Assembly Mechanism and the Morphological Analysis of the Robust Superhydrophobic Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrophobic Solution
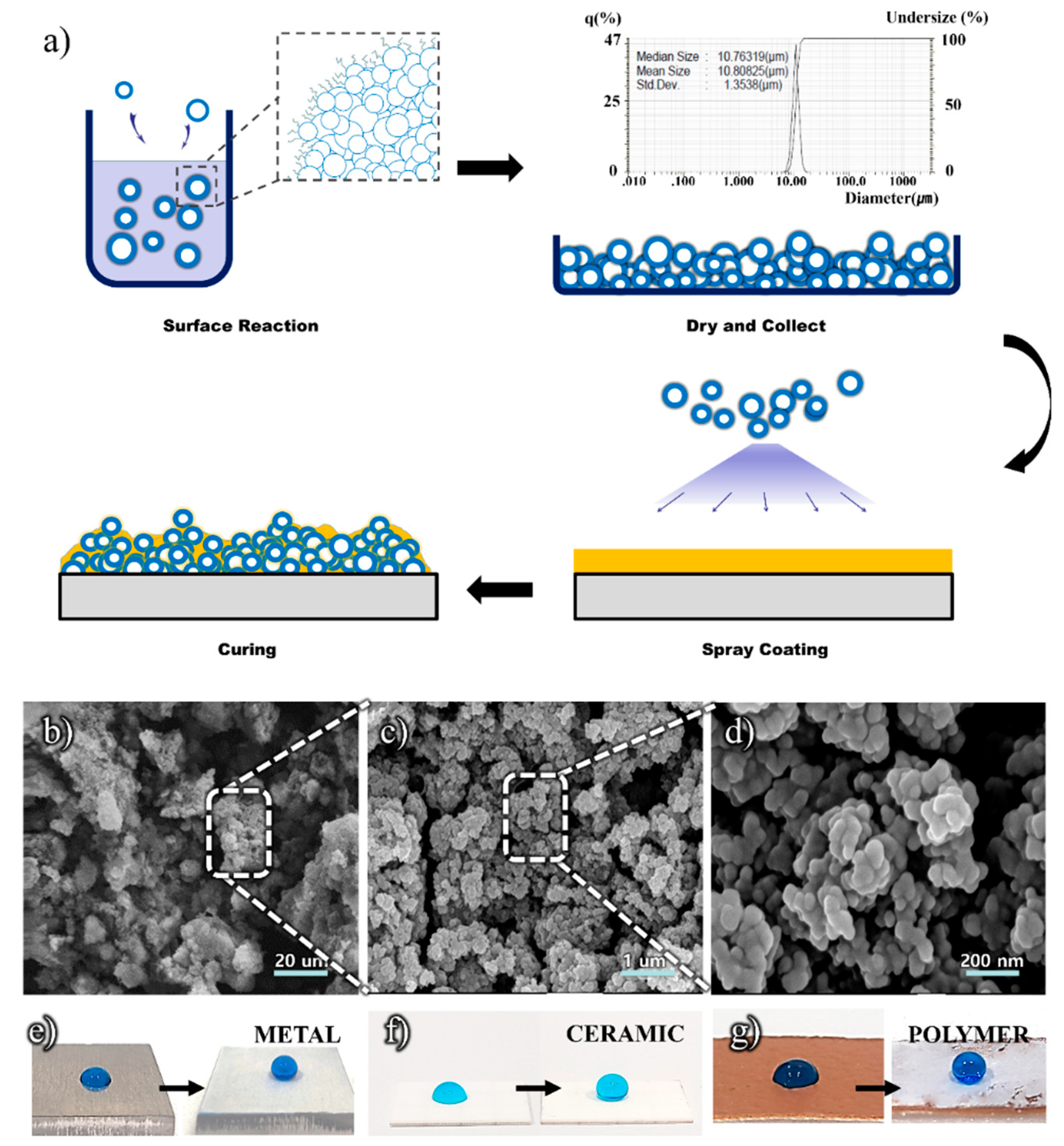
2.2. Preparation of Superhydrophobic Coating
2.3. Characterization
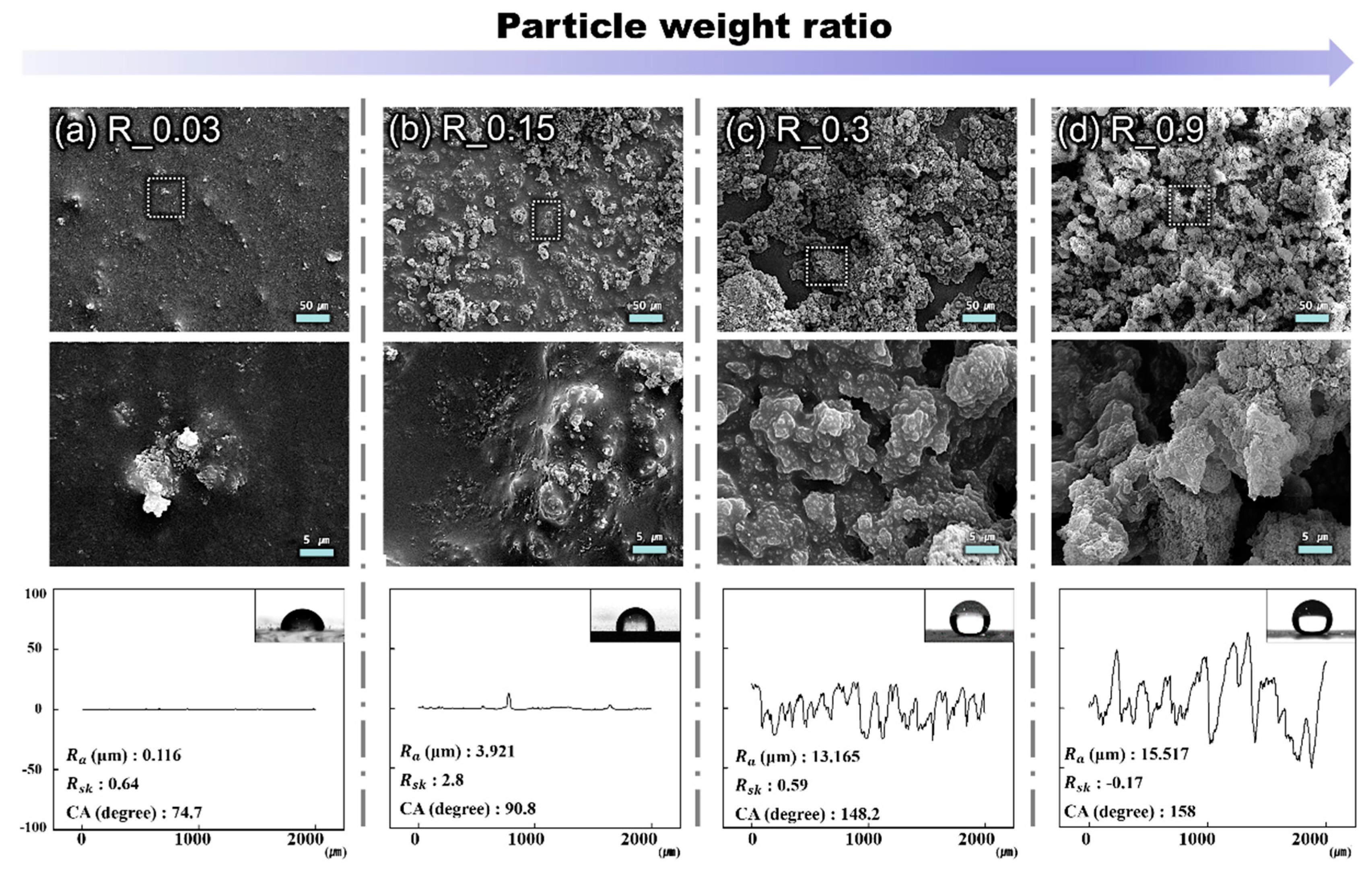
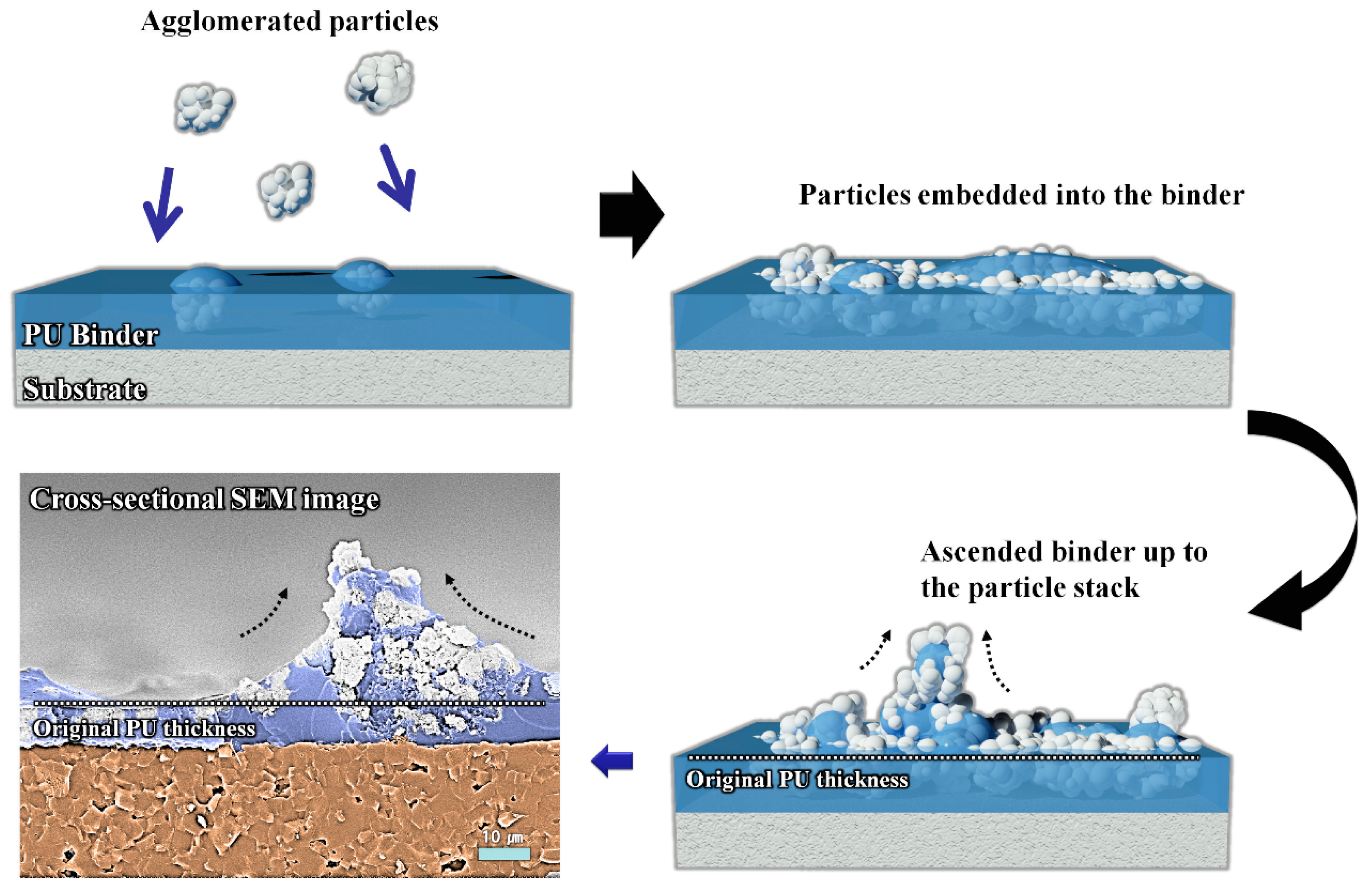
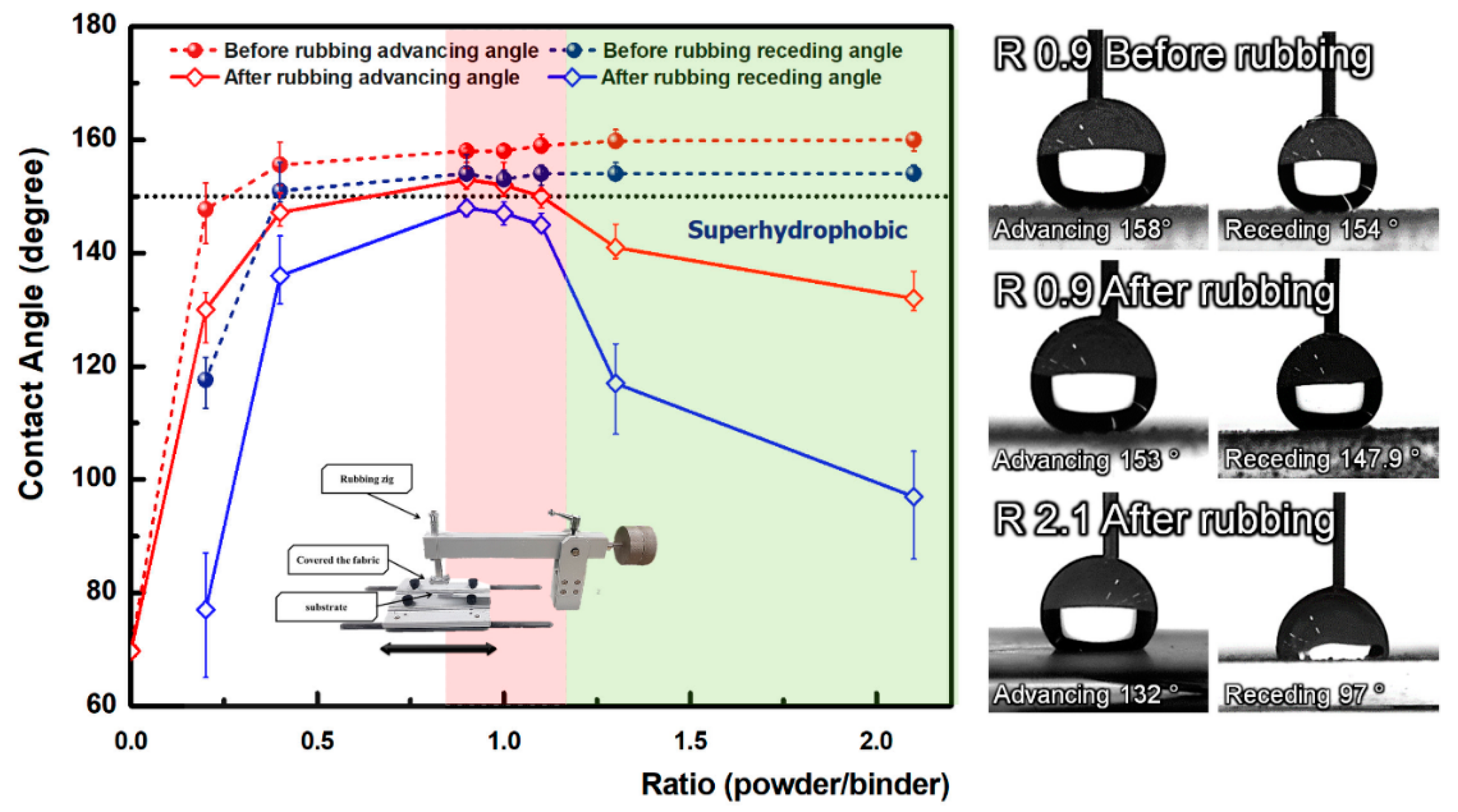
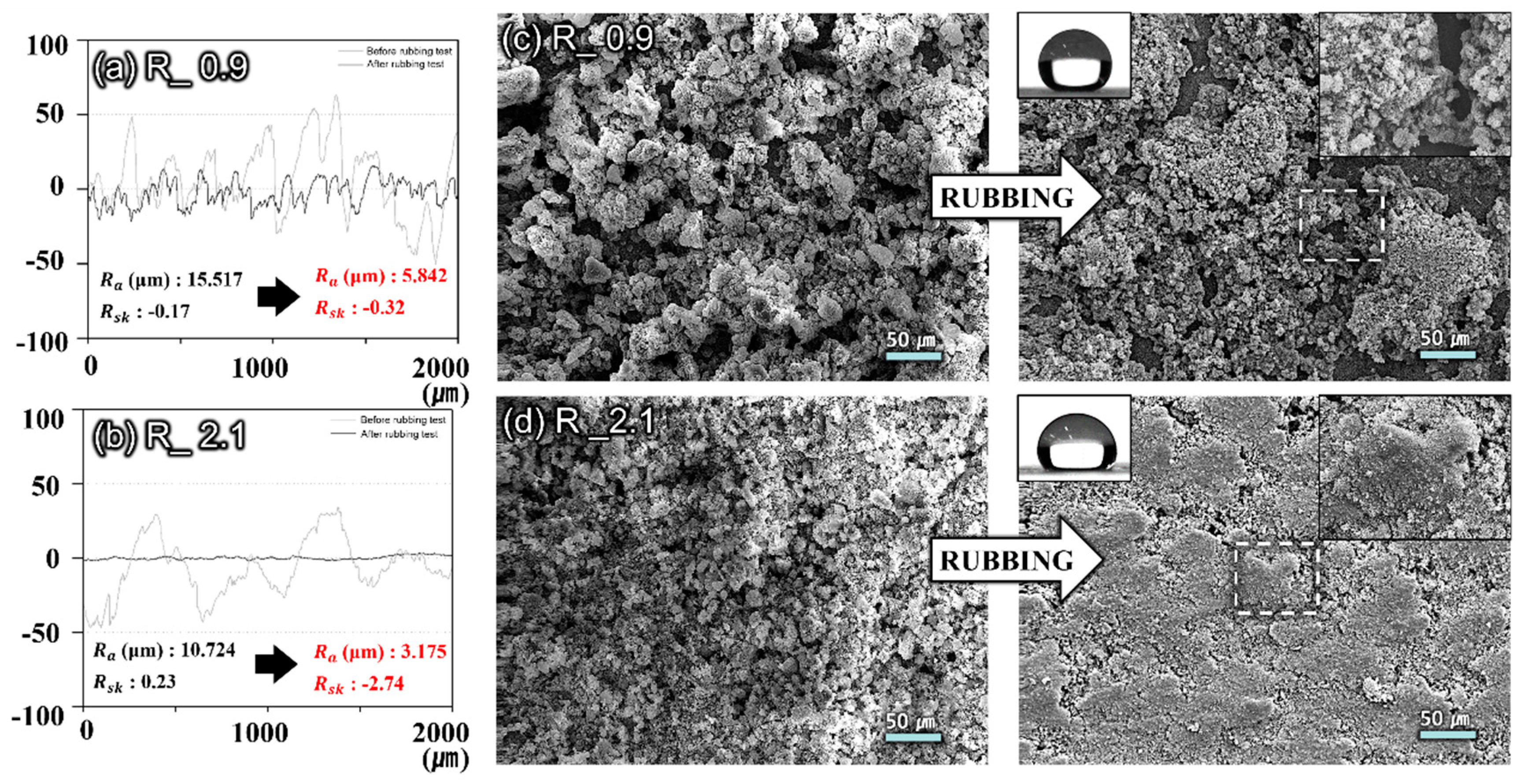
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, B.J.; Zhang, Z.; Baek, S.; Kim, S.; Kim, D.; Yong, K. Bio-inspired dewetted surfaces based on SiC/Si interlocked structures for enhanced-underwater stability and regenerative-drag reduction capability. Sci. Rep. 2016, 6, 24653. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Zheng, X.; Chen, Z.; Zhou, Q.; Chen, Y. 3D-printed biomimetic super-hydrophobic structure for microdroplet manipulation and oil/water separation. Adv. Mater. 2018, 30, 1704912. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Chen, Z.; Tiwari, M.K. All-organic superhydrophobic coatings with mechanochemical robustness and liquid impalement resistance. Nat. Mater. 2018, 17, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Jankauskaitė, V.; Narmontas, P.; Lazauskas, A. Control of polydimethylsiloxane surface hydrophobicity by plasma polymerized hexamethyldisilazane deposition. Coatings 2019, 9, 36. [Google Scholar]
- Sakai, M.; Song, J.-H.; Yoshida, N.; Suzuki, S.; Kameshima, Y.; Nakajima, A. Relationship between sliding acceleration of water droplets and dynamic contact angles on hydrophobic surfaces. Surf. Sci. 2006, 600, L204–L208. [Google Scholar] [CrossRef]
- Han, M.H.; Park, Y.H.; Hyun, J.W.; Ahn, Y.H. Facile method for fabricating superhydrophobic surface on magnesium. Bull. Korean Chem. Soc. 2010, 31, 1067–1069. [Google Scholar] [CrossRef][Green Version]
- Dai, X.; Sun, N.; Nielsen, S.O.; Stogin, B.B.; Wang, J.; Yang, S.; Wong, T.-S. Hydrophilic directional slippery rough surfaces for water harvesting. Sci. Adv. 2018, 4, eaaq0919. [Google Scholar] [CrossRef]
- Karmouch, R.; Ross, G.G. Superhydrophobic wind turbine blade surfaces obtained by a simple deposition of silica nanoparticles embedded in epoxy. Appl. Surf. Sci. 2010, 257, 665–669. [Google Scholar] [CrossRef]
- Feng, L.; Che, Y.; Liu, Y.; Qiang, X.; Wang, Y. Fabrication of superhydrophobic aluminium alloy surface with excellent corrosion resistance by a facile and environment-friendly method. Appl. Surf. Sci. 2013, 283, 367–374. [Google Scholar] [CrossRef]
- Lee, S.; Kim, W.; Yong, K. Overcoming the water vulnerability of electronic devices: A highly water-resistant ZnO nanodevice with multifunctionality. Adv. Mater. 2011, 23, 4398–4402. [Google Scholar] [CrossRef]
- Bayer, S.I. On the durability and wear resistance of transparent superhydrophobic coatings. Coatings 2017, 7, 12. [Google Scholar] [CrossRef]
- Zeng, Y.; Qin, Z.; Hua, Q.; Min, Y.; Xu, Q. Sheet-like superhydrophobic surfaces fabricated on copper as a barrier to corrosion in a simulated marine system. Surf. Coat. Technol. 2019, 362, 62–71. [Google Scholar] [CrossRef]
- Jokinen, V.; Kankuri, E.; Hoshian, S.; Franssila, S.; Ras, R.H.A. Superhydrophobic blood-repellent surfaces. Adv. Mater. 2018, 30, 1705104. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Wang, M.; Wang, H. Separating small amount of water and hydrophobic solvents by novel superhydrophobic copper meshes. Appl. Surf. Sci. 2008, 254, 6002–6006. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Aromaa, M.; Mäkelä, J.M.; Stepien, M.; Saarinen, J.J.; Toivakka, M.; Kuusipalo, J. Development of superhydrophobic coating on paperboard surface using the liquid flame spray. Surf. Coat. Technol. 2010, 205, 436–445. [Google Scholar] [CrossRef]
- Lai, Y.; Gao, X.; Zhuang, H.; Huang, J.; Lin, C.; Jiang, L. Designing superhydrophobic porous nanostructures with tunable water adhesion. Adv. Mater. 2009, 21, 3799–3803. [Google Scholar] [CrossRef]
- Lim, H.S.; Han, J.T.; Kwak, D.; Jin, M.; Cho, K. Photoreversibly switchable superhydrophobic surface with erasable and rewritable pattern. J. Am. Chem. Soc. 2006, 128, 14458–14459. [Google Scholar] [CrossRef] [PubMed]
- Saleema, N.; Sarkar, D.K.; Gallant, D.; Paynter, R.W.; Chen, X.-G. Chemical nature of superhydrophobic aluminum alloy surfaces produced via a one-step process using fluoroalkyl-silane in a base medium. ACS Appl. Mater. Interfaces 2011, 3, 4775–4781. [Google Scholar] [CrossRef] [PubMed]
- Han, J.T.; Xu, X.; Cho, K. Diverse access to artificial superhydrophobic surfaces using block copolymers. Langmuir 2005, 21, 6662–6665. [Google Scholar] [CrossRef]
- Xue, C.H.; Jia, S.T.; Chen, H.Z.; Wang, M. Superhydrophobic cotton fabrics prepared by sol-gel coating of TiO2 and surface hydrophobization. Sci. Technol. Adv. Mater. 2008, 9, 35001. [Google Scholar] [CrossRef]
- Senesi, G.S.; D’Aloia, E.; Gristina, R.; Favia, P.; d’Agostino, R. Surface characterization of plasma deposited nano-structured fluorocarbon coatings for promoting in vitro cell growth. Surf. Sci. 2007, 601, 1019–1025. [Google Scholar] [CrossRef]
- Pan, G.; Xiao, X.; Yu, N.; Ye, Z. Fabrication of superhydrophobic coatings on cotton fabric using ultrasound-assisted in-situ growth method. Prog. Org. Coat. 2018, 125, 463–471. [Google Scholar] [CrossRef]
- Rivero, J.P.; Iribarren, A.; Larumbe, S.; Palacio, F.J.; Rodríguez, R. A comparative study of multifunctional coatings based on electrospun fibers with incorporated ZnO nanoparticles. Coatings 2019, 9, 367. [Google Scholar] [CrossRef]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H.A. Mechanically durable superhydrophobic surfaces. Adv. Mater. 2010, 23, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, E.; Takkunen, L.; Korpela, T.; Suvanto, M.; Pakkanen, T.T.; Pakkanen, T.A. Mechanically robust superhydrophobic polymer surfaces based on protective micropillars. Langmuir 2014, 30, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.; Liu, Y.; Hess, D.W.; Wong, C.P. Mechanically robust superhydrophobicity on hierarchically structured Si surfaces. Nanotechnology 2010, 21, 155705. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Lazauskas, A.; Grigaliūnas, V.; Jucius, D. Recovery behavior of microstructured thiol-ene shape-memory film. Coatings 2019, 9, 267. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Superhydrophobic, superoleophobic coatings for the protection of silk textiles. Prog. Org. Coat. 2016, 97, 44–52. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N.; Savva, A.; Panayiotou, C. Superhydrophobic polymer-particle composite films produced using various particle sizes. Surf. Interface Anal. 2012, 44, 870–875. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Superhydrophobic composite films produced on various substrates. Langmuir 2008, 24, 11225–11232. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.; Emelyanenko, A. Principles of design of superhydrophobic coatings by deposition from dispersions. Langmuir 2009, 25, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Sriramulu, D.; Reed, E.L.; Annamalai, M.; Venkatesan, T.V.; Valiyaveettil, S. Synthesis and characterization of superhydrophobic, self-cleaning nir-reflective silica nanoparticles. Sci. Rep. 2016, 6, 35993. [Google Scholar] [CrossRef] [PubMed]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Sagiv, J. Organized monolayers by adsorption. 1. Formation and structure of oleophobic mixed monolayers on solid surfaces. J. Am. Chem. Soc. 1980, 102, 92–98. [Google Scholar] [CrossRef]
- Rashvand, M.; Ranjbar, Z.; Rastegar, S. Nano zinc oxide as a UV-stabilizer for aromatic polyurethane coatings. Prog. Org. Coat. 2011, 71, 362–368. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Gaines, G.L., Jr. Surface and interfacial tension of polymer liquids—A review. Polym. Eng. Sci. 1972, 12, 1–11. [Google Scholar] [CrossRef]
- Dee, G.T.; Sauer, B.B. The surface tension of polymer liquids. Adv. Phys. 1998, 47, 161–205. [Google Scholar] [CrossRef]
- Llaneza, V.; Belzunce, F.J. Study of the effects produced by shot peening on the surface of quenched and tempered steels: Roughness, residual stresses and work hardening. Appl. Surf. Sci. 2015, 356, 475–485. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Su, C.; Xu, Y.; Gong, F.; Wang, F.; Li, C. The abrasion resistance of a superhydrophobic surface comprised of polyurethane elastomer. Soft Matter 2010, 6, 6068–6071. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Coll. Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Naderizadeh, S.; Athanassiou, A.; Bayer, I.S. Interfacing superhydrophobic silica nanoparticle films with graphene and thermoplastic polyurethane for wear/abrasion resistance. J. Coll. Interface Sci. 2018, 519, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, J.; Yin, L.; Chen, B.; Tang, H.; Liu, C.; Li, C. Fabrication of superhydrophobic polyurethane/MoS2 nanocomposite coatings with wear-resistance. Coll. Surf. A Physicochem. Eng. Asp. 2014, 459, 261–266. [Google Scholar] [CrossRef]
- Milionis, A.; Ruffilli, R.; Bayer, I.S. Superhydrophobic nanocomposites from biodegradable thermoplastic starch composites (Mater-Bi®), hydrophobic nano-silica and lycopodium spores. RSC Adv. 2014, 4, 34395–34404. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Wu, L. Facile fabrication of self-repairing superhydrophobic coatings. Chem. Commun. 2014, 50, 11891–11894. [Google Scholar] [CrossRef]
- Tenjimbayashi, M.; Shiratori, S. Highly durable superhydrophobic coatings with gradient density by movable spray method. J. Appl. Phys. 2014, 116, 114310. [Google Scholar] [CrossRef]
- Zhang, H.-S.; Endrino, J.L.; Anders, A. Comparative surface and nano-tribological characteristics of nanocomposite diamond-like carbon thin films doped by silver. Appl. Surf. Sci. 2008, 255, 2551–2556. [Google Scholar] [CrossRef]
- Horváth, R.; Czifra, Á.; Drégelyi-Kiss, Á. Effect of conventional and non-conventional tool geometries to skewness and kurtosis of surface roughness in case of fine turning of aluminium alloys with diamond tools. Int. J. Adv. Manuf. Technol. 2015, 78, 297–304. [Google Scholar] [CrossRef]
- Patel, K.; Doyle, C.S.; Yonekura, D.; James, B.J. Effect of surface roughness parameters on thermally sprayed PEEK coatings. Surf. Coat. Technol. 2010, 204, 3567–3572. [Google Scholar] [CrossRef]
| Superhydrophobic Material/Method | Total Abrasion Cycles | Load | Reference |
|---|---|---|---|
| MoS2/PU (spray coating) | Over 100 m rubbing distance | 500 g | Tang et al. [46] |
| SiO2/Starch (spray coating) | 17 | 1.1–2.1 kPa | Milionis et al. [47] |
| SiO2/Siloxane (spray coating) | 10 | <10 kPa | Chen et al. [48] |
| SiO2/EAC (spray coating) | 10 | 40 kPa | Tenjimbayashi and Shiratori [49] |
| Grephene/PU (spray coating) | 30 | 15 kPa | Naderizadeh et al. [45] |
| SiO2/PU (spray coating) | 100 | 3.138 kPa | Our study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Sasidharanpillai, A.; Yun, K.H.; Lee, Y.; Yun, D.-J.; Park, W.I.; Bang, J.; Lee, S. Assembly Mechanism and the Morphological Analysis of the Robust Superhydrophobic Surface. Coatings 2019, 9, 472. https://doi.org/10.3390/coatings9080472
Kim D, Sasidharanpillai A, Yun KH, Lee Y, Yun D-J, Park WI, Bang J, Lee S. Assembly Mechanism and the Morphological Analysis of the Robust Superhydrophobic Surface. Coatings. 2019; 9(8):472. https://doi.org/10.3390/coatings9080472
Chicago/Turabian StyleKim, Doeun, Arun Sasidharanpillai, Ki Hoon Yun, Younki Lee, Dong-Jin Yun, Woon Ik Park, Jiwon Bang, and Seunghyup Lee. 2019. "Assembly Mechanism and the Morphological Analysis of the Robust Superhydrophobic Surface" Coatings 9, no. 8: 472. https://doi.org/10.3390/coatings9080472
APA StyleKim, D., Sasidharanpillai, A., Yun, K. H., Lee, Y., Yun, D.-J., Park, W. I., Bang, J., & Lee, S. (2019). Assembly Mechanism and the Morphological Analysis of the Robust Superhydrophobic Surface. Coatings, 9(8), 472. https://doi.org/10.3390/coatings9080472





