Nanofiber Polymers for Coating Titanium-Based Biomedical Implants
Abstract
:1. Introduction
- Three-dimensional (3D);
- Highly porous with an interconnected pore network;
- Fabricated using material that is biodegradable or bioresorbable;
- Controlled degradation rate to match tissue regeneration rate;
- Suitable surface chemistry for cell attachment, proliferation, and differentiation;
- Mechanical properties matching those of the tissues at the site of implantation; and
- Easily fabricated in a variety of morphologies to define the shape and size of the regenerated tissue.
2. Nanofiber Polymers for Biomedical Applications
2.1. Hybridization of Polymers
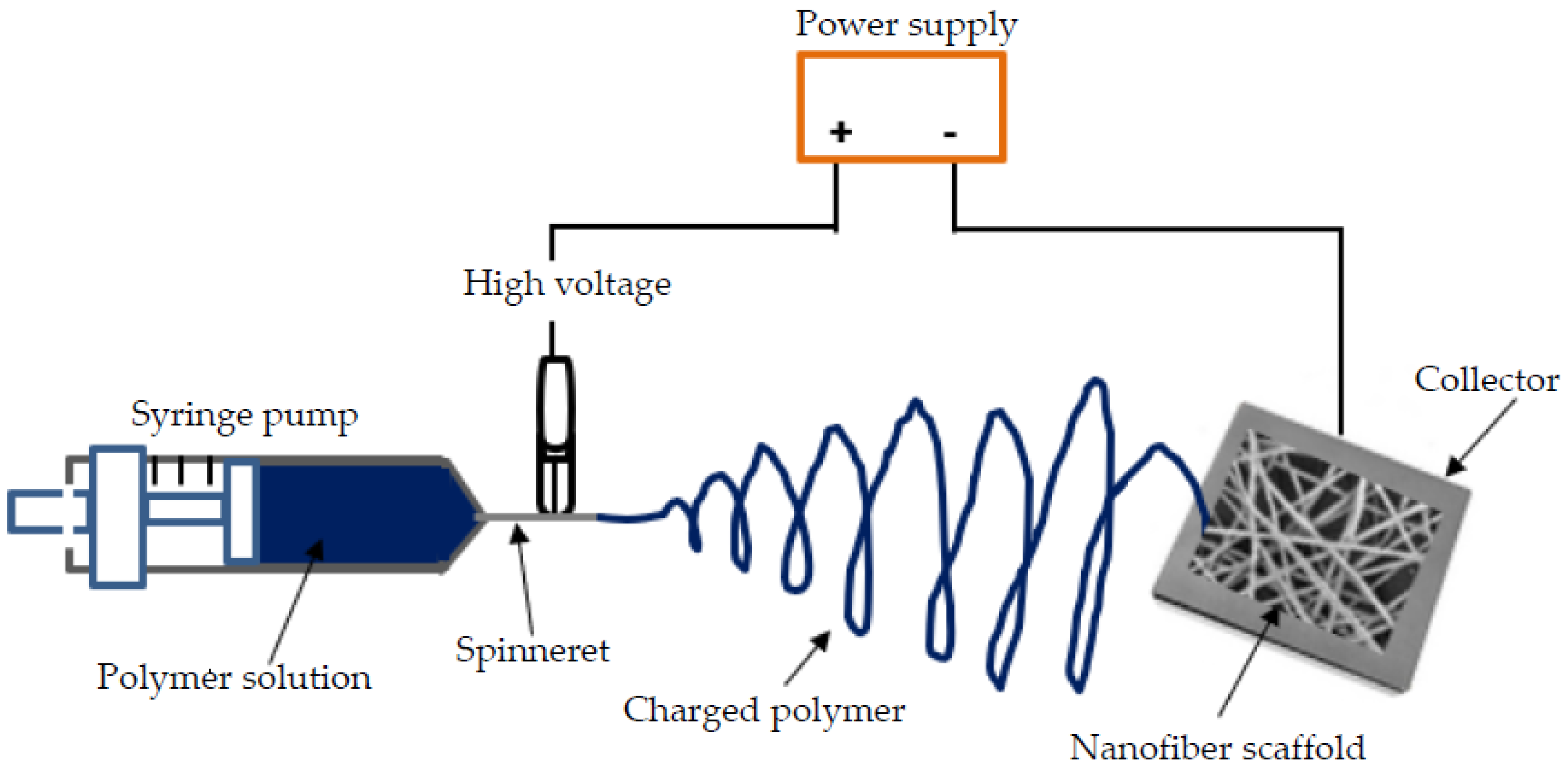
2.2. Electrospinning Technique
3. Physical Characteristics of Electrospun Nanofibers
4. Chemical Implications of Nanofiber-Coated Ti Implants
5. Mechanical Properties of Nanofibers for Ti-Based Implants
Adhesion of Nanofibers on Ti-Based Substrates
- The composition of the coatings and the concentration of the polymer in the NF solution are crucial determining factors of NF adhesion strength. Imbuing the NF polymer solution with an additive (such as nanoparticles of an antibacterial agent) may increase the adhesion strength of the interface by reducing the surface tension of the NF film [81,82].
- High porosity of an NF membrane may cause a faster diffusion of water molecules into the coating. Consequently, the diffusion may cause the layer nearest to a metallic surface to delaminate or completely release from the substrate when the water reaches the implant surface [42].
- The high surface area and the high volume-to-mass ratio of NFs may contribute to the bonding of NFs to the Ti implant surface [79].
- A coating technique during which solvent evaporation may occur could result in poor adhesion performance of the polymer coat [42].
- The application of an interface layer between the polymer NFs and the Ti substrate may result in a tight attachment of otherwise poorly adhering NFs. The interfacial layer (which may be, for example, a polymer hybrid combination or an adhesive) should exhibit ideal adhesion characteristics with an affinity for both the NF film and the substrate surface [42].
6. Biological Properties of Nanofiber-Coated Ti Implants
6.1. Biomineralization Studies
6.2. Osseointegration and Soft Tissue Attachment
6.3. Antimicrobial Properties
6.4. Cytotoxicity
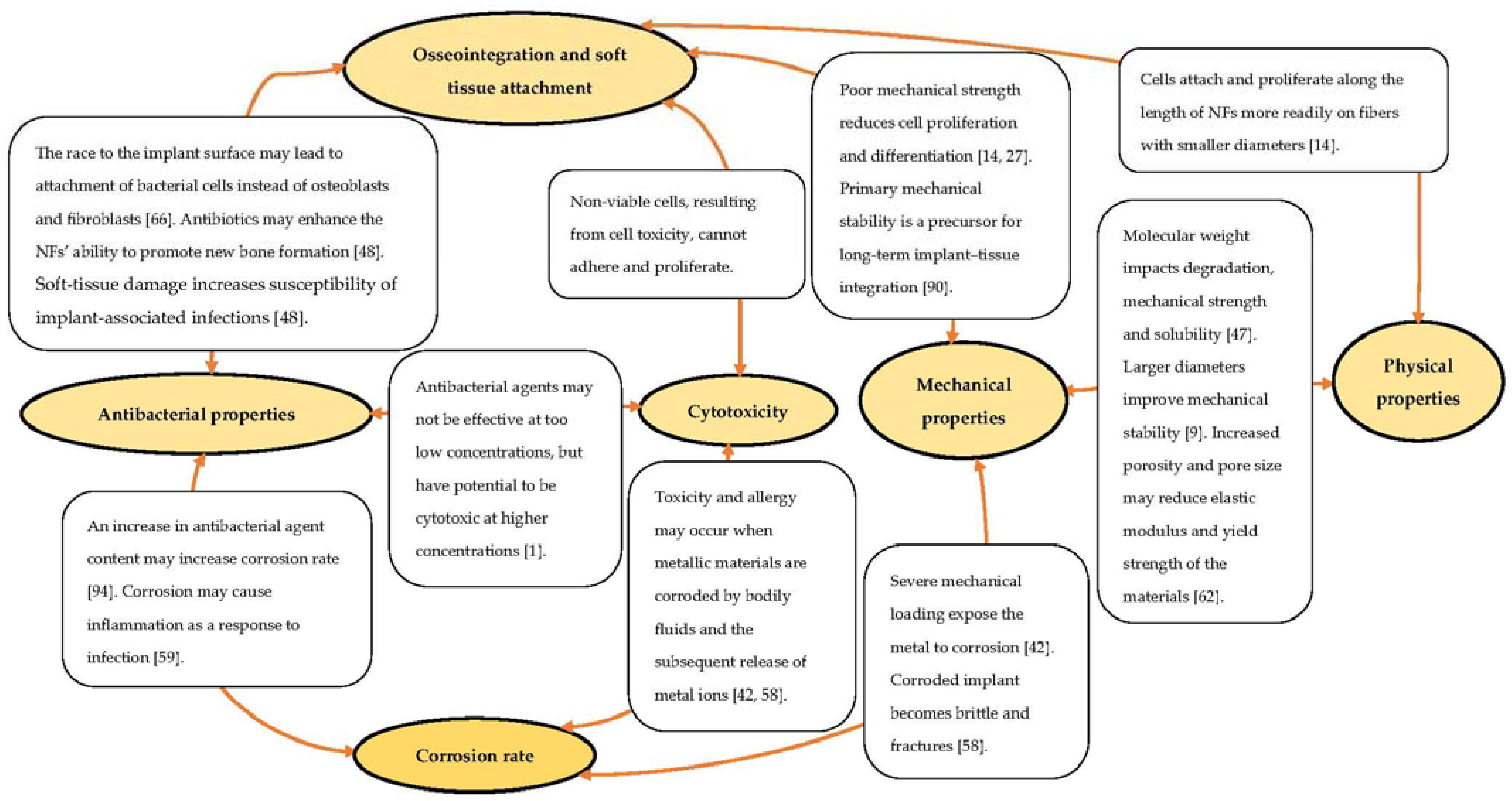
7. Correlation between the Various Properties for Implant Stability
8. Future Recommendations
- Comprehensive observations of the mechanisms at the implant–tissue and implant–coating interfaces should be prioritized.
- Further research is necessary to clarify the nature of the ECM interaction with electrospun NFs at the nanoscale.
- In vitro interactions between the tissue and the implant should be continually observed once the biodegradable NFs have been completely degraded in simulated human body conditions.
- Additional polymeric biomaterials should be evaluated for approval to be utilized in conjunction with implants to overcome the limitation of polymers when addressing critical issues, for instance, tensile strength and Young’s modulus, for load-bearing bone implants.
- With the various polymers available and human body complexities, polymer combinations, along with the nature and concentrations of additives required, should be explored.
- The various demands on NF coating toward the surface modification of Ti implants are mutually dependent and should be investigated holistically for the implants to attain clinical and commercial viability.
- In essence, the development of smart, functional materials exhibiting a synergy of chemical, physical, mechanical and biological properties should be the priority of implant-related studies.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiran, A.S.K.; Kumar, T.S.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and bioactive surface modifications of titanium implants by PCL/TiO2 nanocompo-site coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef] [Green Version]
- Boschetto, F.; Doan, H.N.; Vo, P.P.; Zanocco, M.; Zhu, W.; Sakai, W.; Adachi, T.; Ohgitani, E.; Tsutsumi, N.; Mazda, O.; et al. Antibacterial and osteoconductive effects of chitosan/polyethylene oxide (PEO)/bioactive glass nanofibers for orthopedic applications. Appl. Sci. 2020, 10, 2360. [Google Scholar] [CrossRef] [Green Version]
- Jahanmard, F.; Croes, M.; Castilho, M.; Majed, A.; Steenbergen, M.J.; Lietaert, K.; Vogely, H.C.; van der Wal, B.C.H.; Stapel, D.A.C.; Malda, J.; et al. Bactericidal coating to prevent early and delayed implant-related infections. J. Control. Release 2020, 326, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Zaman, H.A.; Sharif, S.; Idris, M.H.; Kamarudin, A. Metallic biomaterials for medical implant applications: A review. Appl. Mech. Mater. 2015, 735, 19–25. [Google Scholar] [CrossRef]
- Das, S.; Dholam, K.; Gurav, S.; Bendale, K.; Ingle, A.; Mohanty, B.; Chaudhari, P.; Bellare, J.R. Accentuated osseointegration in osteogenic nanofibrous coated titanium implants. Sci. Rep. 2019, 9, 17638. [Google Scholar] [CrossRef] [Green Version]
- Kiran, A.S.K.; Kumar, T.S.S.; Perumal, G.; Sanghavi, R.; Doble, M.; Ramakrish-na, S. Dual nanofibrous bioactive coating and antimicrobial surface treatment for infection resistant titanium implants. Prog. Org. Coat. 2018, 121, 112–119. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Bosco, R.; Van Den Beucken, J.; Leeuwenburgh, S.; Jansen, J. Surface engineering for bone implants: A trend from passive to active surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef] [Green Version]
- Kadavil, H.; Zagho, M.; Elzatahry, A.; Altahtamouni, T. Sputtering of electrospun polymer-based nanofibers for biomedical applications: A perspective. Nanomaterials 2019, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Li, H.M.; Zhang, Q.G.; Guo, N.N.; Zhu, A.M.; Liu, Q.L. Ultrafine polystyrene nanofibers and its application in nanofibrous membranes. Chem. Eng. Sci. 2015, 264, 329–335. [Google Scholar] [CrossRef]
- Weng, L.; Xie, J. Smart electrospun nanofibers for controlled drug release: Recent advances and new perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qiu, Y.; Wang, H.; Chen, Y.; Jin, S.; Chen, S. Preparation of nanofibers with renewable polymers and their application in wound dressing. Int. J. Polym. Sci. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- AlFalah, M.G.K.; Kamberli, E.; Abbar, A.H.; Kandemirli, F.; Saracoglu, M. Corrosion performance of electrospinning nanofiber ZnO-NiO-CuO/polycaprolactone coated on mild steel in acid solution. Surf. Interfaces 2020, 21, 100760. [Google Scholar] [CrossRef]
- Tian, F.; Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Yokoyama, Y.; Estrada, G.G.; Kobayashi, H. Quantitative analysis of cell adhesion on aligned micro- and nanofibers. J. Biomed. Mater. Res. A 2008, 84, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.N.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.K.; Tuan, R.S. Tissue engineering with mesenchymal stem cells. IEEE Eng. Med. Biol. Mag. 2003, 22, 51–56. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [Green Version]
- Nemati, S.; Kim, S.-J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Şimşek, M.; Aldemir, S.D.; Gümüşderelioğlu, M. Anticellular PEO coatings on titanium surfaces by sequential electrospinning and crosslinking processes. Emergent Mater. 2019, 2, 169–179. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative re-view of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Chong, S.-F.; Smith, A.A.A.; Zelikin, A.N. Microstructured, functional PVA hydrogels through bioconjugation with oligopeptides under physiological conditions. Small 2013, 9, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Briceño, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar]
- Montoro, S.R.; Medeiros, S.d.F.; Alves, G.M. Nanostructured hydrogel. In Nanostructured Polymer Blends; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; Elsevier Inc.: Oxford, UK, 2014; pp. 325–355. [Google Scholar]
- Boia, R.; Dias, P.A.N.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; de Sousa, H.C.; Ambrósio, A.F.; Bragad, M.E.M.; et al. Po-rous poly(ε-caprolactone) implants: A novel strategy for efficient intraocular drug delivery. J. Control Release 2019, 316, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Perumal, G.; Sivakumar, P.M.; Nandkumar, A.M.; Doble, M. Synthesis of magnesium phosphate nanoflakes and its PCL composite electrospun nanofiber scaffolds for bone tissue regeneration. Mater. Sci. Eng. C 2020, 109, 110527. [Google Scholar] [CrossRef]
- Chen, P. A Preliminary Discourse on Adhesion of Nanofibers Derived from Electrospun Polymers. Ph.D. Thesis, University of Akron, Akron, OH, USA, 2013. [Google Scholar]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.I.; Kim, B.-S.; Kang, S.W.; Kwon, J.H.; Lee, Y.M.; Kim, S.H.; Kim, Y.H. In vivo biocompatibilty and degradation behavior of elastic poly(L-lactide-co-epsilon-caprolactone) scaffolds. Biomaterials 2004, 25, 5939–5946. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oh, T.H. Biopolymer coatings for biomedical applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef]
- Ravichandran, R. Biomimetic Surface Modification of Dental Implant for Enhanced Osseointegration. Master’s Thesis, National University of Singapore, Singapore, 2009. [Google Scholar]
- Wang, Y.; Li, M.; Rong, J.; Nie, G.; Qiao, J.; Wang, H.; Wu, D.; Su, Z.; Niu, Z.; Huang, Y. Enhanced orientation of PEO polymer chains induced by nanoclays in electrospun PEO/clay composite nanofibers. Colloid Polym. Sci. 2013, 291, 1541–1546. [Google Scholar] [CrossRef]
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a key functional requirement of next-generation medical devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Tao, J. Effects of Molecular Weight and Solution Concentration on Electrospinning of PVA. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2003. [Google Scholar]
- Al Aboody, M.S. Electrospun fabrication and direct coating of bio-degradable fibrous composite on orthopedic titanium implant: Synthesis and characterizations. Mater. Res. Express 2021, 8, 015307. [Google Scholar] [CrossRef]
- Chen, S.; Hao, Y.; Cui, W.; Chang, J.; Zhou, Y. Biodegradable electrospun PLLA/chitosan membrane as guided tissue regeneration membrane for treating periodontitis. J. Mater. Sci. 2013, 48, 6567–6577. [Google Scholar] [CrossRef] [Green Version]
- Miele, D.; Catenacci, L.; Rossi, S.; Sandri, G.; Sorrenti, M.; Terzi, A.; Giannini, C. Collagen/PCL nanofibers electrospun in green solvent by DOE assisted process. An in-sight into collagen contribution. Materials 2020, 13, 4698. [Google Scholar] [CrossRef]
- Ravichandran, R.; Ng, C.C.H.; Liao, S.; Pliszka, D.; Raghunath, M.; Ramakrishna, S.; Chan, C.K. Biomimetic surface modification of titanium surfaces for early cell capture by advanced electrospinning. Biomed. Mater. 2012, 7, 015001. [Google Scholar] [CrossRef]
- Teo, W.-E. Electrospun Coated Metal Implants. Available online: http://electrospintech.com/coatedmetal.html (accessed on 14 January 2022).
- Lee, B.-Y.; Behler, K.; Kurtoglu, M.E.; Wynosky-Dolfi, M.A.; Rest, R.F.; Gogotsi, Y. Titanium dioxide-coated nanofibers for advanced filters. J. Nanoparticle Res. 2010, 12, 2511–2519. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Hwang, M.-G.; Lim, J.K. In vitro bioactivity of titanium implants coated with bicomponent hybrid biodegradable polymers. J. Sol-Gel Sci. Technol. 2012, 64, 756–764. [Google Scholar] [CrossRef]
- Khandaker, M.; Riahinezhad, S.; Sultana, F.; Morris, T.; Wolf, R.; Vaughan, M. Effect of collagen-polycaprolactone nanofibers matrix coating on the In vitro cytocompatibility and in vivo bone responses of titanium. J. Med. Biol. Eng. 2018, 38, 197–210. [Google Scholar] [CrossRef]
- Khandaker, M.; Riahinezhad, S.; Williams, W.R.; Wolf, R. Microgroove and collagen-poly(ε-caprolactone) nanofiber mesh coating improves the mechanical stability and osseointegration of titanium implants. Nanomaterials 2017, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Nitti, P.; Gallo, N.; Natta, L.; Scalera, F.; Palazzo, B.; Sannino, A.; Gervaso, F. Influence of nanofiber orientation on morphological and mechanical properties of electrospun chitosan mats. J. Healthc. Eng. 2018, 2018, 3651480. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y.; Li, D.; Wei, B.; Xu, Y.; Gu, Q.; Wang, L. Electrospun vancomycin-loaded coating on titanium implants for the prevention of im-plant-associated infections. Int. J. Nanomed. 2014, 9, 3027–3036. [Google Scholar]
- Yang, D.; Li, Y.; Nie, J. Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs. Carbohydr. Polym. 2007, 69, 538–543. [Google Scholar] [CrossRef]
- Morel, A.; Domaschke, S.; Urundolil Kumaran, V.; Alexeev, D.; Sadeghpour, A.; Ramakrishna, S.N.; Ferguson, S.J.; Rossi, R.M.; Mazza, E.; Ehret, A.E.; et al. Correlating diameter, mechanical and structural properties of poly(L-lactide) fibres from needleless electrospinning. Acta Biomater. 2018, 8, 169–183. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef] [Green Version]
- Colmenares-Roldán, G.J.; Quintero-Martínez, Y.; Agudelo-Gómez, L.M.; Rodríguez-Vinasco, L.F.; Hoyos-Palacio, L.M. Influence of the molecular weight of polymer, solvents and operational condition in the electrospinning of polycaprolactone. Rev. Fac. Ing. Univ. Antioq. 2017, 84, 35–45. [Google Scholar] [CrossRef]
- Leong, M.F.; Chian, K.S.; Mhaisalkar, P.S.; Ong, W.F.; Ratner, B.D. Effect of electrospun poly(D,L-lactide) fibrous scaffold with nanoporous surface on attachment of por-cine esophageal epithelial cells and protein adsorption. J. Biomed. Mater. Res. A 2009, 89, 1040–1048. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface modifications of titanium materials for developing corrosion behavior in human body environment: A review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Sasikumar, Y.; Karuppusamy, I.; Nallaiyan, R. Surface modification methods for titanium and its alloys and their corrosion behavior in biological environment: A review. J. Bio-Tribo-Corros. 2019, 5, 36. [Google Scholar] [CrossRef]
- Shaterian, M.; Khoobi, A.; Enhessari, M.; Ozaee, K. A new strategy based on thermodiffusion of ceramic nanopigments into metal surfaces and formation of anti-corrosion coatings. Microporous Mesoporous Mater. 2015, 218, 62–68. [Google Scholar] [CrossRef]
- Hanas, T.; Sampath Kumar, T.S. Tailoring biodegradation of fine grained AZ31 alloy implants by nanofibrous coatings. Mater. Today Proc. 2017, 4, 6697–6703. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention—A review. Recent Pat. Mater. Sci. 2010, 2, 40–54. [Google Scholar]
- Gilbert, J.L.; Kubacki, G.W. Oxidative stress, inflammation, and the corrosion of metallic biomaterials: Corrosion causes biology and biology causes corrosion. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Elsevier Inc.: London, UK, 2016; pp. 59–88. [Google Scholar]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases. 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Fernandes, D.J.; de Souza, F.M.; Monteiro, E.d.S.; de Biasi, R.S. Mechanical and clinical properties of titanium and titanium-based alloys (Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. J. Mater. Res. Technol. 2019, 8, 1060–1069. [Google Scholar] [CrossRef]
- Grzeskowiak, R.M.; Schumacher, J.; Dhar, M.S.; Harper, D.P.; Mulon, P.-Y.; Anderson, D.E. Bone and cartilage interfaces with orthopedic implants: A literature review. Front. Surg. 2020, 7, 601244. [Google Scholar] [CrossRef]
- Nakano, T. Mechanical properties of metallic biomaterials. In Metals for Biomedical Devices; Niinomi, M., Ed.; Woodhead Publishing Limited: Oxford, UK, 2010; pp. 71–98. [Google Scholar]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Niinomi, M. Recent progress in research and development of metallic structural biomaterials with mainly focusing on mechanical biocompatibility. Mater. Trans. 2018, 59, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dzogbewu, T.C.; du Preez, W.B. Additive manufacturing of titanium-based implants with metal-based antimicrobial agents. Metals 2021, 11, 453. [Google Scholar] [CrossRef]
- Whisnant, D. Polymer Chemistry: Mechanical Properties. Available online: https://eng.libretexts.org/Bookshelves/Materials_Science/Supplemental_Modules_(Materials_Science)/Polymer_Chemistry/Polymer_Chemistry%3A_Mechanical_Properties (accessed on 21 October 2021).
- Fernandes, D.J.; Elias, C.N.; Valiev, R.Z. Properties and performance of ultrafine grained titanium for biomedical applications. Mater. Res. 2015, 18, 1163–1175. [Google Scholar] [CrossRef]
- American Elements. Titanium—Commercially Pure (CP). Available online: https://www.americanelements.com/titanium-commercially-pure-cp-7440-32-6 (accessed on 14 January 2022).
- AZoMaterials. Titanium (Ti)—Properties, Applications. Available online: https://www.azom.com/properties.aspx?ArticleID=712 (accessed on 30 August 2021).
- Hosseini, S. Fatigue of Ti-6Al-4V. In Biomedical Engineering—Technical Applications in Medicine; Hudak, R., Penhaker, M., Majernik, J., Eds.; BoD—Books on Demand: Norderstedt, Germany, 2012; pp. 75–92. [Google Scholar]
- MatWeb. Titanium, Ti. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=66a15d609a3f4c829cb6ad08f0dafc01 (accessed on 30 August 2021).
- AZoMaterials. Titanium Alloys—Ti6Al4V Grade 5. Available online: https://www.azom.com/properties.aspx?ArticleID=1547 (accessed on 8 June 2021).
- MatWeb. Titanium Ti-6Al-4V ELI (Grade 23), Annealed. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=c4297fb8f1094da189732c224e3be1ed (accessed on 30 August 2021).
- Poinern, G.E.J.; Brundavanam, R.K.; Fawcett, D. Nanometre scale hydroxyapatite ceramics for bone tissue engineering. Am. J. Biomed. Eng. 2013, 3, 148–168. [Google Scholar]
- Boyan, B.D.; Lossdörfer, S.; Wang, L.; Zhao, G.; Lohmann, C.H.; Cochran, D.L. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur. Cells Mater. 2003, 6, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bumgardner, J.D.; Cavin, R.; Carnes, D.L.; Ong, J.L. Osteoblast precursor cell attachment on heat-treated calcium phosphate coatings. J. Dent. Res. 2003, 82, 449–453. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Seta, J.; Chen, L.; Bergum, C.; Zhou, Z.; Kanneganti, P.; Kast, R.E.; Auner, G.W.; Shen, M.; Markel, D.C.; et al. Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus aureus infection. Biomed. Mater. 2017, 12, 45008. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, K.; Lu, X.; Lam, Y.C. A review on the mechanical methods for evaluating coating adhesion. Acta Mech. 2014, 225, 431–452. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Tian, N.; Dong, X.; Cheng, C.-K. Implant coating manufactured by micro-arc oxidation and dip coating in resorbable polylactide for antimicrobial applica-tions in orthopedics. Coatings 2019, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Prolongo, S.G.; Gude, M.R.; Ureña, A. Nanoreinforced adhesives. In Nanofibers; Kumar, A., Ed.; IntechOpen Limited: London, UK, 2010; pp. 39–68. [Google Scholar]
- Maxwell, A.S. Review of Test Methods for Coating Adhesion. Available online: https://eprintspublications.npl.co.uk/2077/1/MATC49.pdf (accessed on 20 September 2021).
- American Society for Testing and Materials (ASTM). Standard Test Method for Evaluation of Scratch Resistance of Polymeric Coatings and Plastics Using an Instrumented Scratch Machine. Available online: https://www.astm.org/Standards/D7027.htm (accessed on 6 September 2021).
- ASTM. Standard Practice for Instrumented Indentation Testing. Available online: https://www.astm.org/Standards/E2546.htm (accessed on 6 September 2021).
- Raita, Y.; Komatsu, K.; Hayakawa, T. Pilot study of gingival connective tissue responses to 3-dimensional collagen nanofiber-coated dental implants. Dent. Mater. J. 2015, 34, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Sadri, M.; Pashmforosh, N.; Samadieh, S. Implants modified with polymeric nanofibers coating containing the antibiotic vancomycin. Nanomed. Res. J. 2017, 2, 208–215. [Google Scholar]
- Aadil, K.R.; Mussatto, S.I.; Jha, H. Synthesis and characterization of silver nanoparticles loaded poly(vinyl alcohol)-lignin electrospun nanofibers and their antimicrobial activity. Int. J. Biol. Macromol. 2018, 120, 763–767. [Google Scholar] [CrossRef] [Green Version]
- des Ligneris, E.; Dumée, L.F.; Al-Attabi, R.; Castanet, E.; Schütz, J.; Kong, L. Mixed matrix poly(vinyl alcohol)-copper nanofibrous anti-microbial air-microfilters. Membranes 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swami, V.; Vijayaraghavan, V.; Swami, V. Current trends to measure implant stability. J. Indian Prosthodont. Soc. 2016, 16, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, M.F.J.; Al-Jumaily, H.A. The relationship between primary (mechanical) and secondary (biological) implant stability: A new measurement technique. J. Med. Dent. Sci. 2020, 8, 11–15. [Google Scholar]
- Vollmer, A.; Saravi, B.; Lang, G.; Adolphs, N.; Hazard, D.; Giers, V.; Stoll, P. Factors influencing primary and secondary implant stability—A retrospective cohort study with 582 implants in 272 patients. Appl. Sci. 2020, 10, 8084. [Google Scholar] [CrossRef]
- Monje, A.; Ravidà, A.; Wang, H.-L.; Helms, J.A.; Brunski, J.B. Relationship be-tween primary/mechanical and secondary/biological implant stability. Int. J. Oral Maxillofac. Implants. 2019, 34, s7–s23. [Google Scholar] [CrossRef]
- Chen, J.; Peng, W.; Zhu, L.; Tan, L.; Etim, I.P.; Wang, X.; Yang, K. Effect of copper content on the corrosion behaviors and antibacterial properties of binary Mg-Cu alloys. Mater. Technol. 2018, 33, 145–152. [Google Scholar] [CrossRef]
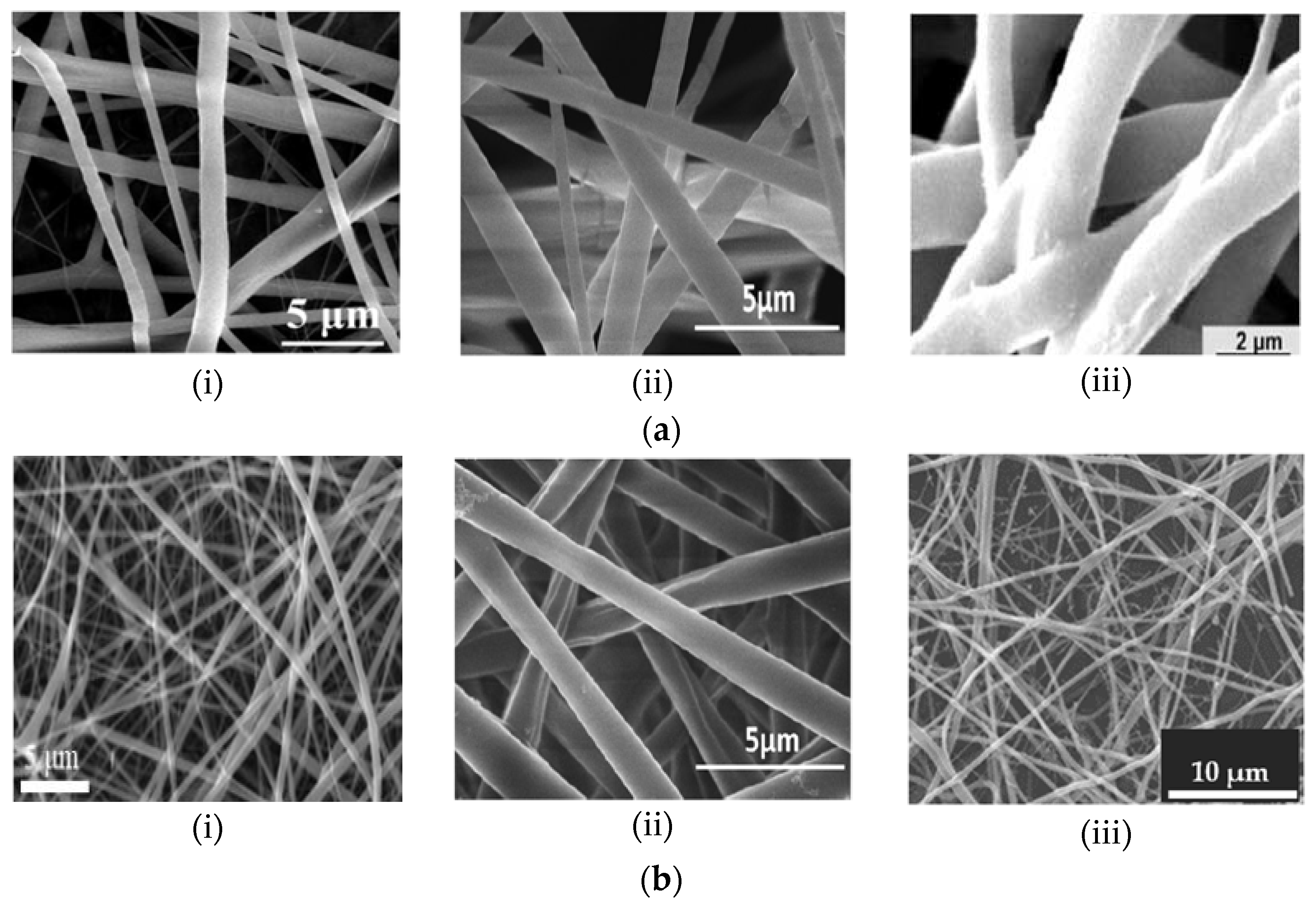
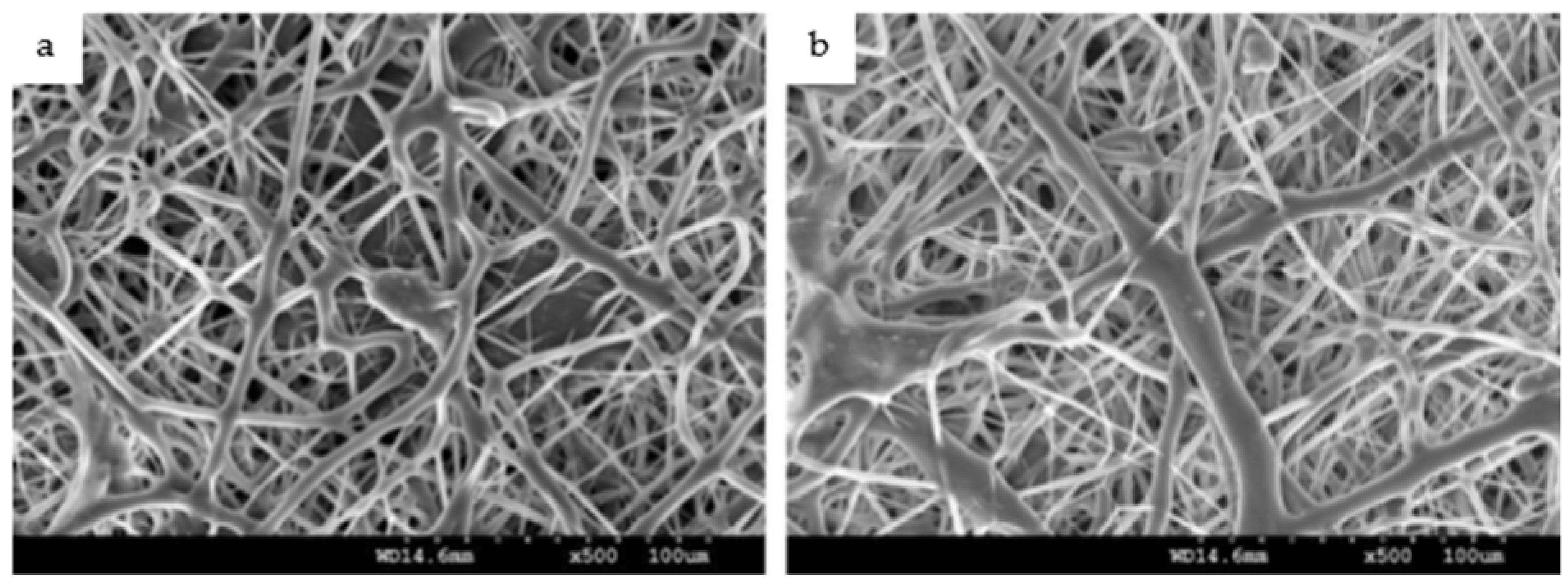
| Polymer | Properties | Applications | Degradation Rate | Ref. |
|---|---|---|---|---|
| Poly(ε-caprolactone) (PCL) | Hydrophobic aliphatic polyester; slow degradation rate; bioactive; flexible mechanical properties; effectively entraps bactericidal material; semi-crystalline; semi-permeable | Long-term implants; bone graft material; tissue engineering scaffolds; drug-delivery systems | 2–4 years | [21,31,32] |
| Poly(lactic acid) (PLA) | Hydrophobic aliphatic polyester; slow degradation rate; bioactive; tunable mechanical properties; crystalline; porous; stereoisomers: poly(L-lactide) (PLLA), poly(D-lactide) (PDLA), and poly(DL-lactide) (PDLLA) | Biomedical coating; load-bearing applications; orthopedic fixation devices; tissue engineering; three-dimensional (3D) printed scaffolds; drug-delivery systems | >24 months | [21,31,32] |
| Poly(lactic-co-glycolic acid) (PLGA) | Hydrophobic/hydrophilic balance; intermediate/adjustable degradation rate; PLA/PGA copolymer; crystalline; semi-permeable; low osteoinductivity | Copolymer for development of bone substitute constructs; bone regeneration; orthopedic implants; tissue engineering | 6–12 months | [9,20,21,32] |
| Poly(glycolic acid) (PGA) | Hydrophilic aliphatic polyester; fast degradation rate; tunable material properties; crystalline; low solubility; semi-permeable | Implants, tissue engineering; drug delivery; biological adhesives; open soft tissue wounds | 2–4 weeks | [14,20,21,32] |
| Poly(ethylene oxide) (PEO) | Hydrophilic; synthetic hydrogel | Composite functional materials; hydrogel coatings; blood contact | - | [2,25,33,34] |
| Poly(vinyl alcohol) (PVA) | Hydrophilic; fast degradation; gel-forming properties; good film-forming; good chemical resistance; semi-crystalline | Implants; tissue engineering | - | [9,23,25,35] |
| Material/Tissue | Density (g/cm3) | Young’s Modulus (GPa) | Yield Strength (MPa) | Tensile Strength (MPa) | Compression Strength (MPa) | Elongation (%) | Fatigue Limit (MPa) | Vickers Hardness (MPa) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| cpTi | 4.54 | 108–115 | 170–483 | 240–550 | 130–170 | 15–24 | 200–234 | 60 | [68,69,70,71,72] |
| Ti–6Al–4V | 4.429–4.512 | 110–119 | 760–1103 | 862–1200 | 848–1172 | 10–18 | 90–610 | ~341 | [64,71,73,74] |
| Cortical bone | 1.8–2.0 | 7–30 | - | 35 trans. 283 long. | 160 trans. 240 long. | - | - | - | [64,75] |
| Cancellous bone | 1.0–1.4 | 0.01–3.0 | - | 1.5–38 | 1.5–12 | - | - | - | [64,75] |
| Collagen | - | 1.0–11.5 | - | 60 | - | - | - | - | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nhlapo, N.; Dzogbewu, T.C.; de Smidt, O. Nanofiber Polymers for Coating Titanium-Based Biomedical Implants. Fibers 2022, 10, 36. https://doi.org/10.3390/fib10040036
Nhlapo N, Dzogbewu TC, de Smidt O. Nanofiber Polymers for Coating Titanium-Based Biomedical Implants. Fibers. 2022; 10(4):36. https://doi.org/10.3390/fib10040036
Chicago/Turabian StyleNhlapo, Nthabiseng, Thywill Cephas Dzogbewu, and Olga de Smidt. 2022. "Nanofiber Polymers for Coating Titanium-Based Biomedical Implants" Fibers 10, no. 4: 36. https://doi.org/10.3390/fib10040036
APA StyleNhlapo, N., Dzogbewu, T. C., & de Smidt, O. (2022). Nanofiber Polymers for Coating Titanium-Based Biomedical Implants. Fibers, 10(4), 36. https://doi.org/10.3390/fib10040036






