Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
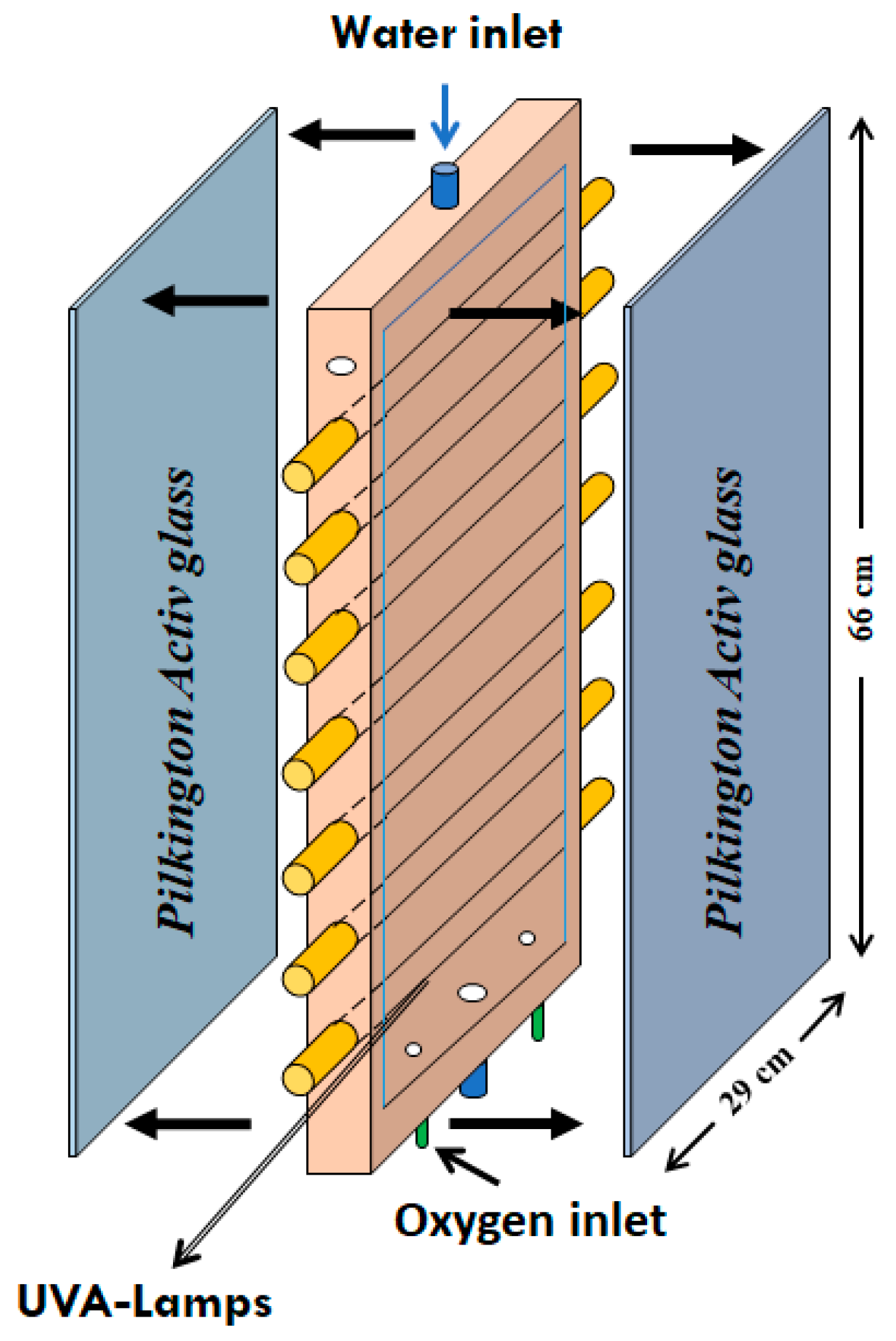
2.2. Experimental Setup and Procedure
2.3. Average Thickness of the Liquid Falling Film
2.4. Analysis
3. Results and Discussion
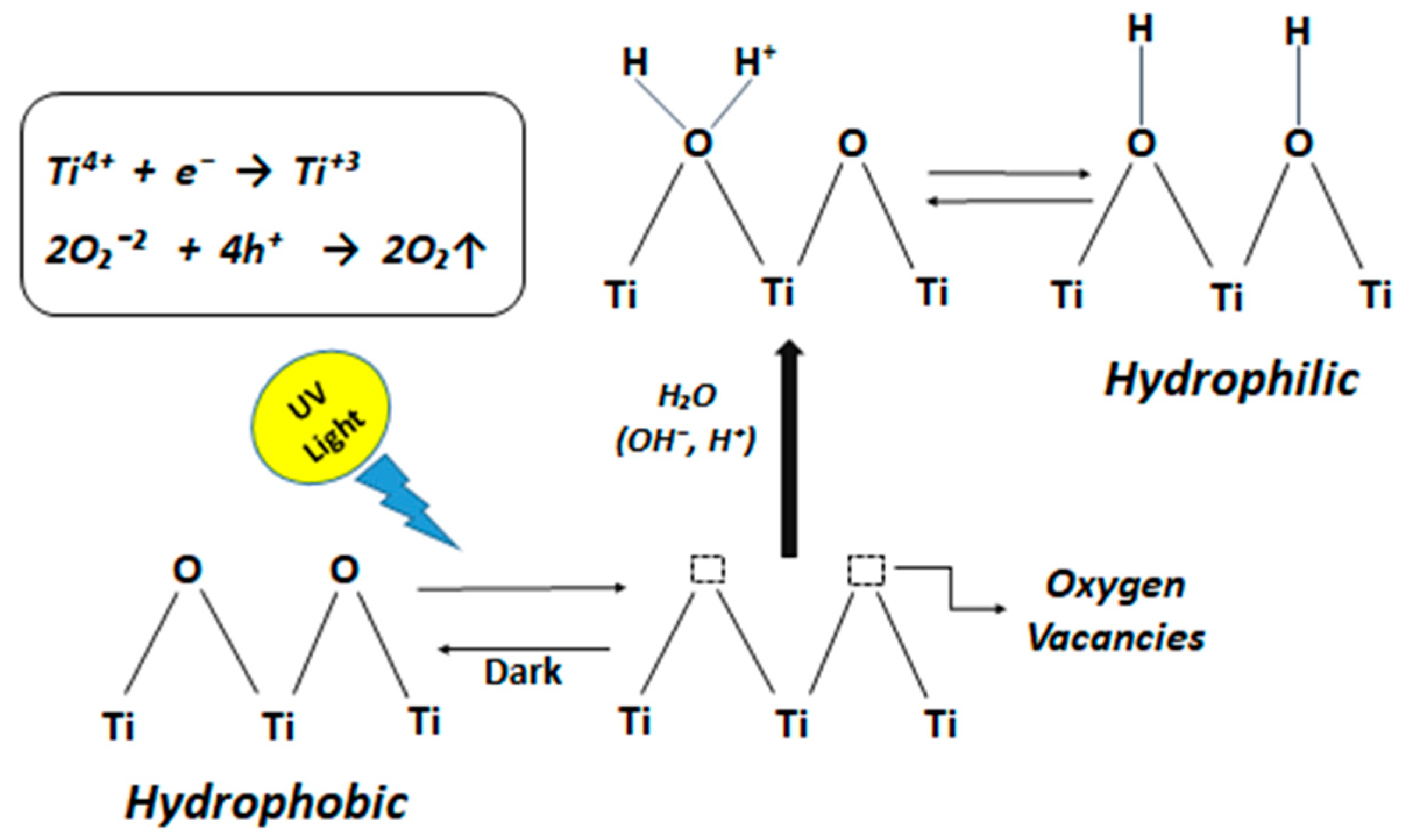
3.1. Photocatalytic Mechanism and Superhydrophilicity of TiO2
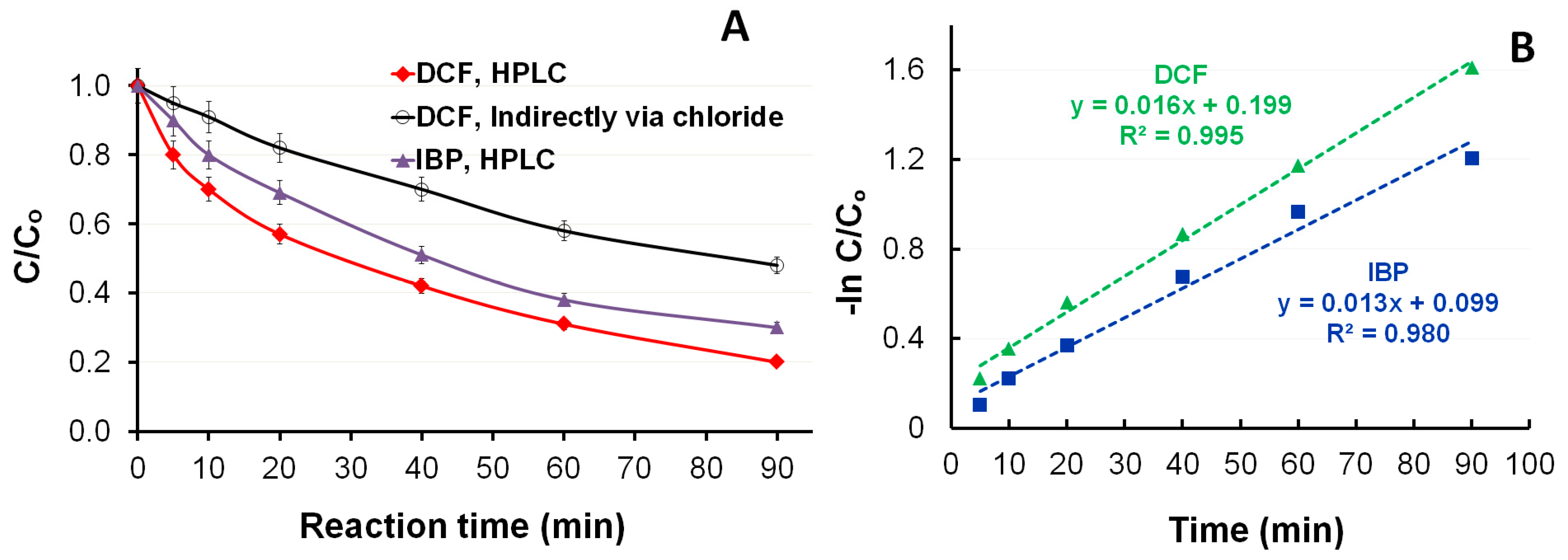
3.2. Photocatalytic Degradation of DCF and IBP
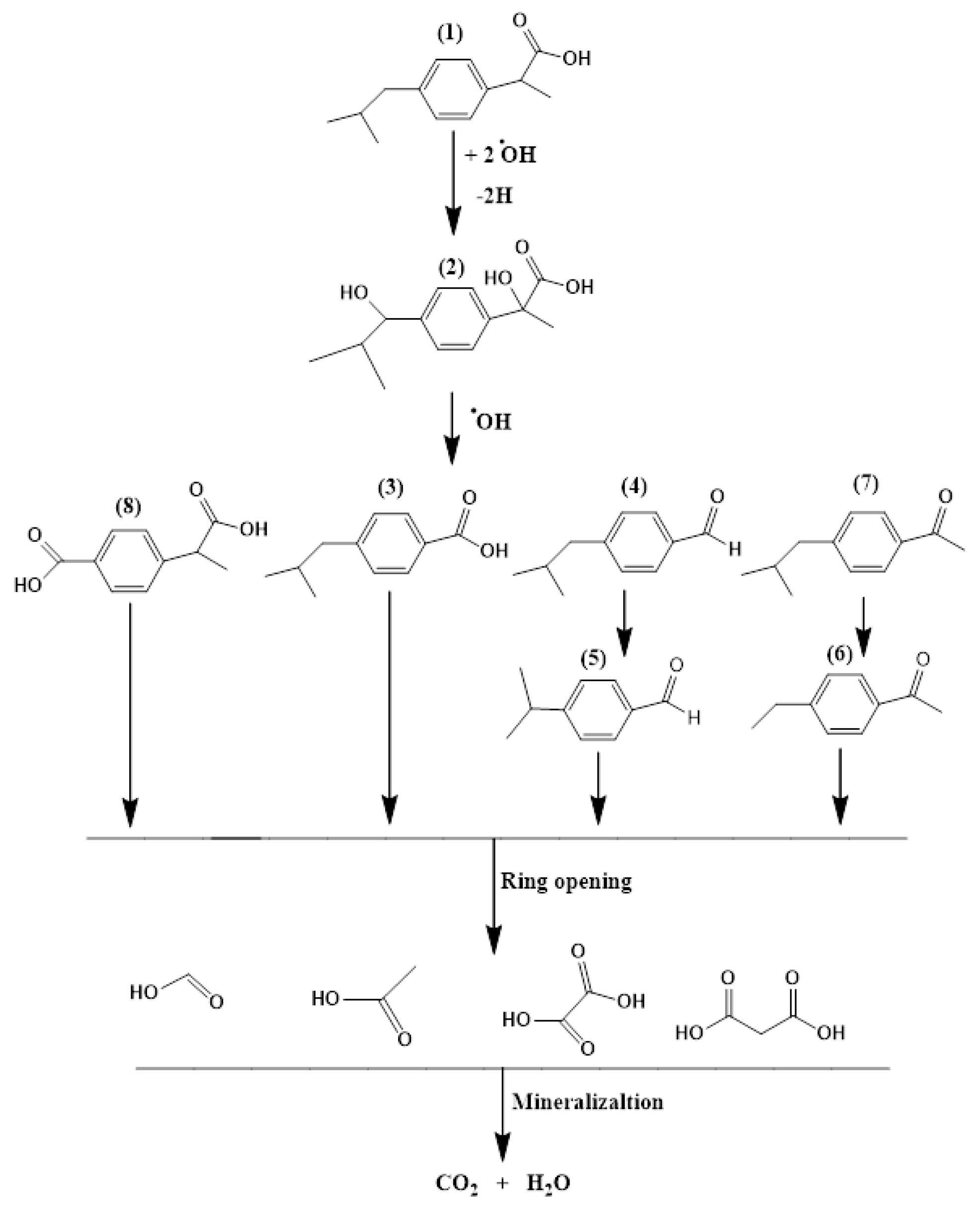
3.3. Degradation Products and Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banaschik, R.; Jablonowski, H.; Bednarski, P.J.; Kolb, J.F. Degradation and intermediates of diclofenac as instructive example for decomposition of recalcitrant pharmaceuticals by hydroxyl radicals generated with pulsed corona plasma in water. J. Hazard. Mater. 2018, 342, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.H.H.; Miessner, H.; Mueller, S.; Mahyar, A.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Comparative study on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J. Hazard. Mater. 2018, 343, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.H.H.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Degradation of pharmaceutical diclofenac and ibuprofen in aqueous solution, a direct comparison of ozonation, photocatalysis, and non-thermal plasma. Chem. Eng. J. 2017, 313, 1033–1041. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D.; Fatta-Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, K.; Naghashkar, N.J.; El-Din, M.G. Degradation of Aqueous Pharmaceuticals by Ozonation and Advanced Oxidation Processes: A Review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Hernando, M.D.; Heath, E.; Petrovic, M.; Barceló, D. Trace-level determination of pharmaceutical residues by LC-MS/MS in natural and treated waters. A pilot-survey study. Anal. Bioanal. Chem. 2006, 385, 985–991. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total. Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef]
- Vieno, N.M.; Härkki, H.; Tuhkanen, T.; Kronberg, L. Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environ. Sci. Technol. 2007, 41, 5077–5084. [Google Scholar] [CrossRef]
- Zwiener, C. Oxidative treatment of pharmaceuticals in water. Water Res. 2000, 34, 1881–1885. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Al-Muhtaseb, A.H.; Kumar, A.; Hira, I.; Ahamad, T.; Ghfar, A.A.; Stadler, F.J. Visible photodegradation of ibuprofen and 2,4-D in simulated waste water using sustainable metal free-hybrids based on carbon nitride and biochar. J. Environ. Manag. 2019, 231, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Motti, C.A.; Glass, B.D.; Oelgemöller, M.; Oelgemoeller, M. Photolysis and TiO2-catalysed degradation of diclofenac in surface and drinking water using circulating batch photoreactors. Environ. Chem. 2014, 11, 51–62. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.H.H. Application of different advanced oxidation processes for the removal of chloroacetic acids using a planar falling film reactor. Chemosphere 2019, 228, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Miessner, H.; Mahyar, A.; Mueller, S.; Kalass, D.; Moeller, D.; Omer, K.M. Removal of dichloroacetic acid from aqueous solution using non-thermal plasma generated by dielectric barrier discharge and nano-pulse corona discharge. Sep. Purif. Technol. 2019, 216, 51–57. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mahyar, A.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Application of a planar falling film reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: A comparative study. Process. Saf. Environ. Prot. 2018, 113, 319–329. [Google Scholar] [CrossRef]
- Mahyar, A.; Miessner, H.; Mueller, S.; Aziz, K.H.H.; Kalass, D.; Moeller, D.; Kretschmer, K.; Manuel, S.R.; Noack, J. Development and application of different non-thermal plasma reactors for the removal of perfluorosurfactants in water: A comparative study. Plasma Chem. Plasma Process. 2019, 39, 531–544. [Google Scholar] [CrossRef]
- Moreira, N.F.; Orge, C.A.; Ribeiro, A.R.; Faria, J.L.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.; Pereira, M.F. Fast mineralization and detoxification of amoxicillin and diclofenac by photocatalytic ozonation and application to an urban wastewater. Water Res. 2015, 87, 87–96. [Google Scholar] [CrossRef]
- Gou, J.; Ma, Q.; Cui, Y.; Deng, X.; Zhang, H.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q.; Liu, H. Visible light photocatalytic removal performance and mechanism of diclofenac degradation by Ag3PO4 sub-microcrystals through response surface methodology. J. Ind. Eng. Chem. 2017, 49, 112–121. [Google Scholar] [CrossRef]
- Aguinaco, A.; Beltrán, F.J.; García-Araya, J.F.; Oropesa, A.L. Photocatalytic ozonation to remove the pharmaceutical diclofenac from water: Influence of variables. Chem. Eng. J. 2012, 189, 275–282. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Munoz, M.; De Pedro, Z.M.; Casas, J.A. Fast degradation of diclofenac by catalytic hydrodechlorination. Chemosphere 2018, 213, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Khabbaz, M.; Entezari, M. Degradation of Diclofenac by sonosynthesis of pyrite nanoparticles. J. Environ. Manag. 2017, 187, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, P.; Liu, H. Visible-light-driven photoelectrocatalytic degradation of diclofenac by N, S–TiO2/TiO2 NTs photoelectrode: performance and mechanism study. J. Environ. Chem. Eng. 2015, 3, 1713–1719. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Napolitano, A.; Andreozzi, R.; D’Ischia, M. Advanced oxidation of the pharmaceutical drug diclofenac with UV/H2O2 and ozone. Water Res. 2004, 38, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Canle, M.; Fernandez, M.I.; Santaballa, J.A.; Faria, J. Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials. Appl. Catal. B Environ. 2011, 107, 110–118. [Google Scholar] [CrossRef]
- Chianese, S.; Iovino, P.; Leone, V.; Musmarra, D.; Prisciandaro, M. Photodegradation of diclofenac sodium salt in water solution: Effect of HA, NO3− and TiO2 on photolysis performance. Water Air Soil Pollut. 2017, 228, 270. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Kciuk, G.; Bobrowski, K.; Gumiela, M.; Koc, A.; Nałęcz-Jawecki, G.; Torun, M.; Ozbay, D. Application of ionizing radiation in decomposition of selected organic pollutants in waters. Eur. Water 2012, 39, 15–26. [Google Scholar]
- Liu, Q.; Luo, X.; Zheng, Z.; Zheng, B.; Zhang, J.; Zhao, Y.; Yang, X.; Wang, J.; Wang, L. Factors that have an effect on degradation of diclofenac in aqueous solution by gamma ray irradiation. Environ. Sci. Pollut. Res. 2011, 18, 1243–1252. [Google Scholar] [CrossRef]
- Tominaga, F.K.; Batista, A.P.D.S.; Teixeira, A.C.S.C.; Borrely, S.I. Degradation of diclofenac by electron beam irradiaton: Toxicitiy removal, by-products identification and effect of another pharmaceutical compound. J. Environ. Chem. Eng. 2018, 6, 4605–4611. [Google Scholar] [CrossRef]
- Xian, G.; Zhang, G.; Chang, H.; Zhang, Y.; Zou, Z.; Li, X. Heterogeneous activation of persulfate by Co3O4-CeO2 catalyst for diclofenac removal. J. Environ. Manag. 2019, 234, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Adityosulindro, S.; Julcour, C.; Barthe, L. Heterogeneous Fenton oxidation using Fe-ZSM5 catalyst for removal of ibuprofen in wastewater. J. Environ. Chem. Eng. 2018, 6, 5920–5928. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, Y.; Nie, W.; Tang, H. Efficient degradation of drug ibuprofen through catalytic activation of peroxymonosulfate by Fe3C embedded on carbon. J. Environ. Sci. 2019, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Choina, J.; Kosslick, H.; Fischer, C.; Flechsig, G.-U.; Frunza, L.; Schulz, A. Photocatalytic decomposition of pharmaceutical ibuprofen pollutions in water over titania catalyst. Appl. Catal. B: Environ. 2013, 129, 589–598. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.; Faria, J.L.; Hentati, O.; Silva, A.M.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Choina, J.; Fischer, C.; Flechsig, G.-U.; Kosslick, H.; Tuan, V.; Tuyen, N.; Tuyen, N.; Schulz, A. Photocatalytic properties of Zr-doped titania in the degradation of the pharmaceutical ibuprofen. J. Photochem. Photobiol. A Chem. 2014, 274, 108–116. [Google Scholar] [CrossRef]
- Uheida, A.; Mohamed, A.; Belaqziz, M.; Nasser, W.S. Photocatalytic degradation of Ibuprofen, Naproxen, and Cetirizine using PAN-MWCNT nanofibers crosslinked TiO2-NH2 nanoparticles under visible light irradiation. Sep. Purif. Technol. 2019, 212, 110–118. [Google Scholar] [CrossRef]
- Ruggeri, G.; Ghigo, G.; Maurino, V.; Minero, C.; Vione, D. Photochemical transformation of ibuprofen into harmful 4-isobutylacetophenone: Pathways, kinetics, and significance for surface waters. Water Res. 2013, 47, 6109–6121. [Google Scholar] [CrossRef]
- Michael, I.; Achilleos, A.; Lambropoulou, D.; Torrens, V.O.; Pérez, S.; Petrović, M.; Barceló, D.; Fatta-Kassinos, D. Proposed transformation pathway and evolution profile of diclofenac and ibuprofen transformation products during (sono) photocatalysis. Appl. Catal. B Environ. 2014, 147, 1015–1027. [Google Scholar] [CrossRef]
- Mahyar, A.; Miessner, H.; Mueller, S.; Moeller, D. Empirical determination and modeling of ozone mass transfer in a planar falling film reactor. Chem. Eng. Res. Des. 2017, 121, 287–294. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Müller, S.; Möller, D. Design and characterization of a multi-phase annular falling-film reactor for water treatment using advanced oxidation processes. J. Environ. Manag. 2013, 120, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
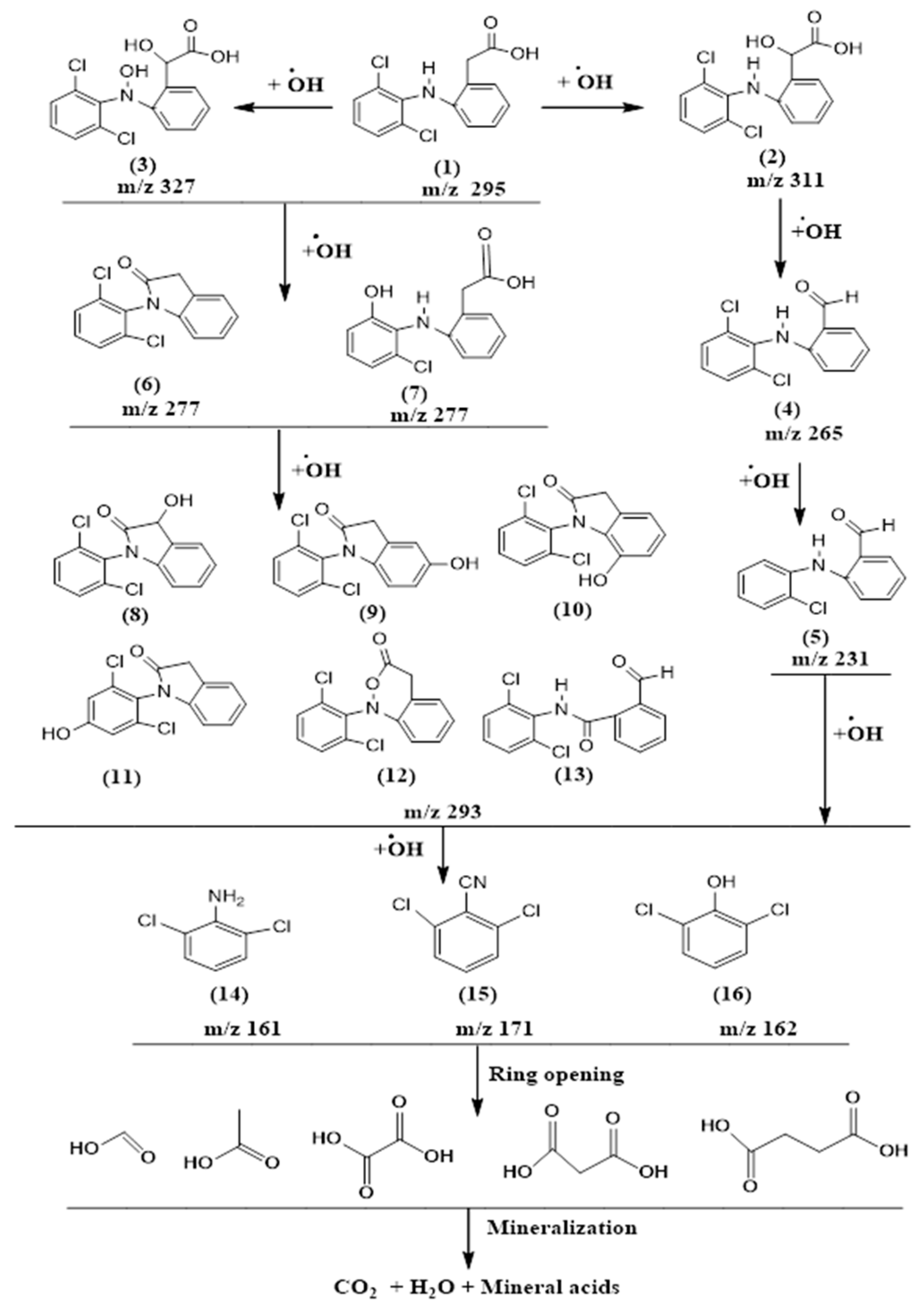
| Number | Compounds | m/z | Rt (min) |
|---|---|---|---|
| (1) | Diclofenac | 295.1 | 12.929 |
| (2) | [2-(2,6-Dichlorophenylamino)-phenyl]-hydroxy-acetic acid | 311 | 11.334 |
| (3) | Dihydroxylation diclofenac | 327.1 | 13.664 |
| (4) | 2-(2,6-Dichlorophenylamino)-benzaldehyde | 265.1 | 11.294 |
| (5) | 2-(2-Chlorophenylamino)-benzaldehyde | 231.1 | 10.777 |
| (6) | 1-(2,6-Dichlorophenyl)-1,3-dihydro-2H-endol-2-one | 277.1 | 11.868 |
| (7) | 2-[2-(2-chloro-6-hydroxy anilino)phenyl]acetic acid | 277.1 | 11.868 |
| (8) | 1-(2,6-Dichlorophenyl)-3-hydroxyindolin-2-one | 293.1 | 13.392 |
| (9) | 1-(2,6-Dichlorophenyl)-5-hydroxyindolin-2-one | 293.1 | 13.964 |
| (10) | 1-(2,6-Dichlorophenyl)-7-hydroxyindolin-2-one | 293.1 | 14.467 |
| (11) | 1-(2,6-Dichloro-4-hydroxyphenyl)indolin-2-one | 293.1 | 13.39 |
| (12) | 1-(2,6-Dichlorophenyl)-1H-benzo [1,2] oxazine-3-4H-one | 293.1 | 13.964 |
| (13) | N-(2,6-Dichlorophenyl)-2-formylbenzamide | 293.1 | 14.467 |
| (14) | 2,6-Dichloroaniline | 161 | 5.068 |
| (15) | 2,6-Dichlorobenzonitrile | 171 | 5.314 |
| (16) | 2,6-Dichlorophenol | 162 | 4.768 |
| Number | Compounds | m/z | Rt (min) |
|---|---|---|---|
| (1) | Ibuprofen | 206.1 | 8.65 |
| (2) | 2-Hydroxy-2-(4-(1-hydroxy-2-methylpropyl) phenyl)propanoic acid | 235.1 | 7.140 |
| (3) | 4-Isobutylbenzoic acid | 178 | 6.704 |
| (4) | 4-Isobutylbenzaldehyde | 162 | 6.063 |
| (5) | 4-Isopropylbenzaldehyde | 148 | 4.636 |
| (6) | 4-Ethylacetophenone | 148 | 6.165 |
| (7) | 4-Isobutylacetophenone | 176 | 6.690 |
| (8) | 4-(1-carboxyethyl)benzoic acid | 190 | 8.140 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hama Aziz, K.H.; Omer, K.M.; Mahyar, A.; Miessner, H.; Mueller, S.; Moeller, D. Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions. Coatings 2019, 9, 465. https://doi.org/10.3390/coatings9080465
Hama Aziz KH, Omer KM, Mahyar A, Miessner H, Mueller S, Moeller D. Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions. Coatings. 2019; 9(8):465. https://doi.org/10.3390/coatings9080465
Chicago/Turabian StyleHama Aziz, Kosar Hikmat, Khalid M. Omer, Ali Mahyar, Hans Miessner, Siegfried Mueller, and Detlev Moeller. 2019. "Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions" Coatings 9, no. 8: 465. https://doi.org/10.3390/coatings9080465
APA StyleHama Aziz, K. H., Omer, K. M., Mahyar, A., Miessner, H., Mueller, S., & Moeller, D. (2019). Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions. Coatings, 9(8), 465. https://doi.org/10.3390/coatings9080465






