oxidation of cellulose nanofibers, showing that the hydroxyl groups at the C-6 position of cellulose molecules were converted to sodium carboxylate. After treating TOCN with an aqueous AgNO3 solution, the C=O vibrations of the carboxylate anion slightly shifted from 1601 to 1590 cm−1, indicating that the sodium salt was converted to silver salt [22]. In our study, the C=O vibrations of the carboxylate anion shifted from 1583 to 1586-1587 cm−1 after ascorbic acid reduction or heat treatment.
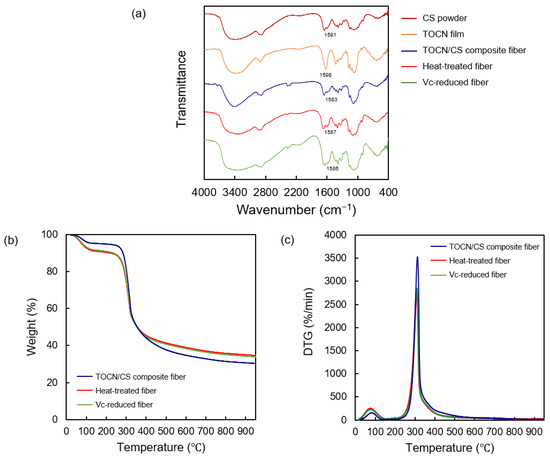
Figure 8.
(a) FT-IR spectra of the CS powder, TOCN film, TOCN/CS composite fiber, heat-treated fiber, and Vc-reduced fiber. (b) TGA curves and (c) differential thermogravimetric curves of the AgNP/TOCN/CS composite fibers.
The mass-loss profiles and degradation rates obtained from TGA reflected the thermal stabilities of the TOCN/CS composite, heat-treated, and Vc-reduced fibers. The TGA and differential thermogravimetric curves for each fiber are shown in Figure 8b,c, respectively. From the initial stage until 110 °C, broad weight loss peaks were observed for all samples, representing water evaporation. Moreover, the TOCN/CS composite fibers decomposed at 314 °C, while the same composition temperature of heat-treated and Vc-reduced fibers at 304 °C was detected, corresponding to the decomposition of cellulose fibers [27]. This lower decomposition temperature of heat-treated and Vc-reduced fibers is caused by the degradation from heat treatment or ascorbic acid during the production.
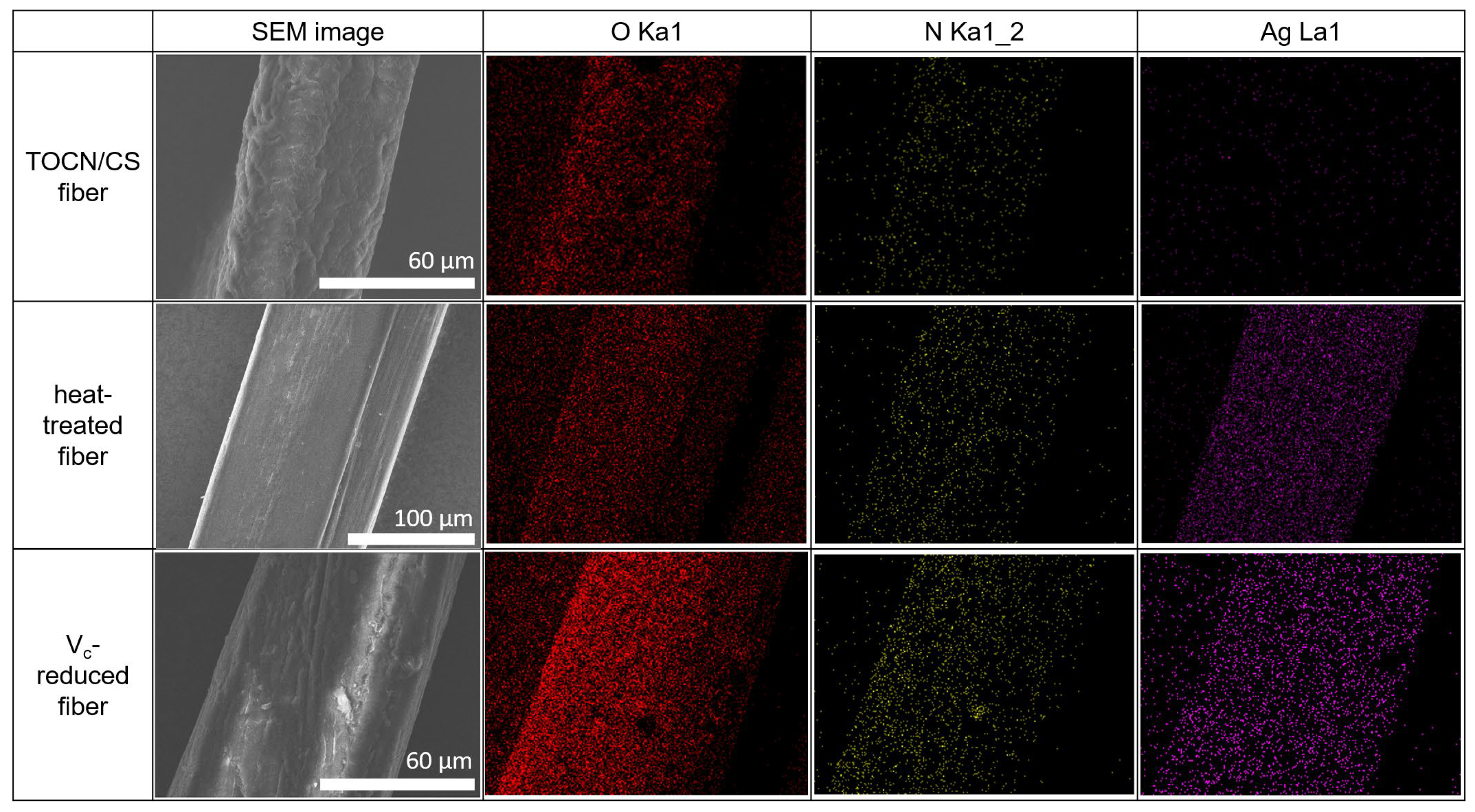
3.4. Confirmation of the Presence of AgNPs on the Fiber Surface
The SEM-EDS results for the TOCN/CS composite, heat-treated, and Vc-reduced fibers are shown in Figure 9. The O and N elements on the fiber surfaces of all the samples were confirmed via EDS mapping. Ag was detected in the heat-treated and Vc-reduced fibers, as expected. These results confirm that the AgNP/TOCN/CS composite fibers were successfully synthesized in this study.
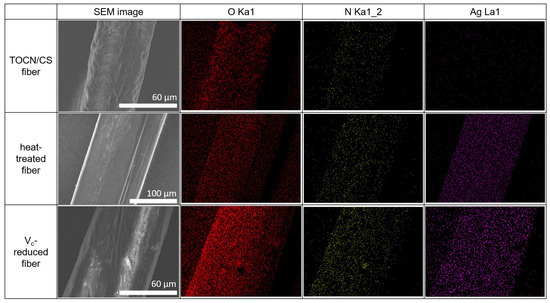
Figure 9.
SEM-EDS results of the TOCN/CS composite, heat-treated, and Vc-reduced fibers.
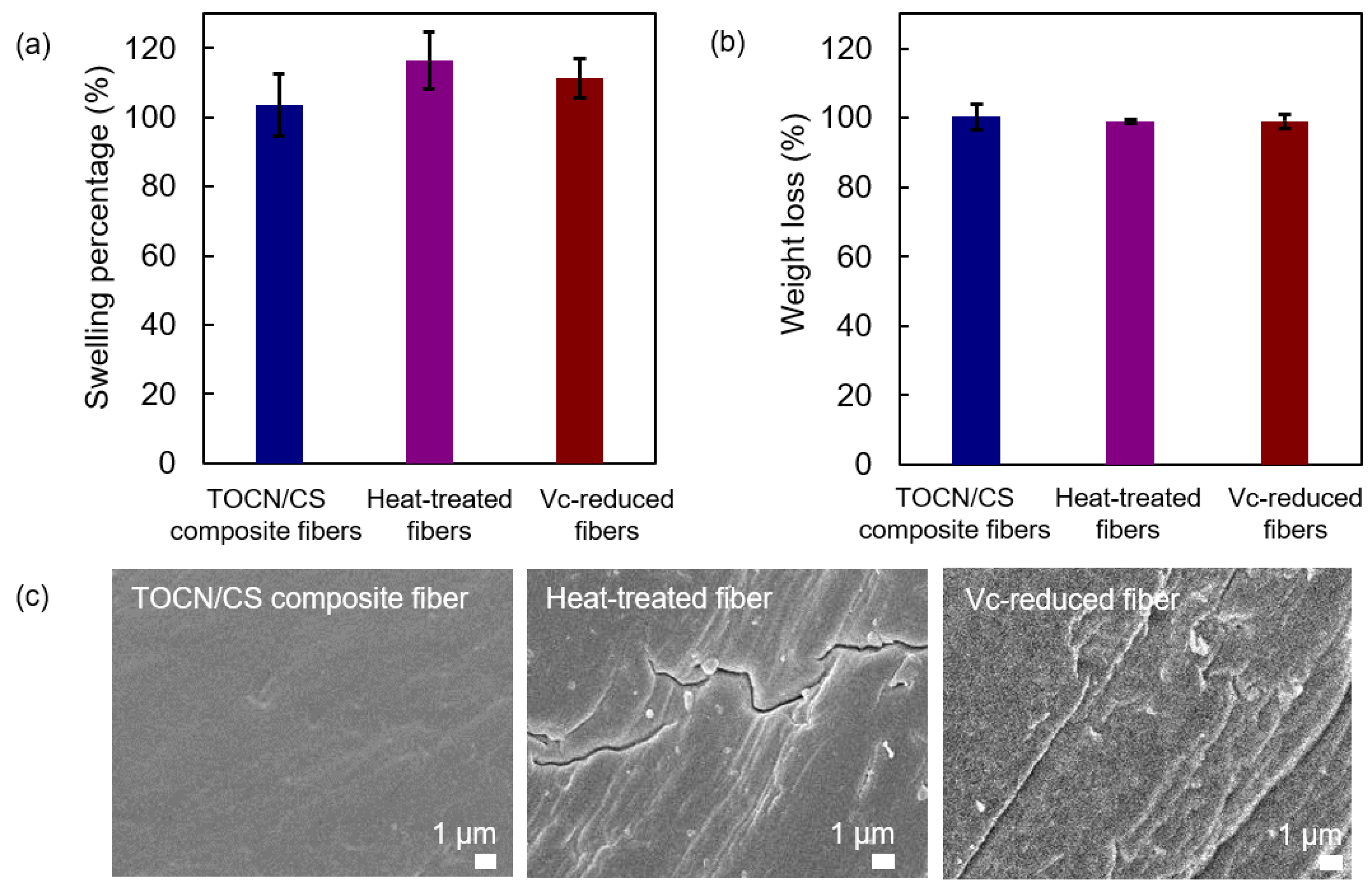
3.5. Swelling and Weight Loss Measurements
The swelling percentage and the remaining weight percentage after the swelling test of each sample are shown in Figure 10a and Figure 10b, respectively. The swelling percentages of the heat-treated fibers were similar to those of the Vc-reduced fibers and slightly higher than those of the TOCN/CS composite fibers. The high swelling percentages of the heat-treated and Vc-reduced fibers correspond to the cracks found on the surface of the fiber after heat treatment and ascorbic acid reduction, respectively (Figure 10c). In our experiments, the TOCN/CS composite fibers were immersed in an AgNO3 aqueous solution. After soaking, the aqueous solution was absorbed and remained inside the fibers. When the fibers dried, the swollen fibers shrank, suggesting that cracks were generated on the fiber surface after drying. Regarding the remaining weight percentage after the swelling test, the differences in the elution of each sample cannot be confirmed.
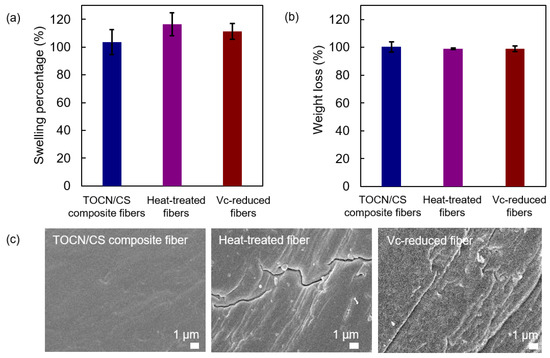
Figure 10.
(a) Swelling percentage, (b) the remaining weight percentage after the swelling test, and (c) FE-SEM micrographs of the TOCN/CS composite, heat-treated, and Vc-reduced fibers after the swelling test.
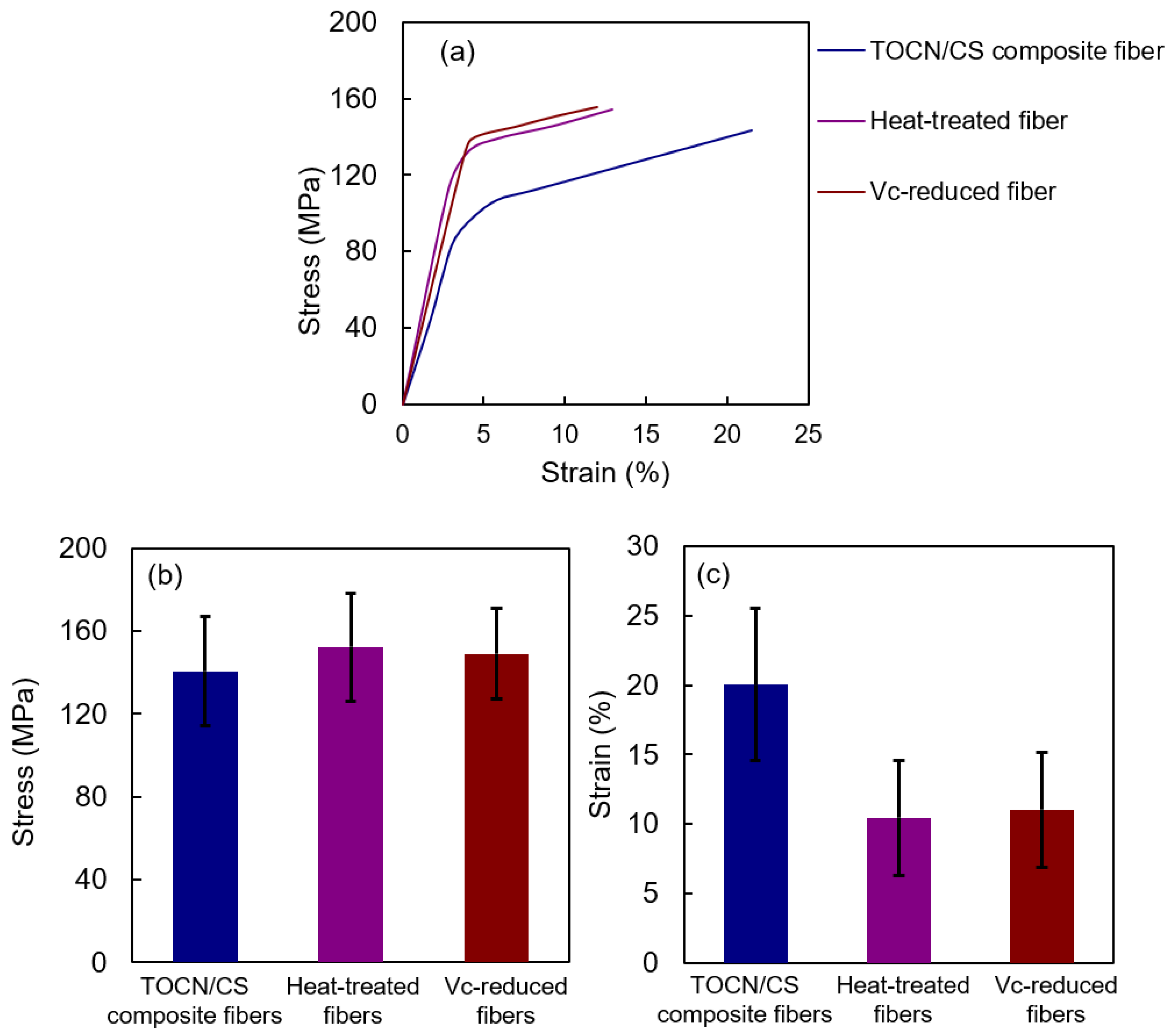
3.6. Tensile Properties of the Fiber Samples
Figure 11 shows the tensile properties of the samples. The stresses of TOCN/CS composite, heat-treated, and Vc-reduced fibers were 140.7 ± 26.24, 152.4 ± 26.10, and 149.1 ± 21.80 MPa, respectively. The stress of the heat-treated fibers was slightly higher than that of the Vc-reduced fibers and significantly higher than that of TOCN/CS composite fibers. However, the strain of TOCN/CS composite fibers decreased from 20.0 ± 5.47% to 10.5 ± 4.14% and 11.0 ± 4.15% after heat treatment and ascorbic acid reduction, respectively. The strain values decreased because the fibers swelled once and shrank during drying, increasing their rigidity.
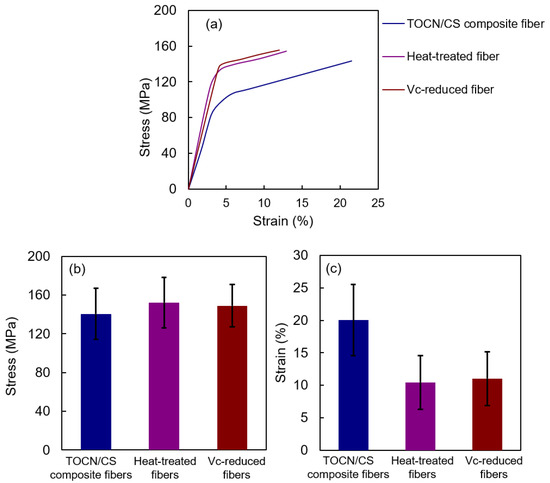
Figure 11.
Tensile properties of each fiber: (a) stress-strain curve, (b) stress, and (c) strain.
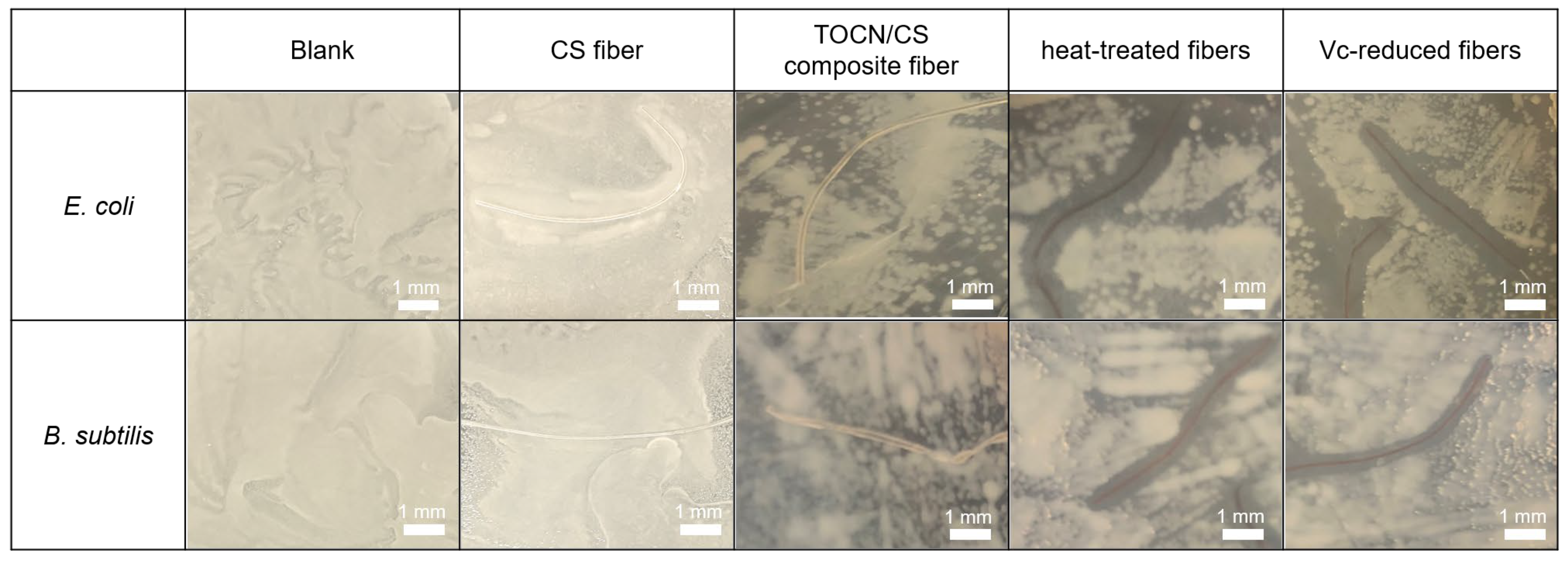
3.7. Antibacterial Properties of the AgNP/TOCN/CS Composite Fibers
The antibacterial test method used in this study was according to JIS L 1920. The inhibition zones using the CS, TOCN/CS composite, heat-treated, and Vc-reduced fibers against two types of bacteria, E. coli and B. subtilis, compared with a blank (no fiber), are shown in Figure 12. The average of the inhibition zone from triplicate tests is shown in Table 1. The inhibition zones were not observed against E. coli and B. subtilis when CS fiber and TOCN/CS composite fibers without AgNPs were used. However, antimicrobial activities were confirmed using the AgNP/TOCN/CS composite fibers. The heat-treated fibers showed an inhibition zone of 0.9 mm with E. coli, similar to that of B. subtilis (0.9 mm).
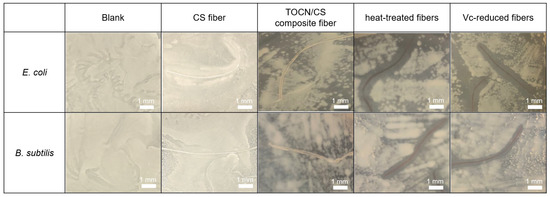
Figure 12.
Inhibition zones of each fiber against E. coli and B. subtilis.

Table 1.
Average values of the inhibition zones of blank (no fiber), CS, TOCN/CS composite, heat-treated, and Vc-reduced fibers.
Additionally, the inhibition zone using the Vc-reduced fiber against E. coli was slightly larger than that against B. subtilis. These results may be because E. coli, a Gram-negative bacterium, has a thinner cell wall than B. subtilis, facilitating the easy diffusion of Ag+ into the bacterial cell [33]. These findings are consistent with the inhibition zone observed against E. coli and S. aureus using TEMPO-oxidized cotton fibers deposited with silver in a previous study. The inhibition zones ranged from 1.0 to 2.5 mm when the silver content ranged from 0.3 to 0.8 mmol·g per gram of fiber for both bacteria [13]. These results suggest that the AgNPs that formed on the fiber surface have antibacterial properties, activated after being released from the fibers.
Apart from the antimicrobial activity test, the TGA under oxygen atmosphere was performed to analyze the amount of AgNPs in the composite fibers. The flow rate of oxygen was 200 mL min−1, the heating rate was 20 °C min−1, and the measurement temperature range was 22–900 °C. The residue mass from the heat-treated and Vc-reduced fibers referring to AgNPs in the fibers were 1.4 and 2.7%, respectively. This higher amount of AgNPs in Vc-reduced fiber corresponds to more color change. In summary, the reduction by ascorbic acid is suggested to be the optimal method to prepare the AgNP/TOCN/CS composite fibers due to the larger amount of AgNPs in the Vc-reduced fiber leading to the larger inhibition zone.
4. Conclusions
In this study, AgNP/TOCN/CS composite fibers were prepared using two different methods: heat treatment and ascorbic acid reduction. Ag/TOCN/CS composite fibers were prepared by immersing them in a 5 mM AgNO3 aqueous solution for 3 h and washed five times with 100 mL of DI water. The color of the fibers, either after heat treatment or ascorbic acid reduction, changed from yellow to orange. The binding energy peak of the Ag 3d orbital was confirmed via XPS measurements, whereas the presence of AgNPs on the fiber surface was confirmed via EDS mapping. The swelling rate of the treated fibers slightly increased, owing to cracks generated by the treatment. After heat treatment or ascorbic acid reduction, the elongation of the fibers was reduced by approximately half, whereas the stress was unaffected. Moreover, the AgNP/TOCN/CS composite fibers showed antibacterial activity against E. coli and B. subtilis because of the AgNPs present on the fiber surface. In conclusion, the reduction by ascorbic acid is suggested to be the optimal method to prepare the AgNP/TOCN/CS composite fibers. Collectively, the results of this study confirm the successful addition of antibacterial properties to TOCN/CS composite fibers.
Author Contributions
D.D. contributed to writing—original draft, review, and editing. K.K. and A.S. contributed to the methodology and investigation. H.T. and T.F. contributed to conceptualization, funding acquisition, resources, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Private University Research Branding Project: Matching Fund Subsidy from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) Japan (2016–2020).
Data Availability Statement
Data will be made available on request.
Acknowledgments
We appreciate Nagahiro SAITO at Chemical Systems Engineering, Graduate School of Engineering, Nagoya University for the SEM-EDS test and DKS Co., Ltd. for providing TOCN.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Kim, S. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Zhao, X.; Luo, Y.; Zheng, K.; Wu, M. Highly effective antibacterial AgNPs@hinokitiol grafted chitosan for construction of durable antibacterial fabrics. Int. J. Biol. Macromol. 2022, 209, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Bandatang, N.; Pongsomboon, S.; Jumpapaeng, P.; Suwanakood, P.; Saengsuwan, S. Antimicrobial electrospun nanofiber mats of NaOH-hydrolyzed chitosan (HCS)/PVP/PVA incorporated with in-situ synthesized AgNPs: Fabrication, characterization, and antibacterial activity. Int. J. Biol. Macromol. 2021, 190, 585–600. [Google Scholar] [CrossRef]
- Jung, J.; Kasi, G.; Seo, J. Development of functional antimicrobial papers using chitosan/starch-silver nanoparticles. Int. J. Biol. Macromol. 2018, 112, 530–536. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.J.; Moon, J.; Kim, J.H.; Heo, D.N.; Bang, J.B.; Lim, H.; Kwon, I.K. Preparation of antibacterial chitosan membranes containing silver nanoparticles for dental barrier membrane applications. J. Ind. Eng. Chem. 2018, 66, 196–202. [Google Scholar] [CrossRef]
- Dutta, T.; Ghosh, N.N.; Chattopadhyay, A.P.; Das, M. Chitosan encapsulated water-soluble silver bionanocomposite for size-dependent antibacterial activity. Nano-Struct. Nano-Objects 2019, 20, 100393. [Google Scholar] [CrossRef]
- Gopinath, V.; MubarakAli, D.; Vadivelu, J.; Kamath, S.M.; Syed, A.; Elgorban, A.M. Synthesis of biocompatible chitosan decorated silver nanoparticles biocomposites for enhanced antimicrobial and anticancer property. Process Biochem. 2020, 99, 348–356. [Google Scholar] [CrossRef]
- Thinakaran, S.; Loordhuswamy, A.; Rengaswami, G. Electrophoretic deposition of chitosan/nano silver embedded microsphere on centrifugal spun fibrous matrices—A facile biofilm resistant biocompatible material. Int. J. Biol. Macromol. 2020, 148, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xia, Z.; Qi, C.; He, M.; Yu, T.; Shi, L. Construction of chitosan/Ag nanocomposite sponges and their properties. Int. J. Biol. Macromol. 2021, 192, 272–277. [Google Scholar] [CrossRef]
- Milanović, J.; Mihajlovski, K.; Nikolić, T.; Kostić, M. Antimicrobial cotton fibers prepared by TEMPO-mediated oxidation and subsequent silver deposition. Cellul. Chem. Technol. 2016, 50, 905–914. Available online: https://www.cellulosechemtechnol.ro/pdf/CCT9-10(2016)/p.905-914.pdf (accessed on 27 July 2023).
- Huang, M.; Chen, F.; Jiang, Z.; Li, Y. Preparation of TEMPO-oxidized cellulose/amino acid/nanosilver biocomposite film and its antibacterial activity. Int. J. Biol. Macromol. 2013, 62, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, B.; Yu, J.; Al-Deyab, S.S. In situ growth of silver nanoparticles on TEMPO-oxidized jute fibers by microwave heating. Carbohydr. Polym. 2013, 92, 571–576. [Google Scholar] [CrossRef]
- Isogai, A.; Kato, Y. Preparation of Polyuronic Acid from Cellulose by TEMPO-mediated Oxidation. Cellulose 1998, 5, 153–164. [Google Scholar] [CrossRef]
- Saito, T.; Shibata, I.; Isogai, A.; Suguri, N.; Sumikawa, N. Distribution of carboxylate groups introduced into cotton linters by the TEMPO-mediated oxidation. Carbohydr. Polym. 2005, 61, 414–419. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Soni, B.; Hassan, E.B.; Schilling, M.W.; Mahmoud, B. Transparent bionanocomposite films based on chitosan and TEMPO-oxidized cellulose nanofibers with enhanced mechanical and barrier properties. Carbohydr. Polym. 2016, 151, 779–789. [Google Scholar] [CrossRef]
- Endo, R.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose nanofibril/poly(vinyl alcohol) composite drawn fibers. Polymer 2013, 54, 935–941. [Google Scholar] [CrossRef]
- Ifuku, S.; Tsuji, M.; Morimoto, M.; Saimoto, H.; Yano, H. Synthesis of silver nanoparticles templated by TEMPO-mediated oxidized bacterial cellulose nanofibers. Biomacromolecules 2009, 10, 2714–2717. [Google Scholar] [CrossRef]
- Ito, H.; Sakata, M.; Hongo, C.; Matsumoto, T.; Nishino, T. Cellulose nanofiber nanocomposites with aligned silver nanoparticles. Nanocomposites 2018, 4, 167–177. [Google Scholar] [CrossRef]
- Pawcenis, D.; Chlebda, D.K.; Jędrzejczyk, R.J.; Leśniak, M.; Sitarz, M.; Łojewska, J. Preparation of silver nanoparticles using different fractions of TEMPO-oxidized nanocellulose. Eur. Polym. J. 2019, 116, 242–255. [Google Scholar] [CrossRef]
- Dechojarassri, D.; Nishida, K.; Ozakiya, R.; Furuike, T.; Tamura, H. Adsorption studies of ammonia, protein, and phytic acid using chitosan fiber coated with oxidized cellulose nanofiber. Fibers 2023, 11, 32. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Salama, A.; El-Ziaty, A.K.; Hazem Hassan, H. Preparation of Tempo-cellulose nanofiber/zinc oxide as antimicrobial and methylene blue photo-degrading nanocomposite. Cellul. Chem. Technol. 2021, 55, 365–373. [Google Scholar] [CrossRef]
- Darder, M.; Karan, A.; del Real, G.; DeCoster, M.A. Cellulose-based biomaterials integrated with copper-cystine hybrid structures as catalysts for nitric oxide generation. Mater. Sci. Eng. C 2020, 108, 110369. [Google Scholar] [CrossRef] [PubMed]
- Oe, T.; Dechojarassri, D.; Kakinoki, S.; Kawasaki, H.; Furuike, T.; Tamura, H. Microwave-assisted incorporation of AgNP into chitosan–alginate hydrogels for antimicrobial applications. J. Funct. Biomater. 2023, 14, 199. [Google Scholar] [CrossRef]
- Nam, S.; Hillyer, M.B.; Condon, B.D.; Lum, J.S.; Richards, M.N.; Zhang, Q. Silver nanoparticle-infused cotton fiber: Durability and aqueous release of silver in laundry water. J. Agric. Food Chem. 2020, 68, 13231–13240. [Google Scholar] [CrossRef]
- Song, L.; Takahashi, K.; Ito, Y.; Aita, T. Preparation of oriented gold plate/cellulose nanofiber composite films by using TEMPO-oxidized cellulose nanofiber as a reducing agent. Microsyst. Technol. 2021, 27, 1039–1049. [Google Scholar] [CrossRef]
- Chekin, F.; Ghasemi, S. Silver nanoparticles prepared in presence of ascorbic acid and gelatin, and their electrocatalytic application. Bull. Mater. Sci. 2014, 37, 1433–1437. [Google Scholar] [CrossRef]
- Qin, Y.; Ji, X.; Jing, J.; Liu, H.; Wu, H.; Yang, W. Size control over spherical silver nanoparticles by ascorbic acid reduction. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 172–176. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).