Flax Noils as a Source of Cellulose for the Production of Lyocell Fibers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of Solutions
2.2.2. Rheological Measurements
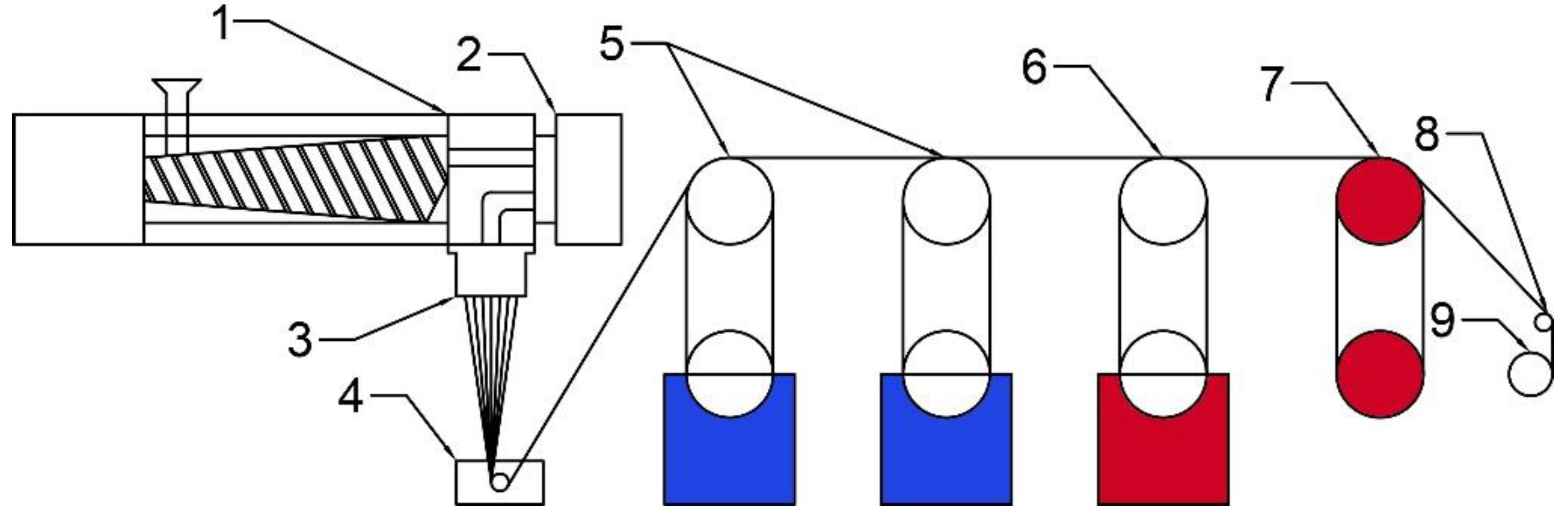
2.2.3. Fiber Spinning
2.2.4. Mechanical Testing
2.2.5. Structural and Morphological Characterization
2.2.6. Thermal Characterization
3. Results and Discussion
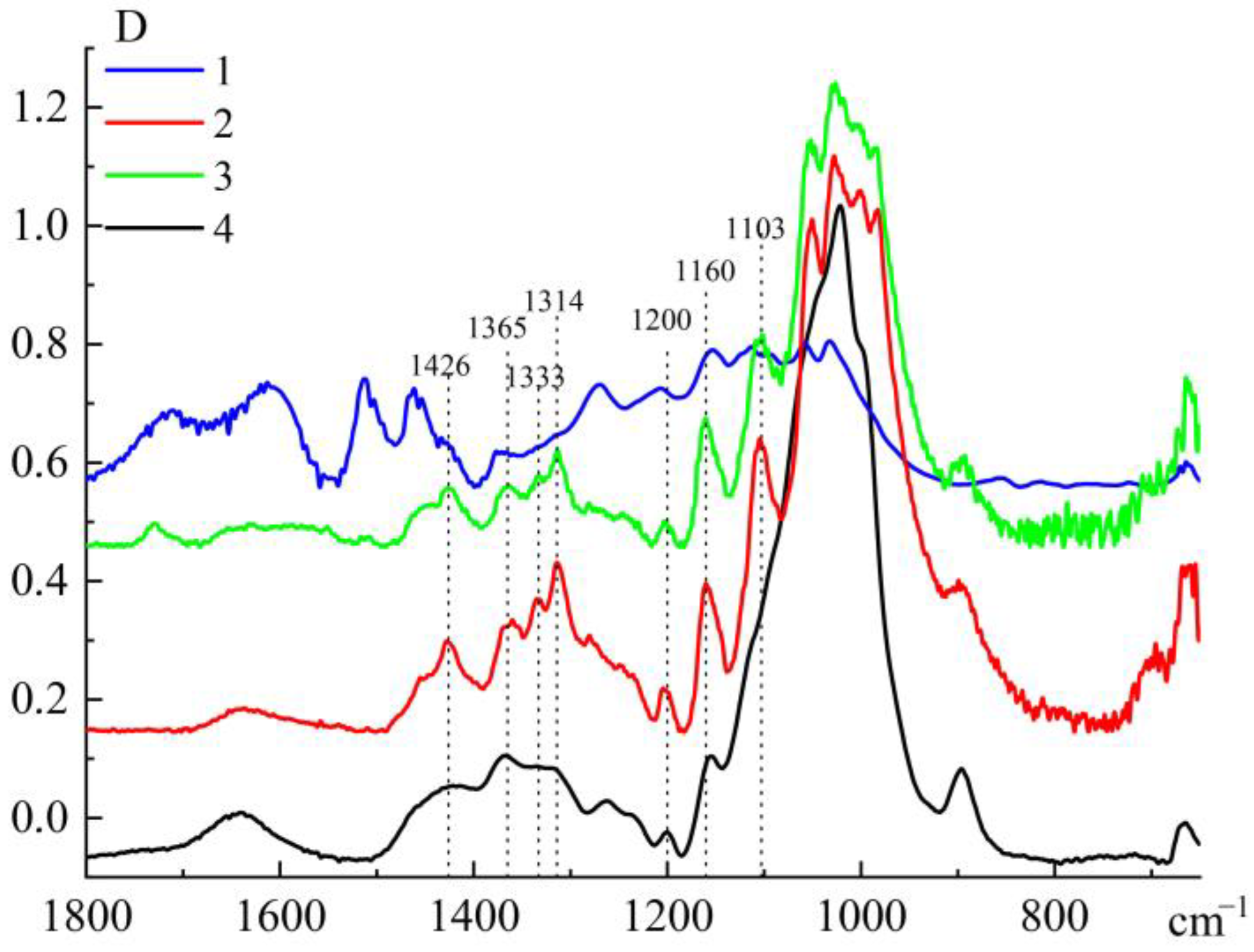
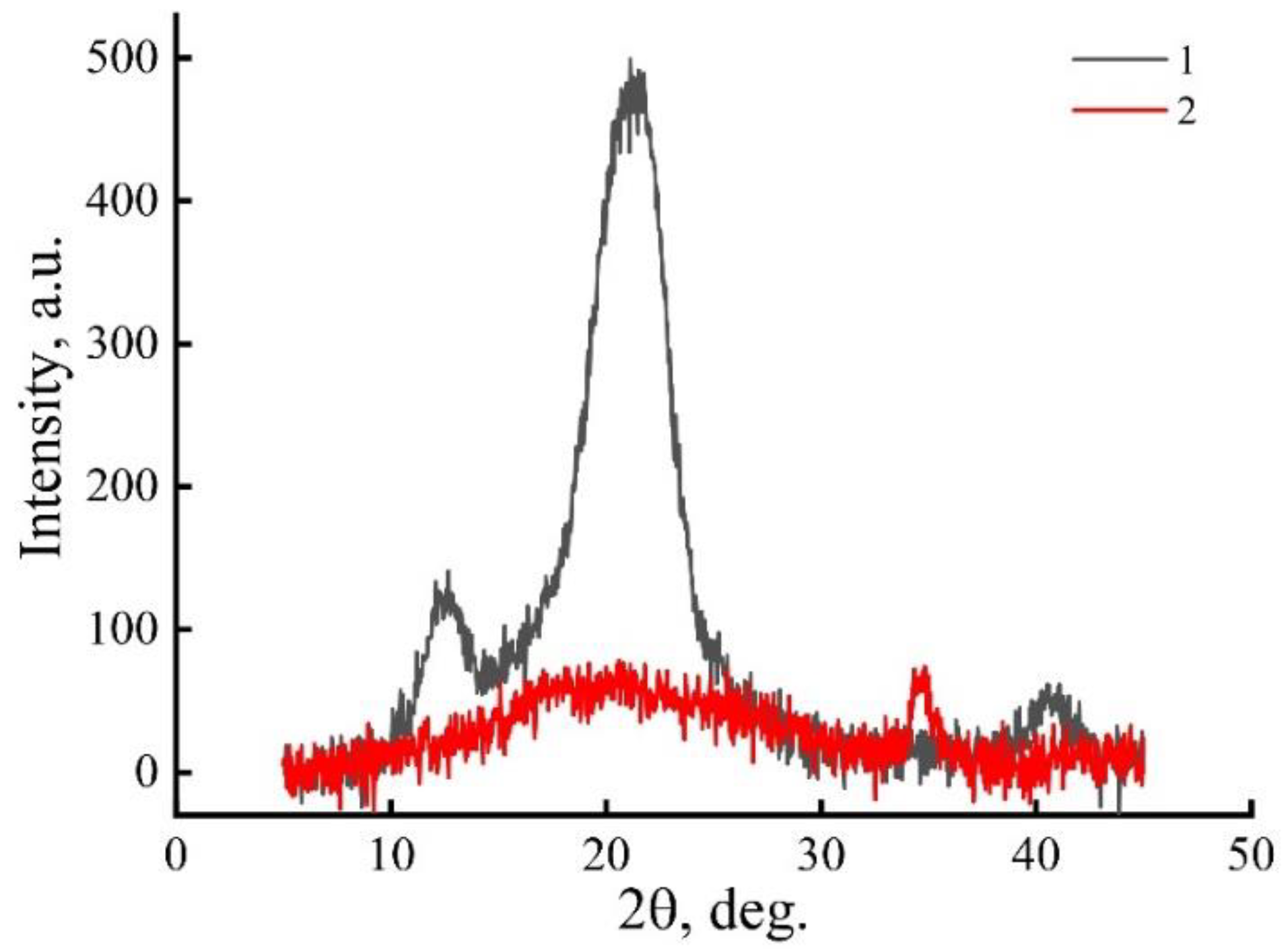
3.1. Structural Analysis
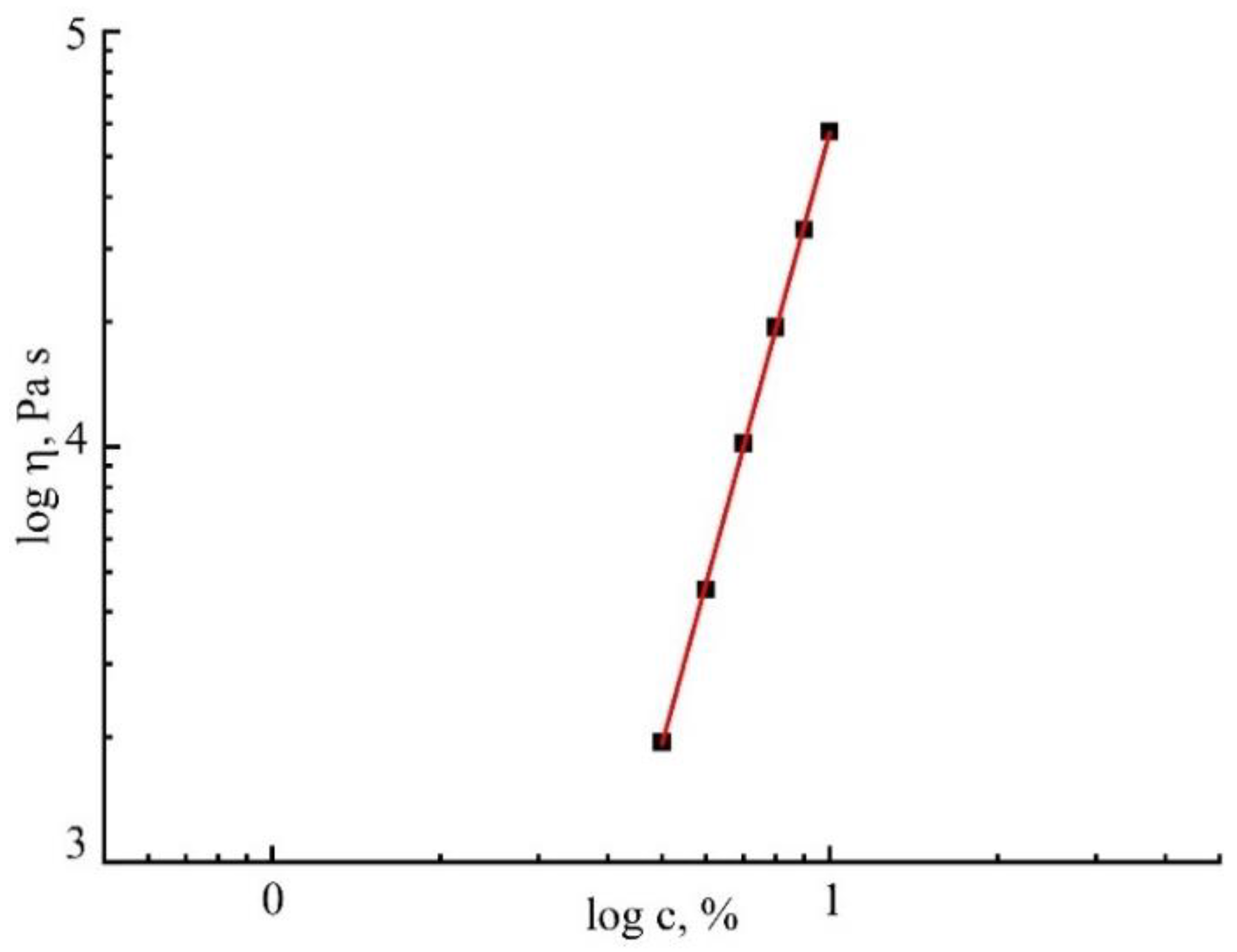
3.2. Rheology
3.3. Fiber Spinning
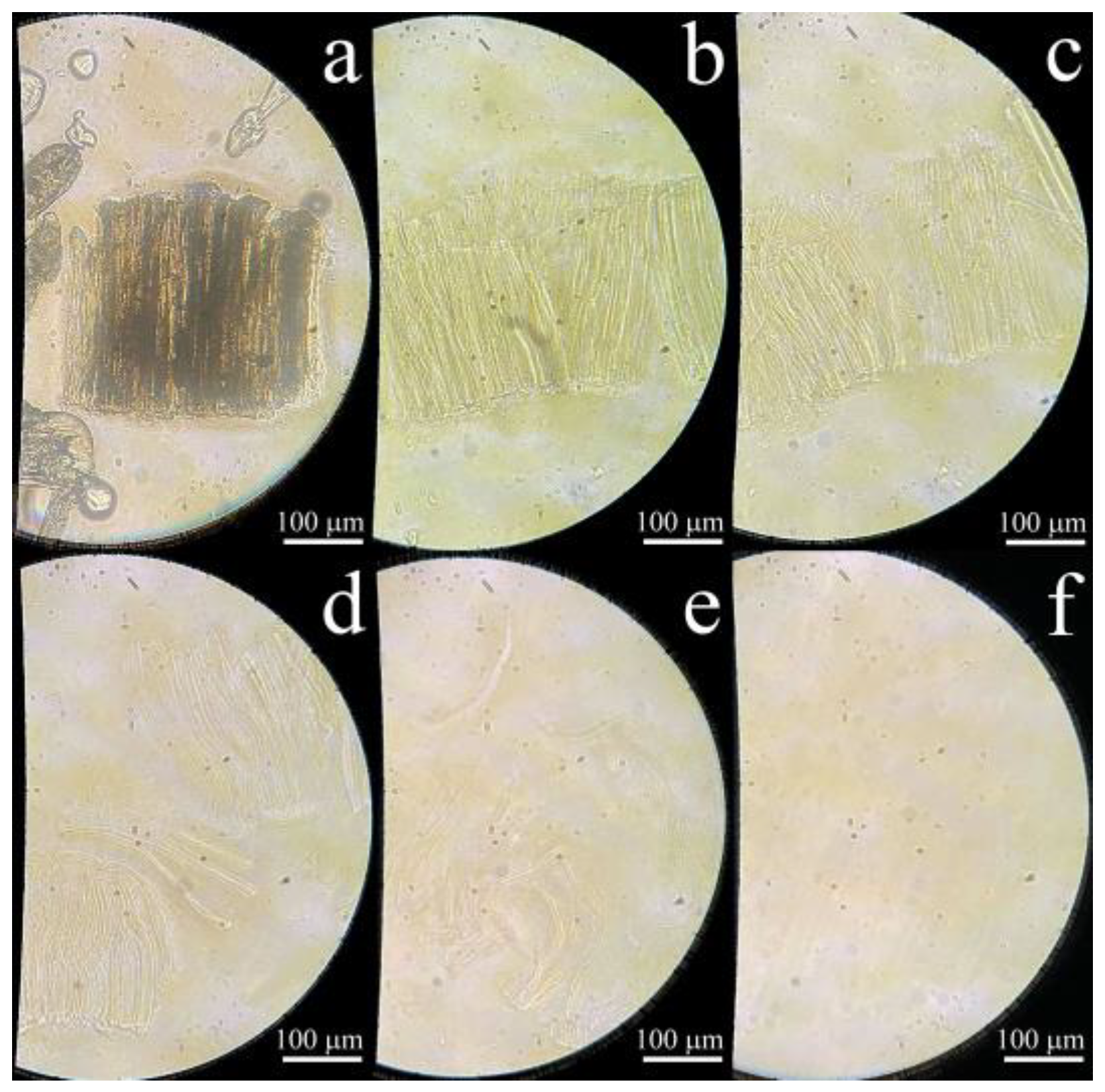
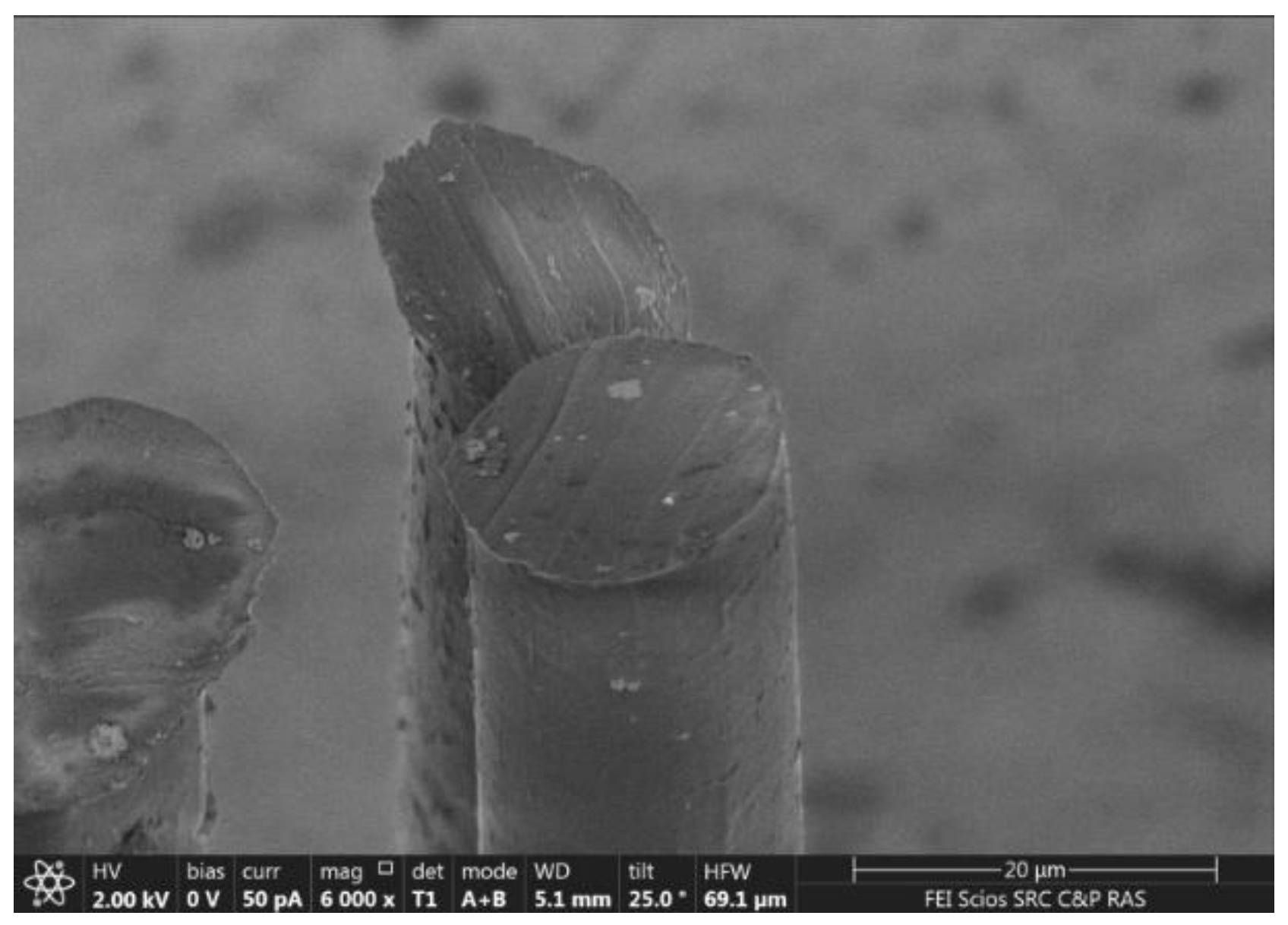
3.4. Structure and Morphology of Fibers
3.5. Mechanical Properties
3.6. Thermal Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taurbekov, A.T.; Mansurov, Z.A.; Chernoglazova, T.V. Obtaining Cellulose Fibers from the Fallen Leaves of the Elm. Ann. Chem. Sci. Res. 2021, 2, 1–5. [Google Scholar]
- Krasovskii, A.; Maus, V.; Yowargana, P.; Pietsch, S.; Rautiainen, M. Monitoring Deforestation in Rainforests Using Satellite Data: A Pilot Study from Kalimantan, Indonesia. Forests 2018, 9, 389. [Google Scholar] [CrossRef] [Green Version]
- Baccini, A.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A.; et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2012, 3, 182–185. [Google Scholar] [CrossRef]
- Gukezheva, M.K. Prospects of cotton import substitution in the national textile industry. Eurasian Sci. J. 2020, 1, 1–9. Available online: https://esj.today/PDF/80ECVN120.pdf (accessed on 10 April 2022).
- Christie, R.M. Colour Chemistry; Royal Society of Chemistry: Cambridge, UK, 2001; p. 205. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, F. Advanced Hierarchical Nanostructured Materials; John Wiley & Sons: Hoboken, NJ, USA, 2014; p. 512. [Google Scholar] [CrossRef]
- Kudela, J.; Kurjatko, S. Wood Structure and Properties ’02; Arbora Publishers: Zvolen, Slovakia, 2002; p. 221. [Google Scholar]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Egorov, Y.A.; Kulichikhin, V.G.; Mikhailov, Y.M. New Hydrated Cellulose Fiber Based on Flax Cellulose. Russ. J. Gen. Chem. 2021, 91, 1807–1815. [Google Scholar] [CrossRef]
- Dai, J.; Dong, H. Intensive cotton farming technologies in China: Achievements, challenges and countermeasures. Field Crops Res. 2014, 155, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Bowyer, J.L.; Stockmann, V.E. An exciting bio-based raw material for the global panels industry. For. Prod. J. 2001, 49, 10–21. [Google Scholar]
- Gismatulina, Y.A. Obtaining cellulose by the nitrate method directly from flax straw. Polzunov. Vestn. 2014, 3, 160–163. [Google Scholar]
- Ainullov, R.K.; Kostochko, A.V.; Valishina, Z.T.; Popov, D.V.; Aleksandrov, A.A. Method for Obtaining Bleached Hemp. Pulp. Patent RF 2735263, 3 April 2020. [Google Scholar]
- Shulzhenko, D.V.; Bessonova, I.Y.; Azanov, M.V.; Dyachenko, L.R.; Fadeev, B.A.; Tyurin, E.T.; Zuykov, A.A. Method for Obtaining Cellulose from Miscanthus for Chemical. Processing. Patent RF 2763880C1, 11 January 2022. [Google Scholar]
- Izgorodin, A.K.; Konoplev, Y.V.; Zakharov, A.G.; Prusov, A.N.; Voronova, M.I.; Volkova, I. Study of the possible use of intermediate flax as raw material for production of cellulose. Fibre Chem. 2004, 36, 343–347. [Google Scholar] [CrossRef]
- Levdansky, V.A.; Levdansky, A.V.; Kuznetsov, B.N. A method for obtaining a cellulose product from flax with a high content of alpha cellulose. J. Sib. Fed. Univ. Chem. 2014, 7, 63–70. [Google Scholar]
- Wiener, J.; Kovacic, V.; Dejlova, P. Differences between flax and hemp. AUTEX Res. J. 2003, 3, 58–63. [Google Scholar]
- Lukanin, E.A.; Egorov, D.A. Cellulosic Product with an alpha-Cellulose Content of 98.5% or More and an Industrial Method for Its. Production. Patent RF 2703250, 15 October 2019. [Google Scholar]
- Galashina, V.N.; Moryganov, A.P.; Zakharov, A.G.; Danilov, A.R.; Gataulin, A.M. Method for Obtaining Purified Flax. Fiber. Patent RF 2347862, 27 February 2009. [Google Scholar]
- Prusov, A.N.; Prusova, S.M.; Zakharov, A.G.; Bazanov, A.V.; Smirnov, P.R.; Radugin, M.V. Chemical transformation of technical fiber of flax, hemp and jute to cellulose and their pyrolysis. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 2016, 59, 97–104. [Google Scholar] [CrossRef]
- Veselov, V.M.; Abramov, Y.K.; Zalevsky, V.M.; Tamurka, V.G.; Volodin, V.S.; Evdokimov, V.D.; Mironov, B.I.; Vatueva, O.B.; Gukasov, N.A.; Marshannikova, L.M.; et al. Method for Obtaining. Cellulose. Patent RF 2574958, 10 February 2016. [Google Scholar]
- Sjostrom, E. Wood Chemistry: Fundamentals and Applications; Elsevier: London, UK, 2013; p. 293. [Google Scholar]
- Bochek, A.M.; Shevchuk, I.L.; Lavrent’ev, V.N. Fabrication of Microcrystalline and Powdered Cellulose from Short Flax Fiber and Flax Straw. Russ. J. Appl. Chem. 2003, 76, 1679–1682. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Mironova, M.V.; Arkharova, N.A.; Klechkovskaya, V.V.; Kulichikhin, V.G. Morphological Transformations in the Process of Coagulation of Cellulose Solution in N-Methylmorpholine N-Oxide with Isobutanol. Polym. Sci. Ser. C 2021, 63, 161–169. [Google Scholar] [CrossRef]
- Yudianti, R.; Syampurwadi, A.; Onggo, H.; Karina, M.; Uyama, H.; Azuma, J. Properties of bacterial cellulose transparent film regenerated from dimethylacetamide–LiCl solution. Polym. Adv. Technol. 2016, 27, 1102. [Google Scholar] [CrossRef]
- Lu, X.; Shen, X. Solubility of bacteria cellulose in zinc chloride aqueous solutions. Carbohydr. Polym. 2011, 86, 239–244. [Google Scholar] [CrossRef]
- Gericke, M.; Schlufter, K.; Liebert, T.; Heinze, T.; Budtova, T. Rheological properties of cellulose/ionic liquid solutions: From dilute to concentrated states. Biomacromolecules 2009, 10, 1188–1194. [Google Scholar] [CrossRef]
- Pavlyuchenko, M.M.; Kaputsky, F.N.; Grinshpan, D.D. Effect of organic solvent nature on the interaction of cellulose with nitrogen tetroxide. J. Appl. Chem. 1975, 48, 1822–1825. [Google Scholar]
- Hammer, R.B.; Turbak, A.F. Production of Rayon from Solutions of Cellulose in N2O4-DMF in Solvent Spun Rayon, Modified Cellulose Fibers and Derivatives; American Chemical Society: Washington, DC, USA, 1977; Chapter 4. [Google Scholar]
- Budtova, T.; Navard, P. Cellul. NaOH-Water Based Solvents A Rev. Cellul. 2016, 23, 5–55. [Google Scholar]
- Grinshpan, D.D.; Gonchar, A.N.; Tsygankova, N.G.; Makarevich, S.E.; Savitskaya, T.A.; Sheimo, E.V. Реoлoгические свoйства кoнцентрирoванных раствoрoв целлюлoзы и ее смесей с другими пoлимерами в oртoфoсфoрнoй кислoте. J. Eng. Phys. Thermophys. 2011, 84, 594. [Google Scholar] [CrossRef]
- Boerstoel, H.; Maatman, H.; Westerink, J.B.; Koenders, B.M. Liquid crystalline solutions of cellulose in phosphoric acid. Polymer 2001, 42, 7371. [Google Scholar] [CrossRef]
- Graenacher, C.; Sallmann, R. Cellulose Solutions and Process of Making Same. U.S. Patent 2179181, 7 November 1939. [Google Scholar]
- Johnson, D.L. Compounds Dissolved in Cyclic Amine Oxides. U.S. Patent 3447939, 2 September 1966. [Google Scholar]
- Golova, L.K.; Romanov, V.V.; Lunina, O.B.; Platonov, V.A.; Papkov, S.P.; Khorozova, O.D.; Yakshin, V.V.; Belasheva, T.P.; Sokira, A.N. Method for Obtaining a Solution for Spinning. Fibers. Patent RF 1645308, 30 April 1991. [Google Scholar]
- Golova, L.K. New Cellulose Fiber Lyocell. Rus. J. Gen. Chem. 2002, XLVI, 49–57. [Google Scholar]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Budaeva, V.V.; Gismatulina, Y.A.; Zolotukhin, V.N.; Rogovoi, M.S.; Melnikov, A.V. A Method for Producing Cellulose from Flax-Mezheumka for the Paper. Industry. Patent RF 2566275, 20 October 2015. [Google Scholar]
- Kopania, E.; Wietecha, J.; Ciechańska, D. Studies on Isolation of Cellulose Fibres from Waste Plant Biomass. Fibres Text. East. Eur. 2012, 20, 167–172. [Google Scholar]
- Hummel, M.; Ma, Y.; Michud, A.; Asaadi, S.; Roselli, A.; Stepan, A.; Hellstén, S.; Sixta, H. Lignocellulosic Multicomponent Fibers Spun from Superbase-Based Ionic Liquids. Lenzing. Ber. 2018, 94, 67–76. [Google Scholar]
- Zhang, H.; Tong, M.; Shao, H.; Hu, X. Comparison of the structures and properties of Lyocell fibers from high hemicellulose pulp and high α-cellulose pulp. J. Appl. Polym. Sci. 2008, 107, 636–641. [Google Scholar] [CrossRef]
- Golova, L.K. Processing of cellulose via highly concentrated “solid solutions”. Fibre Chem. 1996, 28, 5–16. [Google Scholar] [CrossRef]
- Golova, L.K.; Borodina, O.E.; Kuznetsova, L.K.; Lyubova, T.A.; Krylova, T.B. The Solid-Phase MMO Process. Fibre Chem. 2000, 32, 243–251. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Kuznetsova, L.K.; Rebrov, A.V.; Berkovich, A.K.; Skvortsov, I.Y.; Kulichikhin, V.G. Composite fibers based on cellulose and polyacrylonitrile copolymers. Russ. J. Gen. Chem. 2017, 87, 1351–1356. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer After Sixty Years: A Survey and Some New Result in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Bazarnova, N.G. Methods for the Study of Wood and Its Derivatives: Textbook; Alt. State Univ.: Barnaul, Russia, 2002; p. 160. [Google Scholar]
- Ivanova, N.V.; Korolenko, E.A.; Korolik, E.V.; Zhbankov, R.G. Mathematical processing of the IR spectrum of cellulose. Zhurnal Prikl. Spektrosk. 1989, 51, 301–306. [Google Scholar]
- Dehant, J.; Danz, R.; Kimmer, W.; Schmolke, R. Infrared Spectroscopy of Polymers; Oleinik, E.F., Ed.; Chemistry: Moscow, Russia, 1976; p. 471. [Google Scholar]
- O’Connor, R.T.; Du Prè, E.; Mitcham, D. Application of infrared absorption spectroscopy to investigations of cotton and modified cottons. Part I. Physical and crystalline modification and oxidation. Text. Res. J. 1958, 28, 382–392. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Bondarenko, G.N.; Anokhina, T.S.; Dmitrieva, E.S.; Levin, I.S.; Makhatova, V.E.; Galimova, N.Z.; Shambilova, G.K. Structure, Morphology, and Permeability of Cellulose Films. Membranes 2022, 12, 297. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Kuznetsova, L.K.; Mironova, M.V.; Vinogradov, M.I.; Bermeshev, M.V.; Levin, I.S.; Kulichikhin, V.G. Composite Fibers from Cellulose Solutions with Additives of Bis (Trimethylsilyl) Acetylene and Alkoxysilanes: Rheology, Structure and Properties. Fibre Chem. 2019, 51, 26–31. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Nuruddin, M.; Clarkson, C.; Montes, F.; Howarter, J.; Youngblood, J.P. Cellulose nanocrystal (CNC) coatings with controlled anisotropy as high-performance gas barrier films. ACS Appl. Mater. Interfaces 2018, 11, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.L. Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 2013; p. 420. [Google Scholar]
- Kozlowski, R.M.; Mackiewicz-Talarczyk, M. Handbook of Natural Fibres: Volume 1: Types, Properties and Factors Affecting Breeding and Cultivation; Woodhead Publishing: Duxford, UK, 2020; p. 838. [Google Scholar]
- Charlet, K.; Jernot, J.P.; Gomina, M. Mechanical properties of flax fibers and of the derived unidirectional composites. J. Compos. Mater. 2010, 44, 2887–2896. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Bondarenko, G.N.; Skvortsov, I.Y.; Berkovich, A.K.; Bermeshev, M.V.; Mironova, M.V. Carbon—Silicon-Carbide Fibers Prepared from Solid Solutions of Cellulose in N-Methylmorpholine-N-Oxide with Added Tetraethoxysilane. Fibre Chem. 2017, 49, 231–236. [Google Scholar] [CrossRef]







| Composition of Noils | % wt |
|---|---|
| Shives and weed impurities | 3 ± 1.5 |
| Cellulose fibers up to 5 mm | 17 ± 9 |
| Cellulose fibers 5–15 mm | 57 ± 3 |
| Cellulose fibers 15–30 mm | 18 ± 5 |
| Cellulose fibers 30–50 mm | 3 ± 2.5 |
| Cellulose fibers 50–70 mm | 2 ± 0.5 |
| Csolution, % | Diameter, μm | Strength, MPa | Modulus, GPa | Elongation, % |
|---|---|---|---|---|
| 7 (flax noils) | 16–18 | 950–1070 | 9–15 | 6–9 |
| 16 (wood pulp) | 16–20 | 610–730 | 14–20 | 6–9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarov, I.S.; Golova, L.K.; Smyslov, A.G.; Vinogradov, M.I.; Palchikova, E.E.; Legkov, S.A. Flax Noils as a Source of Cellulose for the Production of Lyocell Fibers. Fibers 2022, 10, 45. https://doi.org/10.3390/fib10050045
Makarov IS, Golova LK, Smyslov AG, Vinogradov MI, Palchikova EE, Legkov SA. Flax Noils as a Source of Cellulose for the Production of Lyocell Fibers. Fibers. 2022; 10(5):45. https://doi.org/10.3390/fib10050045
Chicago/Turabian StyleMakarov, Igor S., Lyudmila K. Golova, Alexander G. Smyslov, Markel I. Vinogradov, Ekaterina E. Palchikova, and Sergei A. Legkov. 2022. "Flax Noils as a Source of Cellulose for the Production of Lyocell Fibers" Fibers 10, no. 5: 45. https://doi.org/10.3390/fib10050045
APA StyleMakarov, I. S., Golova, L. K., Smyslov, A. G., Vinogradov, M. I., Palchikova, E. E., & Legkov, S. A. (2022). Flax Noils as a Source of Cellulose for the Production of Lyocell Fibers. Fibers, 10(5), 45. https://doi.org/10.3390/fib10050045







