Isolation and Properties of Cellulose Nanocrystals Fabricated by Ammonium Persulfate Oxidation from Sansevieria trifasciata Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
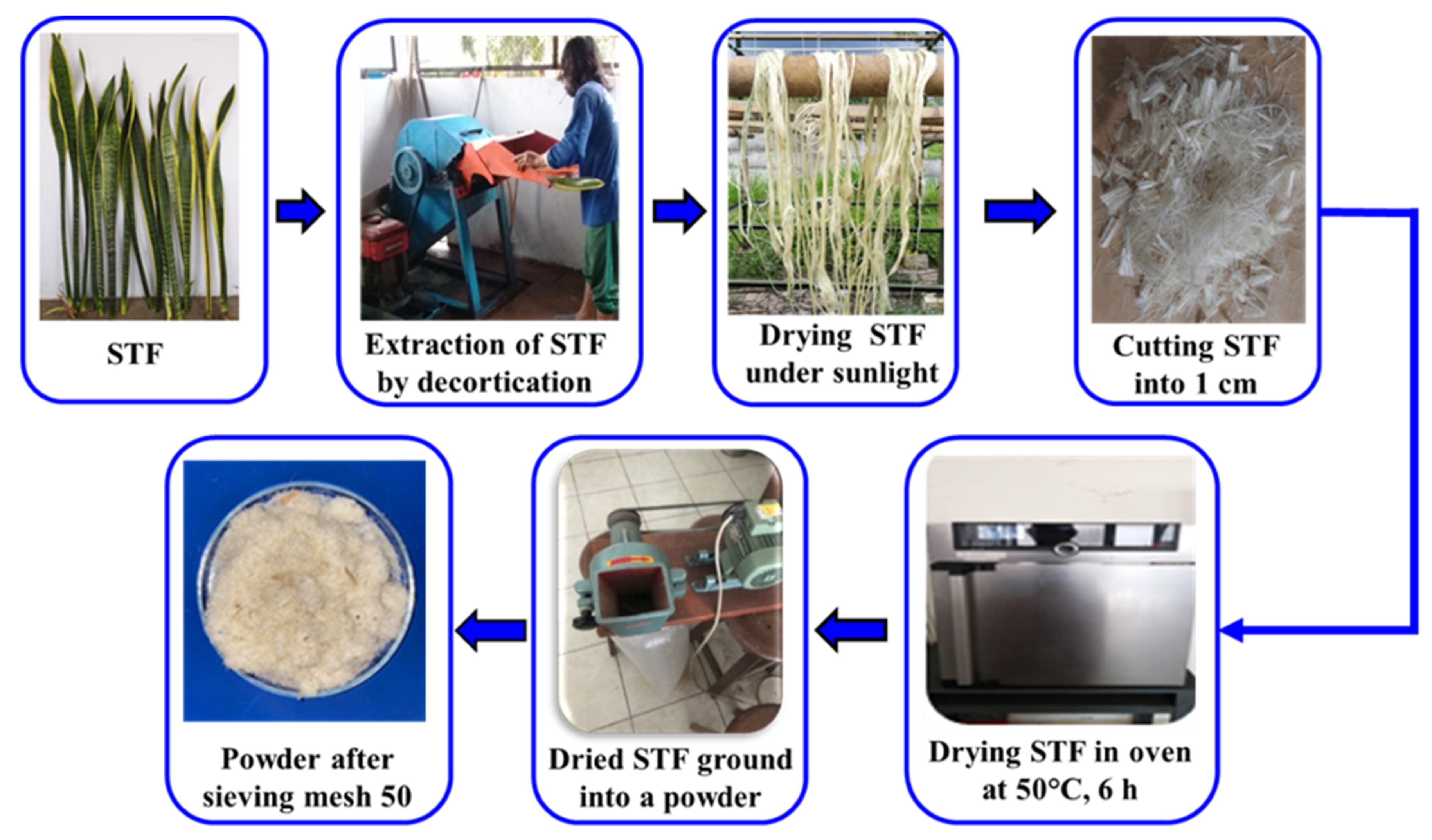
2.2. Extraction of Sansevieria trifasciata Fibers
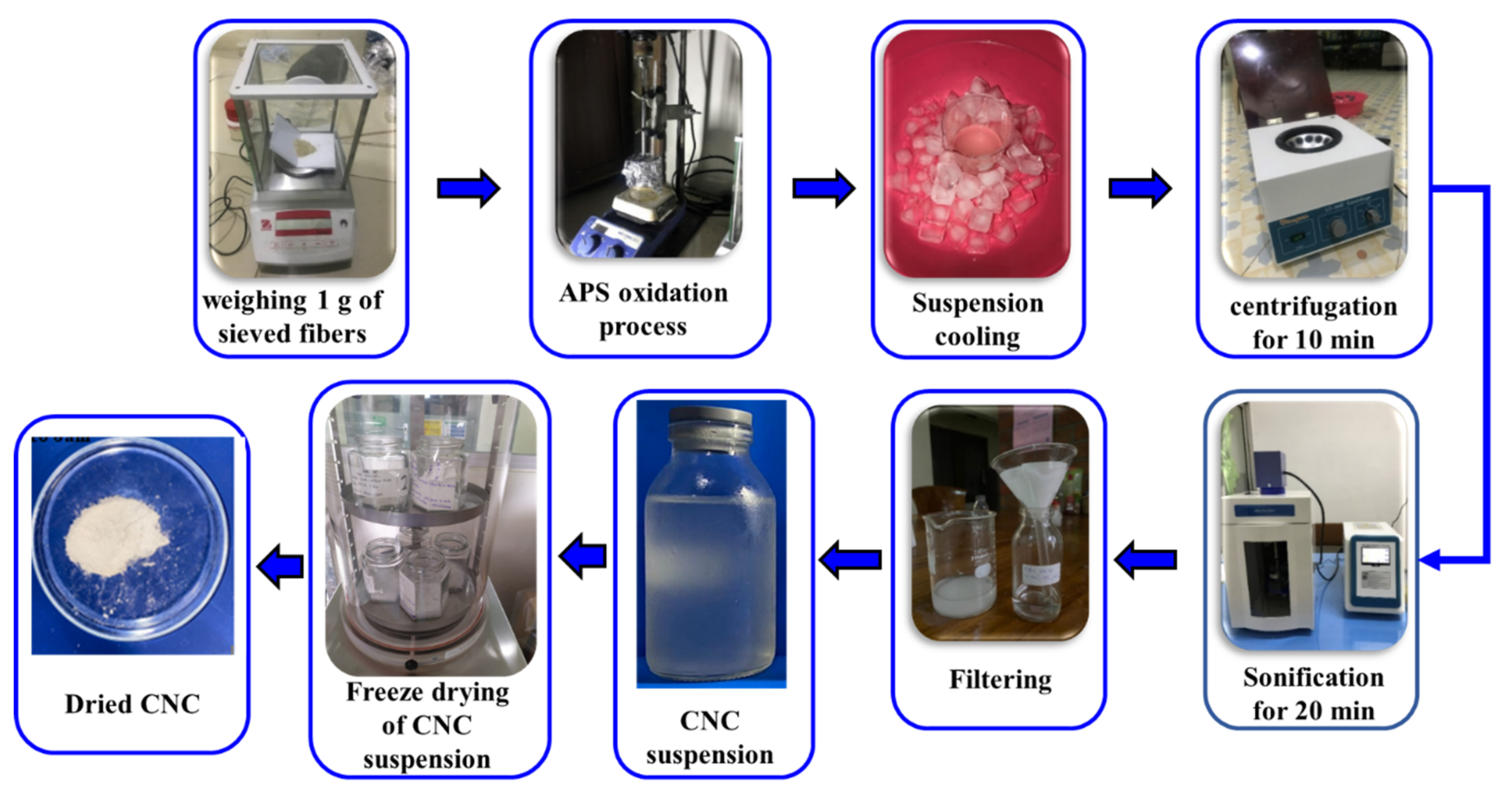
2.3. Isolation of Cellulose Nanocrystals
2.4. Characterization
2.4.1. SEM/EDS Analysis
2.4.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.3. X-ray Diffraction (XRD) Analysis
2.4.4. Transmission Electron Microscopy (TEM)
2.4.5. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Morphological Analysis by SEM/EDS
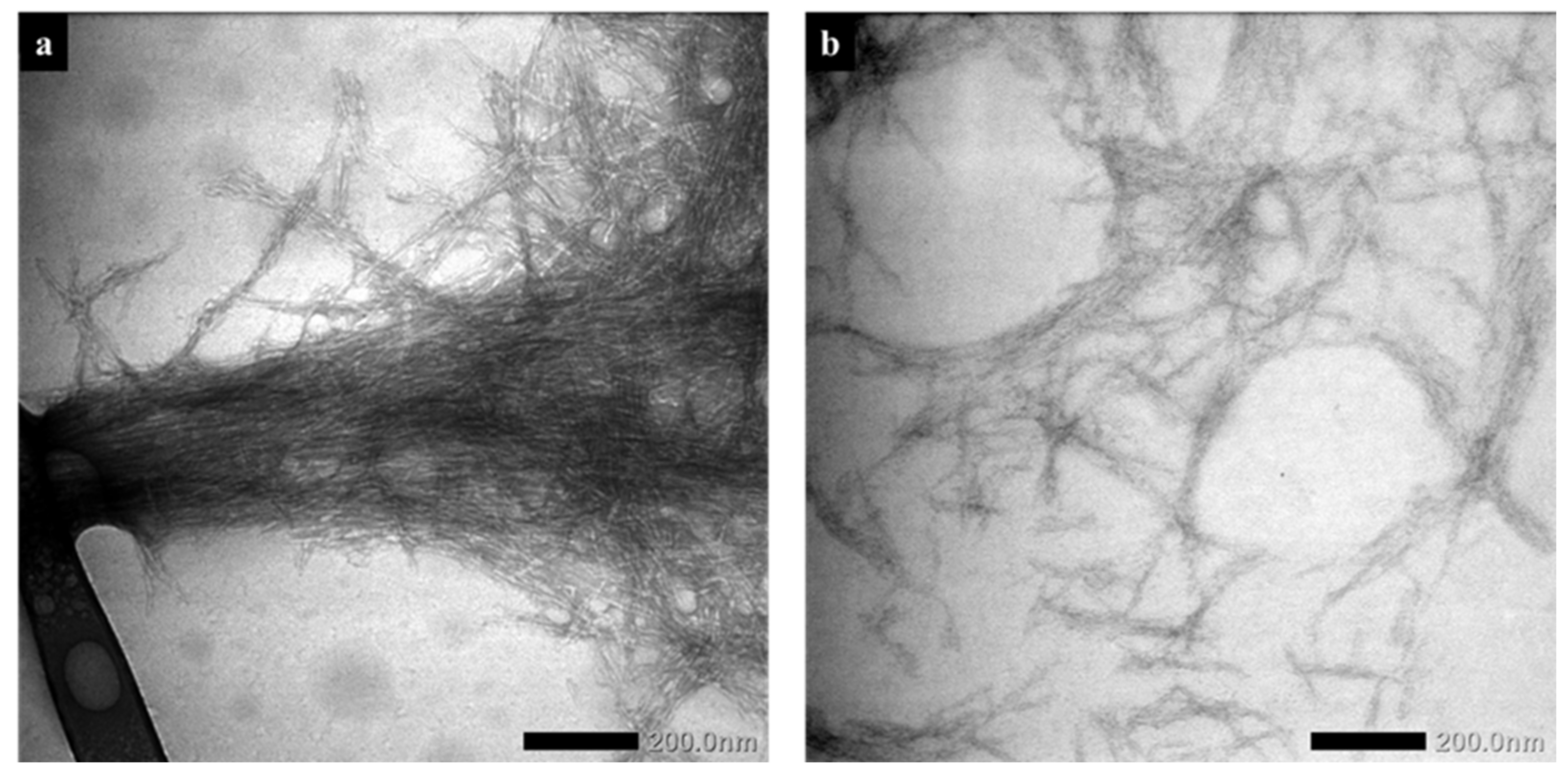
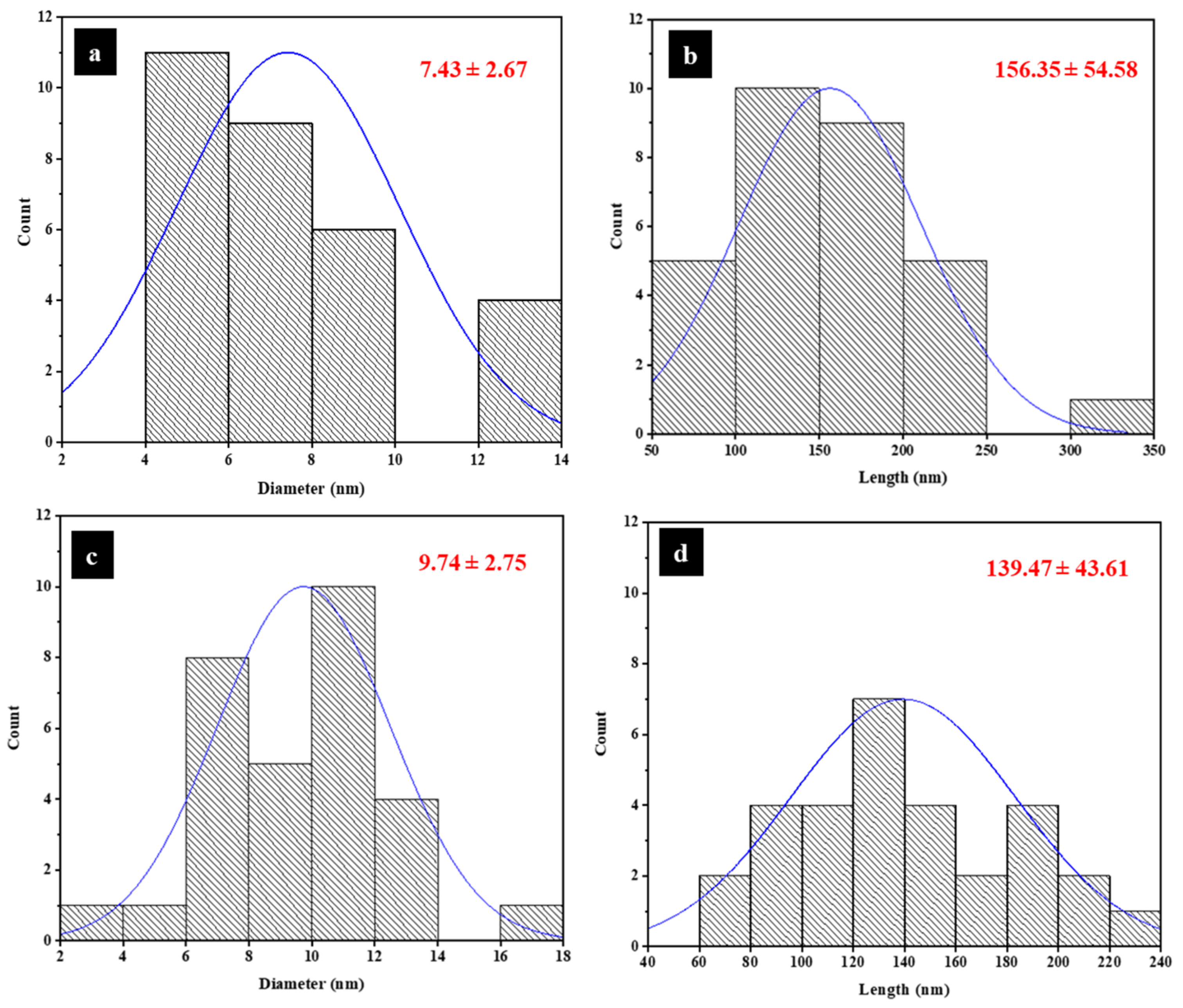
3.2. Transmission Electron Microscopy
3.3. Fourier Transform Infrared (FT-IR) Spectroscopy
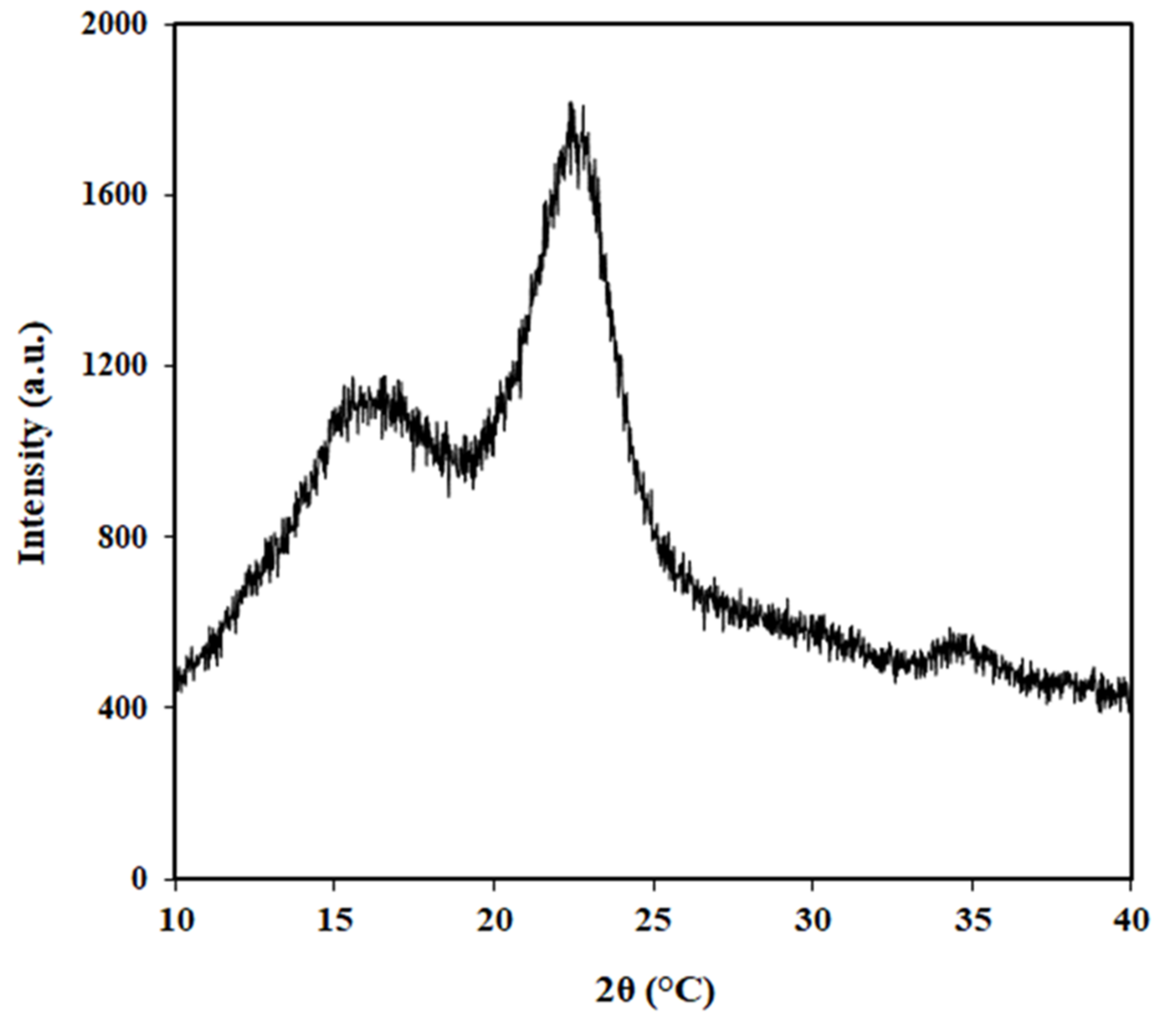
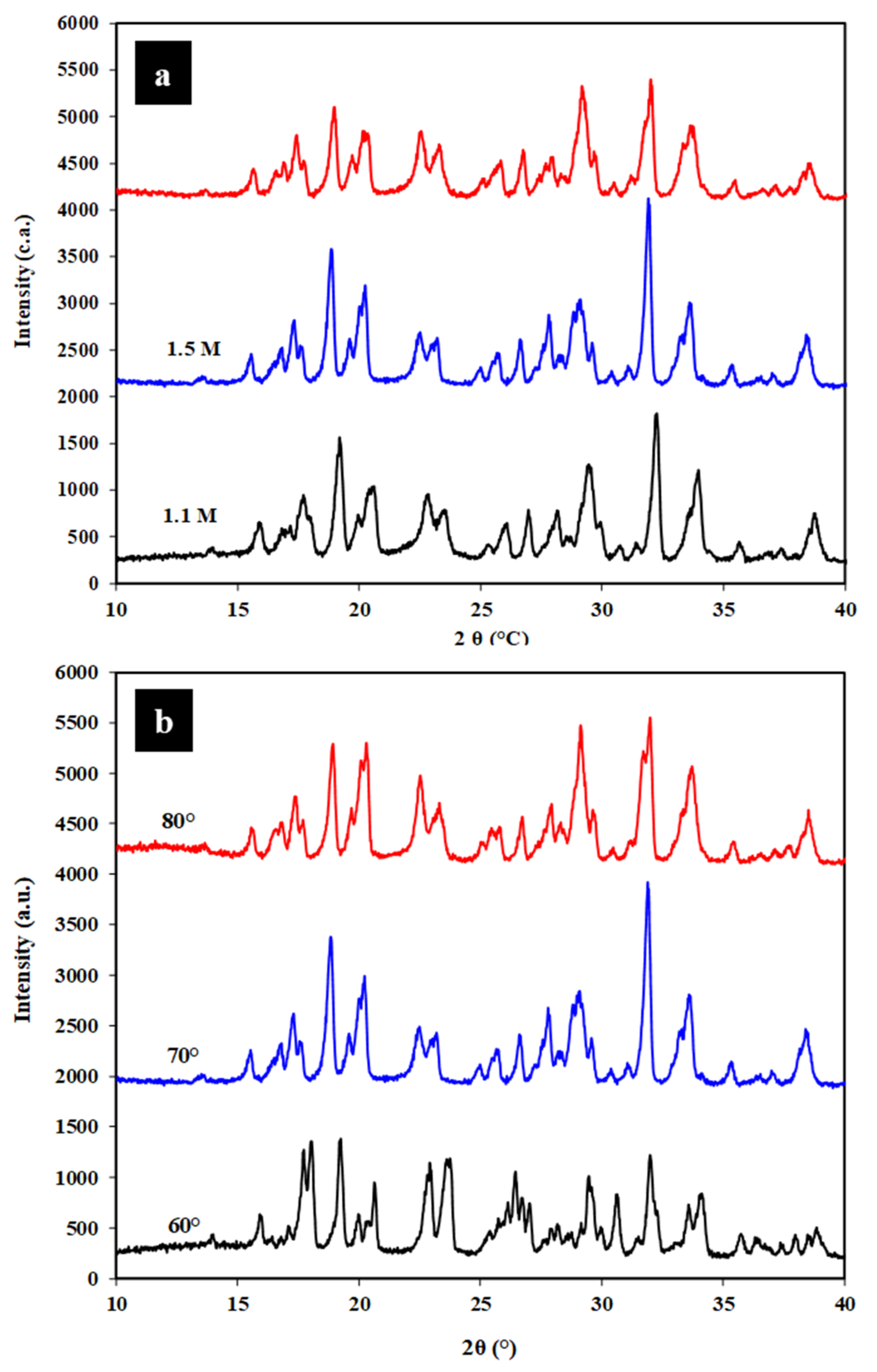
3.4. X-ray Diffraction (XRD)
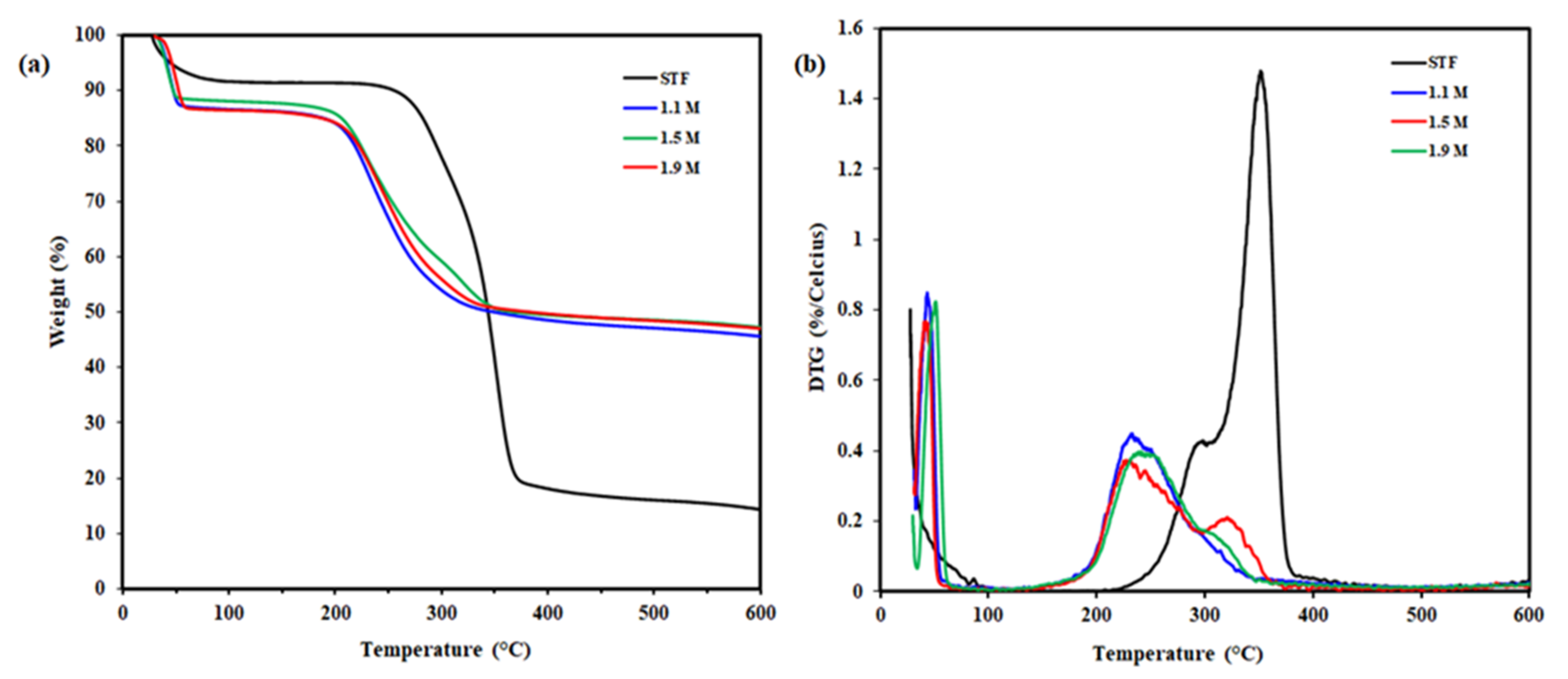
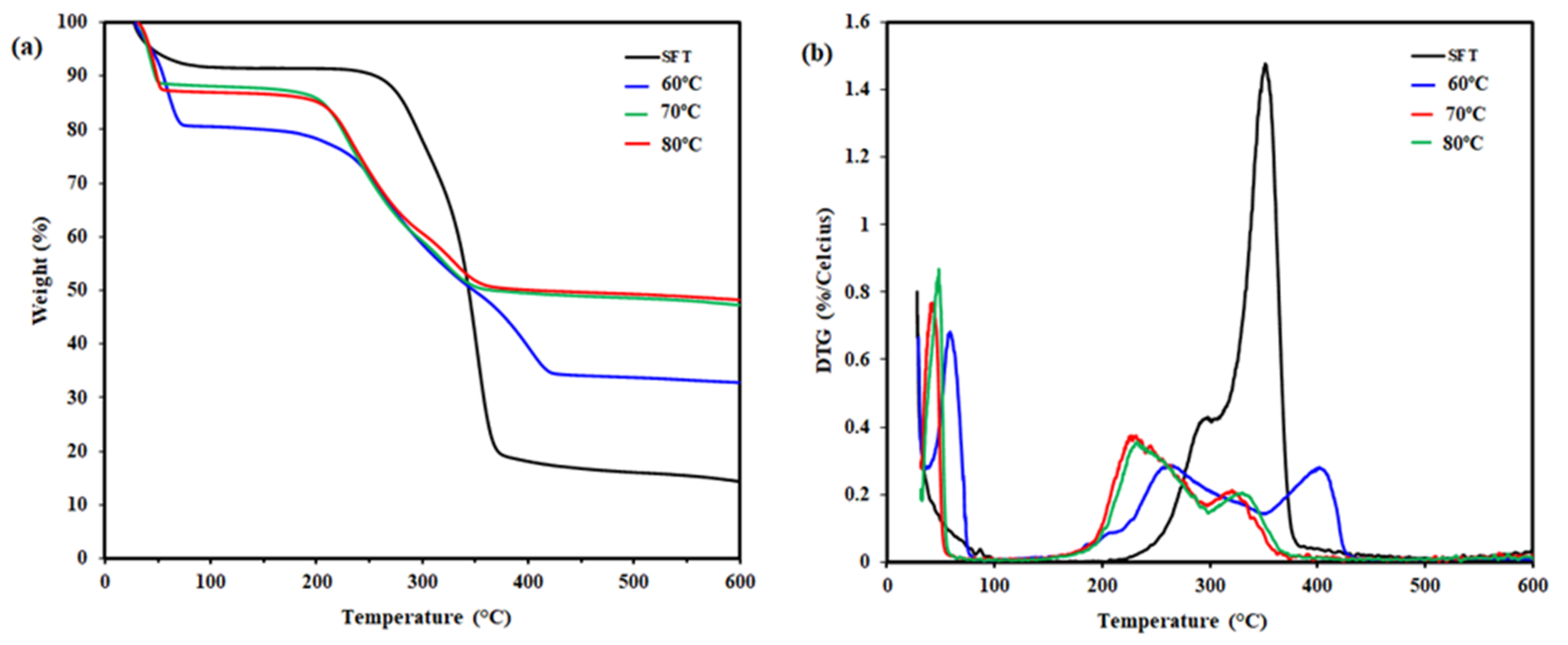
3.5. Thermogravimetry Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanimozhi, M. Investigating the physical characteristics of sansevieria trifasciata fibre. Int. J. Sci. Res. Publ. 2011, 1, 1–4. [Google Scholar]
- Rwawiire, S.; Tomkova, B. Morphological, thermal, and mechanical characterization of Sansevieria trifasciata fibers. J. Nat. Fibers 2015, 12, 201–210. [Google Scholar] [CrossRef]
- Keng, H.; Chin, S.C.; Tan, H.T.W. The Concise Flora of Singapore: Monocotyledons; Singapore University Press: Singapore, 1998; Volume II. [Google Scholar]
- Mardiyati; Steven; Rizkiansyah, R.R.; Senoaji, A.; Suratman, R. Effects of alkali treatment on the mechanical and thermal properties of Sansevieria trifasciata fiber. AIP Conf. Proc. 2016, 1725, 020043. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties, and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Csiszar, E.; Kalic, P.; Kobol, A.; Eduardo de Paulo, F. The effect of low frequency ultrasound on the production and properties of nanocrystalline cellulose suspensions and films. Ultrason. Sonochem. 2016, 31, 473–480. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griessere, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Li, W.; Yue, J.Q.; Liu, S.X. Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly(vinyl alcohol) composites. Ultrason. Sonochemistry 2012, 19, 479–485. [Google Scholar] [CrossRef]
- Khan, A.; Vu, K.D.; Chauve, G.; Bouchard, J.; Riedl, B.; Lacroix, M. Optimization of microfluidization for the homogeneous distribution of cellulose nanocrystals (CNCs) in biopolymeric matrix. Cellulose 2014, 21, 3457–3468. [Google Scholar] [CrossRef] [Green Version]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Kusmono; Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and characterization of cellulose nanocrystal extracted from ramie fibers by sulfuric acid hydrolysis. Heliyon 2020, 6, e05486. [Google Scholar] [CrossRef]
- Ribeiro, R.S.A.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N., Jr. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Wu, Q.; Ren, S.; Lei, T. Comparison of highly transparent all-cellulose nanopaper prepared using sulfuric acid and TEMPO-mediated oxidation methods. Cellulose 2015, 22, 1123–1133. [Google Scholar] [CrossRef]
- Espino, E.; Cakir, M.; Domenek, S.; Roma´n-Gutie´rrez, A.D.; Belgacem, N.; Bras, J. Isolation and characterization of cellulose nanocrystals from industrial by-products of Agave tequilana and barley. Ind. Crops Prod. 2014, 62, 552–559. [Google Scholar] [CrossRef]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and properties of carboxylated cellulose nanocrystals prepared from a novel one-step procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Liu, C.; Mu, X.; Gong, W.; Lv, D.; Hong, Y.; Si, C.; Li, B. Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose 2016, 23, 2389–2407. [Google Scholar] [CrossRef]
- Bashar, M.M.; Zhu, H.; Yamamoto, S.; Mitsuishi, M. Highly carboxylated and crystalline cellulose nanocrystals from jute fiber by facile ammonium persulfate oxidation. Cellulose 2019, 26, 3671–3684. [Google Scholar] [CrossRef]
- Ji, H.; Xiang, Z.; Qi, H.; Han, T.; Pranovich, A.; Song, T. Strategy towards one-step preparation of carboxylic cellulose nanocrystals and nanofibrils with high yield, carboxylation and highly stable dispersibility using innocuous citric acid. Green Chem. 2019, 21, 1956–1964. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Liu, H.; Shang, S.; Song, J.; Wang, W. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016, 138, 237–243. [Google Scholar] [CrossRef]
- Mascheroni, E.; Rampazzo, R.; Ortenzi, M.A.; Piva, G.; Bonetti, S.; Piergiovanni, L. Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 2016, 23, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Zaini, L.H.; Febrianto, F.; Wisata, N.J.; Maulana, M.M.I.; Lee, S.H.; Kim, N.H. Effect of ammonium persulfate concentration on characteristics of cellulose nanocrystals from oil palm frond. J. Korean Wood Sci. Technol. 2019, 47, 597–606. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Kato, R.; Rowan, S.J. Preparation of cellulose nanofibers from Miscanthus x. Giganteus by ammonium persulfate oxidation. Carbohydr. Polym. 2019, 212, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Wang, K.; Zhang, H.; Yuan, Y.; Wei, H.X.; Wang, X.; Duan, Y.; Zhuo, L.; Zhang, J. Modified ammonium persulfate oxidations for efficient preparation of carboxylated cellulose nanocrystals. Carbohydr. Polym. 2020, 229, 115572. [Google Scholar] [CrossRef]
- Culsum, N.T.U.; Melinda, C.; Leman, I.; Wibowo, A.; Budhi, Y.W. Isolation and characterization of cellulose nanocrystals (CNCs) from industrial denim waste using ammonium persulfate. Mater. Today Commun. 2021, 26, 101817. [Google Scholar] [CrossRef]
- Hu, S.; Qin, Z.; Cheng, M.; Chen, Y.; Liu, J.; Zhang, Y. Improved properties and drug delivery behaviors of electrospun cellulose acetate nanofibrous membranes by introducing carboxylated cellulose nanocrystals. Cellulose 2018, 25, 1883–1898. [Google Scholar] [CrossRef]
- Marwanto, M.; Maulana, M.I.; Febrianto, F.; Wistara, N.J.; Nikmatin, S.; Masruchin, N.; Zaini, L.H.; Lee, S.H.; Kim, N.H. Effect of oxidation time on the properties of cellulose nanocrystals prepared from Balsa and Kapok fibers using ammonium persulfate. Polymers 2021, 13, 1894. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Park, B.-D. Optimum oxidation for direct and efficient extraction of carboxylated cellulose nanocrystals from recycled MDF fibers by ammonium persulfate. Carbohydr. Polym. 2021, 251, 117029. [Google Scholar] [CrossRef]
- Indirasetyo, N.L. Production and Characterization of Cellulose Nanocrystal from Sansevieria Fibers via Ammonium Persulfate Oxidation method. Bachelor’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2021. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray Diffractometer. Textil. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Khanjanzadeh, H.; Park, B.-D. Characterization of carboxylated cellulose nanocrystals from recycled fiberboard fibers using ammonium persulfate oxidation. J. Korean Wood Sci. Technol. 2020, 48, 231–244. [Google Scholar] [CrossRef]
- Filipova, L.; Fridrihsone, V.; Cabulis, U.; Berzins, A. Synthesis of nanofibrillated cellulose by combined ammonium persulphate treatment with ultrasound and mechanical processing. Nanomaterials 2018, 8, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oun, A.A.; Rhim, J.W. Characterization of carboxymethyl cellulose-based nanocomposite films reinforced with oxidized nanocellulose isolated using ammonium persulfate method. Carbohydr. Polym. 2017, 174, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qin, Z.; Liu, Y.; Qin, Y.; Li, T.; Chen, L.; Zhu, M. Efficient extraction of carboxylated spherical cellulose nanocrystals with narrow distribution through hydrolysis of lyocell fibers by using ammonium persulfate as an oxidant. J. Mater. Chem. A 2014, 2, 251–258. [Google Scholar] [CrossRef]
- Goh, K.Y.; Ching, Y.C.; Chuah, C.H.; Abdullah, L.C.; Liou, N.S. Individualization of microfibrillated celluloses from oil palm empty fruit bunch: Comparative studies between acid hydrolysis and ammonium persulfate oxidation. Cellulose 2016, 23, 79–390. [Google Scholar]
- Hu, Y.; Tang, L.; Lu, Q.; Wang, S.; Chen, X.; Huang, B. Preparation of cellulose nanocrystals and carboxylated cellulose nanocrystals from borer powder of bamboo. Cellulose 2014, 21, 1611–1618. [Google Scholar] [CrossRef]
- Fortunati, E.; Benincasa, P.; Balestra, G.M.; Luzi, F.; Mazzaglia, A.; Buono, D.D.; Pugliaa, D.; Torre, L. Revalorization of barley straw and husk as precursors for cellulose nanocrystals extraction and their effect on PVA_CH nanocomposites. Ind. Crops Prod. 2016, 92, 201–217. [Google Scholar] [CrossRef]
- Hafemann, E.; Battisti, R.; Marangoni, C.; Machado, R.A.F. Valorization of royal palm tree agroindustrial waste by isolating cellulose nanocrystals. Carbohydr. Polym. 2019, 218, 188–198. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Sarasini, F.; Tirillò, J.; Maffei, G.; Zuorro, A.; Lavecchia, R.; Kenny, J.M.; Torre, L. Valorization and extraction of cellulose nanocrystals from North African grass: Ampelodesmos mauritanicus (Diss). Carbohydr. Polym. 2019, 209, 328–337. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.R.; Xu, F.; Sun, R.C. Simple approach to reinforce hydrogels with cellulose nanocrystals. Nanoscale 2014, 6, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Moosavinejad, S.M.; Madhoushi, M.; Vakili, M.; Rasouli, D. Evaluation of degradation in chemical compounds of wood in historical buildings using FT-IR and FT-Raman vibrational spectroscopy. Maderas Cienc. Y Tecnol. 2019, 21, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Nascimento, S.A.; Rezende, C.A. Combined approaches to obtain cellulose nanocrystals, nanofibrils and fermentable sugars from elephant grass. Carbohydr. Polym. 2018, 180, 38–45. [Google Scholar] [CrossRef]
- Al-Dulaimi, A.A.; Wanrosli, W.D. Isolation and characterization of nanocrystalline cellulose from totally chlorine free oil palm empty fruit bunch pulp. J. Polym. Environ. 2017, 25, 192–202. [Google Scholar] [CrossRef]
- Yu, H.; Abdalkarim, S.Y.H.; Zhang, H.; Wang, C.; Tam, K.C. Simple process to produce high-yield cellulose nanocrystals using recyclable citric/hydrochloric acids. ACS Sustain. Chem. Eng. 2019, 7, 4912–4923. [Google Scholar] [CrossRef]
- Oudiani, A.E.; Chaabouni, Y.; Msahli, S.; Sakli, F. Crystal transition from cellulose I to cellulose II in NaOH treated Agave americana L. fibre. Carbohydr. Polym. 2011, 86, 1221–1229. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Tecante, A. Extraction and characterization of cellulose nanofibers from Rose stems (Rosa spp.). Carbohydr. Polym. 2019, 220, 53–59. [Google Scholar] [CrossRef]
- Suwantong, O.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing asiaticoside or Centella Asiatica crude extract and the release characteristics of asiaticoside. Polymer 2008, 49, 4239–4247. [Google Scholar] [CrossRef]
- Du, C.; Liu, M.; Li, B.; Li, H.; Meng, Q.; Zhan, H. Cellulose nanocrystals prepared by persulfate one-step oxidation of bleached bagasse pulp. BioResources 2016, 11, 4017–4024. [Google Scholar] [CrossRef]
- Montane, D.; Farriol, X.; Salvado, J.; Jollez, P.; Chornet, E. Application of steamexplosion to the fabrication and rapid vapour-phase alkaline pulping of Wheat straw. Biomass Bioenergy 1998, 14, 261–278. [Google Scholar] [CrossRef]
- Thambiraj, S.; Ravi Shankaran, D. Preparation and physicochemical characterization of cellulose nanocrystals from industrial waste cotton. Appl. Surf. Sci. 2017, 412, 405–416. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Devi, R.R.; Dhar, P.; Kalamdhad, A.; Katiyar, V. Fabrication of cellulose nanocrystals from agricultural compost. Compos. Sci. Util. 2015, 23, 104–116. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Pinkl, S.; Gindl-Altmutter, W. Application of surface chemical functionalized cellulose nanocrystals to improve the performance of UF adhesives used in wood-based composites-MDF type. Carbohydr. Polym. 2019, 206, 11–20. [Google Scholar] [CrossRef]
- Kasiri, N.; Fathi, M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2018, 106, 1023–1031. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Characterization of nanocelluloses isolated from Ushar (Calotropis procera) seed fiber: Effect of isolation method. Mater. Lett. 2016, 168, 146–150. [Google Scholar] [CrossRef]
- Kaffashsaie, E.; Yousefi, H.; Nishino, T.; Matsumoto, T.; Mashkour, M.; Madhoushi, M.; Kawaguchi, H. Direct conversion of raw wood to TEMPO-oxidized cellulose nanofibers. Carbohydr. Polym. 2021, 262, 117938. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Adel, A.; El-Shafei, A.; Ibrahim, A.; Al-Shemy, M. Extraction of oxidized nanocellulose from date palm (Phoenix dactylifera L.) sheath fibers: Influence of CI and CII polymorphs on the properties of chitosan/bionanocomposite films. Ind. Crops Prod. 2018, 124, 155–165. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, Y.; Han, B.; Zhang, Y. Effect of oxidation time on the properties of cellulose nanocrystals from hybrid poplar residues using the ammonium persulfate. Carbohydr. Polym. 2017, 174, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Rhim, J.W. Isolation of oxidized nanocellulose from rice straw using the ammonium persulfate method. Cellulose 2018, 25, 2143–2149. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Preparation of multifunctional carboxymethyl cellulose-based films incorporated with chitin nanocrystal and grapefruit seed extract. Int. J. Biol. Macromol. 2020, 152, 1038–1046. [Google Scholar] [CrossRef]



| Element (wt%) | ||||
|---|---|---|---|---|
| C | N | O | Na | S |
| 8.93 | 16.27 | 47.35 | 10.66 | 16.79 |
| Sample | Crystallinity Index (%) |
|---|---|
| STFs | 52.4 |
| CNC 1.1 M | 80.0 |
| CNC 1.5 M | 87.4 |
| CNC 1.9 M | 83.4 |
| CNC 60 °C | 76.2 |
| CNC 70 °C | 87.4 |
| CNC 80 °C | 61.4 |
| Sample | Tonset (°C) | Tmax1 (°C) | Tmax2 (°C) | Wresidue (%) |
|---|---|---|---|---|
| STF | 270 | 295 | 352 | 14.3 |
| CNC 1.1 M | 203 | 233 | - | 45.6 |
| CNC 1.5 M | 206 | 229 | 320 | 47.3 |
| CNC 1.9 M | 209 | 244 | 318 | 46.9 |
| CNC 60 °C | 220 | 260 | 402 | 32.8 |
| CNC 70 °C | 206 | 229 | 320 | 47.3 |
| CNC 80 °C | 207 | 232 | 329 | 48.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indirasetyo, N.L.; Kusmono. Isolation and Properties of Cellulose Nanocrystals Fabricated by Ammonium Persulfate Oxidation from Sansevieria trifasciata Fibers. Fibers 2022, 10, 61. https://doi.org/10.3390/fib10070061
Indirasetyo NL, Kusmono. Isolation and Properties of Cellulose Nanocrystals Fabricated by Ammonium Persulfate Oxidation from Sansevieria trifasciata Fibers. Fibers. 2022; 10(7):61. https://doi.org/10.3390/fib10070061
Chicago/Turabian StyleIndirasetyo, Nafiis Lazuardi, and Kusmono. 2022. "Isolation and Properties of Cellulose Nanocrystals Fabricated by Ammonium Persulfate Oxidation from Sansevieria trifasciata Fibers" Fibers 10, no. 7: 61. https://doi.org/10.3390/fib10070061
APA StyleIndirasetyo, N. L., & Kusmono. (2022). Isolation and Properties of Cellulose Nanocrystals Fabricated by Ammonium Persulfate Oxidation from Sansevieria trifasciata Fibers. Fibers, 10(7), 61. https://doi.org/10.3390/fib10070061





