Corrosion and Magnetization Analyses of Iron Encapsulated Aluminum Particles by Numerical Simulations
Abstract
:1. Introduction
2. Methods
2.1. MD Simulation System and Settings
2.2. Magnetization Simulations
3. Results and Discussion
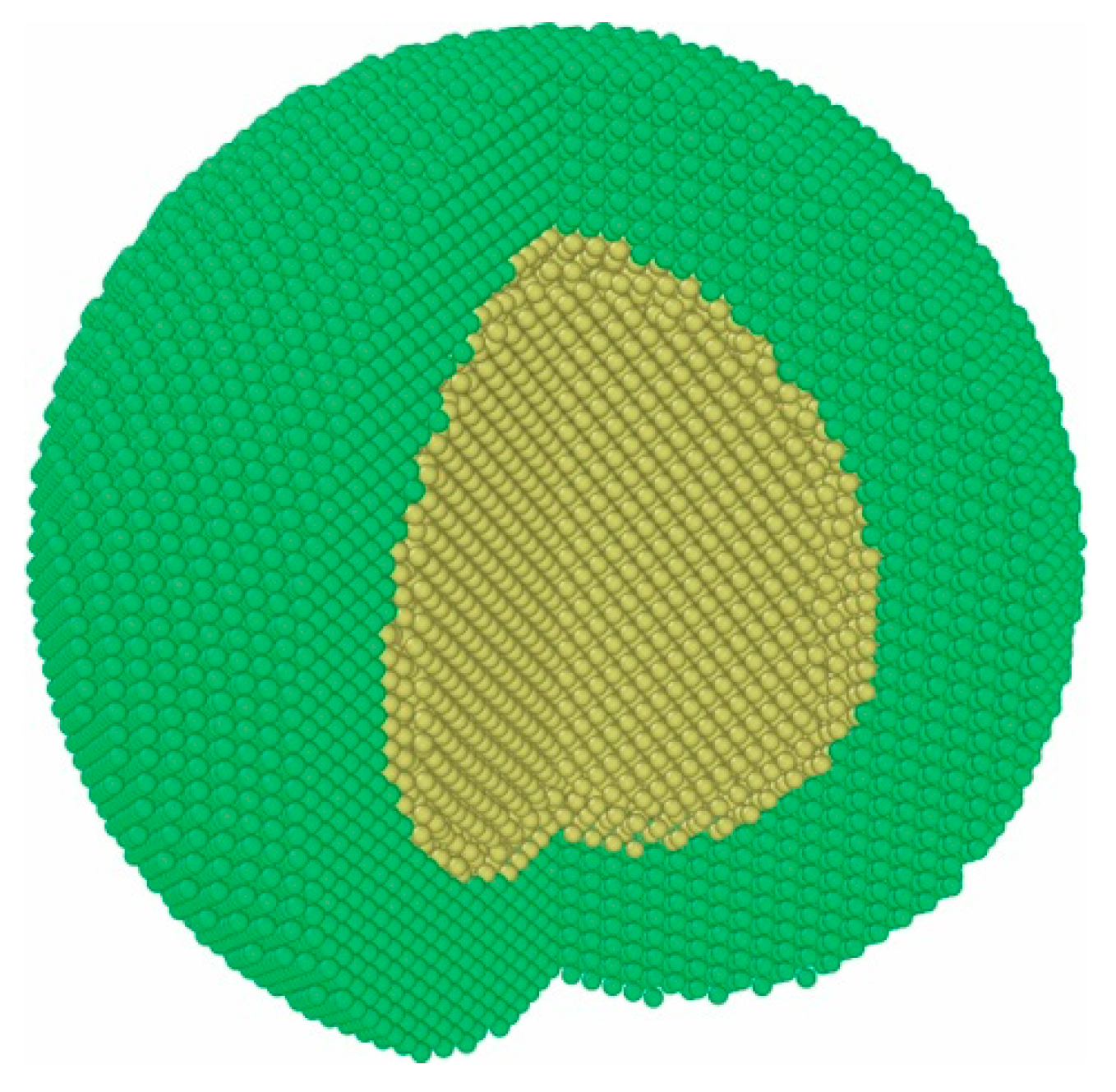
3.1. Oxygen Involved Corrosion Behaviors for Fe Encapsulated Al Particle
3.2. Water Involved Corrosion Behaviors for Fe Encapsulated Al Particle
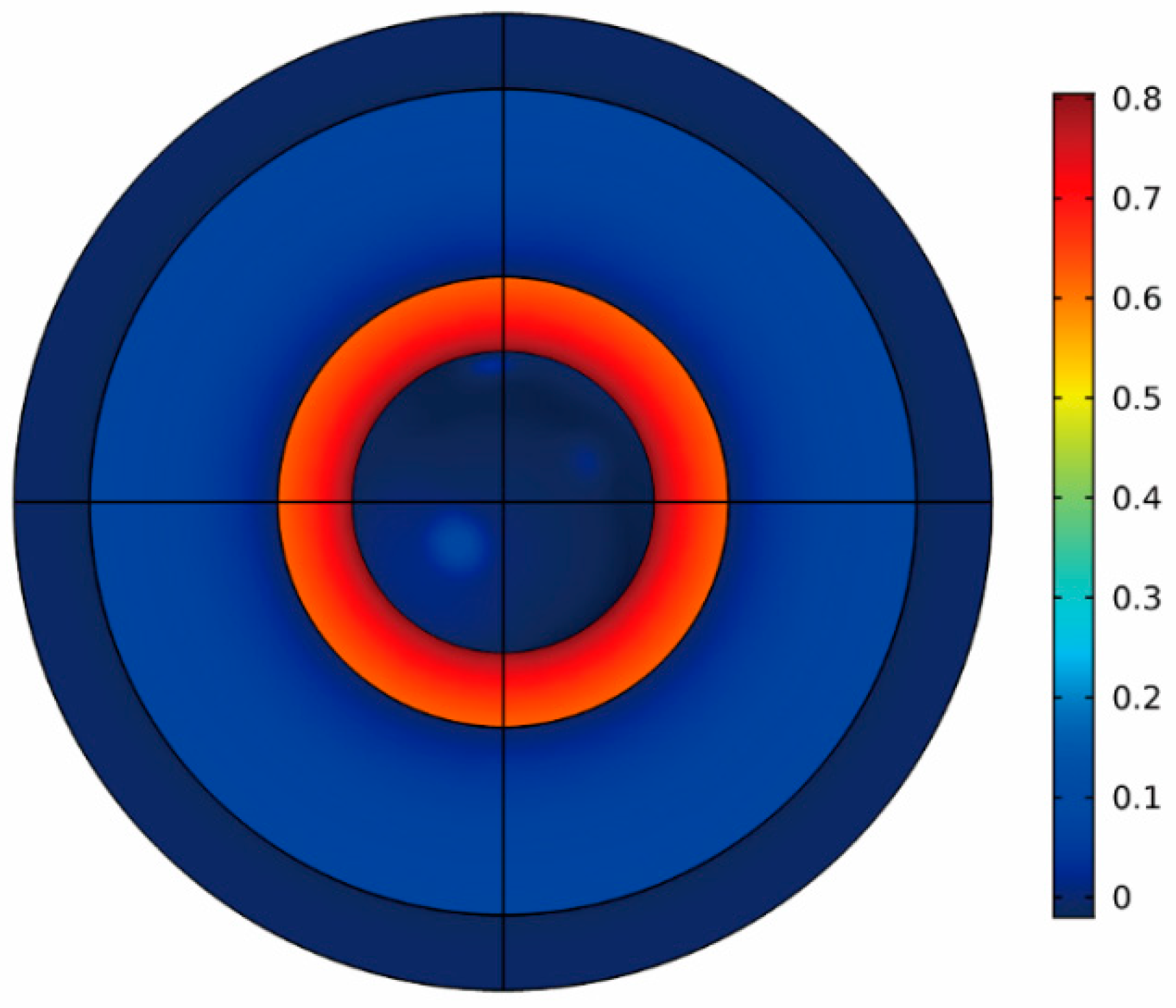
3.3. Magnetization Properties of Fe Encapsulated Al Particles
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Fe | Iron |
| Al | Aluminum: |
| MD | Molecular dynamic |
| FEA | Finite element analysis |
| LPFG | Long period fiber gratings |
| EAM | Embedded atom method |
| NVT | Canonical ensemble |
| MSD | Mean square displacement |
References
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar]
- Plachý, T.; Kutalkova, E.; Sedlacik, M.; Vesel, A.; Masar, M.; Kuritka, I. Impact of corrosion process of carbonyl iron particles on magnetorheological behavior of their suspensions. J. Ind. Eng. Chem. 2018, 66, 362–369. [Google Scholar] [CrossRef]
- Wang, J.; Shao, X.; Tian, G.; Li, Z.; Bao, W. Preparation and properties of a-Fe microparticles with high stability. Mater. Lett. 2017, 192, 36–39. [Google Scholar] [CrossRef]
- Motozuka, S.; Ikeda, T.; Miyagawa, T.; Sato, H.; Morinaga, M. Formation process of the {001} fiber texture on iron particles using simple ball milling. Powder Technol. 2017, 321, 9–12. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Romero, A.; Fernandez, J.; Santos, A. In situ chemical reduction of chlorinated organic compounds from lindane production wastes by zero valent iron microparticles. J. Water Process. Eng. 2018, 26, 146–155. [Google Scholar] [CrossRef]
- Yap, L.W.; Shi, Q.; Gong, S.; Wang, Y.; Chen, Y.; Zhu, C.; Gu, Z.; Suzuki, K.; Zhu, Y.; Cheng, W. Bifunctional Fe3O4@AuNWs particle as wearable bending and strain sensor. Inorg. Chem. Commun. 2019, 104, 98–104. [Google Scholar] [CrossRef]
- Monamary, A.; Vijayalakshmi, K.; Jereil, S.D. Fe overlayered hybrid TiO2/ITO nanocomposite sensor for enhanced hydrogen sensing at room temperature by novel two step process. Sens. Actuators B Chem. 2019, 287, 278–289. [Google Scholar] [CrossRef]
- Guo, C.; Fan, L.; Wu, C.; Chen, G.; Li, W. Ultrasensitive LPFG corrosion sensor with Fe-C coating electroplated on a Gr/AgNW film. Sens. Actuators B Chem. 2019, 283, 334–342. [Google Scholar] [CrossRef]
- Hwang, J.H.; Pathak, P.; Wang, X.; Rodriguez, K.L.; Park, J.; Cho, H.J.; Lee, W.H. A novel Fe-chitosan-coated carbon electrode sensor for in situ As(III) detection in mining wastewater and soil leachate. Sens. Actuators B Chem. 2019, 294, 89–97. [Google Scholar] [CrossRef]
- Yi, Q.; Xu, J.; Liu, Y.; Zhai, D.; Zhou, K.; Pan, D. Molecular dynamics study on core-shell structure stability of aluminum encapsulated by nano-carbon materials. Chem. Phys. Lett. 2017, 669, 192–195. [Google Scholar] [CrossRef]
- Wang, H.; Leung, D.Y.; Leung, M.K.; Ni, M. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Ilyukhina, A.; Kravchenko, O.; Bulychev, B. Studies on microstructure of activated aluminum and its hydrogen generation properties in aluminum/water reaction. J. Alloy. Compd. 2017, 690, 321–329. [Google Scholar] [CrossRef]
- Thampi, K.R.; Newell, A. Novel amorphous aluminum hydroxide catalysts for aluminumewater reactions to produce H2 on demand. Int. J. Hydrog. Energy 2017, 42, 23446–23454. [Google Scholar]
- Zou, H.; Chen, S.; Zhao, Z.; Lin, W. Hydrogen production by hydrolysis of aluminum. J. Alloy. Compd. 2013, 578, 380–384. [Google Scholar] [CrossRef]
- Liu, H.; Yang, F.; Yang, B.; Zhang, Q.; Chai, Y.; Wang, N. Rapid hydrogen generation through aluminum-water reaction in alkali solution. Catal. Today 2018, 318, 52–58. [Google Scholar] [CrossRef]
- Elitzur, S.; Rosenband, V.; Gany, A. Study of hydrogen production and storage based on aluminum–water reaction. Int. J. Hydrogen Energy 2014, 39, 6328–6334. [Google Scholar] [CrossRef]
- Shmelev, V.; Nikolaev, V.; Lee, J.H.; Yim, C. Hydrogen production by reaction of aluminum with water. Int. J. Hydrogen Energy 2016, 41, 16664–16673. [Google Scholar] [CrossRef]
- Shimojo, F.; Ohmura, S.; Kalia, R.K.; Nakano, A.; Vashishta, P. Molecular dynamics simulations of rapid hydrogen production from water using aluminum clusters as catalyzers. Phys. Rev. Lett. 2010, 104. [Google Scholar] [CrossRef]
- Russo, M.F.; Li, R.; Mench, M.; Van Duin, A.C. Molecular dynamic simulation of aluminum–water reactions using the ReaxFF reactive force field. Int. J. Hydrogen Energy 2011, 36, 5828–5835. [Google Scholar] [CrossRef]
- Sun, R.; Liu, P.; Qi, H.; Liu, J.; Ding, T. Molecular dynamic simulations of ether-coated aluminum nano-particles as a novel hydrogen source. J. Nanoparticle Res. 2019, 21, 72. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, D.W.; Kim, S.H.; Kim, C.K.; Choi, Y.J. Synthesis and improved explosion behaviors of aluminum powders coated with nano-sized nickel film. Appl. Surf. Sci. 2017, 415, 104–108. [Google Scholar] [CrossRef]
- Vorozhtsov, A.; Lerner, M.; Rodkevich, N.; Nie, H.; Abraham, A.; Schoenitz, M.; Dreizin, E.; Dreizin, E. Oxidation of nano-sized aluminum powders. Thermochim. Acta 2016, 636, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Timothy, A.; Shafirovich, E.A.; Varma, A. Ignition of iron-coated and nickel-coated aluminum particles under normal and reduced-gravity conditions. J. Propuls. Power 2008, 24, 805–813. [Google Scholar]
- Sundaram, D.S.; Puri, P.; Yang, V. Thermochemical behavior of nickel-coated nanoaluminum particles. J. Phys. Chem. C 2013, 117, 7858–7869. [Google Scholar] [CrossRef]
- Sundaram, D.S.; Puri, P.; Yang, V. Thermochemical behavior of nano-sized aluminum-coated nickel particles. J. Nanoparticle Res. 2014, 16, 2392. [Google Scholar] [CrossRef]
- Hong, S.; Van Duin, A.C.T. Atomistic-scale analysis of carbon coating and its effect on the oxidation of aluminum nanoparticles by reaxff-molecular dynamics simulations. J. Phys. Chem. C 2016, 120, 9464–9474. [Google Scholar] [CrossRef]
- Lin, P.; Colina, C.M. Molecular simulation of protein–polymer conjugates. Curr. Opin. Chem. Eng. 2019, 23, 44–50. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Wang, N.; Peng, J.; Pang, A.; He, T.; Du, F.; Jaramillo-Botero, A. Thermodynamic simulation of the RDX–aluminum interface using ReaxFF molecular dynamics. J. Phys. Chem. C 2017, 121, 14597–14610. [Google Scholar] [CrossRef]
- Kwak, H.; Zou, C.; Vasenkov, A.V.; Van Duin, A.C.T.; Shin, Y.K. Development and validation of a ReaxFF reactive force field for Fe/Al/Ni alloys: Molecular dynamics study of elastic constants, diffusion, and segregation. J. Phys. Chem. A 2012, 116, 12163–12174. [Google Scholar]
- Mendelev, M.; Srolovitz, D.; Ackland, G.; Han, S.; Ackland, G. Effect of Fe segregation on the migration of a non-symmetric Σ5 tilt grain boundary in Al. J. Mater. Res. 2005, 20, 208–218. [Google Scholar] [CrossRef]
- Alexander Stukowski, Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 1–7.
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511. [Google Scholar] [CrossRef]
- William, G. Hoover, Canonical dynamics: Equilibrium phase-space distributions. Am. Phys. Soc. 1984, 31, 1695–1697. [Google Scholar]
- Butler, S.; Zhang, Z. Forward modeling of geophysical electromagnetic methods using Comsol. Comput. Geosci. 2016, 87, 1–10. [Google Scholar] [CrossRef]
- Soltanova, D.; Baranov, P.; Baranova, V.; Chudinova, A. Simulating magnetic field of a ferromagnetic pipe underwater in COMSOL Multiphysics. IOP Conf. Ser. Mater. Sci. Eng. 2015, 93, 012023. [Google Scholar] [CrossRef]
- Liu, P.; Sun, R.; Liu, J. Adsorption behaviors of ether and aluminum surface: A molecular dynamics study. Int. J. Mod. Phys. B 2019, 33, 1950028. [Google Scholar] [CrossRef]

















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Sun, R.; Qi, H.; Lv, F. Corrosion and Magnetization Analyses of Iron Encapsulated Aluminum Particles by Numerical Simulations. Coatings 2019, 9, 557. https://doi.org/10.3390/coatings9090557
Guo J, Sun R, Qi H, Lv F. Corrosion and Magnetization Analyses of Iron Encapsulated Aluminum Particles by Numerical Simulations. Coatings. 2019; 9(9):557. https://doi.org/10.3390/coatings9090557
Chicago/Turabian StyleGuo, Jing, Ruochen Sun, Hui Qi, and Fangwei Lv. 2019. "Corrosion and Magnetization Analyses of Iron Encapsulated Aluminum Particles by Numerical Simulations" Coatings 9, no. 9: 557. https://doi.org/10.3390/coatings9090557
APA StyleGuo, J., Sun, R., Qi, H., & Lv, F. (2019). Corrosion and Magnetization Analyses of Iron Encapsulated Aluminum Particles by Numerical Simulations. Coatings, 9(9), 557. https://doi.org/10.3390/coatings9090557



