The Influence of Polymer Blends on Regulating Chondrogenesis
Abstract
1. Introduction
2. Materials and Methods
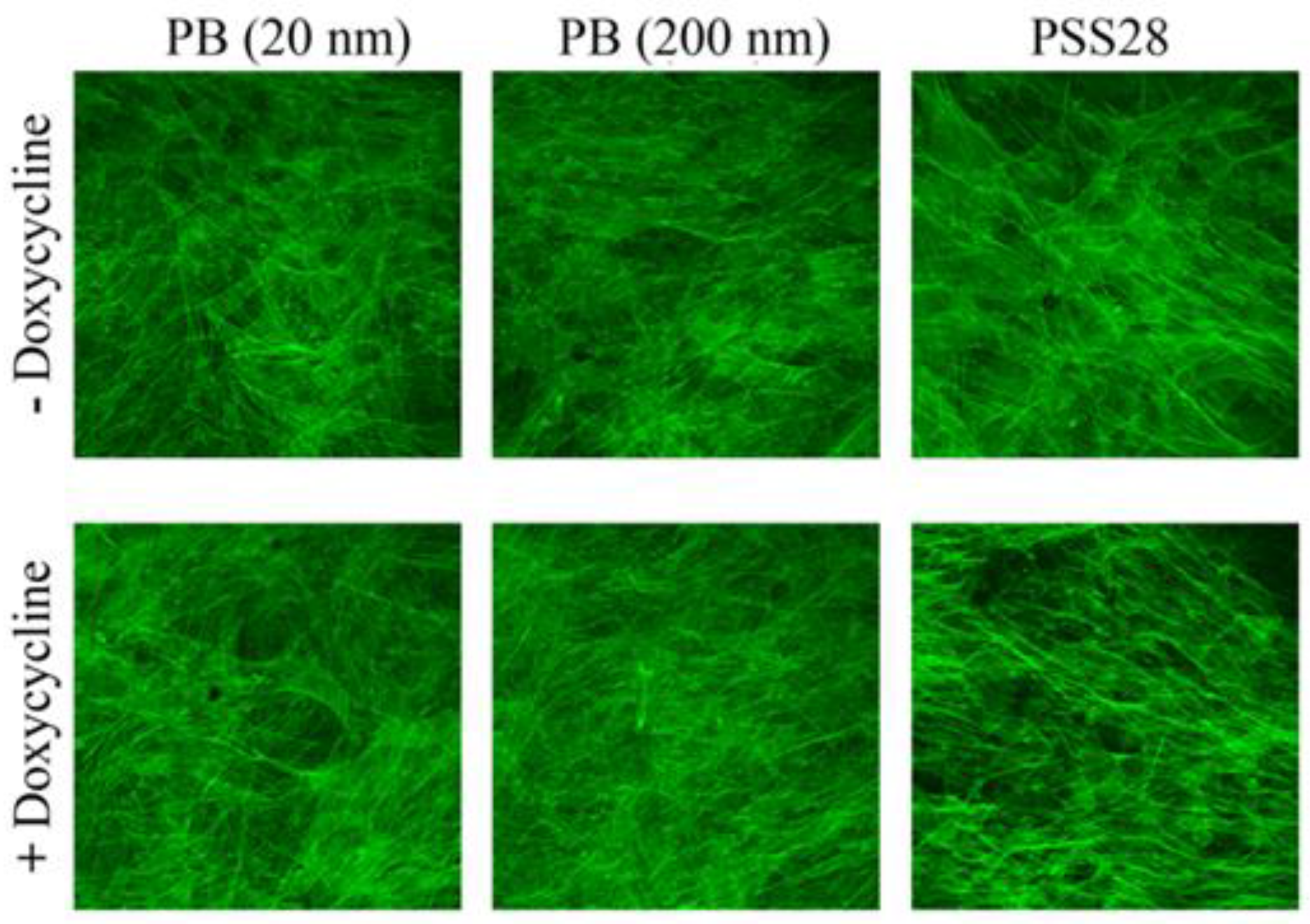
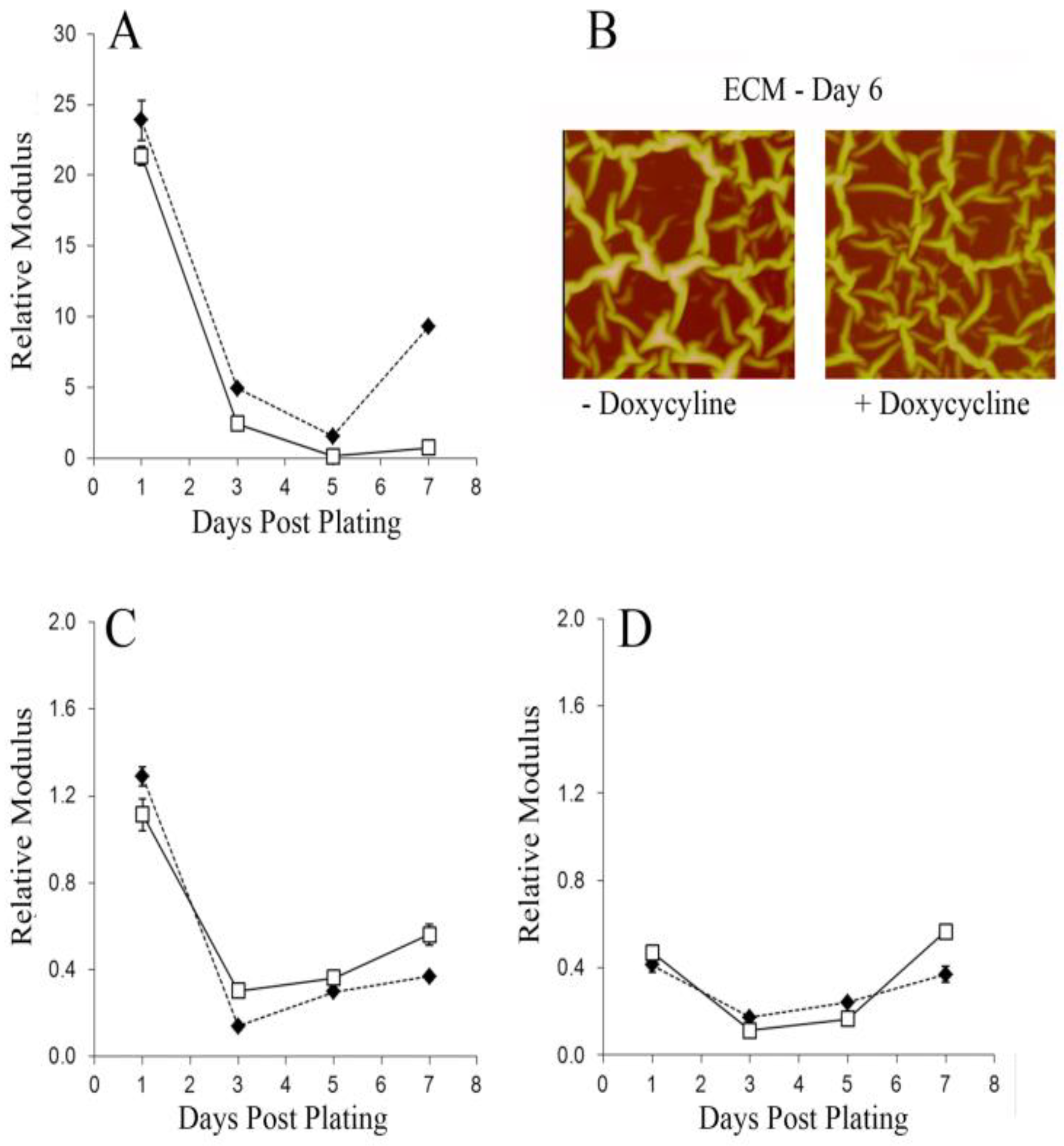
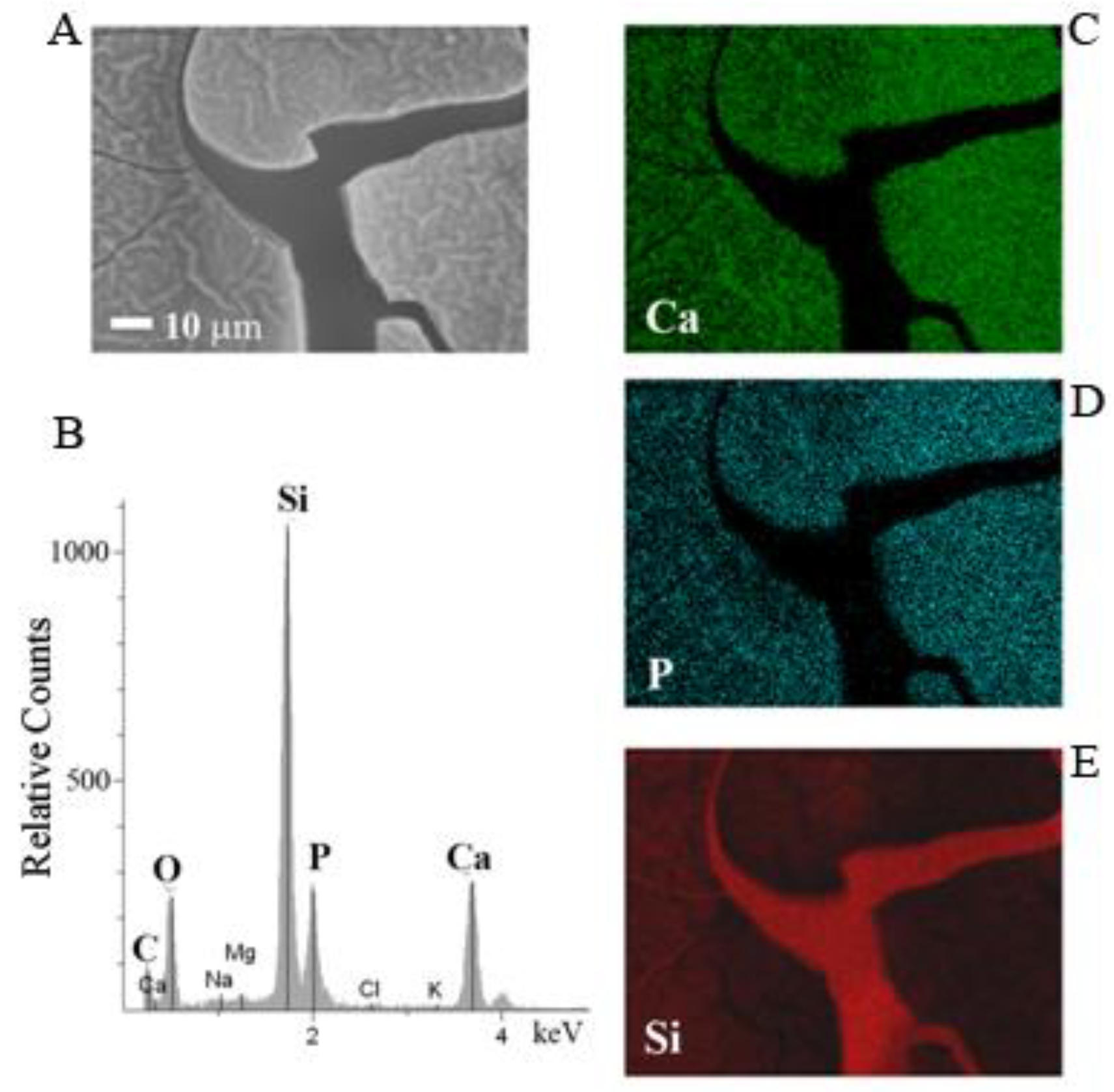
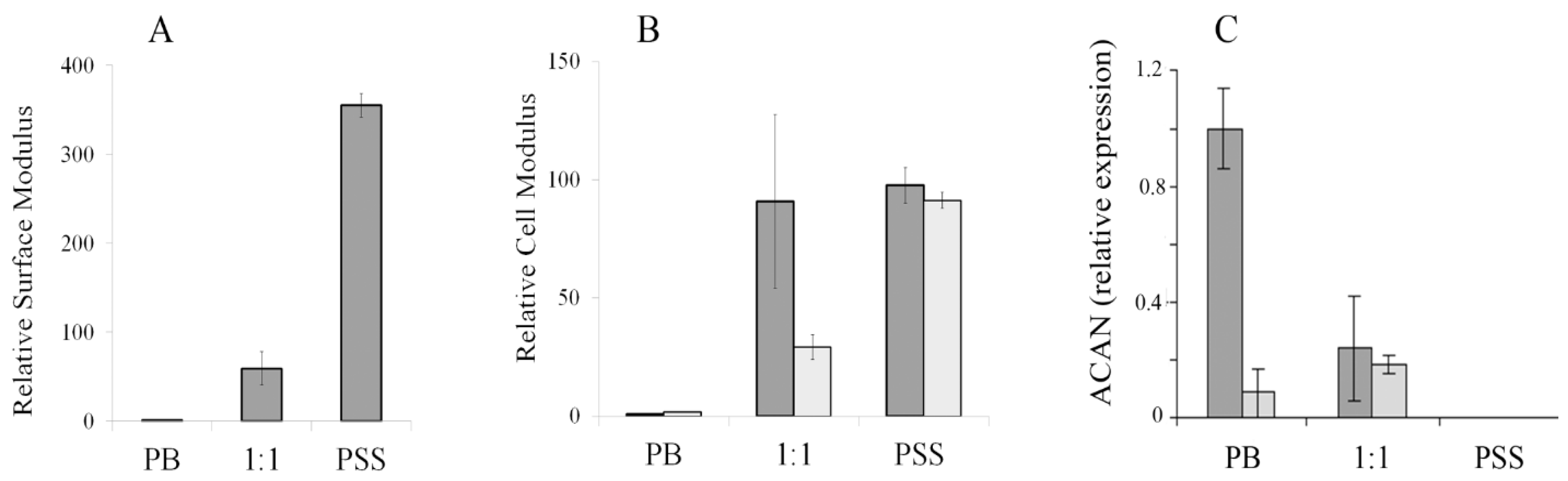
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garrison, K.R.; Donell, S.; Ryder, J.; Shemilt, I.; Mugford, M.; Harvey, I.; Song, F. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: A systematic review. Health Technol. Assess 2007, 11, 1–150. [Google Scholar] [CrossRef] [PubMed]
- Lissenberg-Thunnissen, S.N.; De Gorter, D.J.J.; Sier, C.F.M.; Schipper, I.B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int. Orthop. 2011, 35, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Estrada, K.D.; Lyons, K.M. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009, 20, 379–388. [Google Scholar] [CrossRef]
- Rosen, V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009, 20, 475–480. [Google Scholar] [CrossRef]
- Mi, M.; Jin, H.; Wang, B.; Yukata, K.; Sheu, T.-J.; Ke, Q.H.; Tong, P.; Im, H.-J.; Guozhi, X.; Chen, D. Chondrocyte BMP2 signaling plays an essential role in bone fracture healing. Gene 2013, 512, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Edgar, C.M.; Chakravarthy, V.; Barnes, G.; Kakar, S.; Gerstenfeld, L.C.; Einhorn, T.J. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone 2009, 40, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424. [Google Scholar] [CrossRef]
- Wong, D.A.; Kumar, A.; Jatana, S.; Ghiselli, G.; Wong, K. Neurologic impairment from ectopic bone in the lumbar canal: A potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008, 8, 1011–1018. [Google Scholar] [CrossRef]
- Kaneko, H.; Arakawa, T.; Mano, H.; Kaneda, T.; Kaneda, T.; Ogasawara, A.; Nakagawa, M.; Nakagawa, M.; Toyama, Y.; Yabe, Y.; et al. Direct stimulation of osteoclasticbone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 2000, 27, 479–486. [Google Scholar] [CrossRef]
- Smucker, J.D.; Rhee, J.M.; Singh, K.; Yoon, S.T.; Heller, J.G. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine 2006, 31, 2813–2819. [Google Scholar] [CrossRef]
- Vaidya, R.; Weir, R.; Sethi, A.; Meisterling, S.; Hakeos, W.; Wybo, C.D. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J. Bone Jt. Surg. Br. 2007, 89, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Denker, A.E.; Haas, A.R.; Nicoll, S.B.; Tuan, R.S. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: I. Stimulation by bone morphogenetic protein-2 in high-density micromass cultures. Differentiation 1999, 64, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hasharoni, A.; Zilberman, Y.; Turgeman, G.; Helm, G.A.; Liebergall, M.; Gazit, D. Murine spinal fusion induced by engineered mesenchymal stem cells that conditionally express bone morphogenetic protein-2. J. Neurosurg. Spine. 2005, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsos, I.K.; Turgeman, G.; Zhou, S.; Kurkalli, B.G.; Pelled, G.; Tzur, L.; Kelley, P.; Stumm, N.; Mi, S.; Müller, R.; et al. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol. Ther. 2001, 3, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Sheyn, D.; Ruthemann, M.; Mizrahi, O.; Kallai, I.; Zilberman, Y.; Tawackoli, W.; Kanim, L.E.; Zhao, L.; Bae, H.; Pelled, G.; et al. Genetically modified mesenchymal stem cells induce mechanically stable posterior spine fusion. Tissue Eng. Part A 2010, 16, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Noel, D.; Gazit, D.; Bouquet, C.; Apparailly, F.; Bony, C.; Plence, P.; Millet, V.; Turgeman, G.; Perricaudet, M.; Sany, J.; et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells 2004, 22, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Yang, X. Mesenchymal stem cells and bone regeneration: Current status. Injury 2011, 42, 562–568. [Google Scholar] [CrossRef]
- Griffin, M.; Igbal, S.A.; Bayat, A. Exploring the application of mesenchymal stem cells in bone repair and regeneration. J. Bone Jt. Surg. Britain. 2011, 93, 427–434. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, S.M.; Jeon, O.; Choi, Y.C.; Kim, B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef]
- Frohlich, M.; Grayson, W.L.; Wan, L.Q.; Marolt, D.; Drobnic, M.; Vunjak-Novakovic, G. Tissue engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Curr. Stem Cell Res. Ther. 2008, 3, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hong, J.; Zheng, Q.; Guo, X.; Lan, S.; Cui, F.; Pan, H.; Zou, Z.; Chen, C. Repair of rat cranial bone defects with nHAC/PLLA and BMP-2-related peptide or rhBMP-2. J. Orthop. Res. 2011, 29, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Y.; Yuan, X.; Lu, L. Enhanced healing of rat calvarial defects with MSCs loaded on BMP-2 releasing chitosan/alginate/hydroxyapatite scaffolds. PLoS ONE 2014, 9, e104061. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2012, 37, 2491–2498. [Google Scholar] [CrossRef]
- Ingber, D.E. Mechanosensation through integrins: Cells act locally but think globally. Proc. Natl. Acad. Sci. USA 2003, 100, 1472–1474. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, L.; Sun, Y.; Simmons, C.A. Mesenchymal stem cell mechanobiology and emerging experimental platforms. J. R. Soc. Interface 2013, 10, 20130179. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef]
- Wang, Y.K.; Yu, X.; Cohen, D.M.; Wozniak, M.A.; Yang, M.T.; Gao, L.; Eyckmans, J.; Chen, C.S. Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev. 2011, 21, 1176–1186. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Bherwani, A.; Simon, M.; Rafailovich, M.; Jurukovski, V. Entangled polymer surface confinement, an alternative method to control stem cell differentiation in the absence of chemical mediators. Ann. Mater. Sci. Eng. 2014, 1, 7. [Google Scholar]
- Aranguren, M.I.; Macosko, C.W. Modulus of polybutadiene networks by hydrosilation crosslinking. Macromolecules 1988, 21, 2483–2491. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Rosenwasser, M.P.; Buckwalter, J.A.; Malinin, T.I.; Mow, V.C. Intersecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J. Orthop. Res. 1991, 9, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Pernodet, N.; Rafailovich, M.; Sokolov, J.; Xu, D.; Yang, N.L.; McLeod, K. Fibronectin fibrillogenesis n sulfonated polystyrene surfaces. J. Biomed. Mater. Res. A 2003, 64, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.M.; Qin, Y.-X.; DiMasi, E.; Ba, X.; Rafailovich, M.; Pernodet, N. Biomineralization of a self-assembled ex.racellular matrix for bone tissue engineering. Tissue Eng. Part A 2009, 15, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Pu, Y.; Zhang, W.; Rafailovich, M.; Sokolov, J. Shear modulation force microscopy study of near surface glass transition temperatures. Phys. Rev. Lett. 2000, 85, 2340–2343. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ge, S.; Rafailovich, M.; Sokolov, J.; Duan, Y.; Pearce, E.; Zaitsev, V.; Schwarz, S. Surface transitions by shear modulation force microscopy. Langmuir 2001, 17, 5865–5871. [Google Scholar] [CrossRef]
- Yagi, K.; Tsuji, K.; Nifuji, A.; Shinomiya, K.; Nakashima, K.; DeCrombrugghe, B.; Noda, M. Bone morphogenetic protein-2 enhances osterix gene expression in chondrocytes. J. Cell. Biochem. 2003, 88, 1077–1083. [Google Scholar] [CrossRef]
- Zuscik, M.J.; Hilton, M.J.; Zhang, X.; Chen, D.; O’Keefe, R.J. Regulation of chondrogenesis and chondrocyte differentiation by stress. J. Clin. Investig. 2008, 118, 429–438. [Google Scholar] [CrossRef]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Nurminskaya, M.; Linsenmayer, T.F. Identification and characterization of up-regulated genes during chondrocyte hypertrophy. Dev. Dyn. 1996, 206, 260–271. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required forosteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Jing, J.; Hinton, R.J.; Jing, Y.; Liu, Y.; Zhou, X.; Feng, J.Q. Osterix couples chondrogenesis and osteogenesis in post-natal condylar growth. J. Dent. Res. 2014, 93, 1014–1021. [Google Scholar] [CrossRef]
- Oh, J.H.; Park, S.Y.; de Crombrugghe, B.; Kim, J.E. Chondrocyte-specific ablation of Osterix leads to impaired endochondral ossification. Biochem. Biophys. Res. Commun. 2012, 418, 634–640. [Google Scholar] [CrossRef]
- Matsubara, T.; Kida, K.; Yamaguchi, A.; Hata, K.; Ichida, F.; Meguro, H.; Aburatani, H.; Nishimura, R.; Yoneda, T. BMP2 regulates osterix through Msx2 and Runx2 during osteoblast differentiation. J. Biol. Chem. 2008, 283, 29119–29125. [Google Scholar] [CrossRef]
- Domowicz, M.S.; Cortes, M.; Henry, J.G.; Schwartz, N.B. Aggrecan modulation of growth plate morphogenesis. Dev. Biol. 2009, 329, 242–257. [Google Scholar] [CrossRef]

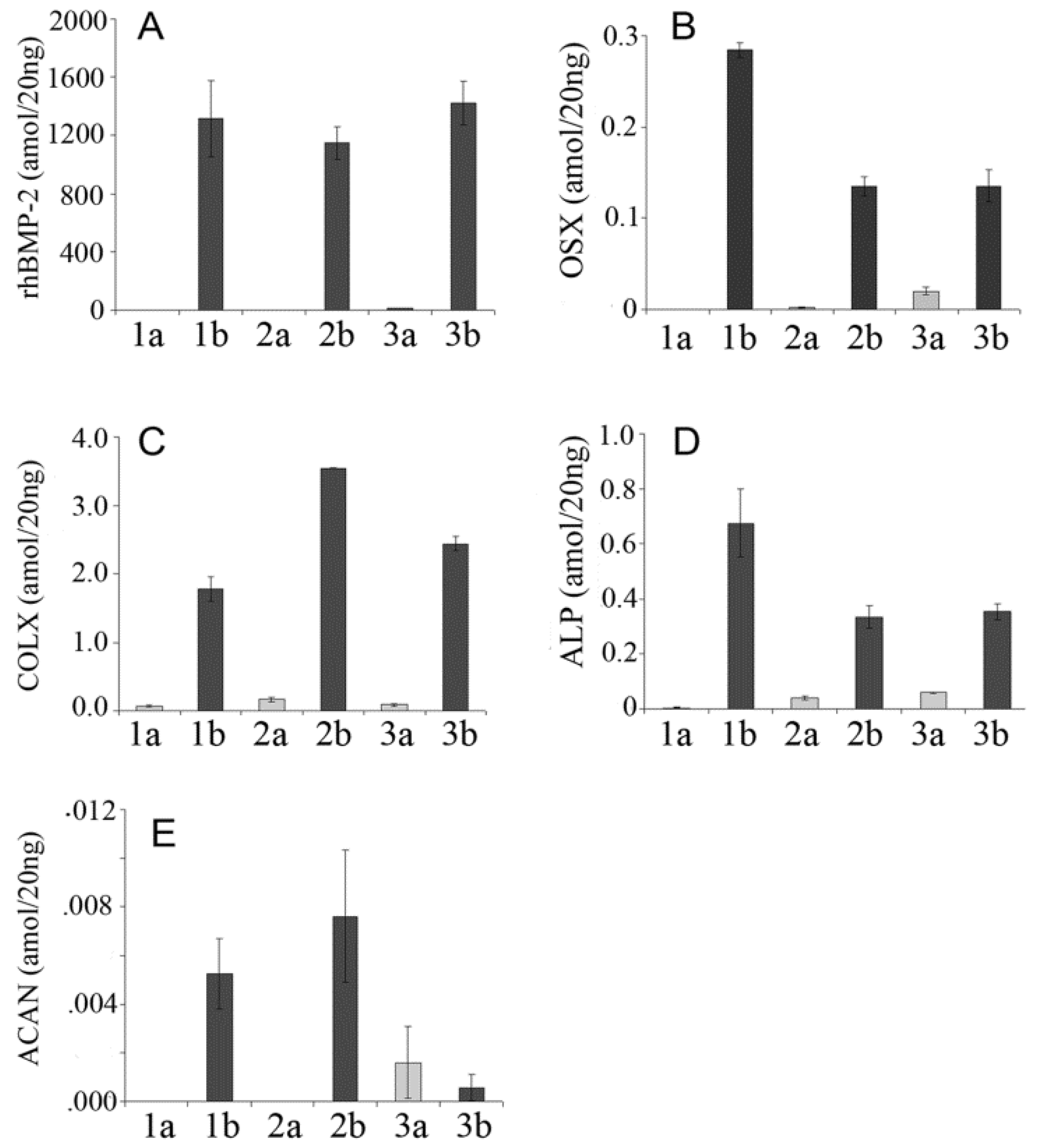

| rhBMP-2 | Forward GACCACCGGTTGGAGAGGGCA, reverse GGTCACGGGGAATTTCGAGTTGG |
| OSX | forward CCTGACTCCTTGGGACCCGGTC, reverse CTGGGTAGGCGTCCCCCATGG |
| ALP | forward TCCTGGGAGATGGTATGGGCGTC, reverse GTTGCATCGCGTGCGCTCTG |
| COL-X | forward CGCATCTCCCAGCACCAGAATC, reverse GGTGTCCTCGAGGTCCGGTTG |
| ACAN | forward TCAACCGTTGCAGACCAGGAGCA, reverse CAGGCTGGTTTGGACGCCACT |
| 18S | forward GTAACCCGTTGAACCCCATT, reverse CCATCCAATCGGTAGTAGCG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bherwani, A.; Chang, C.-C.; Pelled, G.; Gazit, Z.; Gazit, D.; Rafailovich, M.; Simon, M. The Influence of Polymer Blends on Regulating Chondrogenesis. Coatings 2019, 9, 451. https://doi.org/10.3390/coatings9070451
Bherwani A, Chang C-C, Pelled G, Gazit Z, Gazit D, Rafailovich M, Simon M. The Influence of Polymer Blends on Regulating Chondrogenesis. Coatings. 2019; 9(7):451. https://doi.org/10.3390/coatings9070451
Chicago/Turabian StyleBherwani, Aneel, Chung-Chueh Chang, Gadi Pelled, Zulma Gazit, Dan Gazit, Miriam Rafailovich, and Marcia Simon. 2019. "The Influence of Polymer Blends on Regulating Chondrogenesis" Coatings 9, no. 7: 451. https://doi.org/10.3390/coatings9070451
APA StyleBherwani, A., Chang, C.-C., Pelled, G., Gazit, Z., Gazit, D., Rafailovich, M., & Simon, M. (2019). The Influence of Polymer Blends on Regulating Chondrogenesis. Coatings, 9(7), 451. https://doi.org/10.3390/coatings9070451






