Impact of Thermal Stress on Abrasive Dust from a Carbon Fiber-Reinforced Concrete Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Equipment
2.2. Sample Preparation
2.2.1. Thermal Stress
2.2.2. Dry-Cutting Simulation
2.3. Analytical Methods
2.3.1. Online Particle Size Determination
2.3.2. Pyrolysis Gas Chromatography—Mass Spectrometry
2.3.3. Thermal Analysis—Mass Spectrometry
2.3.4. Thermo-Optical Carbon Analyzer—Mass Spectrometry
2.3.5. Scanning Electron Microscopy
2.3.6. Statistical Evaluation
3. Results and Discussion
3.1. Particle Size Distribution
3.2. Organic Composition
3.2.1. Organic Composition of Reinforcement before Cutting
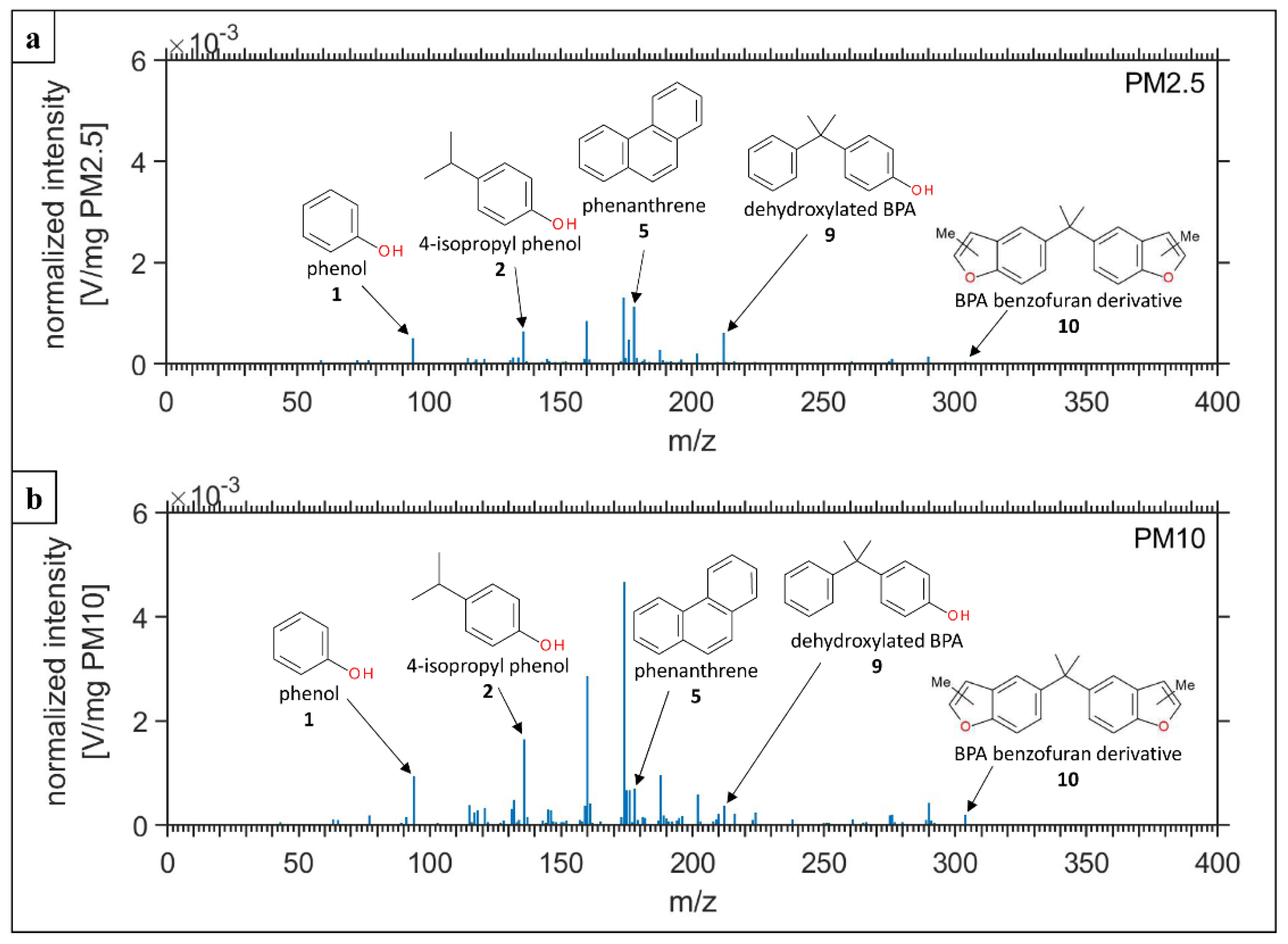
3.2.2. Organic Composition of Abrasive PM
3.3. Fiber Morphology
3.3.1. Fiber Morphology before Cutting
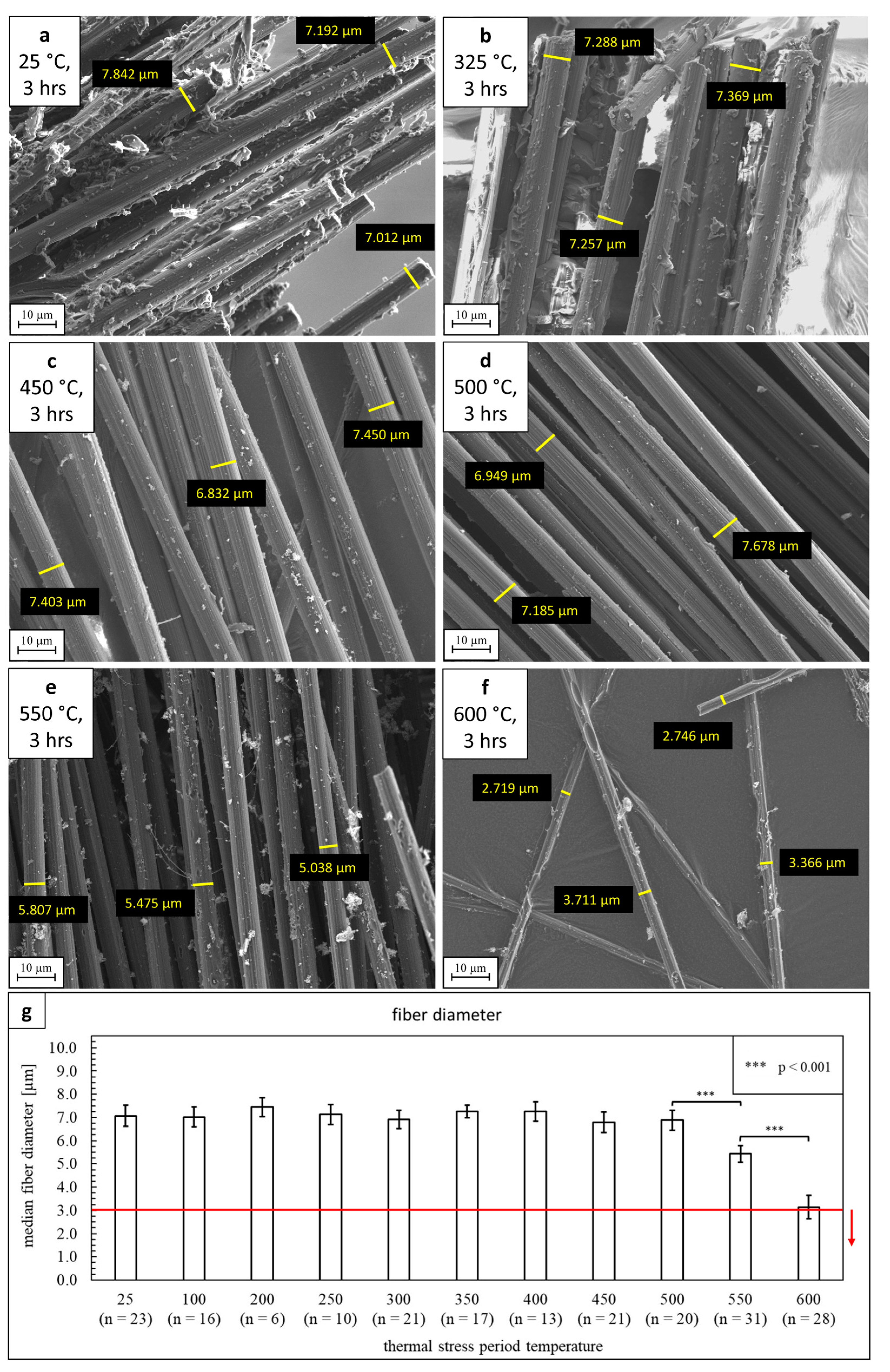
3.3.2. Fiber Morphology/Occurrence in Abrasive PM10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Böhm, R.; Thieme, M.; Wohlfahrt, D.; Wolz, D.; Richter, B.; Jäger, H. Reinforcement Systems for Carbon Concrete Composites Based on Low-Cost Carbon Fibers. Fibers 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Mai, Y.W. Strength and fracture properties of asbestos-cement mortar composites. J. Mater. Sci. 1979, 14, 2091–2102. [Google Scholar] [CrossRef]
- Lenain, J.C.; Bunsell, A.R. The resistance to crack growth of asbestos cement. J. Mater. Sci. 1979, 14, 321–332. [Google Scholar] [CrossRef]
- Ghugal, Y.M.; Deshmukh, S.B. Performance of Alkali-resistant Glass Fiber Reinforced Concrete. J. Reinf. Plast. Compos. 2006, 25, 617–630. [Google Scholar] [CrossRef]
- Kizilkanat, A.B.; Kabay, N.; Akyüncü, V.; Chowdhury, S.; Akça, A.H. Mechanical properties and fracture behavior of basalt and glass fiber reinforced concrete: An experimental study. Constr. Build. Mater. 2015, 100, 218–224. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Di Bella, G.; Valenza, A. A review on basalt fibre and its composites. Compos. Part B Eng. 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Chung, D. Cement reinforced with short carbon fibers: A multifunctional material. Compos. Part B Eng. 2000, 31, 511–526. [Google Scholar] [CrossRef]
- Wang, C.; Li, K.-Z.; Li, H.-J.; Jiao, G.-S.; Lu, J.; Hou, D.-S. Effect of carbon fiber dispersion on the mechanical properties of carbon fiber-reinforced cement-based composites. Mater. Sci. Eng. A 2008, 487, 52–57. [Google Scholar] [CrossRef]
- Sim, J.; Park, C.; Moon, D.Y. Characteristics of basalt fiber as a strengthening material for concrete structures. Compos. Part B Eng. 2005, 36, 504–512. [Google Scholar] [CrossRef]
- Lee, J.J.; Song, J.; Kim, H. Chemical stability of basalt fiber in alkaline solution. Fibers Polym. 2014, 15, 2329–2334. [Google Scholar] [CrossRef]
- Felley-Bosco, E.; MacFarlane, M. Asbestos: Modern Insights for Toxicology in the Era of Engineered Nanomaterials. Chem. Res. Toxicol. 2018, 31, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Hillemann, L.; Stintz, M.; Streibel, T.; Zimmermann, R.; Öder, S.; Kasurinen, S.; Di Bucchianico, S.; Kanashova, T.; Dittmar, G.; Konzack, D.; et al. Charakterisierung von Partikelemissionen aus dem Trennschleifprozess von kohlefaserverstärktem Beton (Carbonbeton). Gefahrstoffe 2018, 230–240. [Google Scholar]
- Scheerer, S. Was ist Textilbeton? Eine kurze Einführung in das Thema. Beton-Stahlbetonbau 2015, 110, 4–7. [Google Scholar] [CrossRef]
- Bienkowski, N.; Hillemann, L.; Streibel, T.; Kortmann, J.; Kopf, F.; Zimmermann, R.; Jehle, P. Bearbeitung von Carbonbeton-eine bauverfahrenstechnische und medizinische Betrachtung. Bauingeneur 2017, 10, 110–117. [Google Scholar]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem. Int. Ed. Engl. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- Jäger, H.; Cherif, C.; Kirsten, M.; Behnisch, T.; Wolz, D.S.; Böhm, R.; Gude, M. Influence of processing parameters on the properties of carbon fibres—An overview. Mater. Werkst. 2016, 47, 1044–1057. [Google Scholar] [CrossRef]
- Morgan, P. Carbon Fibers and Their Composites; Taylor & Francis: Boca Raton, FL, USA, 2005; ISBN 978-0-8247-0983-9. [Google Scholar]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrolysis 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, B.; Sun, L.; Zhang, H. Study on the thermal oxidative stabilization reactions and the formed structures in polyacrylonitrile during thermal treatment. Polym. Degrad. Stab. 2017, 140, 104–113. [Google Scholar] [CrossRef]
- Saha, B.; Schatz, G.C. Carbonization in polyacrylonitrile (PAN) based carbon fibers studied by ReaxFF molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 4684–4692. [Google Scholar] [CrossRef]
- Abelmann, A.; Maskrey, J.R.; Lotter, J.T.; Chapman, A.M.; Nembhard, M.D.; Pierce, J.S.; Wilmoth, J.M.; Lee, R.J.; Paustenbach, D.J. Evaluation of take-home exposure to asbestos from handling asbestos-contaminated worker clothing following the abrasive sawing of cement pipe. Inhal. Toxicol. 2018, 29, 555–566. [Google Scholar] [CrossRef]
- Weiler, L.; Vollpracht, A. Environmental Compatibility of Carbon Reinforced Concrete: Irrigated Construction Elements. Key Eng. Mater. 2019, 809, 314–319. [Google Scholar] [CrossRef]
- Koch, A.; Bergelt, P.; Fiala, P.; Käfer, U.; Orasche, J.; Bauer, S.; Di Bucchianico, S.; Stintz, M.; Gröger, T.; Streibel, T.; et al. Investigation of Chemical Composition and Fiber-Occurrence in Inhalable Particulate Matter Obtained from Dry Cutting Processes of Carbon Fiber Reinforced Concrete Composite, Concrete and the Carbon Fiber Reinforcement Materials. Aerosol Sci. Eng. 2021, 5, 292–306. [Google Scholar] [CrossRef]
- TRGS 905 Verzeichnis Krebserzeugender, Keimzellmutagener Oder Reproduktionstoxischer Stoffe. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRGS/TRGS-905.html (accessed on 19 February 2022).
- Meyer, L.O.; Schulte, K.; Grove-Nielsen, E. CFRP-Recycling Following a Pyrolysis Route: Process Optimization and Potentials. J. Compos. Mater. 2009, 43, 1121–1132. [Google Scholar] [CrossRef] [Green Version]
- Yatim, N.M.; Shamsudin, Z.; Shaaban, A.; Ghafar, J.A.; Khan, M.J.H. Recovery of carbon fiber from carbon fiber reinforced polymer waste via pyrolysis. J. Adv. Manuf. Technol. 2020, 14, 37–47. [Google Scholar]
- Tranchard, P.; Duquesne, S.; Samyn, F.; Estèbe, B.; Bourbigot, S. Kinetic analysis of the thermal decomposition of a carbon fibre-reinforced epoxy resin laminate. J. Anal. Appl. Pyrolysis 2017, 126, 14–21. [Google Scholar] [CrossRef]
- Matielli Rodrigues, G.G.; Faulstich de Paiva, J.M.; Braga do Carmo, J.; Botaro, V.R. Recycling of carbon fibers inserted in composite of DGEBA epoxy matrix by thermal degradation. Polym. Degrad. Stab. 2014, 109, 50–58. [Google Scholar] [CrossRef]
- López, F.A.; Rodríguez, O.; Alguacil, F.J.; García-Díaz, I.; Centeno, T.A.; García-Fierro, J.L.; González, C. Recovery of carbon fibres by the thermolysis and gasification of waste prepreg. J. Anal. Appl. Pyrolysis 2013, 104, 675–683. [Google Scholar] [CrossRef]
- Zöllner, M.; Lieberwirth, H.; Kempkes, P.; Fendel, A. Thermal resistance of carbon fibres/carbon fibre reinforced polymers under stationary atmospheric conditions and varying exposure times. Waste Manag. 2019, 85, 327–332. [Google Scholar] [CrossRef]
- Eibl, S. Potential for the formation of respirable fibers in carbon fiber reinforced plastic materials after combustion. Fire Mater. 2017, 41, 808–816. [Google Scholar] [CrossRef]
- Černý, R.; Němečková, J.; Rovnaníková, P.; Bayer, P. Effect of thermal decomposition processes on the thermal properties of carbon fiber reinforced cement composites in high-temperature range. J. Therm. Anal. Calorim. 2007, 90, 475–488. [Google Scholar] [CrossRef]
- Regnier, N.; Mortaigne, B. Analysis by pyrolysis/gas chromatography/mass spectrometry of glass fibre/vinylester thermal degradation products. Polym. Degrad. Stab. 1995, 49, 419–428. [Google Scholar] [CrossRef]
- Otto, S.; Streibel, T.; Erdmann, S.; Sklorz, M.; Schulz-Bull, D.; Zimmermann, R. Application of pyrolysis-mass spectrometry and pyrolysis-gas chromatography-mass spectrometry with electron-ionization or resonance-enhanced-multi-photon ionization for characterization of crude oils. Anal. Chim. Acta 2015, 855, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Rüger, C.P.; Grimmer, C.; Sklorz, M.; Neumann, A.; Streibel, T.; Zimmermann, R. Combination of Different Thermal Analysis Methods Coupled to Mass Spectrometry for the Analysis of Asphaltenes and Their Parent Crude Oils: Comprehensive Characterization of the Molecular Pyrolysis Pattern. Energy Fuels 2018, 32, 2699–2711. [Google Scholar] [CrossRef]
- Brecht, D.; Uteschil, F.; Schmitz, O.J. Thermogravimetry coupled to an atmospheric pressure photo ionization quadrupole mass spectrometry for the product control of pharmaceutical formulations and the analysis of plasticizers in polymers. Talanta 2019, 198, 440–446. [Google Scholar] [CrossRef]
- Rüger, C.P.; Miersch, T.; Schwemer, T.; Sklorz, M.; Zimmermann, R. Hyphenation of Thermal Analysis to Ultrahigh-Resolution Mass Spectrometry (Fourier Transform Ion Cyclotron Resonance Mass Spectrometry) Using Atmospheric Pressure Chemical Ionization for Studying Composition and Thermal Degradation of Complex Materials. Anal. Chem. 2015, 87, 6493–6499. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Chen, L.-W.A.; Chang, M.O.; Robinson, N.F.; Trimble, D.; Kohl, S. The IMPROVE_A Temperature Protocol for Thermal/Optical Carbon Analysis: Maintaining Consistency with a Long-Term Database. J. Air Waste Manag. Assoc. 2007, 57, 1014–1023. [Google Scholar] [CrossRef] [Green Version]
- Diab, J.; Streibel, T.; Cavalli, F.; Lee, S.C.; Saathoff, H.; Mamakos, A.; Chow, J.C.; Chen, L.-W.A.; Watson, J.G.; Sippula, O.; et al. Hyphenation of a EC/OC thermal—Optical carbon analyzer to photo-ionization time-of-flight mass spectrometry: An off-line aerosol mass spectrometric approach for characterization of primary and secondary particulate matter. Atmos. Meas. Tech. 2015, 8, 3337–3353. [Google Scholar] [CrossRef] [Green Version]
- Grabowsky, J.; Streibel, T.; Sklorz, M.; Chow, J.C.; Watson, J.G.; Mamakos, A.; Zimmermann, R. Hyphenation of a carbon analyzer to photo-ionization mass spectrometry to unravel the organic composition of particulate matter on a molecular level. Anal. Bioanal. Chem. 2011, 401, 3153–3164. [Google Scholar] [CrossRef]
- Miersch, T.; Czech, H.; Stengel, B.; Abbaszade, G.; Orasche, J.; Sklorz, M.; Streibel, T.; Zimmermann, R. Composition of carbonaceous fine particulate emissions of a flexible fuel DISI engine under high velocity and municipal conditions. Fuel 2019, 236, 1465–1473. [Google Scholar] [CrossRef]
- Boesl, U.; Zimmermann, R.; Weickhardt, C.; Lenoir, D.; Schramm, K.-W.; Kettrup, A.; Schlag, E.W. Resonance-enhanced multi-photon ionization: A species-selective ion source for analytical time-of-flight mass spectroscopy. Chemosphere 1994, 29, 1429–1440. [Google Scholar] [CrossRef]
- Streibel, T.; Zimmermann, R. Resonance-enhanced multiphoton ionization mass spectrometry (REMPI-MS): Applications for process analysis. Annu. Rev. Anal. Chem. 2014, 7, 361–381. [Google Scholar] [CrossRef]
- Gehm, C.; Streibel, T.; Passig, J.; Zimmermann, R. Determination of Relative Ionization Cross Sections for Resonance Enhanced Multiphoton Ionization of Polycyclic Aromatic Hydrocarbons. Appl. Sci. 2018, 8, 1617. [Google Scholar] [CrossRef] [Green Version]
- Fachbereich Umweltmesstechnik. VDI 3492: Messen von Innenraumluftverunreinigungen, Messen von Immissionen, Messen Anorganischer Faserförmiger Partikeln, Rasterelektronenmikroskopisches Verfahren; VDI Verein Deutscher Ingenieure e.V.: Düsseldorf, Germany, 2004; Available online: https://www.vdi.de/richtlinien/details/vdi-3492-messen-von-innenraumluftverunreinigungen-messen-von-immissionen-messen-anorganischer-faserfoermiger-partikel-rasterelektronenmikroskopisches-verfahren (accessed on 23 April 2022).
- Harrison, R.M.; Yin, J. Particulate matter in the atmosphere: Which particle properties are important for its effects on health? Sci. Total Environ. 2000, 249, 85–101. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Agimelen, O.S.; Mulholland, A.J.; Sefcik, J. Modelling of artefacts in estimations of particle size of needle-like particles from laser diffraction measurements. Chem. Eng. Sci. 2017, 158, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Rüger, C.P.; Tiemann, O.; Neumann, A.; Streibel, T.; Zimmermann, R. Review on Evolved Gas Analysis Mass Spectrometry with Soft Photoionization for the Chemical Description of Petroleum, Petroleum-Derived Materials, and Alternative Feedstocks. Energy Fuels 2021, 35, 18308–18332. [Google Scholar] [CrossRef]
- Chen, R.; Xu, X.; Lu, S.; Zhang, Y.; Lo, S. Pyrolysis study of waste phenolic fibre-reinforced plastic by thermogravimetry/Fourier transform infrared/mass spectrometry analysis. Energy Convers. Manag. 2018, 165, 555–566. [Google Scholar] [CrossRef]
- Qiu, T.; Ge, F.; Li, C.; Lu, S. Study of the thermal degradation of flame-retardant polyester GFRP using TGA and TG-FTIR-GC/MS. J. Therm. Anal. Calorim. 2022, 147, 5743–5760. [Google Scholar] [CrossRef]
- Fache, M.; Montérémal, C.; Boutevin, B.; Caillol, S. Amine hardeners and epoxy cross-linker from aromatic renewable resources. Eur. Polym. J. 2015, 73, 344–362. [Google Scholar] [CrossRef]
- Wald, F.; Chlouba, J.; Uhlíř, A.; Kallerová, P.; Štujberová, M. Temperatures during fire tests on structure and its prediction according to Eurocodes. Fire Saf. J. 2009, 44, 135–146. [Google Scholar] [CrossRef]
- Alarifi, A.A.; Dave, J.; Phylaktou, H.N.; Aljumaiah, O.A.; Andrews, G.E. Effects of fire-fighting on a fully developed compartment fire: Temperatures and emissions. Fire Saf. J. 2014, 68, 71–80. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Armstrong, B.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: A review and meta-analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef]
- TRGS 519 Asbest: Abbruch-, Sanierungs- oder Instandhaltungsarbeiten. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRGS/TRGS-519.html (accessed on 19 February 2022).
- Gibbs, G.W.; Hwang, C.Y. Physical parameters of airborne asbestos fibres in various work environments-preliminary findings. Am. Ind. Hyg. Assoc. J. 1975, 36, 459–466. [Google Scholar] [CrossRef]
- TRGS 910 Risikobezogenes Maßnahmenkonzept für Tätigkeiten mit Krebserzeugenden Gefahrstoffen. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRGS/TRGS-910.html (accessed on 19 February 2022).
- Donovan, E.P.; Donovan, B.L.; Sahmel, J.; Scott, P.K.; Paustenbach, D.J. Evaluation of bystander exposures to asbestos in occupational settings: A review of the literature and application of a simple eddy diffusion model. Crit. Rev. Toxicol. 2011, 41, 52–74. [Google Scholar] [CrossRef] [PubMed]
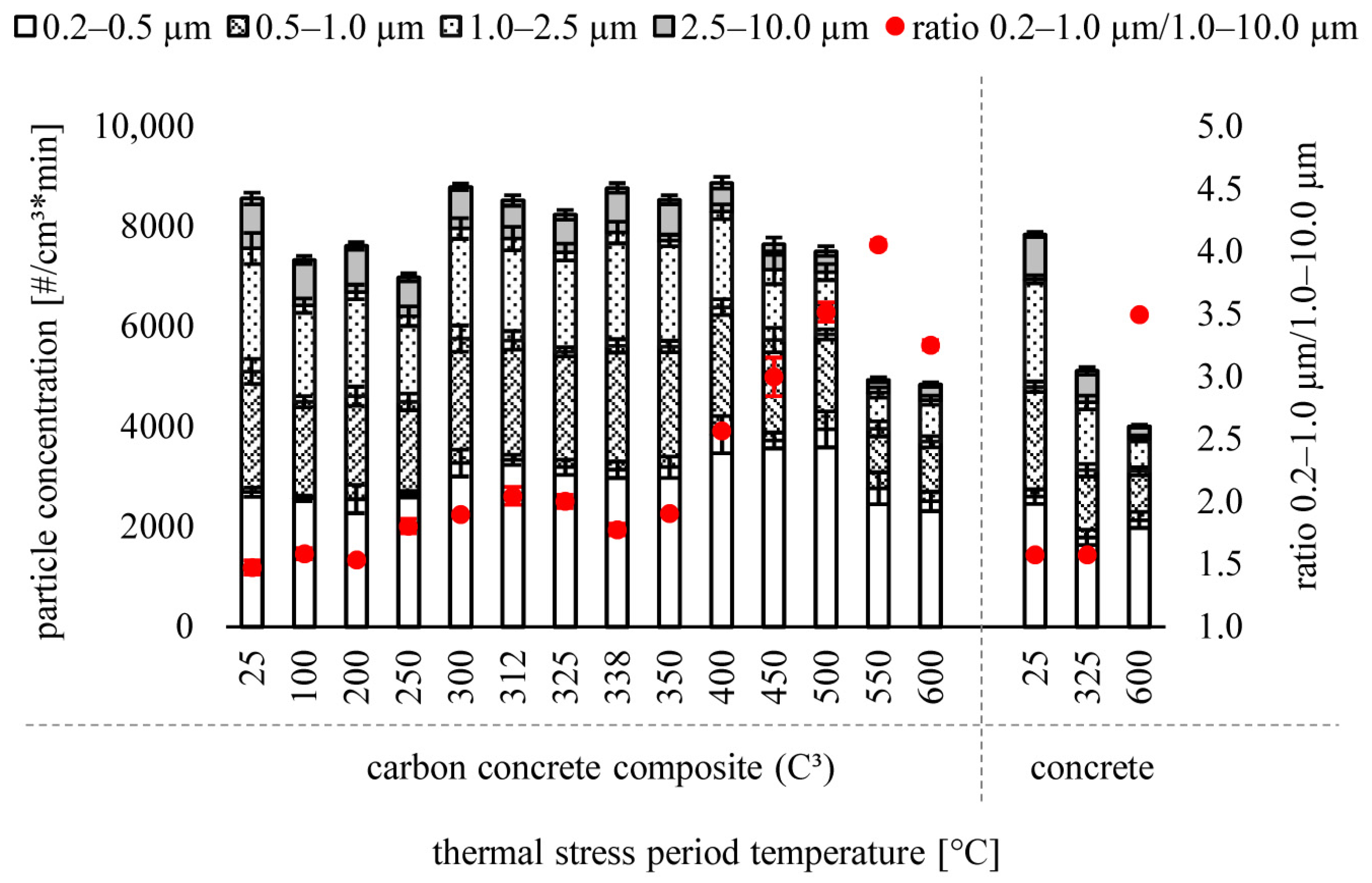


| 0.2–2.5 µm [particles/cm3 * min] | 0.2–10 µm [particles/cm3 * min] | 0.2–40 µm [particles/cm3 * min] | |
|---|---|---|---|
| C3 | |||
| 25 °C | 7600 ± 200 | 8600 ± 300 | 8600 ± 300 |
| 100 °C | 6400 ± 200 | 7300 ± 200 | 7400 ± 200 |
| 200 °C | 6700 ± 200 | 7600 ± 200 | 7600 ± 200 |
| 250 °C | 6200 ± 200 | 7000 ± 200 | 7000 ± 200 |
| 300 °C | 8000 ± 300 | 8800 ± 300 | 8800 ± 300 |
| 312 °C | 7800 ± 200 | 8500 ± 300 | 8500 ± 300 |
| 325 °C | 7500 ± 200 | 8200 ± 100 | 8300 ± 100 |
| 338 °C | 7900 ± 200 | 8800 ± 200 | 8800 ± 200 |
| 350 °C | 7700 ± 200 | 8500 ± 200 | 8600 ± 200 |
| 400 °C | 8300 ± 300 | 8900 ± 200 | 8900 ± 200 |
| 450 °C | 7100 ± 300 | 7600 ± 200 | 7700 ± 300 |
| 500 °C | 7100 ± 200 | 7500 ± 200 | 7500 ± 200 |
| 550 °C | 4700 ± 200 | 4900 ± 200 | 5000 ± 200 |
| 600 °C | 4500 ± 200 | 4800 ± 200 | 4900 ± 200 |
| Concrete | |||
| 25 °C | 6900 ± 100 | 7800 ± 200 | 7900 ± 200 |
| 325 °C | 4500 ± 200 | 5100 ± 200 | 5100 ± 200 |
| 600 °C | 3800 ± 100 | 4000 ± 100 | 4000 ± 100 |
| Exposure Temperature [°C] | Residue [wt%] | Evaporable Fraction [wt%] | Number of Sum Formulae |
|---|---|---|---|
| 25 | 71.2 | 28.8 | 1897 |
| 300 | 76.5 | 23.5 | 2951 |
| 325 | 84.9 | 15.1 | 2274 |
| 350 | 93.2 | 6.8 | 2584 |
| # | Identified Substance | Compound Name | m/z | 25–250 °C | 300–312 °C | 325–350 °C | 400–600 °C |
|---|---|---|---|---|---|---|---|
| 1 | 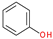 | Phenol | 94 | X | X | - | - |
| 2 | 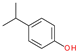 | 4-isopropyl phenol | 136 | - | X | - | - |
| 3 | C11H12O | - | 160 | - | X | X | - |
| 4 | C12H14O | - | 174 | - | X | X | - |
| 5 | 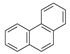 | Phenanthrene | 178 | X | X | X | - |
| 6 | 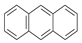 | Anthracene | 178 | X | X | X | - |
| 7 | 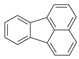 | Fluoranthene | 202 | - | X | - | - |
| 8 | 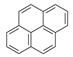 | Pyrene | 202 | - | X | - | - |
| 9 |  | 4-(2-phenyl-2-propanyl) phenol | 212 | X | X | - | - |
| 10 | 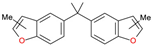 | n-methyl-5-[1-methyl-1-(n-methylbenzofuran-5-yl)ethyl] benzofuran | 304 | - | X | X | - |
| Exposure Temperature [°C]/material | Fiber Length Span [µm] | Fiber Diameter Span [µm] |
|---|---|---|
| 450/C3 | 10.3–769.2 | 2.6–8.1 |
| 500/C3 | 22.3–1573 | 3.8–7.4 |
| 550/C3 | 1.6–3447 | 0.1–7.0 |
| 600/C3 | 5.4–1514 | 0.1–6.0 |
| room temperature/asbestos | 0.2–38.0 [58] | 0.01–2.9 [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, A.; Friederici, L.; Fiala, P.; Springer, A.; Di Bucchianico, S.; Stintz, M.; Frank, M.; Rüger, C.P.; Streibel, T.; Zimmermann, R. Impact of Thermal Stress on Abrasive Dust from a Carbon Fiber-Reinforced Concrete Composite. Fibers 2022, 10, 39. https://doi.org/10.3390/fib10050039
Koch A, Friederici L, Fiala P, Springer A, Di Bucchianico S, Stintz M, Frank M, Rüger CP, Streibel T, Zimmermann R. Impact of Thermal Stress on Abrasive Dust from a Carbon Fiber-Reinforced Concrete Composite. Fibers. 2022; 10(5):39. https://doi.org/10.3390/fib10050039
Chicago/Turabian StyleKoch, Arne, Lukas Friederici, Petra Fiala, Armin Springer, Sebastiano Di Bucchianico, Michael Stintz, Marcus Frank, Christopher Paul Rüger, Thorsten Streibel, and Ralf Zimmermann. 2022. "Impact of Thermal Stress on Abrasive Dust from a Carbon Fiber-Reinforced Concrete Composite" Fibers 10, no. 5: 39. https://doi.org/10.3390/fib10050039
APA StyleKoch, A., Friederici, L., Fiala, P., Springer, A., Di Bucchianico, S., Stintz, M., Frank, M., Rüger, C. P., Streibel, T., & Zimmermann, R. (2022). Impact of Thermal Stress on Abrasive Dust from a Carbon Fiber-Reinforced Concrete Composite. Fibers, 10(5), 39. https://doi.org/10.3390/fib10050039






