1. Introduction
Glass and carbon fibers are widely used as reinforcements in composites owing to their excellent mechanical and thermal properties. Anyway, their production and their disposal still have a great ecological impact. For this reason, researchers in recent years have focused their studies on alternative sustainable solutions, such as basalt fibers, exploring also innovative recycling techniques.
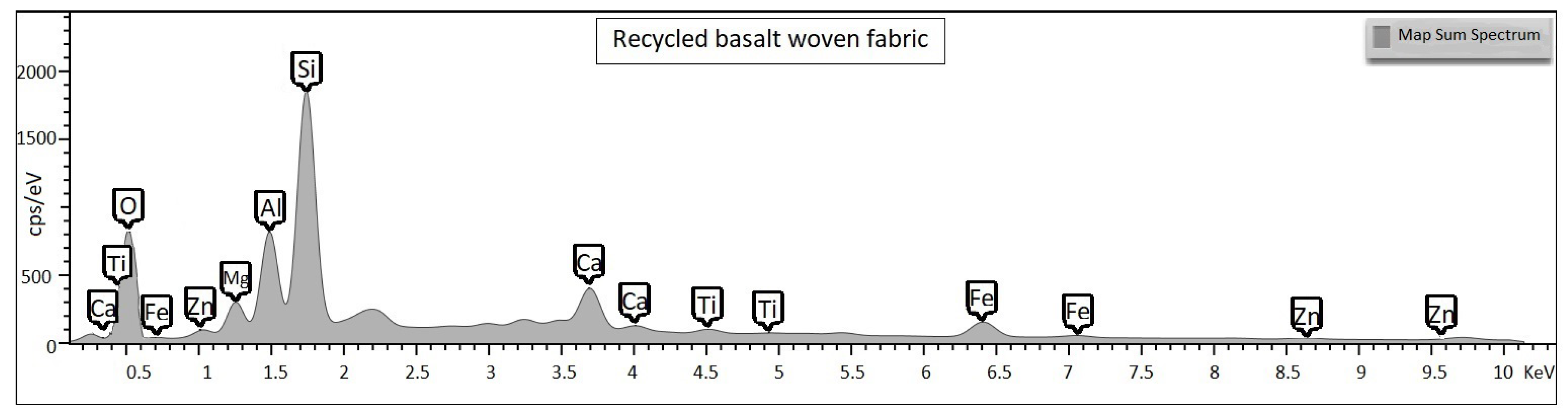
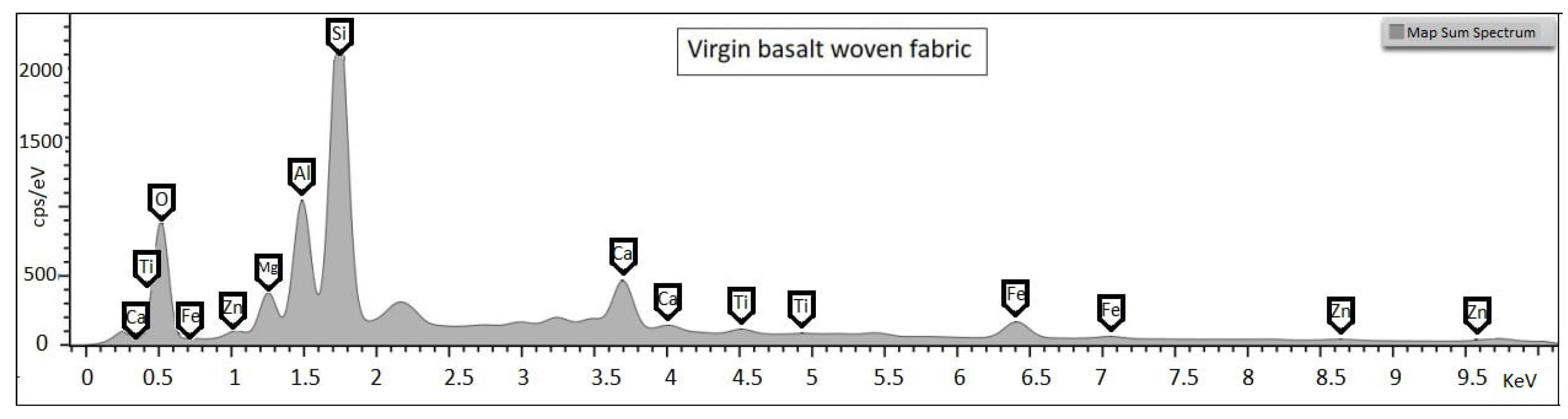
Basalt is a volcanic rock among the most common minerals on the Earth surface. It is mainly composed of oxides such as
,
, CaO, MgO and other oxides in concentrations of less than 5% (
Figure 1) [
1].
Basalt fibers can be directly produced from basalt rock, following a manufacturing process without additives, so they are considered natural fibers, even if they are not effectively biodegradable [
2]. Basalt continuous fibers are extruded through small nozzles directly by crushed and melted basalt rock (melting temperature around 1350–1420 °C). Energy and cost requirements of basalt production are higher than E-glass (E-GF) but similar to S-glass (S-GF); however, the high availability of the raw material and the fact that, unlike GF, it does not require additives, as mentioned before, makes the manufacturing process cheaper and more sustainable [
3,
4,
5].
In comparison with glass and carbon fibers some considerations must be pointed out concerning mechanical and thermal features. Although BFs has al slightly higher density than glass and carbon fibers (CF), tensile strength is higher than E-GFs and S-GFs, while it is still comparable to that of CF (
Table 1). Furthermore, basalt fibers are also stiffer than glass fibers but less stiff than carbon fibers (
Table 1). Elongation at break of basalt fibers (BF) is higher than that of glass and carbon fibers (
Table 1). Recent studies by Plappert et al. investigated tension, compression, and shear of BFs and epoxy specimens and confirmed the abovementioned [
6].
Furthermore, basalt fibers have good thermal and chemical properties, such as resistance to heat and a good chemical inertia [
7,
8]. In fact, basalt products do not generate toxic reactions with air or water, during their life cycle; they are non-combustible and in contact with other chemicals they do not activate chemical reactions that can harm the environment [
5].
Such material is interesting due to its function as reinforcement of thermosetting polymers; examples are epoxy, polyester and vinyl ester resins [
9]. BFs have, indeed, an excellent bonding nature, so when embedded within an organic binder, composite materials with great wear resistance and synergistic effects are obtained [
10]. Additionally, basalt fibers and glass fibers are characterized by the need for a sizing agent to ensure profitability, processability and performance. The sizing agent is designed to form a thin layer to protect, lubricate and hold together fibers during transportation and weaving; it also acts as a coupling agent for a better adhesion of the matrix to the fibers [
11]. Mechanical properties of ceramic fibers are flaw-dependant due to their brittle failure—in fact, the reduction in their properties is often attributed to the growth of surface flaws for handling without appropriate lubrication, degradation of the sizing with thermal or chemical methods [
12].
Basalt fibers are often adopted in marine application thanks to their lightness and their corrosion resistance. As shown in the research of Lu et al., basalt has good durability in seawater; for an immersion time in artificial seawater of 15 days or less at 20 °C, tensile strength is preserved, and elongation at break also slightly decreases after 30 days. The epoxy matrix protects the basalt fibers after salted water desorption thanks to the post-curing process and salt precipitation [
13]. Another piece of research, by Sukur and Onal, on BFRP for marine applications demonstrates the benefits of graphene nanoplatelet modification of the epoxy matrix which leads to less water absorption and basalt fiber’s properties preservation [
14].
Moreover, basalt fibers are often used in manufacturing of protective equipment for firefighters, and of whatsoever requires proper fire resistance and high melting temperature [
15]. Actually, basalt fibers have a wider temperature range of stability than most ceramics implied in composite materials [
16]. Sim et al. have tested basalt fibers at high temperature environment (1200 °C for 2 h), representative of a fire event. Compared to carbon and glass fibers that lose their volumetric stability, basalt fibers maintained their shape and mechanical integrity. Thermal stability and heat resistance is a consequence of basalt mineralogical and chemical composition and presence of micorpores [
17,
18]. The relation between the surface modifications of basalt fibers and their thermal and fire properties in flame-retardant epoxy composites was investigated by Guo et al. in more recent studies. The importance of an optimized pre-treatment of BF surface is highlighted for best performance in flame-retardant epoxy composites [
19].
However, the environmental impact of waste BFRP disposal, such as that of glass and carbon fiber reinforced polymer, is huge. The estimated time of the BFRP degradation process is about 50–100 years. Besides landfilling, incineration treatment could be another solution to dispose of basalt fibers; however, one downside of this method is is that it produces harmful air pollution. Some of the toxic compounds found in the burning smoke are nitrogen oxides, chlorinated hydrocarbons, sulphur dioxide and dioxins [
20].
For this purpose, new eco-friendly methods of recycling have been developed—including methods which guarantee the possibility of reusing basalt fabric—for different applications to reduce the environmental footprint of products. A mechanical recycling technique allows the same original chemical structure of molecules to be retained. This method could be a perfect sustainable option because it does not create or use dangerous substances; in addition, it requires a small amount of energy and air pollution is not generated.
However, short fibers are the final result of this recycling process; chopped fibers can be used as an additive charge for geopolymers and as fillers for products with lower mechanical properties (e.g., reinforced concretes or mats) [
21].
Two other possible approaches are thermal and chemical techniques. The most diffused type of thermal recovery of the fibers consists of incineration of the polymer matrix; microwave pyrolysis is another example that could minimize energy consumption and avoid secondary air pollution. However, recycling methods through pyrolysis may cause a drop in the mechanical properties of basalt fibers up to 65% due to the activation of surface flaw growth and degradation of the sizing [
12]. Previous studies have shown that it is possible to regain up to 94% of initial mechanical strength by a chemical regeneration process in a cloridric acid (HCl) and sodium hydroxide (NaOH) solution [
22]. Basalt fibers remelting is also a possible recycling option, as long as the woven fabrics, rowings, yarns or single fibers are completely free from any residual matrix.
Researchers have developed efficient chemical recycling mechanisms for basalt fiber thermoplastic composites that completely remove the matrix from the woven fabric: methods of solvolysis in acetone with ultrasound and mechanical stirring were applied to an acrylate-based basalt composite obtaining well recycled fibers [
23].
On the other hand, concerning the degradation of thermosetting polymeric matrices, previous analyses had mainly focused on carbon fibers epoxy composites (CFRP) with examples of both chemical degradation, such as acid digestion and depolymerization [
24,
25]. Several studies have investigated recycling through solvolysis of thermosetting matrices but none, to the authors’ knowledge, have investigated the effects of this chemical treatment on the mechanical properties of BFs. The durability of basalt in alkaline environment has been studied by Wu et al. and they confirmed that their resistance is relatively weak due to the reactions between silica of the fibers and hydroxyl groups [
26]. Considering basalt behavior with strong bases, acid treatment is preferable to alkaline treatment. Among all the possible acid options, peracetic acid, obtained from the reaction between acetic acid and hydrogen peroxide is strong enough to allow for matrix degradation.
In this document, an optimized version of the recycling processes by Ma and Nutt and Das et al. has been studied and applied to BF/epoxy composites in order to assess and test the quality of the recovered fibers [
24,
25]. The experiments were based on the oxidative degradation of the epoxy matrix with peracetic acid (PAA), taking advantage of the good chemical resistance of basalt fibers. A chemical pre-treatment was examined, but a thermal pre-treatment was considered way more eco-friendly as it allows for other reagents to be avoided and minimizes energy consumption estimated by an LCA of the process; moreover, it also leads to potential for industrial scaling of the experiment.
The laminated composite specimen (basalt/epoxy) underwent a curing process at room temperature and was then heated above glass transition temperature into a laboratory oven. This pre-treatment ensures the swelling of epoxy to enhance diffusion of PAA into the matrix. The chemical solvolysis was carried out immediately afterwards. At the end of the recycling process tensile tests were performed on the recovered fibers following ASTM D2256 indications, to establish a possible reduction in terms of mechanical properties [
27,
28,
29]. Finally, SEM analyses were carried out to ensure the efficacy of the implemented chemical technique and to verify that there was no trace of epoxy resin on the surface of the single strands.
The outcomes of this work provide information about an efficient way to recover woven basalt fibers. The recycled specimen kept the original weaving after solvolysis method. Further, the breaking tenacity loss registered through mechanical tests was investigated per standard ASTM D2256 and the chemical composition of the fabric was examined. The process scheme is shown in
Figure 2. This research aims to provide a valid recycling alternative for BFRP. The impact of this research is promoting the use of natural fibers with excellent properties—hence basalt fibers—in polymer reinforcement by demonstrating the possibility of fiber recovery. Hereinafter, an efficient chemical recycling method of BFRP is presented: mechanical property maintenance is proved, and a weaving pattern of the specimen tested is retained. The method involves the use of weak acids and peroxides, less toxic to people and less harmful to the environment than the chemicals often used in recycling processes. The possibility of obtaining resin free recycled woven fabrics may allow both the re-melting and re-lamination of the recovered basalt reinforces.
2. Materials and Methods
2.1. Raw Materials
The recycling process is tested on commercial-grade basalt fibers, supplied by Isomatex (Gembloux–Belgium), fabric reference:
WFBSB3_0200.
XXX.0127.
T2/2.
ISAA. The fabric surface weight is 200 g/cm
3 (EN 12127) with elementary diameters from 9.0 to 11.0 µm, volume density of constituent yarns of 2.600 gr/cm
3 (according to ASTM C693), and twill pattern [
30]. The original size of the basalt fabric tested are about 210 × 750 mm
2.
The epoxy resin used is SR GreenPoxy 33 (resin and hardener reference: SZ 8525), produced by Sicomin (Chateauneuf les Martigues—France). The resin has a density of 1.159 g/cm3, while the hardener has a density of 0.94 g/cm3 at 20 °C. Resin and hardner mix viscosity at T = 20 °C is 1300 mPa s. Curing was carried out at a 20 °C temperature: extimated gel time of 1.5 h and completely cured after 6.3 h. Glass transition temperature of the cured resin is 80 °C after a 20 °C curing process. It has a bio-based carbon content of about 34–36%.
2.2. Lamination
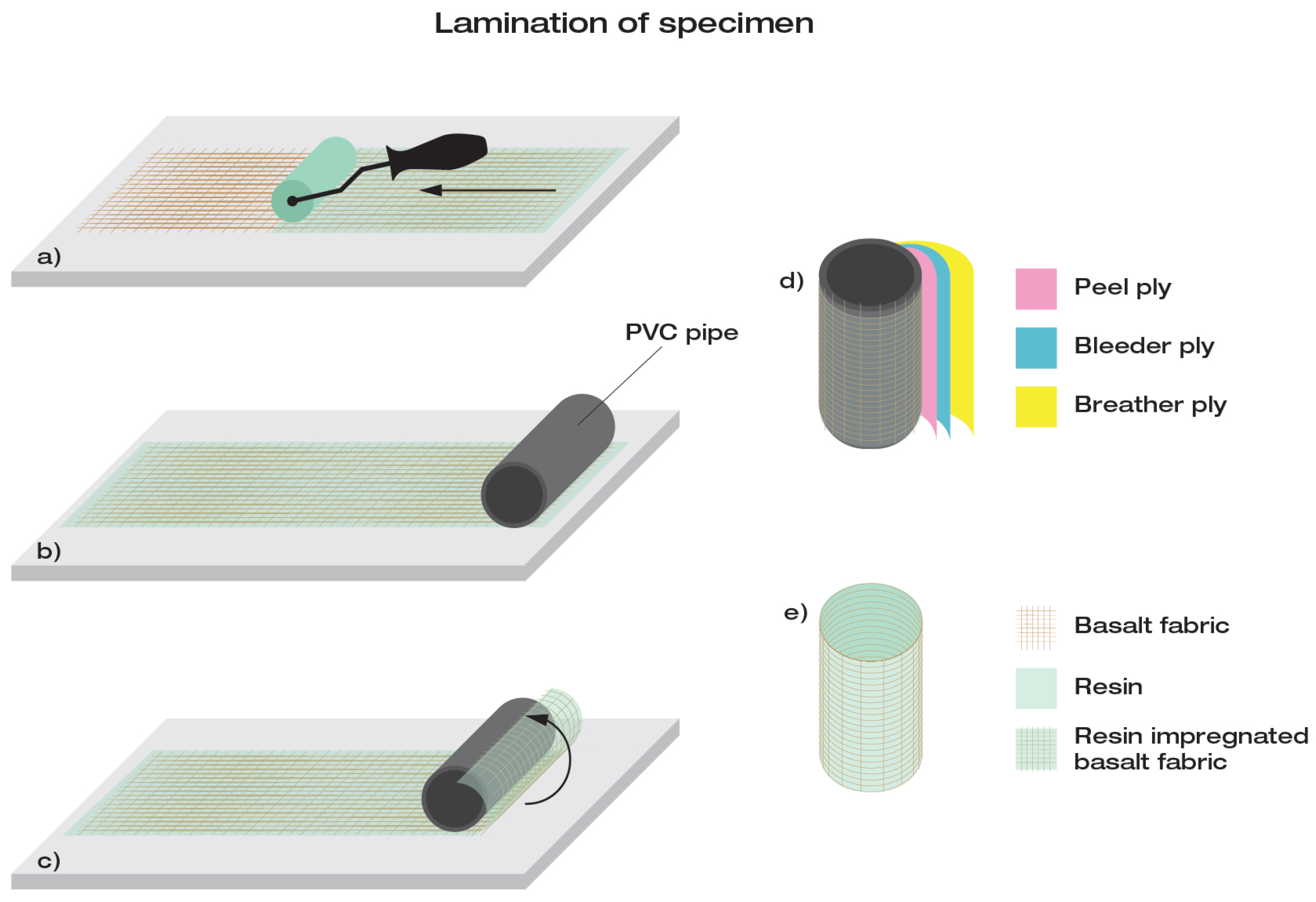
A hand lay-up lamination process was carried out to obtain the composite specimen. A cylindrical mould in PVC, coated with a wax release agent to facilitate the detachment of the sample, was used to shape a specimen suitable for chemical laboratory beakers. The hand lay up (HLU) lamination process, shown in
Figure 3, is a practical and cheaper way to mould a wide range of geometries.
Liquid resin was poured and spread over the 710 × 250 mm2 piece of basalt fabric with rollers and spatulas to ensure a correct and homogeneous fiber wetting. The fabric was then rolled on the mould; peel-ply was applied to guarantee a better surface finish, then bleeding ply and breather ply were applied to absorb the excess resin and shield the specimen from the environment during curing. Curing took place at room temperature for more than 24 h.
The result was a composite sample in a cylindrical shape with an internal diameter of 70 mm and a thickness of 0.65 mm.
2.3. Pre-Treatment
As shown in previous studies, the acid digestion rate was mainly diffusion controlled. Before the immersion of the specimen in the acid treatment solution, a swelling pre-treatment was needed to further enhance the degradation mechanism of the matrix by the acid solution and speed up the process time [
24]. The specimen is heated above the resin glass transition temperature (Tg = 80 °C) in a warmed-up heater for 60 min at 90 °C. This pre-treatment could be used on every type of ceramic fiber because it is relevant to the resin behaviour only. Epoxy resin at room temperature behaves as a compact and fragile solid, while over the Tg, the free volume exponentially increases among the polymeric chains. This phenomenon changes the material appearance, as it becomes a rubbery solid and allows a faster and more efficient diffusion of the treatment solution throughout the matrix.
Immediately after removing the specimen from the heater, it was rapidly immersed in the treatment solution.
2.4. Treatment
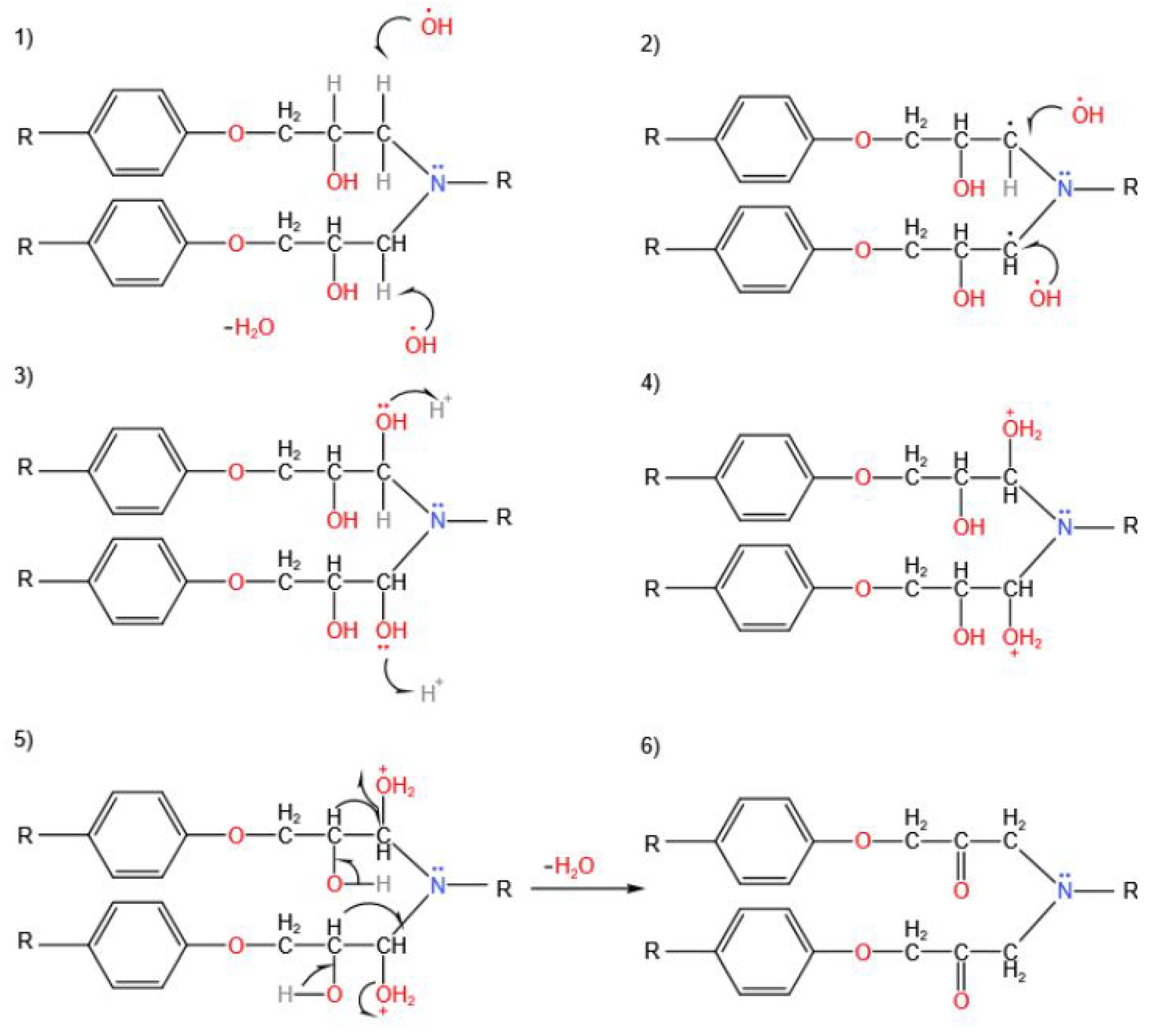
The epoxy decomposition consists of the oxidation of C-OH groups to C=O and in the breaking of the C-N bonds. The free radical mechanism is initiated by both H-O radicals generated by hydrogen peroxide and the acyloxy radicals from the peracetic acid. The mechanism can be described as follows:
The O-O bond in paracetic acid is unstable, so it generates the radicals from the PAA (1):
The hydroxyl radical reacts with the paracetic acid itself (2) and (3):
Acyloxy radicals decompose (4) and (5):
The methyl radical now formed is also unstable, and so it reacts with oxygen to form the peroxy radical (6).
The free radicals re-create the PAA (7):
At this stage, the formation of phenols and phenolic derivatives occurs during the decomposition reaction.
The C–N bond is a weak bond and so it is attacked by the acyloxy (
Figure 4) and the hydroxyl (
Figure 5).
The acid solution, composed of 91% vol of acetic acid (AcOH 14M) and 9% of hydrogen peroxide 30% wt), is prepared in a beaker covered with aluminium foil to avoid extended evaporation. The use of this set up keeps the largest quantity of the solution in the beaker for future recycling processes.
The solution was positioned on a magnetic-stirred thermal plate at maximum power. When a temperature of 110 °C ± 5 °C was reached, the specimen was put inside the beaker and the treatment began.
The solution temperature was maintained at 110 °C for 1.5 h until the polymer matrix was fully dissolved and the matrix was visibly free from it.
2.5. Post-Treatment
After the required time for solvolysis, the beaker is cooled in a water crystallizer at room temperature. The solution is collected for possible future distillation and the recovered fabric was washed first in abundant acetone (98% vol) to remove matrix residues and then in distilled water to neutralize the surface. Subsequently, the specimen was unrolled to obtain a basalt fabric as compact and regular as possible. Then, it was dabbed with absorbent paper. After 24 h of drying at room temperature, the specimen was ready for mechanical testing.
2.6. Mechanical Testing
From the variation of the mechanical properties, it is possible to determine the efficiency of the recycling method: for this reason, tensile tests are performed both on virgin and recycled fiber strands, under the same environmental condition, to obtain concrete reference values in relation to the machinery used.
The indications of ASTM D2256 have been followed, so a preconditioning of the fibers before the tensile tests could be carried out [
27,
28,
29]. Preconditioning consists of placing the fabric in a heater at 110 °C ± 1 °C at atmospheric pressure for 60 min to eliminate every trace of humidity.
Once the basalt fabrics were unrolled, longitudinal fiber strands were carefully pulled out in the direction of extraction with the aid of forceps. Ten fiber strands for both recycled and virgin fabric were weighed and measured in length.
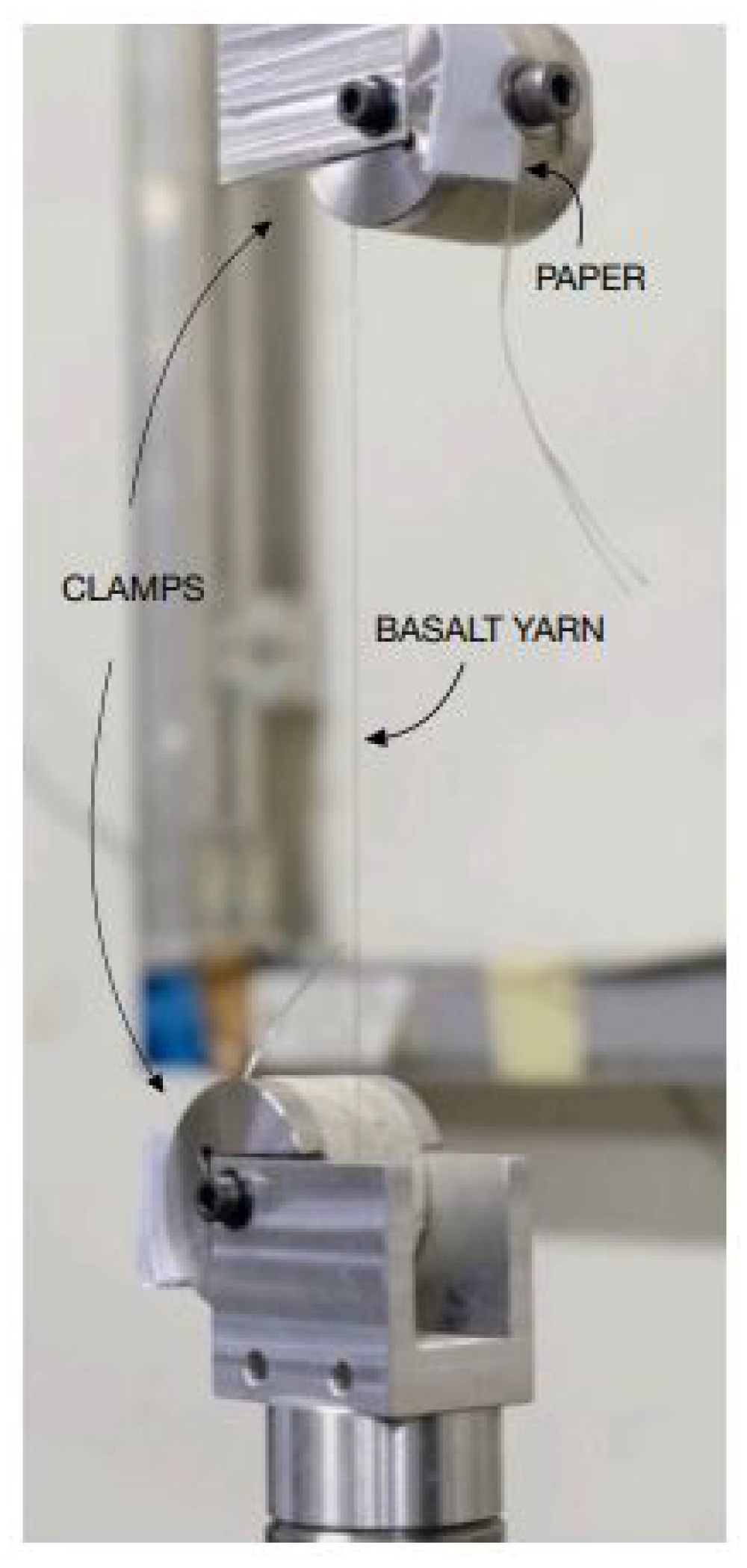
Then, every single yarn was placed in an MTS QTest/10, the tensile testing machine, at a gauge length of 200 mm, aligned exactly parallel to the applied force, and held in position by the pressure of a screw, squeezing it on the surface of the 25 mm-radius cylindrical clamps. Two layers of paper, inserted between the fiber and the surface of the cylinder and the screw, were functional to prevent the fiber from slipping and being damaged by the screw thread (
Figure 6). Each fiber yarn was pre-tensioned with a load of approximately 3.532 N and then subjected to traction at an elongation rate of 45,000 mm/min.
According to ASTM D2256, tensile tests were performed until five valid specimens were obtained for both virgin and recycled yarns. The samples that slipped during the test and/or those that broke near the clamp attachments were excluded from the data processing.
2.7. SEM Analysis
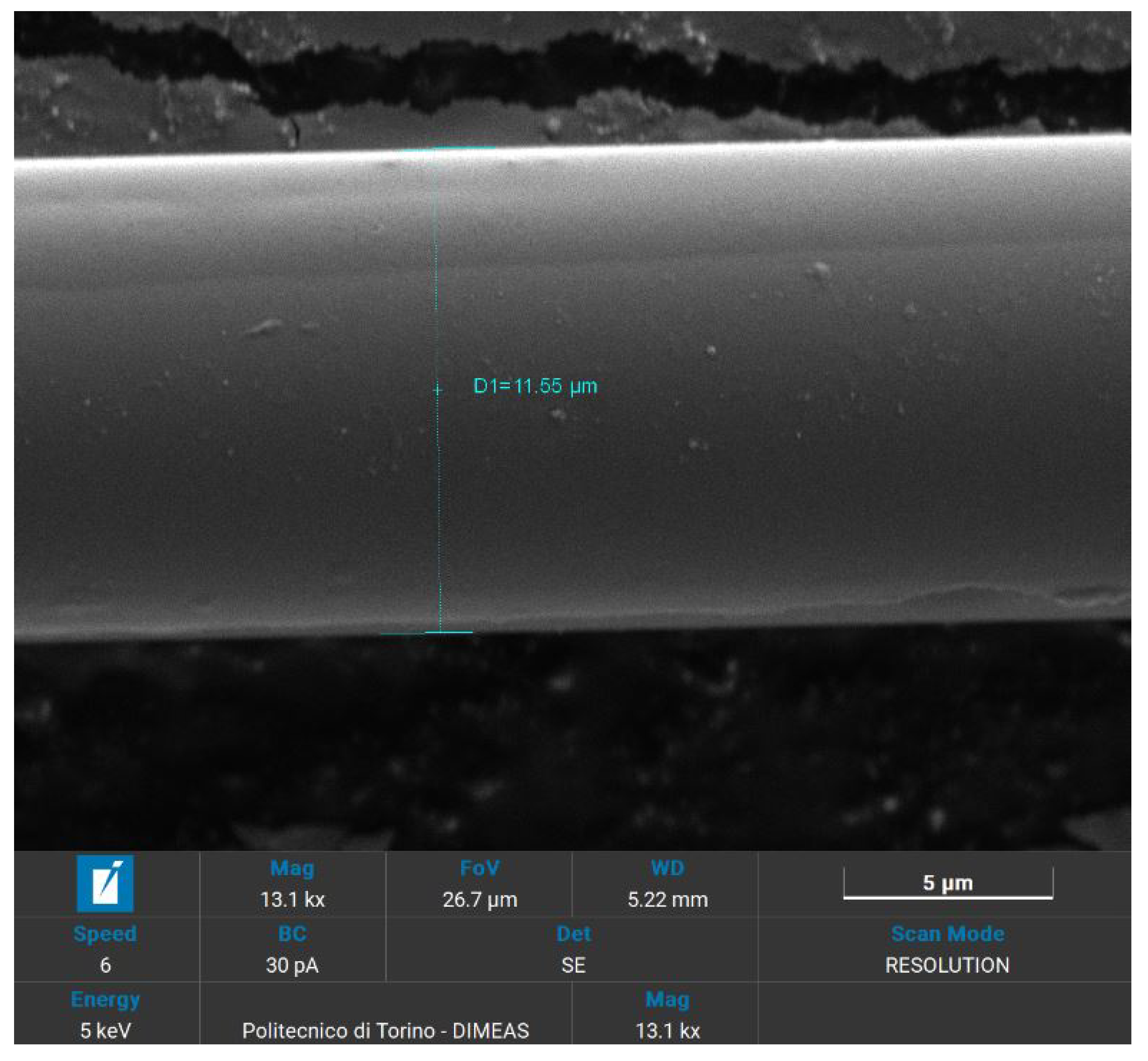
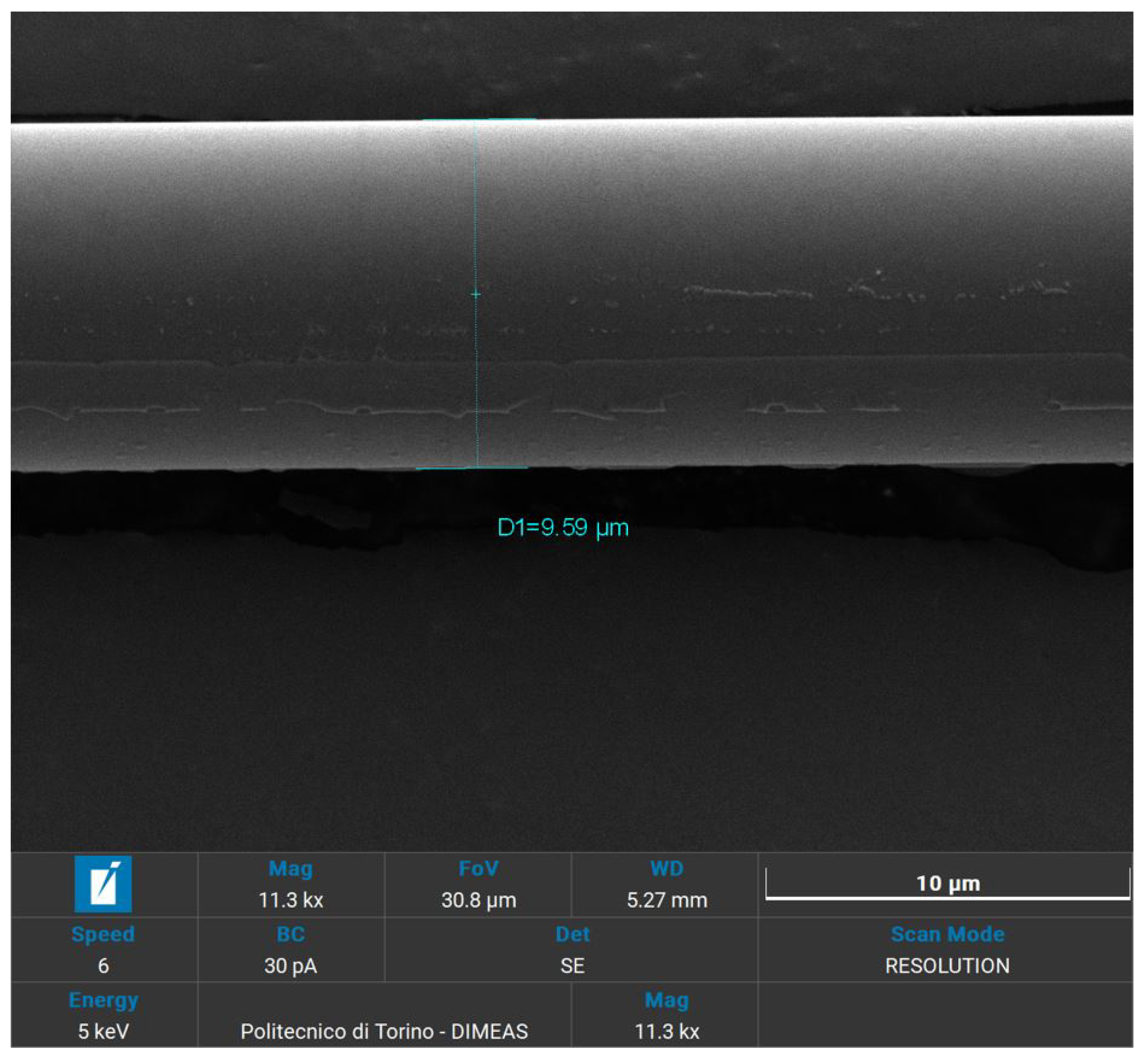
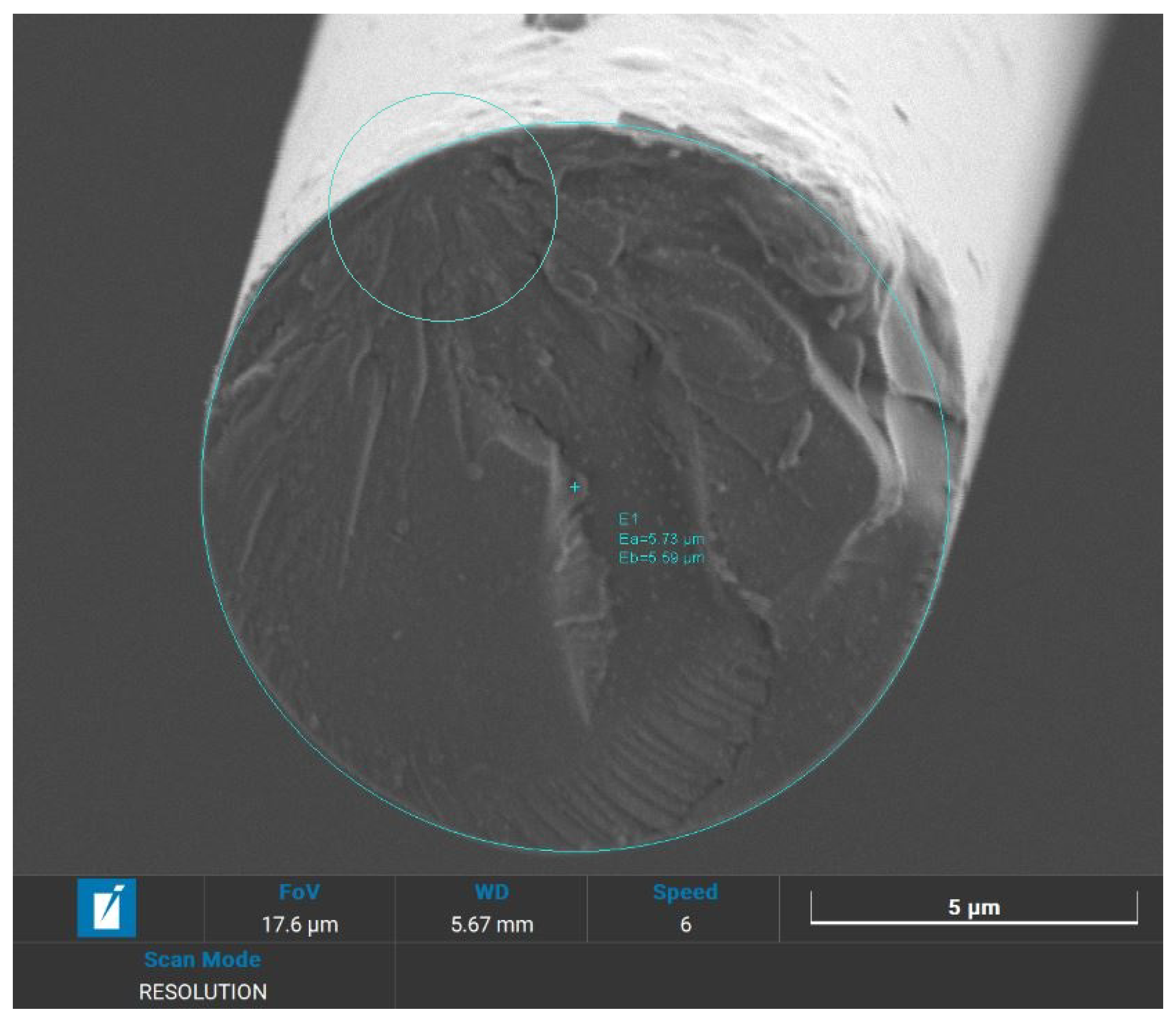
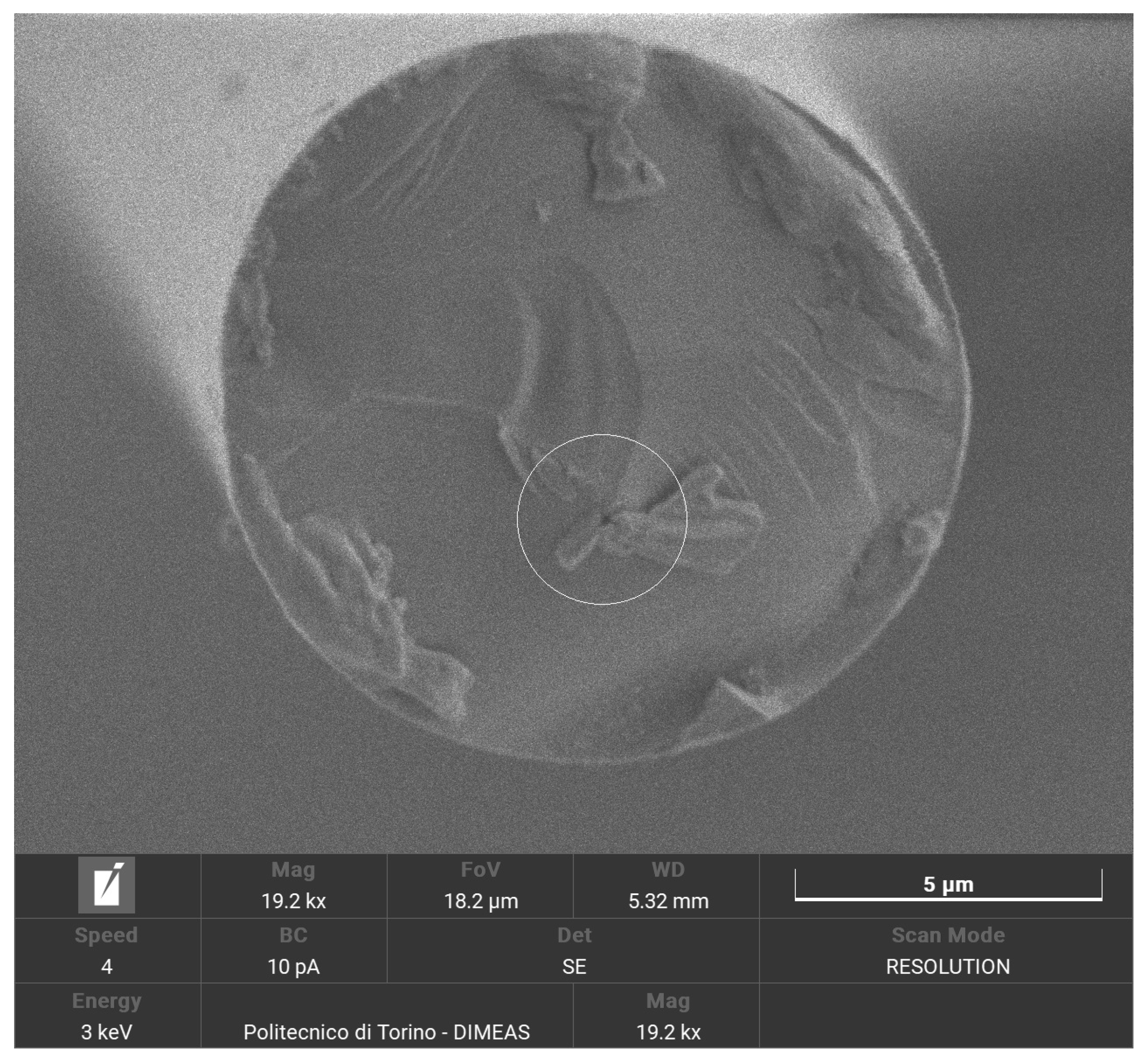
In preparation for SEM analysis, as basalt fibers were not conductive themselves, selected samples were covered with a layer of gold with a thickness of 5 nm, by means of a Quorum Technologies Q150R S/E/ES sputter. To achieve the typical thickness of 5 nm used for SEM applications, a current of 20 mA and sputter time of 15 s were set. The samples chosen for analysis are the recycled and the virgin woven fabrics, the lateral surface of the fibers and their fracture surface.
All the specimens were analysed using a high-resolution SEM (Tescan Vega) that also provides a qualitative chemical EDS analysis. These visual and chemical analyses investigate the surface morphology, the appearance of resin, eventual damages or impurities that caused fracture [
31].
4. Conclusions
Nowadays, many governments place restrictions on landfilling of waste composite material, so investigating different recycling methods could be not only interesting from a scientific standpoint, but also crucial for the environment.
Thermal recycling method is easier and faster, nevertheless basalt fibers demonstrate a significant loss of mechanical properties due to the temperature range usually adopted: high temperatures and sizing pyrolysis cause a massive growth of not negligible surface defects, leading to the loss of 65% of original mechanical properties. On the other hand, a chemical treatment, such as solvolysis, is less invasive to the fibers, allowing a better maintenance of mechanical properties.
The results reported prove the potential of recycling BFRP by solvolysis: breaking tenacity is kept up to 90.5% compared to the untreated corresponding fibers. SEM analyses proved that surface and bulk flaws cause brittle failure. An increase of surface flaws is thought to be the consequence of handling, lamination and only at last of recycling process. Basalt fibers, indeed, have a high acid resistance and are not affected by mild acids, due to their chemical inertia.
The presented experimental study provides accurate information about the possibility to produce a recycled basalt fabric with a clean and resin-free superficial morphology and with the same original weaving. Thanks to the small reduction in mechanical properties, recovered fibers could be re-used for new composites.
The innovative aspect of this recycling process is the usage of a weak acid that could be recovered from the solution through distillation. Future studies will be related to a distillation process of the thermosetting matrix: the authors’ primary intention is developing a complete recycling process of the whole composite produced. Pursuing this further, the circularity of the described process makes it worthwhile for an industrial scale implementation.
In conclusion, concerning the lack of information in the literature, more research on eco-friendly methods for BFRP recycling should be carried out, in preparation for the future widespread use of this natural fiber with high mechanical properties.