Microstructure and Microhardness of Ni/Al-TiB2 Composite Coatings Prepared by Cold Spraying Combined with Postannealing Treatment
Abstract
:1. Introduction
2. Materials and Methods
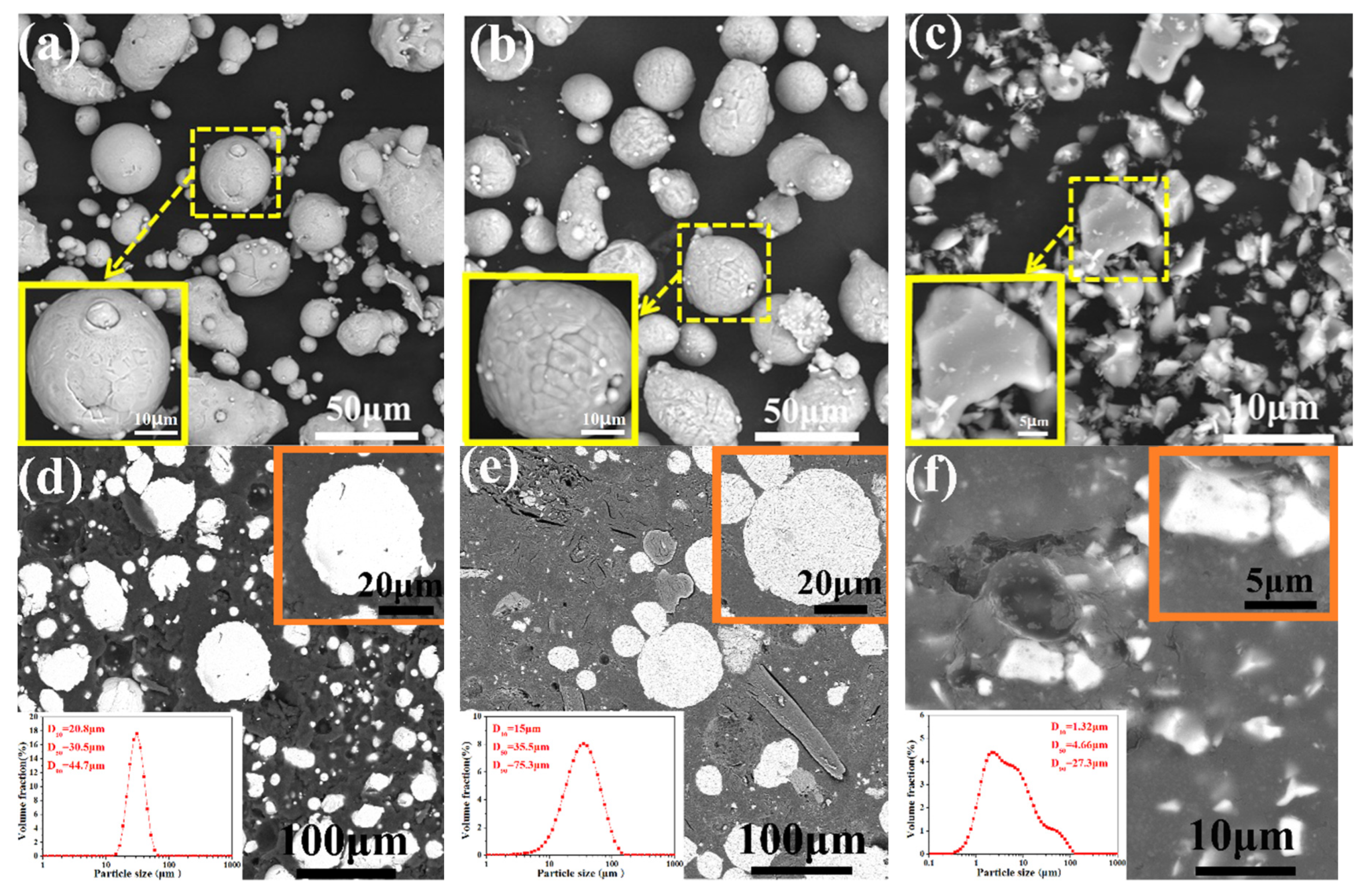
2.1. Powder and Coating Preparation
2.2. Annealing Treatment
2.3. Microstructure Characterization and Microhardness of Coatings
3. Results and Discussion
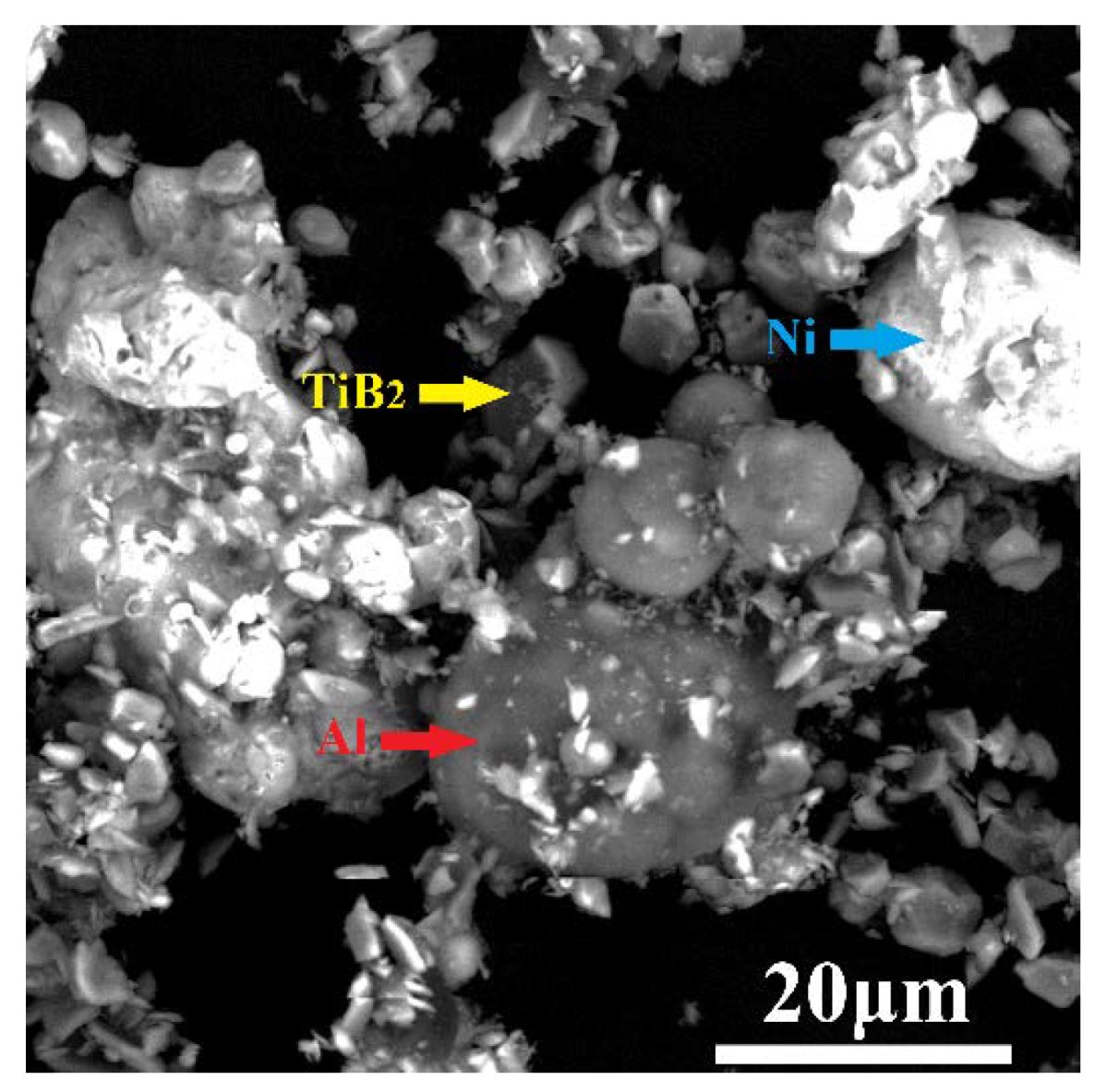
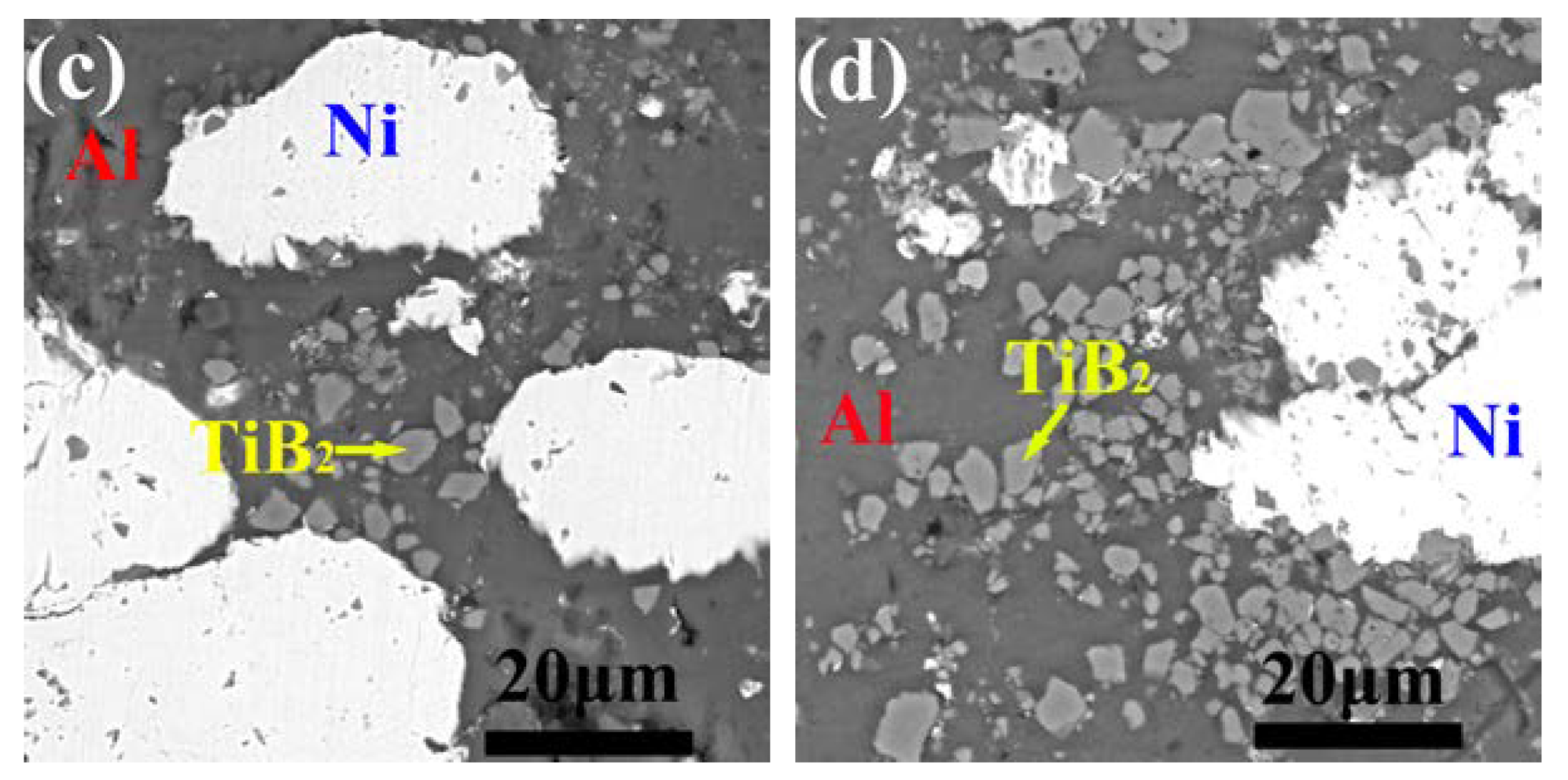
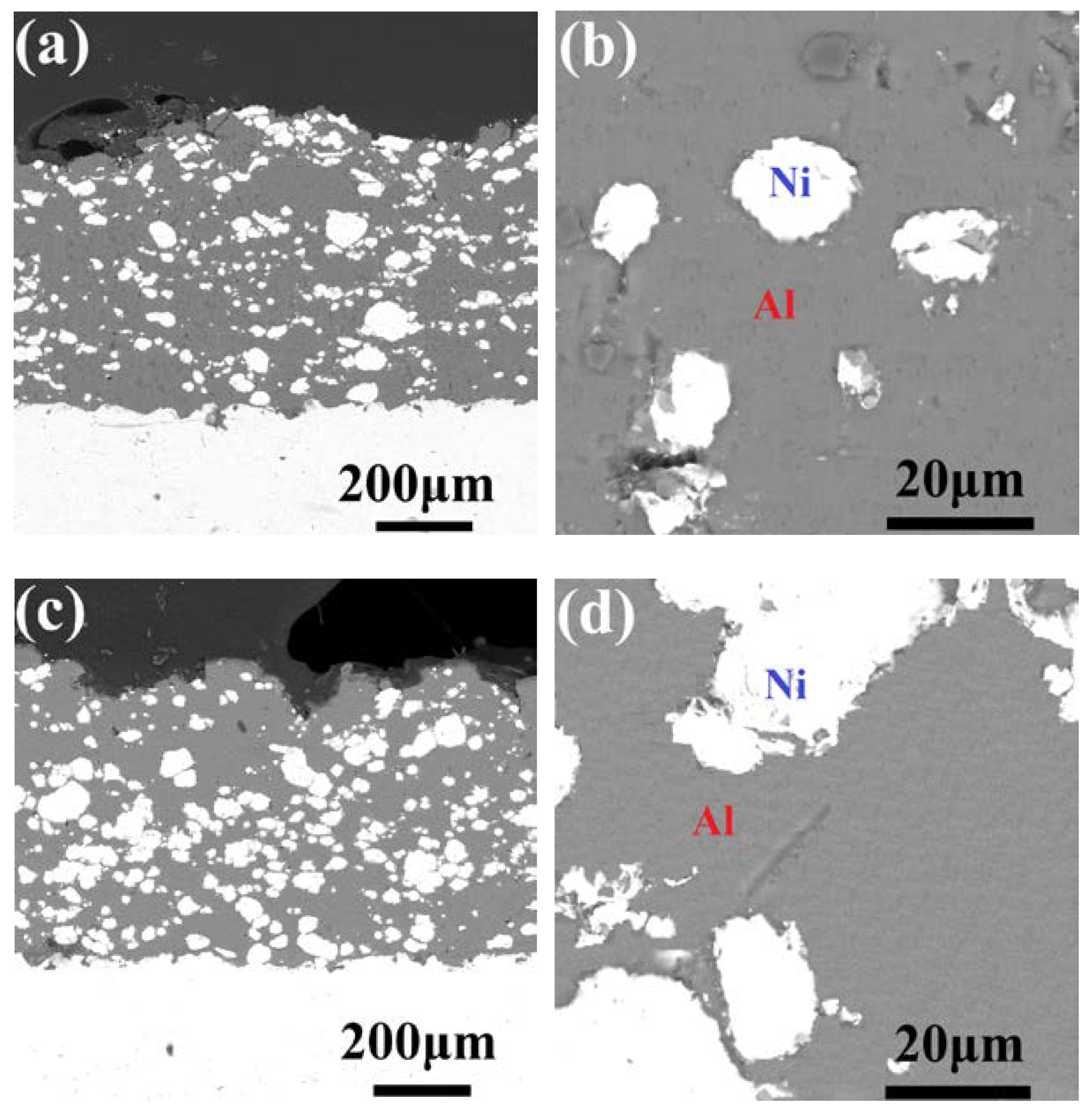
3.1. Microstructure of the As-Sprayed Ni/Al-TiB2 Coatings
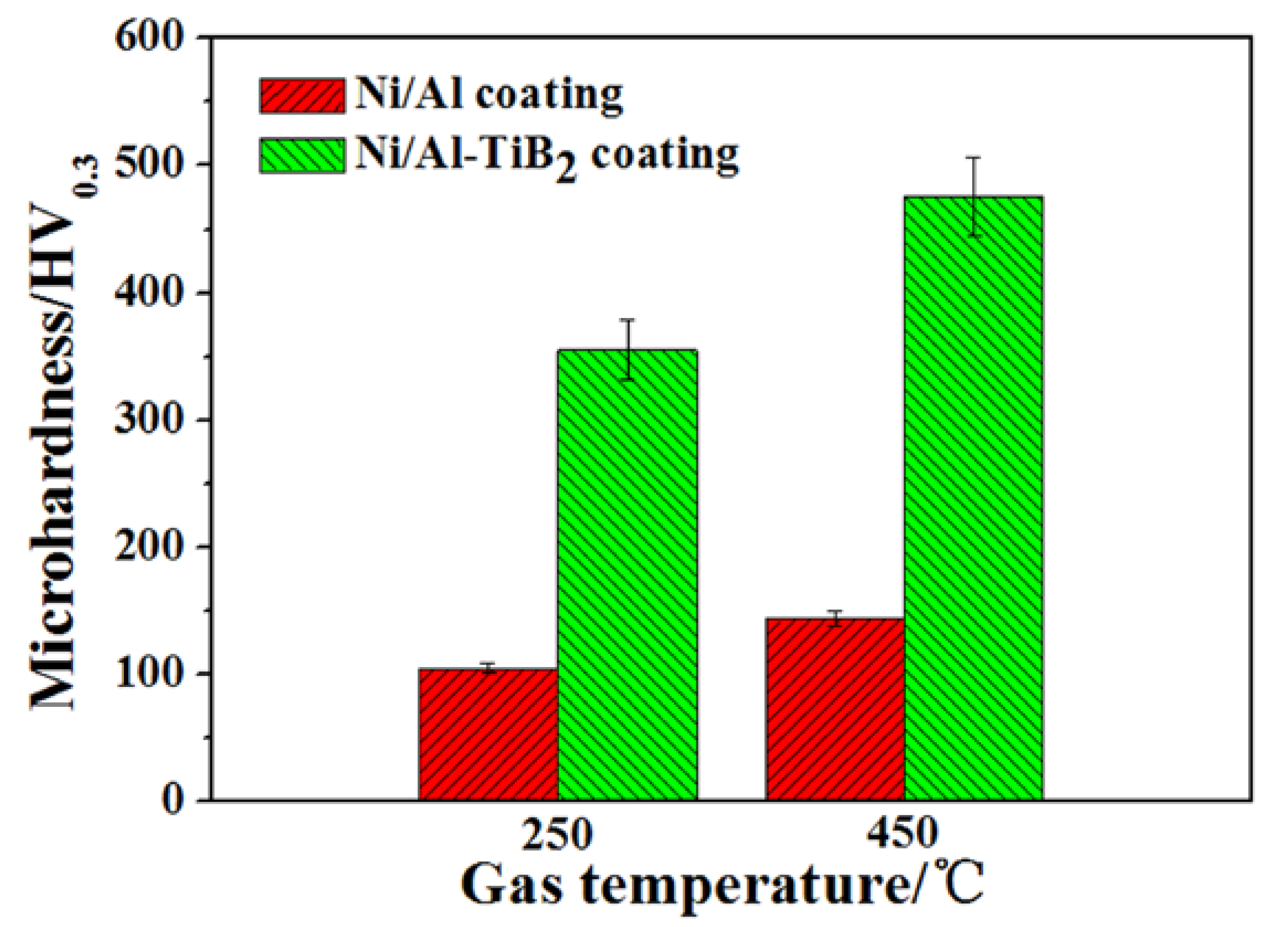
3.2. Microhardness of As-Sprayed Ni/Al-TiB2 Coatings
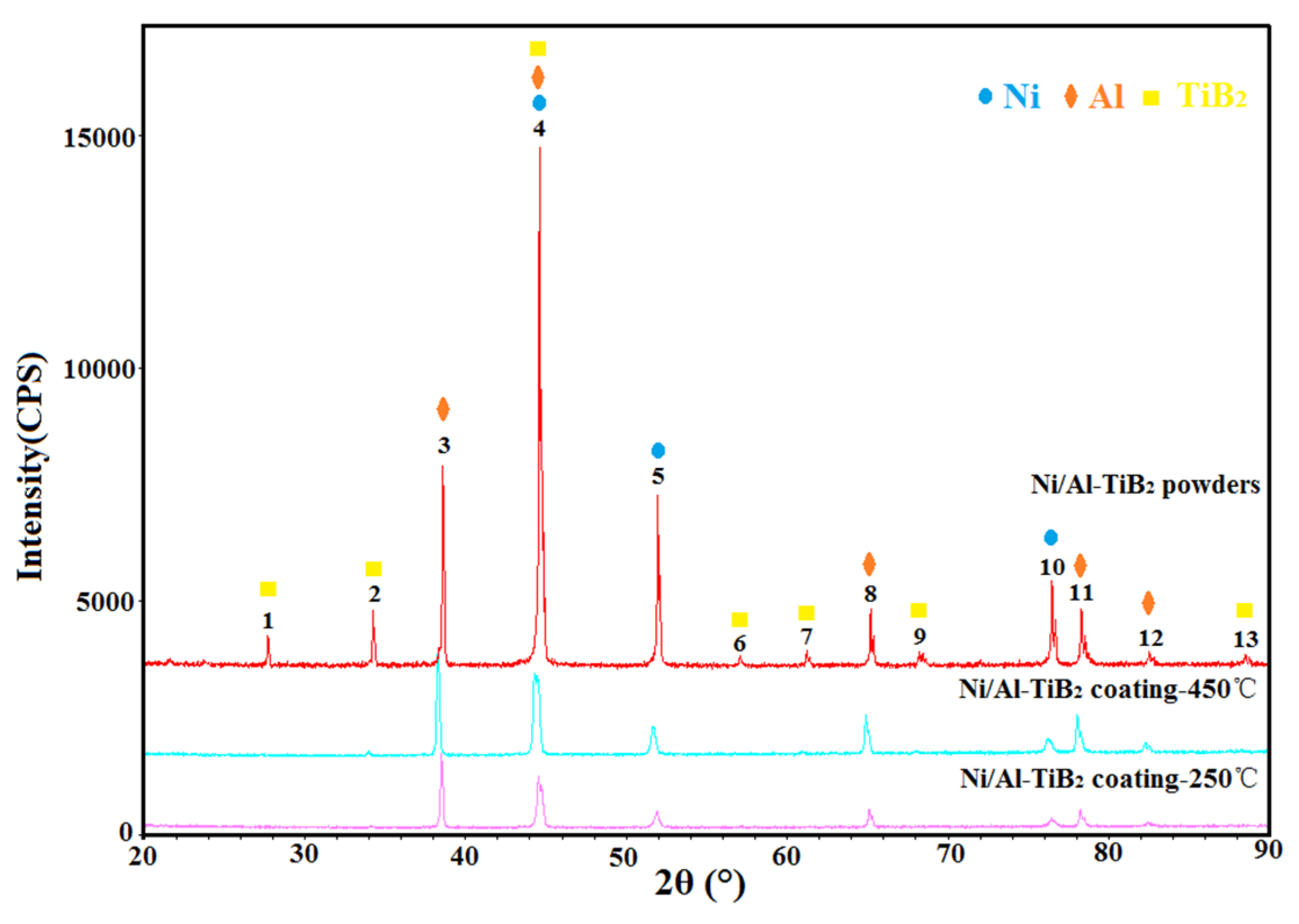
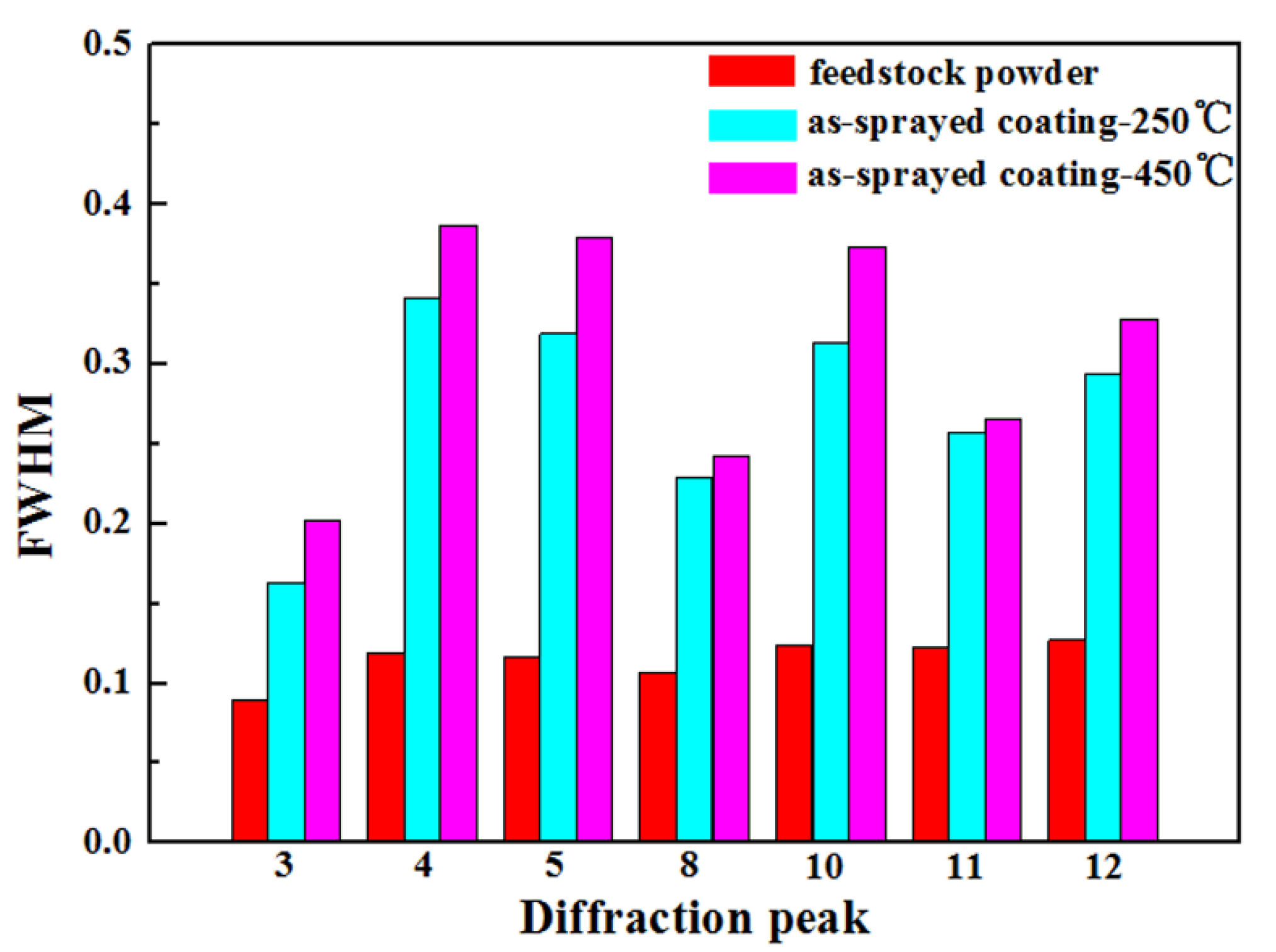
3.3. Phase Compositions of Feedstock Powder and As-Sprayed Ni/Al-TiB2 Coatings
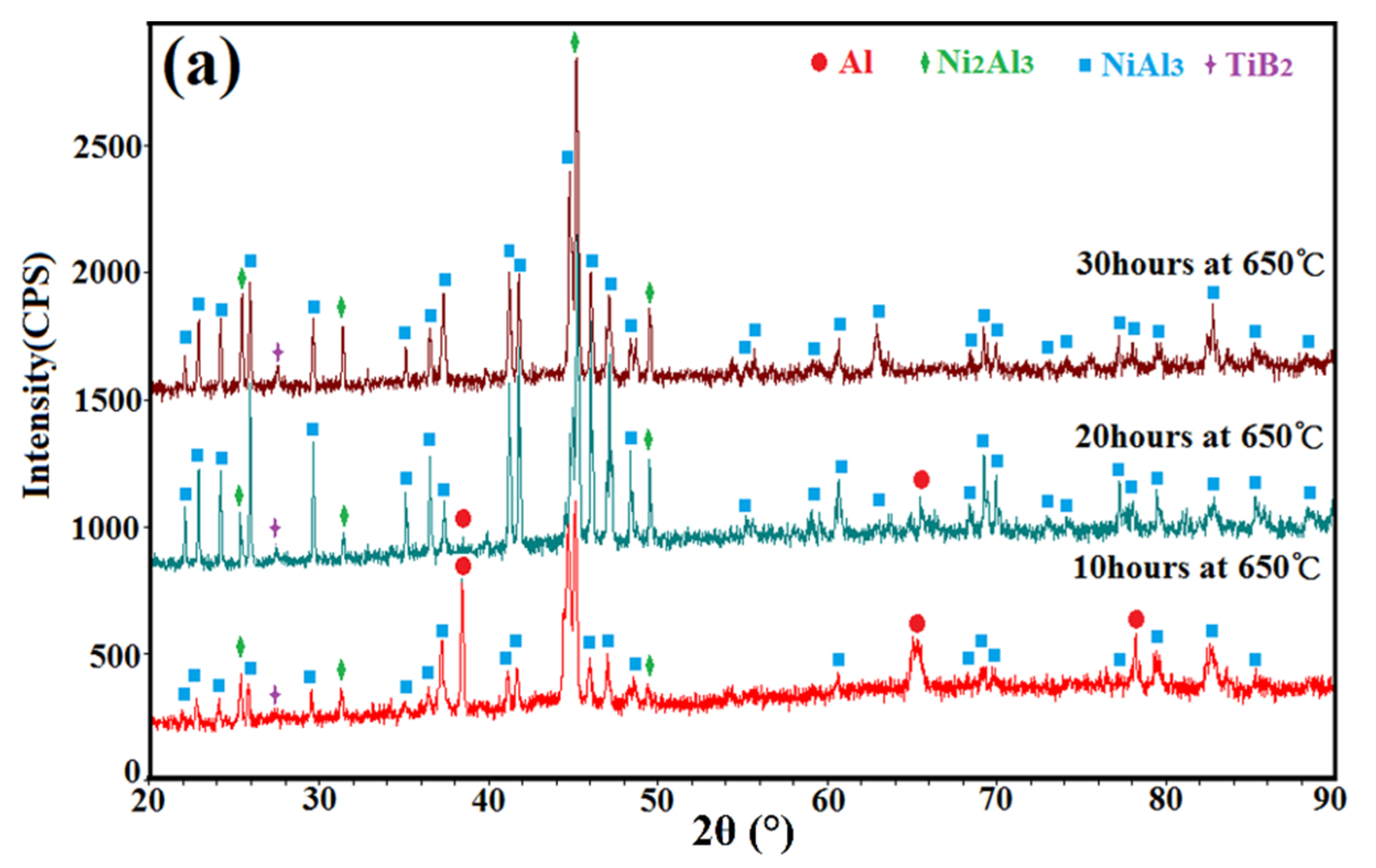
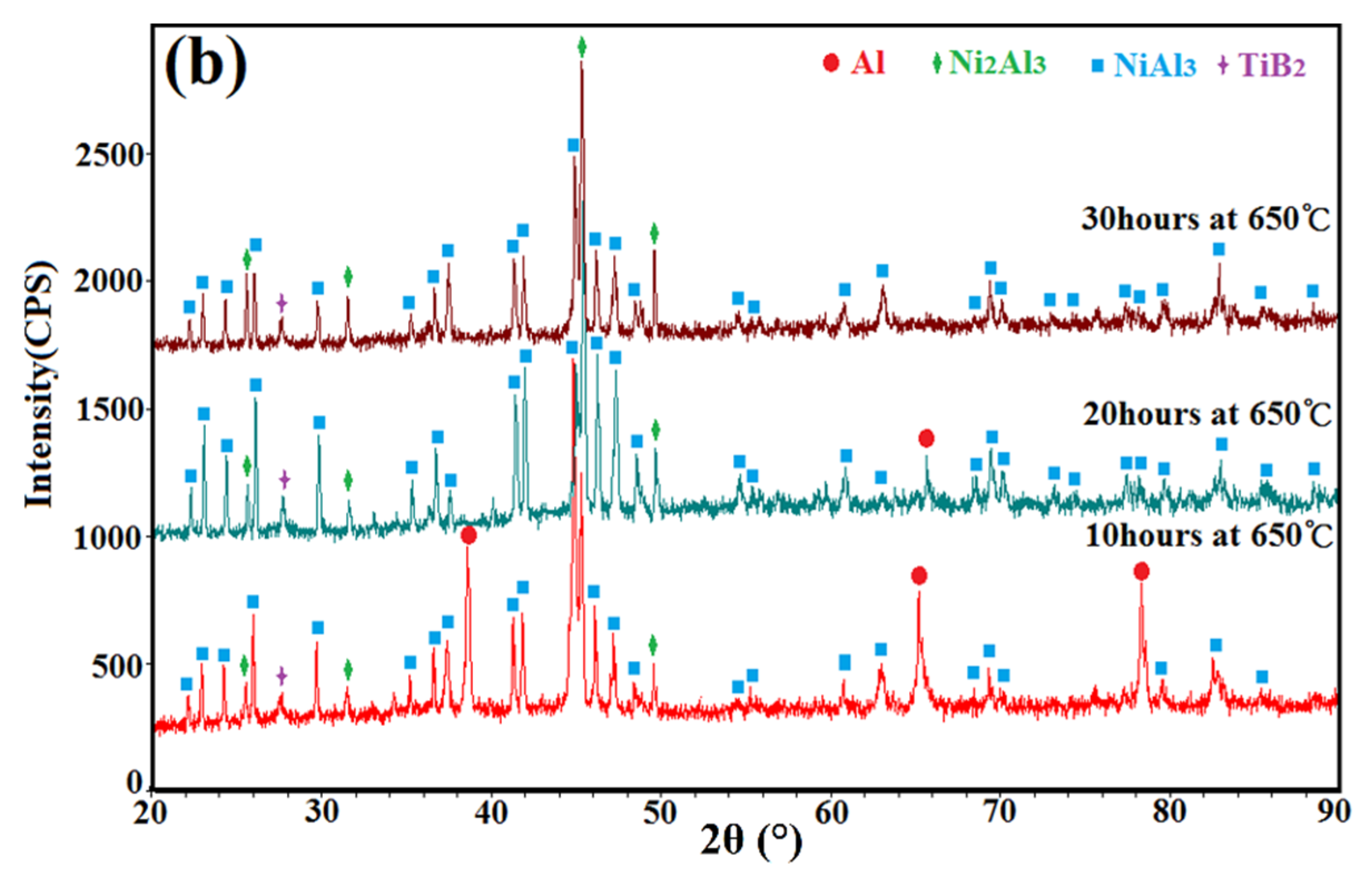
3.4. XRD Patterns of the Annealed Ni/Al-TiB2 Coatings
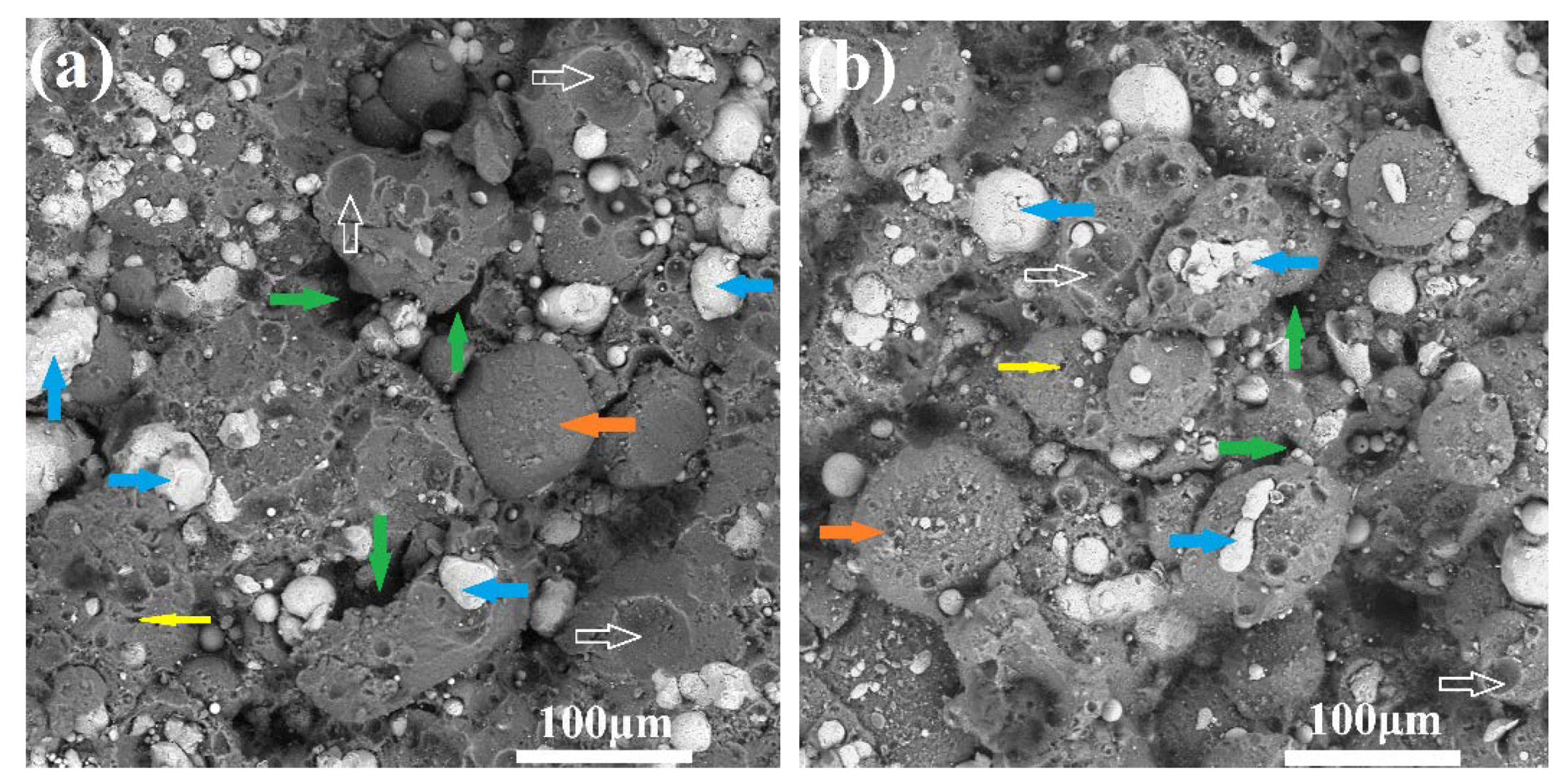
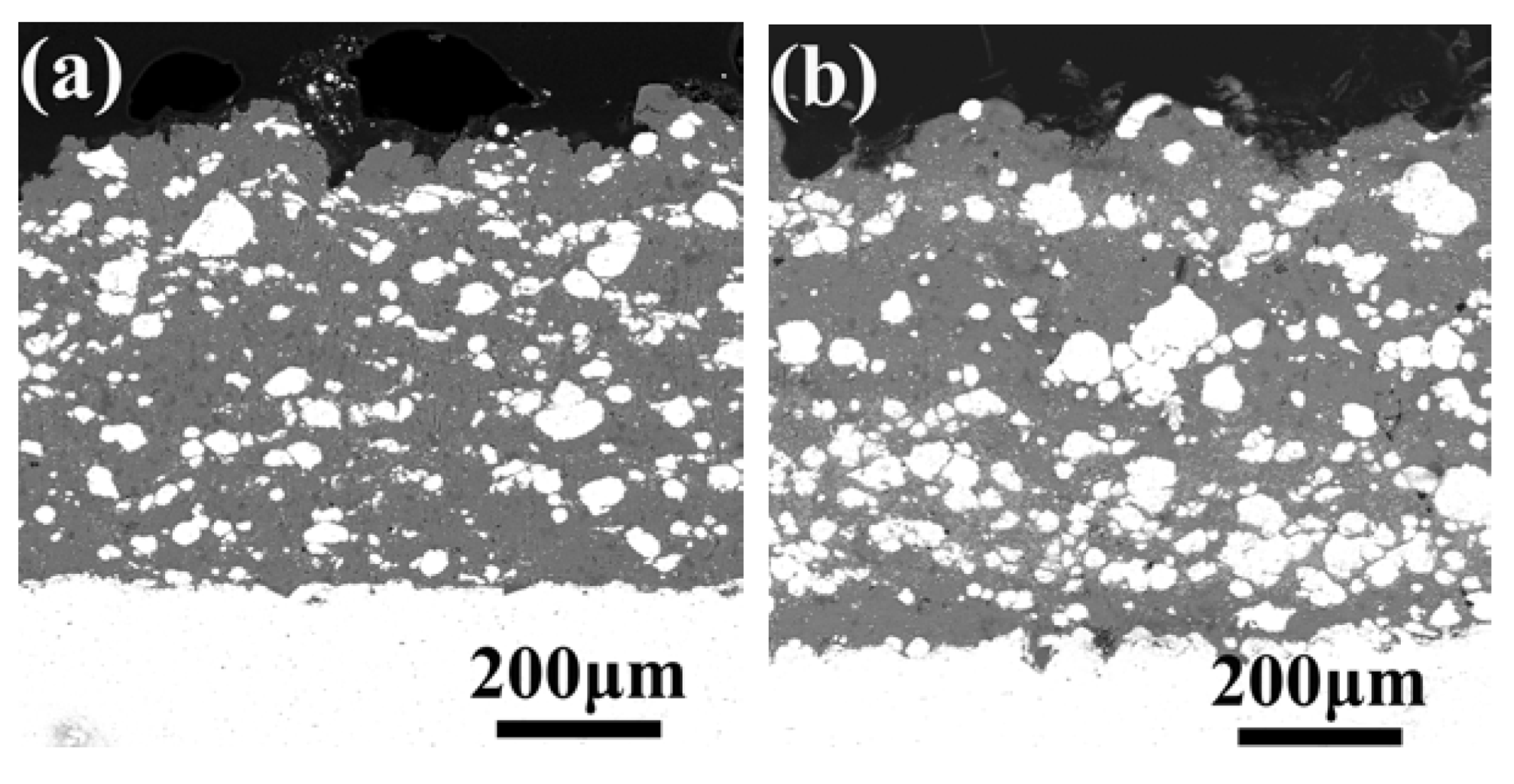
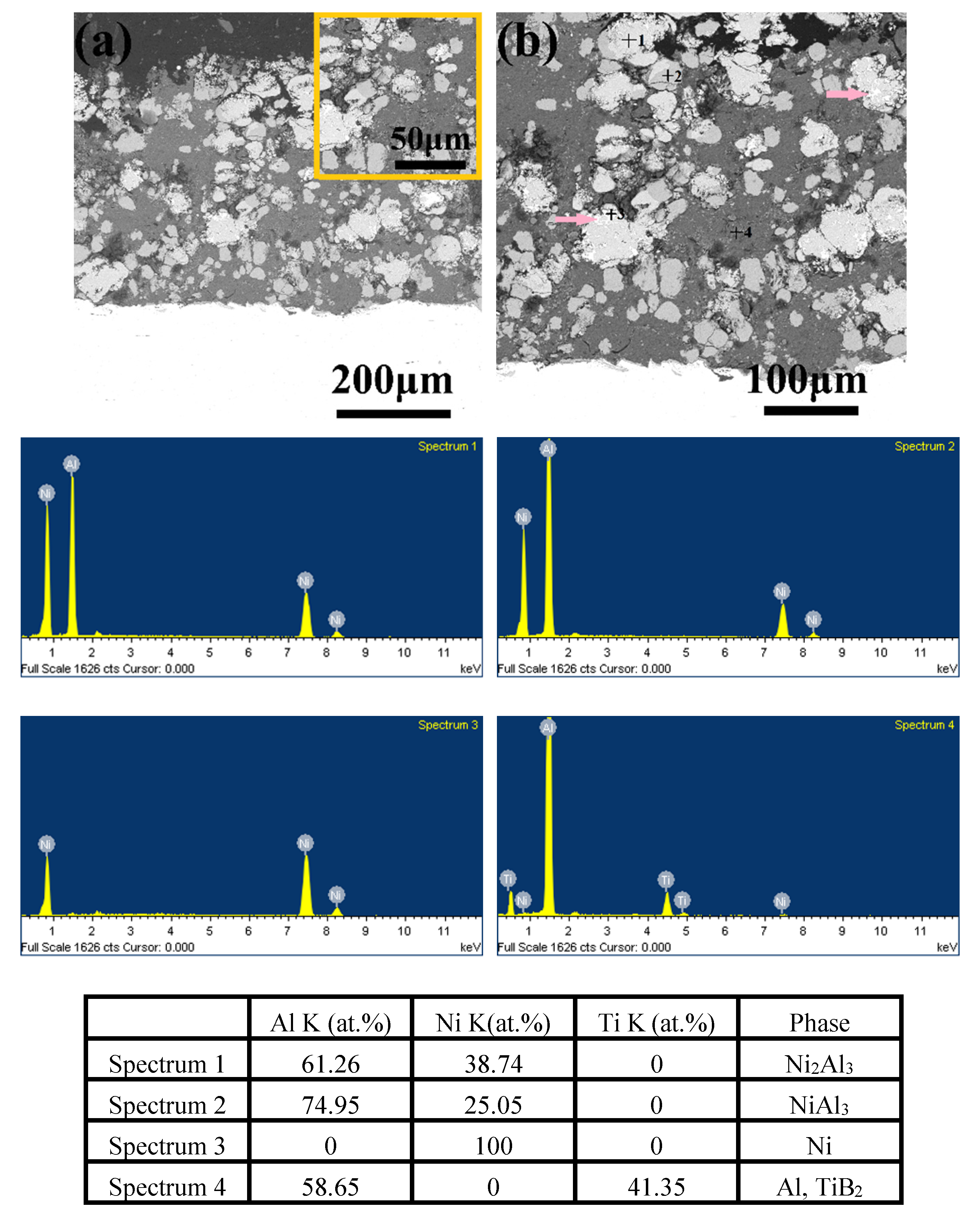
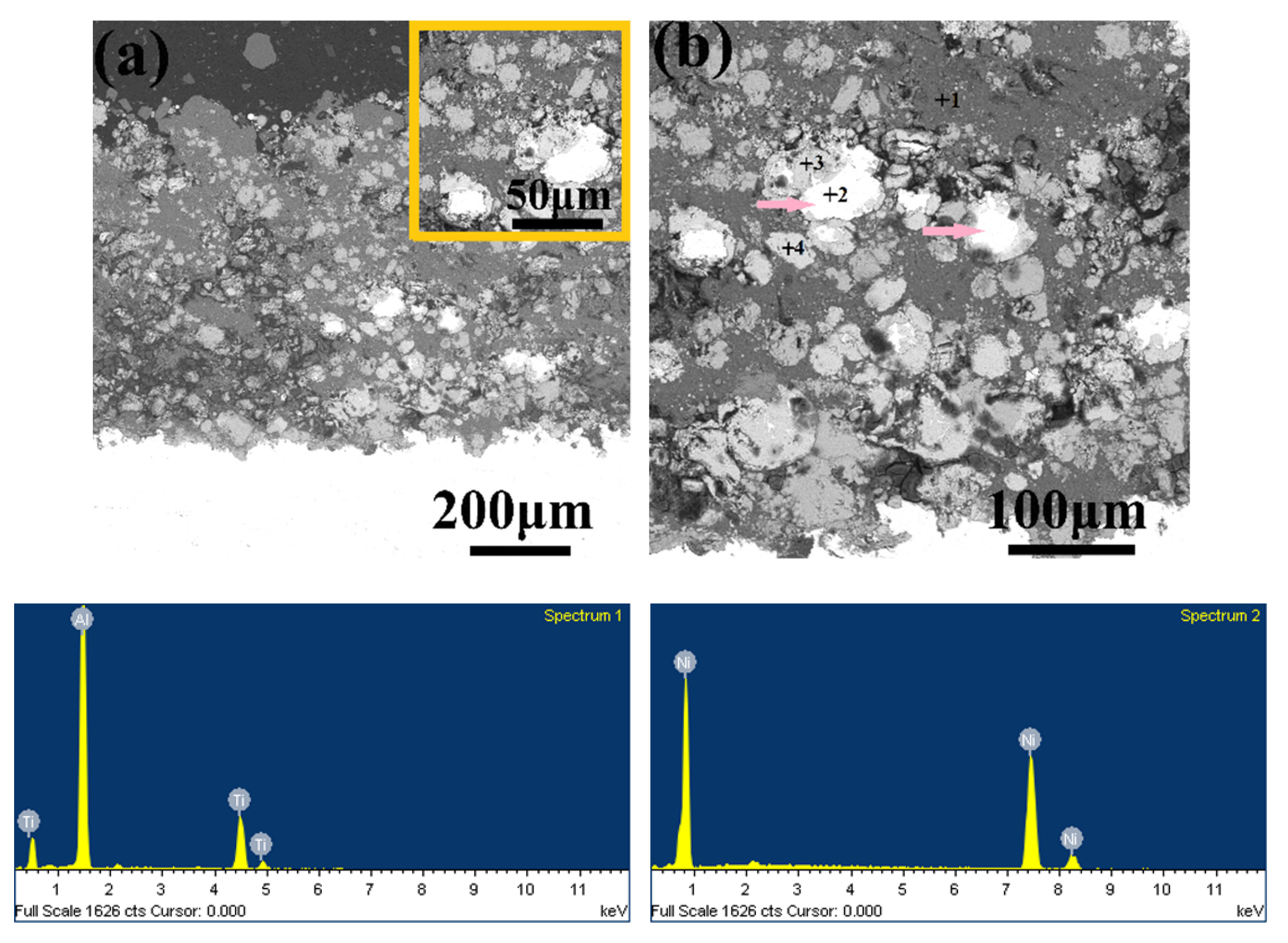
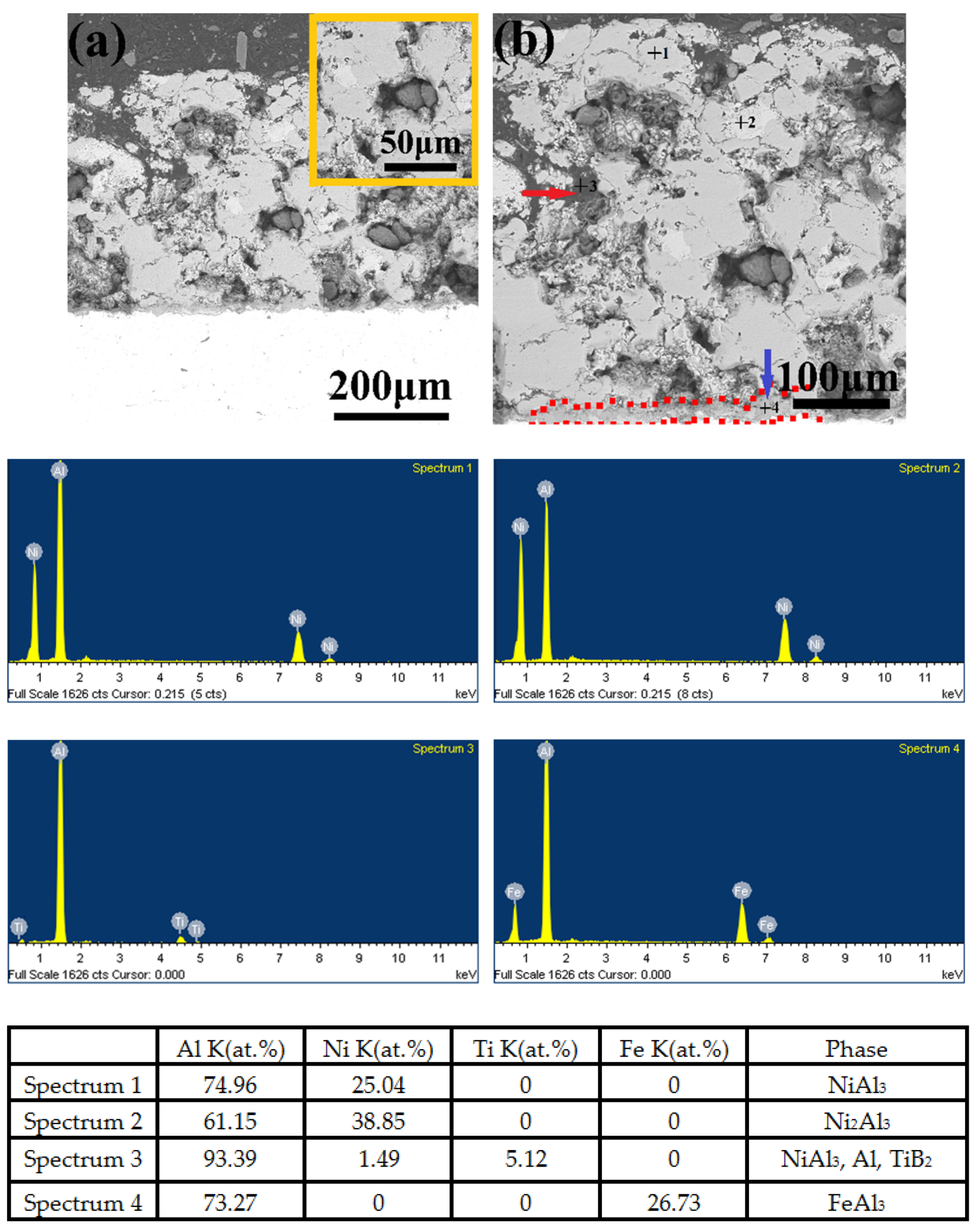
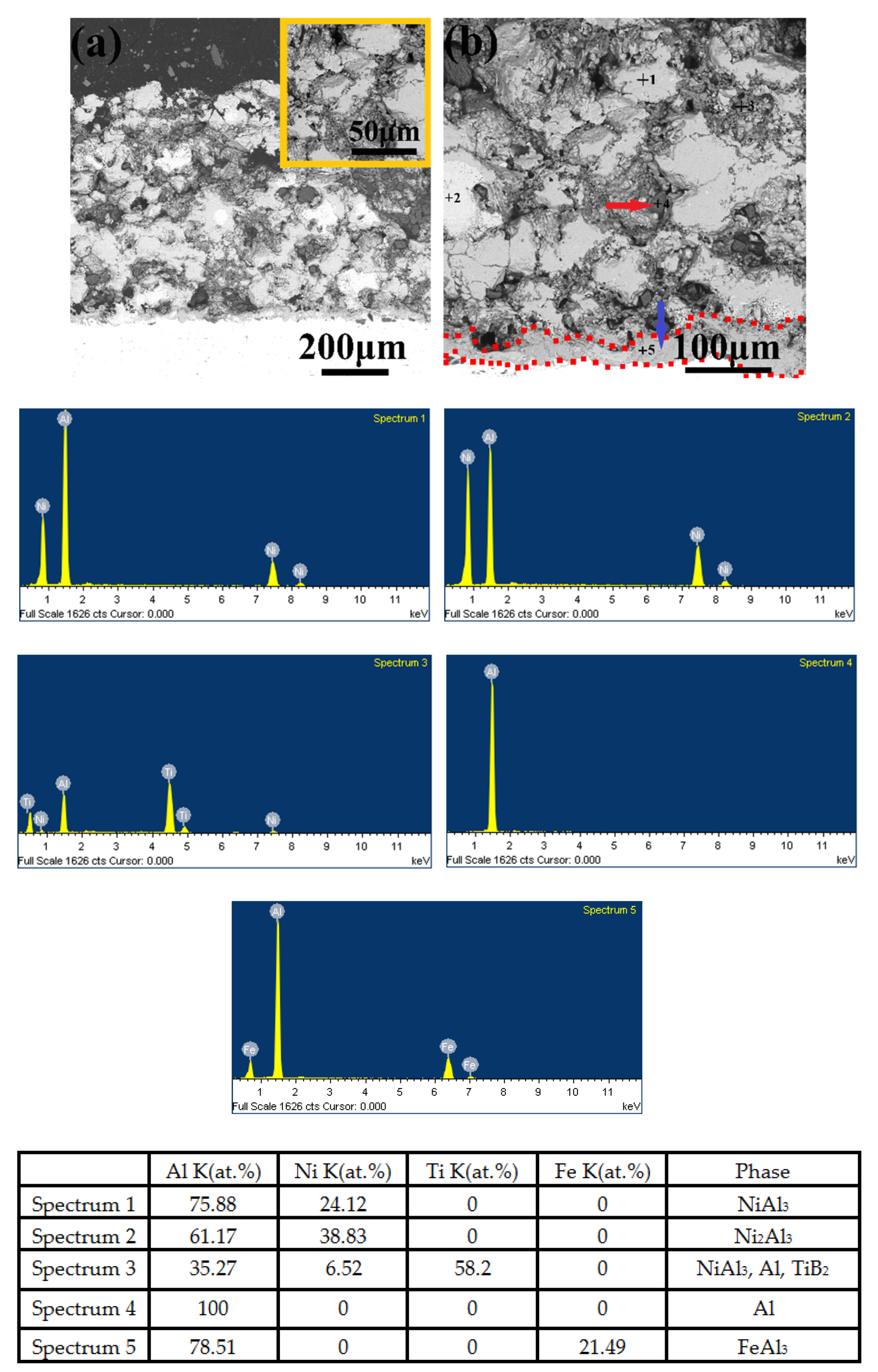
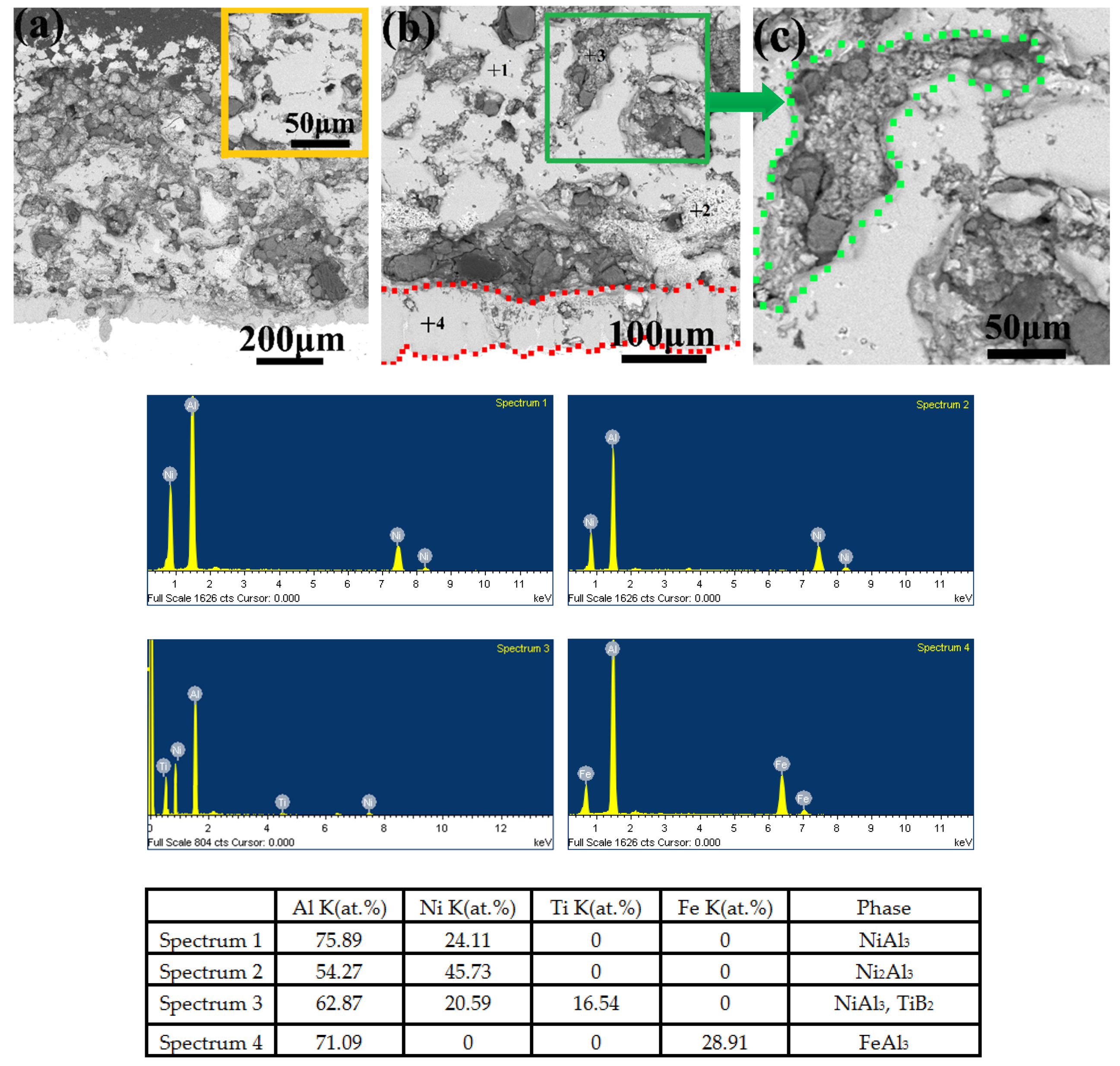
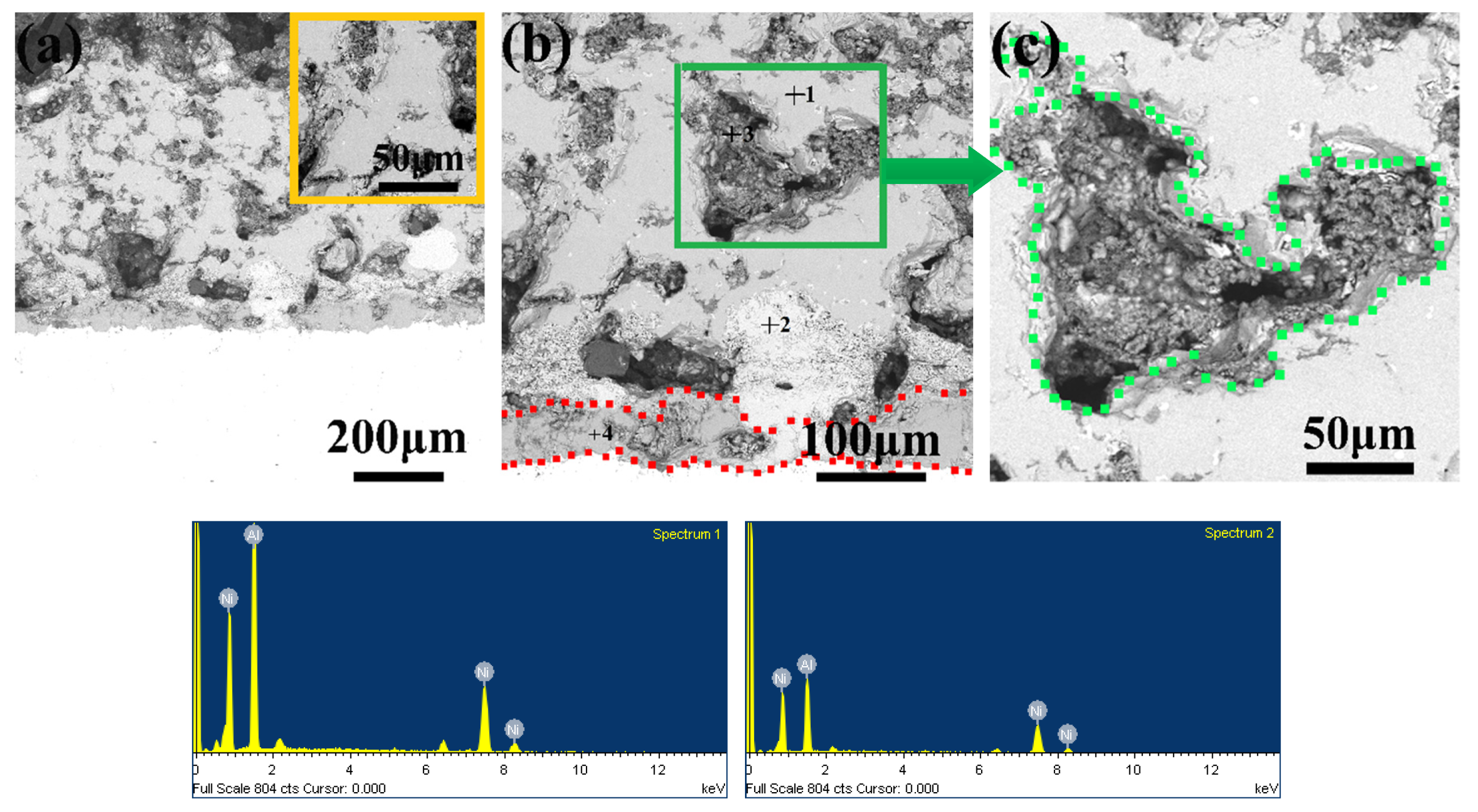
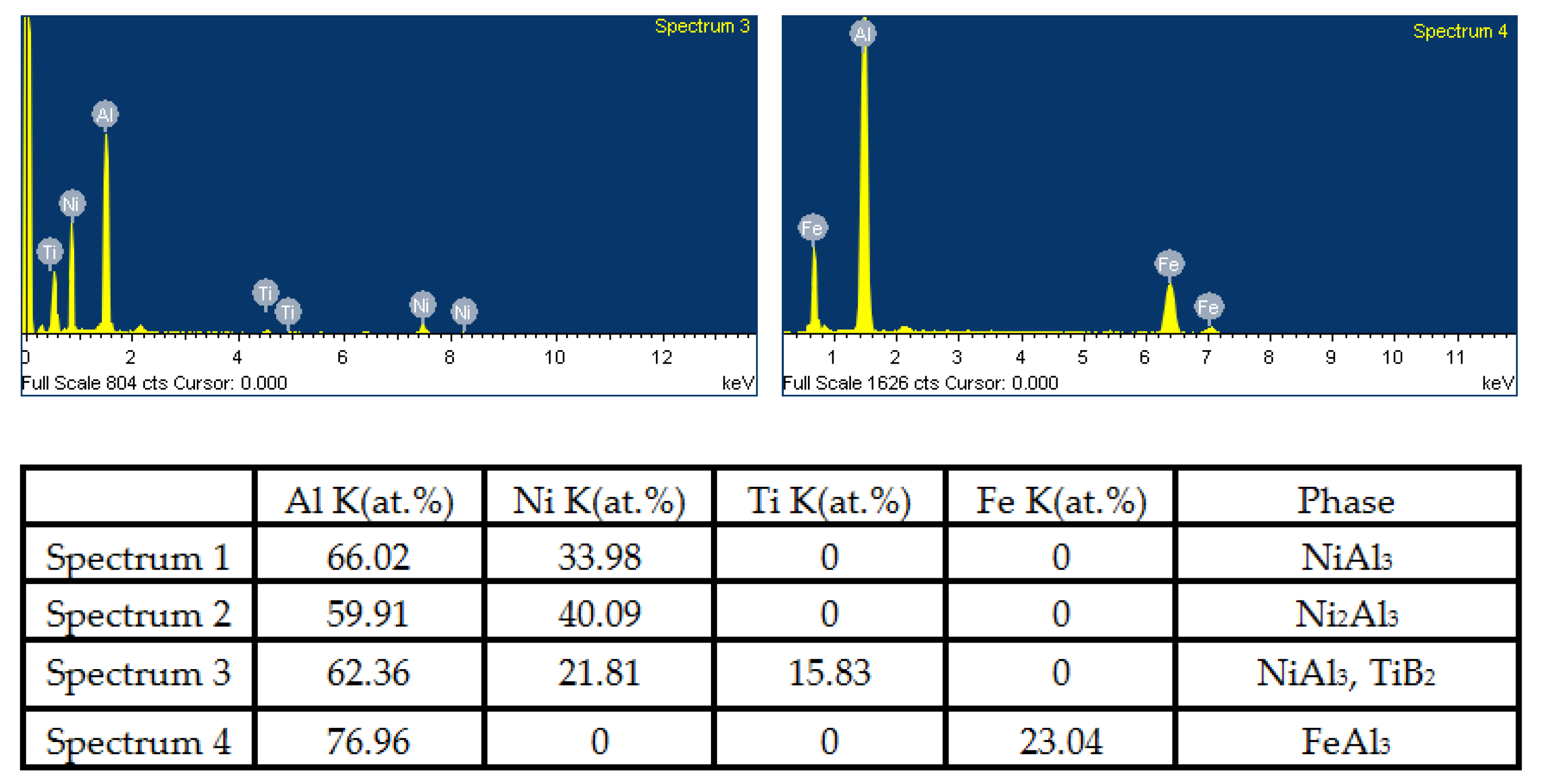
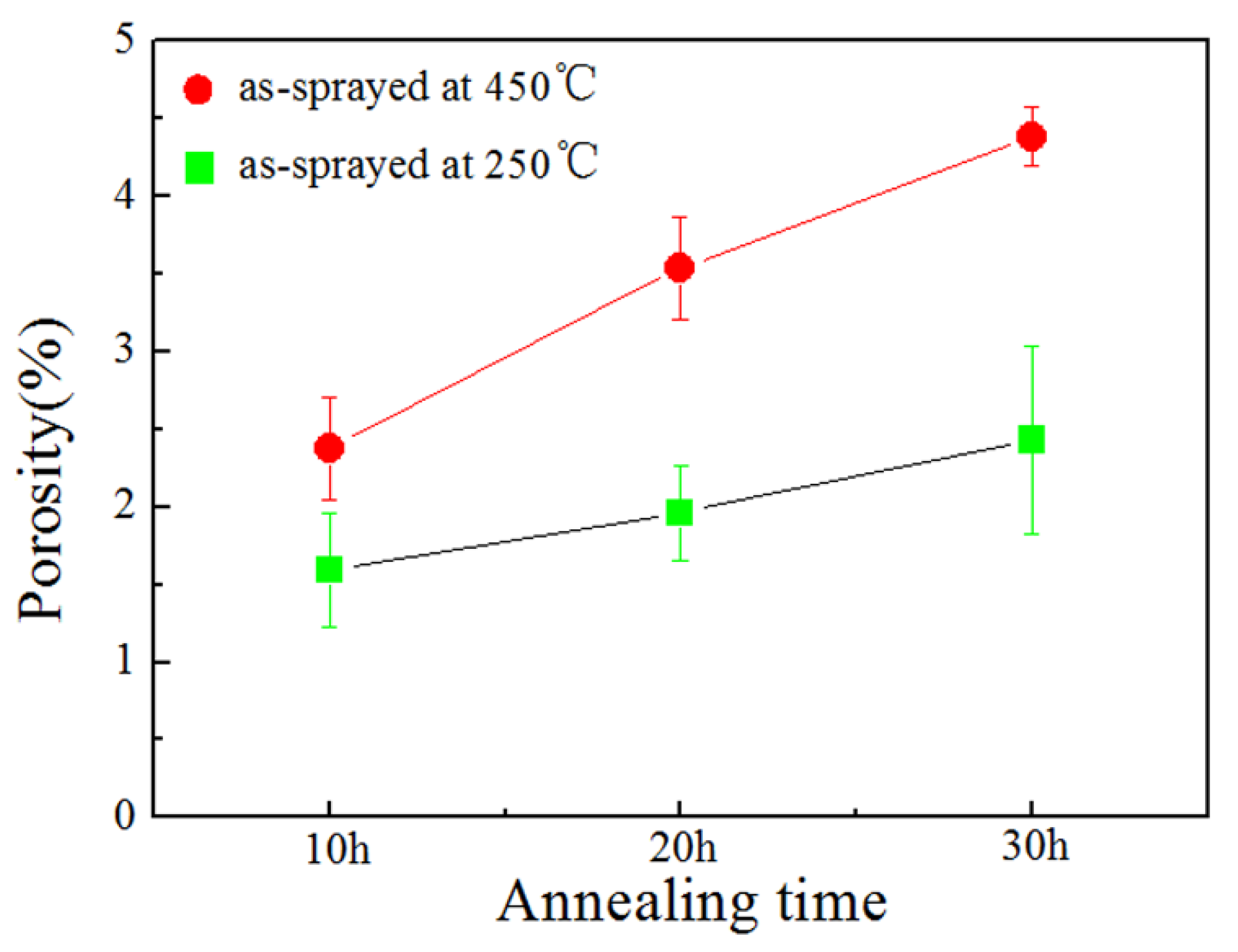
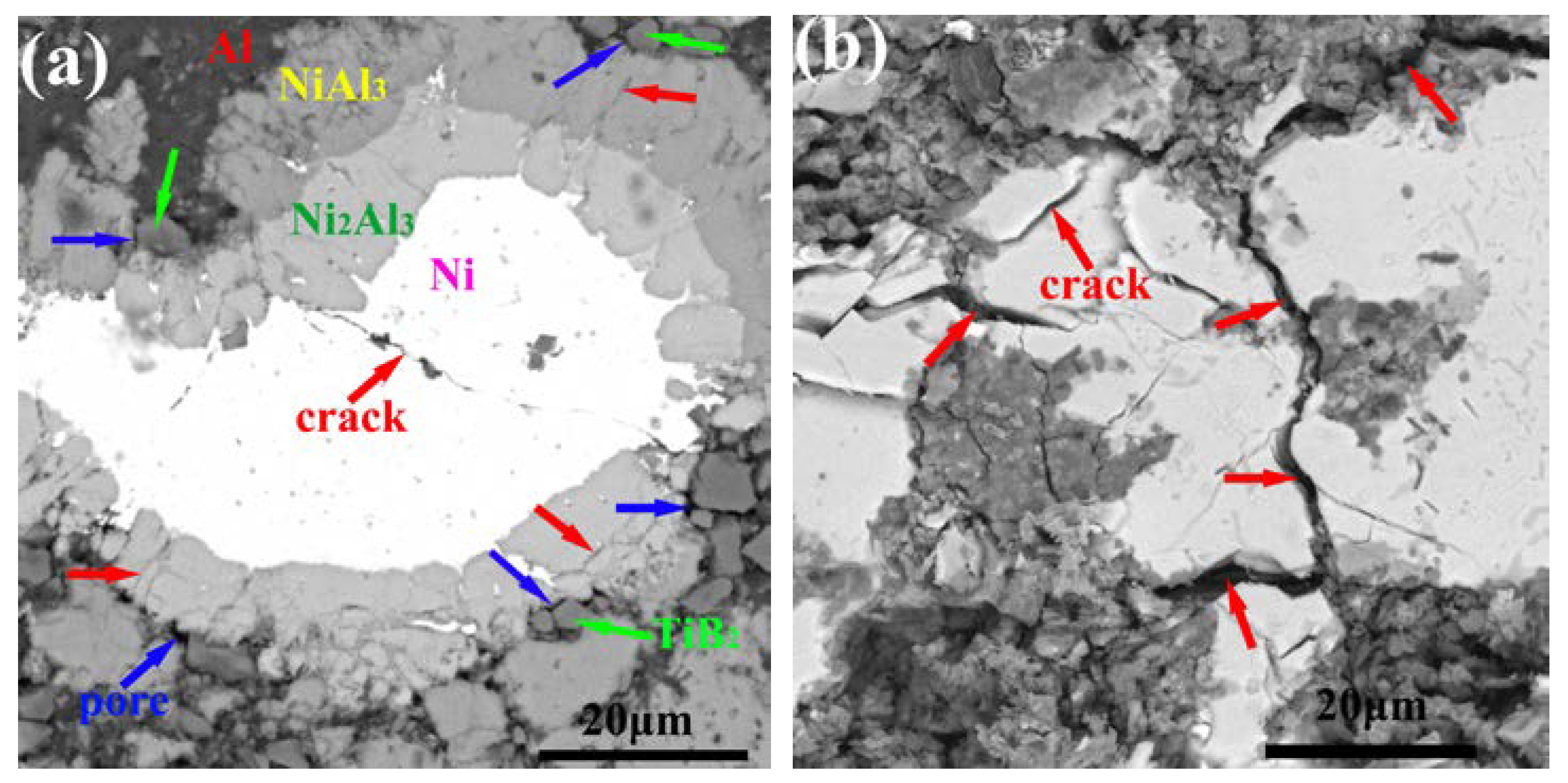
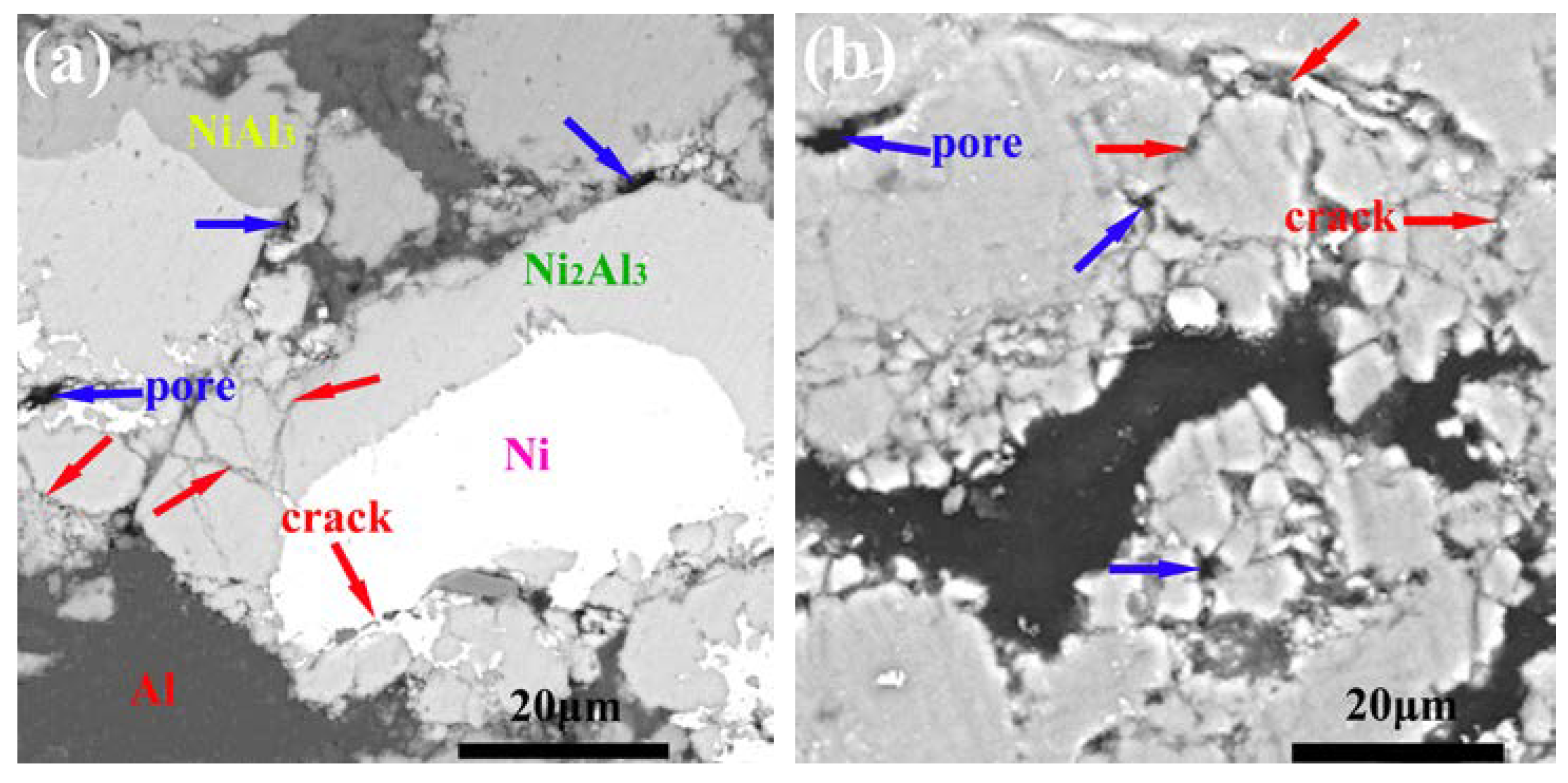
3.5. Microstructure, Compositionand Porosity, and Microhardness of the Annealed Ni/Al-Tib2 coatings
4. Conclusions
- The average surface roughness values of the coatings dropped from 8.66 ± 0.73 to 6.17 ± 0.88 μm as the spraying gas temperature increased.
- The average porosities of the coatings as-sprayed at gas temperatures of 250 and 450 °C were 0.68% and 0.054%, respectively. The average contents of Ni, Al, and TiB2 in the as-sprayed coating increased as the spraying gas temperature increased. The average contents of Ni, Al, and TiB2 in the coating as-sprayed at 450 °C were 32.66 ± 1.87, 54.93 ± 3.61, and 12.41 ± 1.13 vol %, respectively.
- Owing to the reinforcing role of TiB2 particles, the microhardness of Ni/Al-TiB2 coatings was higher than that of Ni/Al coatings. The microhardness of the coatings increased as the spraying gas temperature increased. The highest microhardness of Ni/Al-TiB2 coatings as-sprayed at 450 °C was 475.26 ± 31.21 HV0.3.
- Due to the low processing temperature of cold spraying, the main phase compositions of the as-sprayed Ni/Al-TiB2 coatings were Ni, Al, and TiB2 phases with no oxides or nickel aluminum intermetallic phases. FWHMs of Ni and Al diffraction peaks indicated that Ni and Al particles underwent a certain plastic deformation upon high-speed impact.
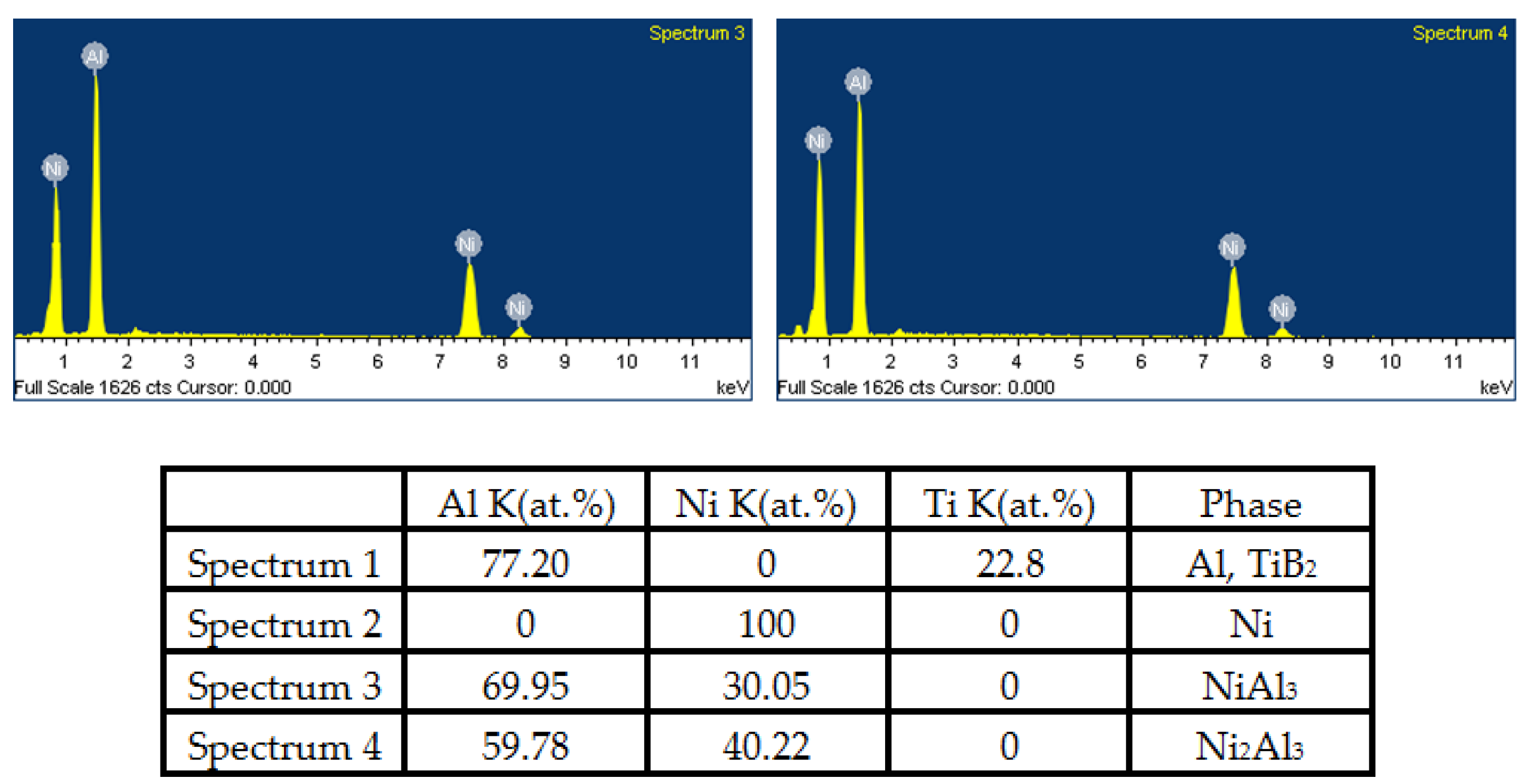
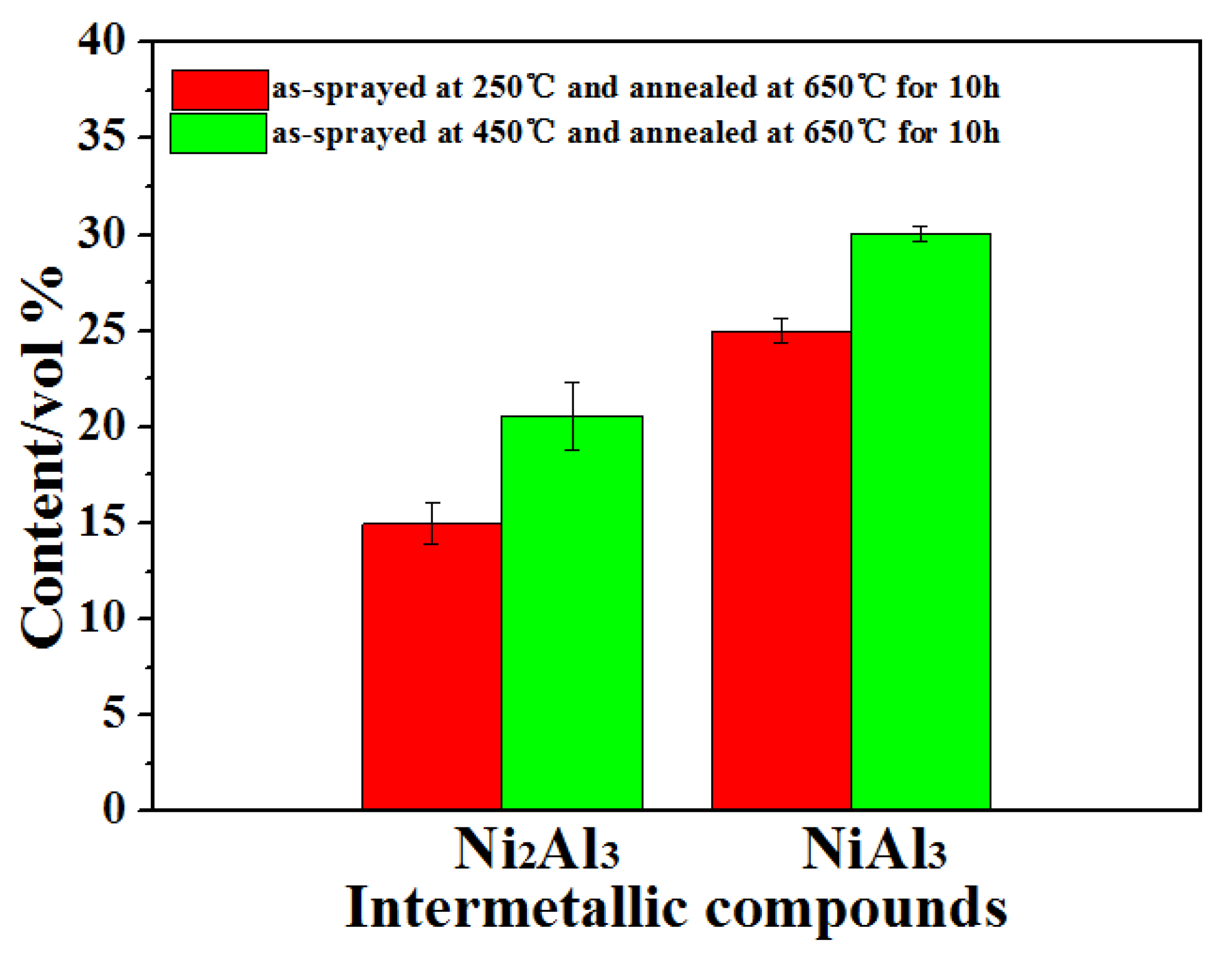
- The combined XRD and EDS analysis results for the annealed coatings after annealing at 650 °C for 10, 20, and 30 h revealed that NiAl3 and Ni2Al3 intermetallic compounds were in situ synthesized in all of the annealed coatings.
- By analyzing the microstructure of the annealed coatings, Al phase gradually decreased with the diffusion reaction occurring. Further, TiB2 particles changed from the comparative more distribution to the accumulation in the coating due to the continuous formation and growth of NiAl intermetallics.
- The porosity of the annealed coatings increased as the annealing times and spraying gas temperatures increased. Due to the increased amount of TiB2-reinforced particles and NiAl3 and Ni2Al3 intermetallic compounds, it led to larger volume change and much more accumulation of TiB2 particles to form more pores in the annealed coating as-sprayed at 450 °C.
- The microhardness of Ni/Al-TiB2 coating as-sprayed at 450 °C and annealed at 650 °C for 10 h was the highest (701 ± 50 HV0.3) compared with the other coatings, however, the microhardness of the annealed coating gradually decreased as the porosity of the annealed coatings increased.
Author Contributions
Funding
Conflicts of Interest
References
- Grabke, H.J. Oxidation of NiAl and FeAl. Intermetallics 1999, 7, 1153–1158. [Google Scholar] [CrossRef]
- Sikka, V.K.; Mavity, J.T.; Anderson, K. Processing of nickel aluminides and their industrial applications. Mater. Sci. Eng. A 1992, 153, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Busso, E.P.; McClintock, F.A. Mechanisms of cyclic deformation of NiAl single crystals at high temperatures. Acta Metall. Mater. 1994, 42, 3263–3275. [Google Scholar] [CrossRef]
- Ansara, I.; Dupin, N.; Lukas, H.L.; Sundman, B. Thermodynamic assessment of the Al-Ni system. J. Alloy. Compd. 1997, 247, 20–30. [Google Scholar] [CrossRef]
- Bochenek, K.; Basista, M. Advances in processing of NiAl intermetallic alloys and composites for high temperature aerospace applications. Prog. Aerosp. Sci. 2015, 79, 136–146. [Google Scholar]
- Movahedi, B. Microstructural evolutions of nickel-aluminide nanocomposites during powder synthesis and thermal spray processes. Adv. Powder Technol. 2014, 25, 871–878. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.M. Microstructure of laser clad TiC/NiAl-Ni3 (Al, Ti, C) wear-resistant intermetallic matrix composite coatings. Mater. Lett. 2003, 57, 2029–2036. [Google Scholar] [CrossRef]
- Guo, J.T.; Wang, Z.S.; Sheng, L.Y.; Zhou, L.Z.; Yuan, C.; Chen, Z.G.; Song, L. Wear properties of NiAl based materials. Prog. Nat. Sci. Mater. Int. 2012, 22, 414–425. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Tan, Y.F.; Wang, X.L.; Tan, H.; Zhou, C.H. Tribological properties of WC and CeO2 particles reinforced in-situ synthesized NiAl matrix composite coatings at elevated temperature. Surf. Coat. Technol. 2014, 244, 123–130. [Google Scholar] [CrossRef]
- Movahedi, B. Fracture toughness and wear behavior of NiAl-based nanocomposite HVOF coatings. Surf. Coat. Technol. 2013, 235, 212–219. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.J.; Liao, H.L.; Coddet, C. Microstructure and wear resistance of FeAl/Al2O3 intermetallic composite coating prepared by atmospheric plasma spraying. Surf. Coat. Technol. 2015, 268, 24–29. [Google Scholar] [CrossRef]
- Ryu, H.C.; Shim, M.K.; Hong, S.H. Reactive processing and mechanical properties of ZrO2/NiAl intermetallic matrix composite. J. Mater. Process. Technol. 1997, 63, 411–416. [Google Scholar] [CrossRef]
- Guo, J.T.; Jiang, D.T.; Xing, Z.P.; Li, G.S. Tensile properties and microstructures of NiAl-20TiB2 and NiAl-20TiC in situ composites. Mater. Des. 1997, 18, 357–360. [Google Scholar] [CrossRef]
- Umanskii, A.P.; Polyarus, E.N.; Ukrainets, M.S.; Kapitanchuk, L.M. Structure and tribotechnical characteristics of NiAl-CrB2 composite materials and coatings. Powder Metall. Met. Ceram. 2015, 54, 53–59. [Google Scholar] [CrossRef]
- Yeh, C.L.; Ke, C.Y.; Chen, Y.C. In sity formation of TiB2/TiC and TiB2/TiN reinforced NiAl by self-propagating combustion synthesis. Vacuum 2018, 151, 185–188. [Google Scholar] [CrossRef]
- Hawk, J.A.; Alman, D.E. Abrasive wear behavior of NiAl and NiAl-TiB2 composites. Wear 1999, 225–229, 544–556. [Google Scholar] [CrossRef]
- Bhaumik, S.K.; Divakar, C.; Rangaraj, L.; Singh, A.K. Reaction sintering of NiAl and TiB2-NiAl composites under pressure. Mater. Sci. Eng. A 1998, 257, 341–348. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Prakash, S. Evaluation of the corrosion behaviour of plasma-sprayed Ni3Al coatings on steel in oxidation andmolten salt environment at 900 °C. Surf. Coat. Technol. 2003, 166, 89–100. [Google Scholar] [CrossRef]
- Movahedi, B. Mechanical and tribological behavior of Ni(Al)-reinforced nanocomposite plasma spray coatings. J. Therm. Spray Technol. 2014, 23, 477–485. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Li, D.Y.; Li, S.M.; Zhang, Z.; Zhang, N.N. High-temperature oxidation resistance of NiAl intermetallic formed in situ by thermal spraying. Coatings 2018, 8, 292. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W. Microstructures, properties and high-temperature carburization resistances of HVOF thermal sprayed NiAl intermetallic-based alloy coatings. Surf. Coat. Technol. 2004, 183, 18–28. [Google Scholar] [CrossRef]
- Enayati, M.H.; Karimzadeh, F.; Tavoosi, M.; Movahedi, B.; Tahvilian, A. Nanocrystalline NiAl coating prepared by HVOF thermal spraying. J. Therm. Spray Technol. 2011, 20, 440–446. [Google Scholar] [CrossRef]
- Culha, O.; Celik, E.; Azem, N.F.A.; Birlik, I.; Toparli, M.; Turk, A. Microstructural, thermal and mechanical properties of HVOF sprayed Ni-Al-based bond coatings on stainless steel substrate. J. Mater. Process. Technol. 2008, 204, 221–230. [Google Scholar] [CrossRef]
- Hearley, J.A.; Little, J.A.; Sturgeon, A.J. The effect of spray parameters on the properties of high velocity oxy-fuel NiAl intermetallic coatings. Surf. Coat. Technol. 2000, 123, 210–218. [Google Scholar] [CrossRef]
- Deshpande, S.; Sampath, S.; Zhang, H. Mechanisms of oxidation and its role in microstructural evolution of metallic thermal spray coatings- case study for Ni-Al. Surf. Coat. Technol. 2006, 200, 5395–5406. [Google Scholar] [CrossRef]
- Hou, S.X.; Liu, Z.D.; Liu, D.Y. The study of NiAl-TiB2 coatings prepared by electro-thermal exposion ultrahigh speed spraying technology. Surf. Coat. Technol. 2011, 205, 4562–4568. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhou, J.S.; Chen, J.M.; Zhou, H.D.; Guo, C.; Wang, L.Q.; Yang, L.B. Preparation, microstructure and tribological behavior of laser cladding NiAl intermetallic compound coatings. Wear 2012, 274–275, 298–305. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhou, J.S.; Ren, S.F.; Wang, L.Q.; Xin, B.B.; Gao, S.L. Tribological properties of laser cladding NiAl intermetallic compound coatings at elevated temperatures. Tribol. Int. 2016, 104, 321–327. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.J.; Wang, X.R.; Ren, Z.L.; Li, C.X.; Yang, G.J. Formation of NiAl intermetallic compound by cold spraying of ball-milled Ni/Al alloy powder through postannealing treatment. J. Therm. Spray Technol. 2008, 17, 715–720. [Google Scholar] [CrossRef]
- Wang, K.K.; Wang, S.R.; Xiong, T.Y.; Wen, D.S.; Wang, G.Q.; Liu, W.T.; Du, H. Protective performance of Zn-Al-Mg-TiO2 coating prepared by cold spraying on marine steel equipment. Coatings 2019, 9, 339. [Google Scholar] [CrossRef]
- Tria, S.; Elkedim, O.; Li, W.Y.; Liao, H.L. Ball milled Ni-Ti powder deposited by cold spraying. J. Alloys Compd. 2009, 483, 334–336. [Google Scholar] [CrossRef]
- Lupoi, R.; O’Neill, W. Deposition of metallic coatings on polymer surfaces using cold spray. Surf. Coat. Technol. 2010, 205, 2167–2173. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Y.; Zhang, C.; Guo, X.P.; Zhang, G.; Liao, H.L.; Li, C.J.; Coddet, C. Effect of standoff distance on coating deposition characteristics in cold spraying. Mater. Des. 2008, 29, 297–304. [Google Scholar] [CrossRef]
- Li, C.J.; Li, W.Y. Deposition characteristics of titanium coating in cold spraying. Surf. Coat. Technol. 2003, 167, 278–283. [Google Scholar] [CrossRef]
- Suhonen, T.; Varis, T.; Dosta, S.; Torrell, M.; Guilemany, J.M. Residual stress development in cold sprayed Al, Cu and Ti coatings. Acta Metall. 2013, 61, 6329–6337. [Google Scholar] [CrossRef]
- Tan, A.W.Y.; Lek, J.Y.; Sun, W.; Bhowmik, A.; Marinescu, I.L.; Song, X.; Zhai, W.; Li, F.; Dong, Z.L.; Boothroyd, C.B.; et al. Influence of particle velocity when propelled using N2 or N2-He mixed gas on the properties of cold-sprayed Ti6Al4V coatings. Coatings 2018, 8, 327. [Google Scholar] [CrossRef]
- Ji, G.C.; Wang, H.T.; Chen, X.; Bai, X.B.; Dong, Z.X.; Yang, F.G. Characterization of cold-sprayed multimodal WC-12Co coating. Surf. Coat. Technol. 2013, 235, 536–543. [Google Scholar] [CrossRef]
- Wang, H.T.; Li, C.J.; Yang, G.J.; Li, C.X.; Zhang, Q.; Li, W.Y. Microstructural characterization of cold-sprayed nanostructured FeAl intermetallic compound coating and its ball-milled feedstock powders. J. Therm. Spray Technol. 2007, 16, 669–676. [Google Scholar] [CrossRef]
- Yang, G.J.; Zhao, S.N.; Li, C.X.; Li, C.J. Effect of phase transformation mechanism on the microstructure of cold-sprayed Ni/Al-Al2O3 composite coatings during post-spray annealing treatment. J. Therm. Spray Technol. 2013, 22, 398–405. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jung, S.H.; Lee, S.Y.; Ko, K.H. Alloying of cold-sprayed Al-Ni composite coating by post-annealing. Appl. Surf. Sci. 2007, 253, 3496–3502. [Google Scholar] [CrossRef]
- Wang, H.T.; Li, C.J.; Ji, G.C.; Yang, G.J. Annealing effect on the intermetallic compound formation of cold sprayed Fe/Al composite coating. J. Therm. Spray Technol. 2012, 21, 571–577. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Ko, K. Annealing effects on the intermetallic compound formation of cold sprayed Ni, Al coatings. J. Mater. Process. Technol. 2009, 209, 937–943. [Google Scholar] [CrossRef]
- Novoselova, T.; Celotto, S.; Morgan, R.; Fox, P.; O’Neill, W. Formation of TiAl intermetallics by heat treatment of cold-sprayed precursor deposits. J. Alloys Compd. 2007, 436, 69–77. [Google Scholar] [CrossRef]
- Kong, L.Y.; Qi, J.Z.; Lu, B.; Yang, R.; Cui, X.Y.; Li, T.F.; Xiong, T.Y. Oxidation resistance of TiAl3-Al composite coating on orthorhombic Ti2AlNb based alloy. Surf. Coat. Technol. 2010, 204, 2262–2267. [Google Scholar] [CrossRef]
- Wang, H.T.; Li, C.J.; Yang, G.J.; Li, C.X. Cold spraying of Fe/Al powder mixture: Coating characteristics and influence of heat treatment on the phase structure. Appl. Surf. Sci. 2008, 255, 2538–2544. [Google Scholar] [CrossRef]
- Kang, H.K.; Kang, S.B. Tungsten/copper composite deposits produced by a cold spray. Scr. Mater. 2003, 49, 1169–1174. [Google Scholar] [CrossRef]
- Susan, D.F.; Misiolek, W.Z.; Marder, A.R. Reaction synthesis of Ni-Al-based particle composite coatings. Matell. Mater. Trans. A 2001, 32, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Torres, R.D.; Lepienski, C.M.; Moore, J.J.; Reimanis, I.E. Influence of the processing route in the microstructure and mechanical properties of NiAl/TiB2 composites produced by combustion synthesis. Metall. Mater. Trans. B 2009, 40, 187–195. [Google Scholar] [CrossRef]
- Biswas, A.; Roy, S.K. Comparison between the microstructural evolutions of two modes of SHS of NiAl: Key to a common reaction mechanism. Acta Mater. 2004, 52, 257–270. [Google Scholar] [CrossRef]

| Coating Types | Specimens | Average Value (μm) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Coating sprayed at 250 °C | 8.56 | 9.15 | 8.78 | 7.46 | 9.33 | 8.66 ± 0.73 |
| Coating sprayed at 450 °C | 6.73 | 7.25 | 5.36 | 6.28 | 5.21 | 6.17 ± 0.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, C.; Xu, S.; Hu, Y.; Ji, G.; Wang, H. Microstructure and Microhardness of Ni/Al-TiB2 Composite Coatings Prepared by Cold Spraying Combined with Postannealing Treatment. Coatings 2019, 9, 565. https://doi.org/10.3390/coatings9090565
Chen X, Li C, Xu S, Hu Y, Ji G, Wang H. Microstructure and Microhardness of Ni/Al-TiB2 Composite Coatings Prepared by Cold Spraying Combined with Postannealing Treatment. Coatings. 2019; 9(9):565. https://doi.org/10.3390/coatings9090565
Chicago/Turabian StyleChen, Xiao, Chengdi Li, Shunjian Xu, Yao Hu, Gangchang Ji, and Hongtao Wang. 2019. "Microstructure and Microhardness of Ni/Al-TiB2 Composite Coatings Prepared by Cold Spraying Combined with Postannealing Treatment" Coatings 9, no. 9: 565. https://doi.org/10.3390/coatings9090565





