Biofunctionalization of Textile Materials.1. Biofunctionalization of Poly(Propylene) (PP) Nonwovens Fabrics by Alafosfalin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Polymers
2.1.2. Chemical Agents
2.1.3. Finishing Agents
2.1.4. Bacterial Strains
2.2. Methods
2.2.1. Nonwoven Fabrics
2.2.2. Dip-Coating of Nonwoven Fabric and Modification of Phosphonodipeptide
2.2.3. Scanning Electron Microscopy
2.2.4. ATR-FTIR
2.2.5. UV–Vis Analysis
2.2.6. Filtration Parameters
2.2.7. Tensile Testing
2.2.8. Antimicrobial Activity
3. Results and Discussion
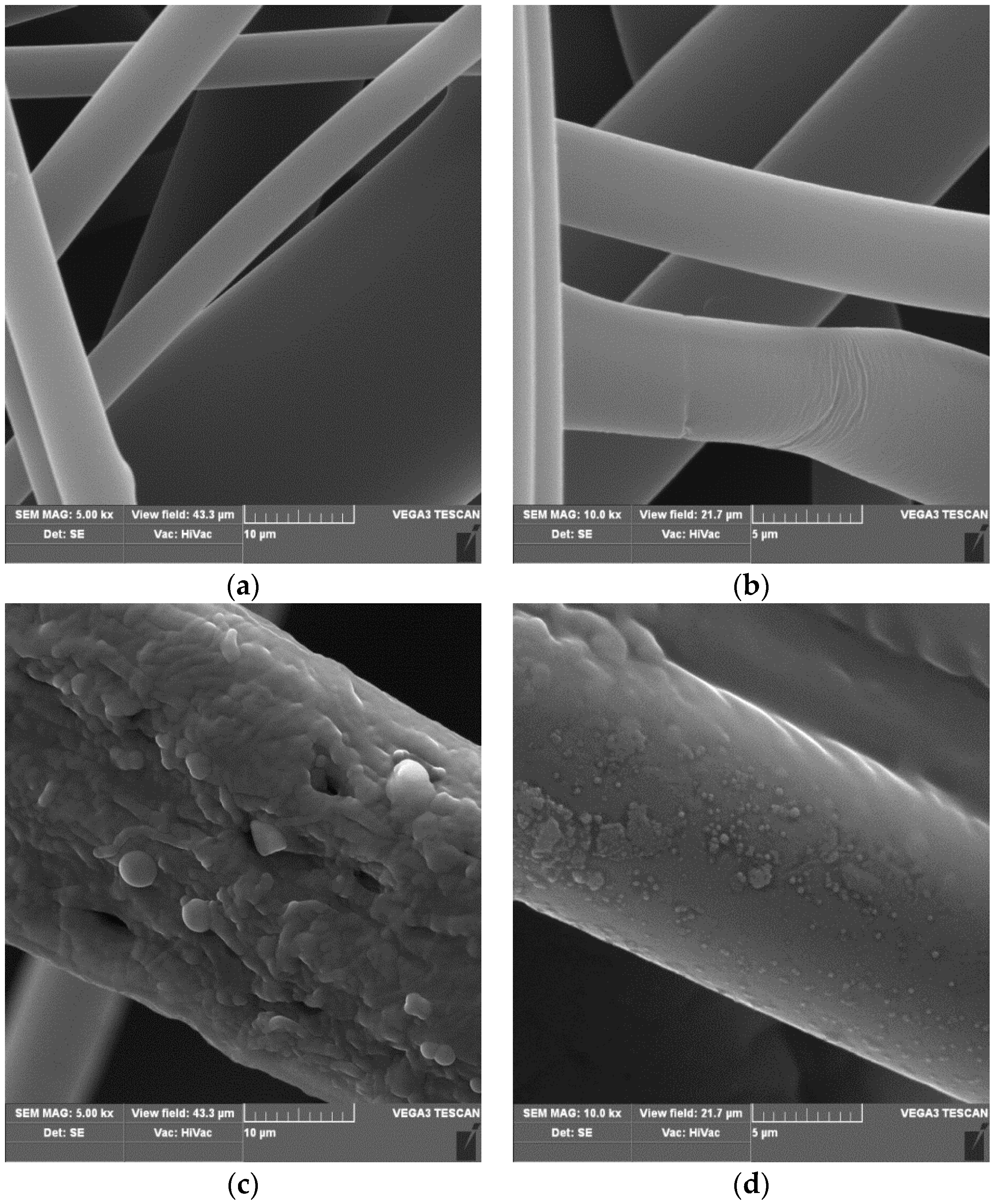
3.1. Scanning Electron Microscopy
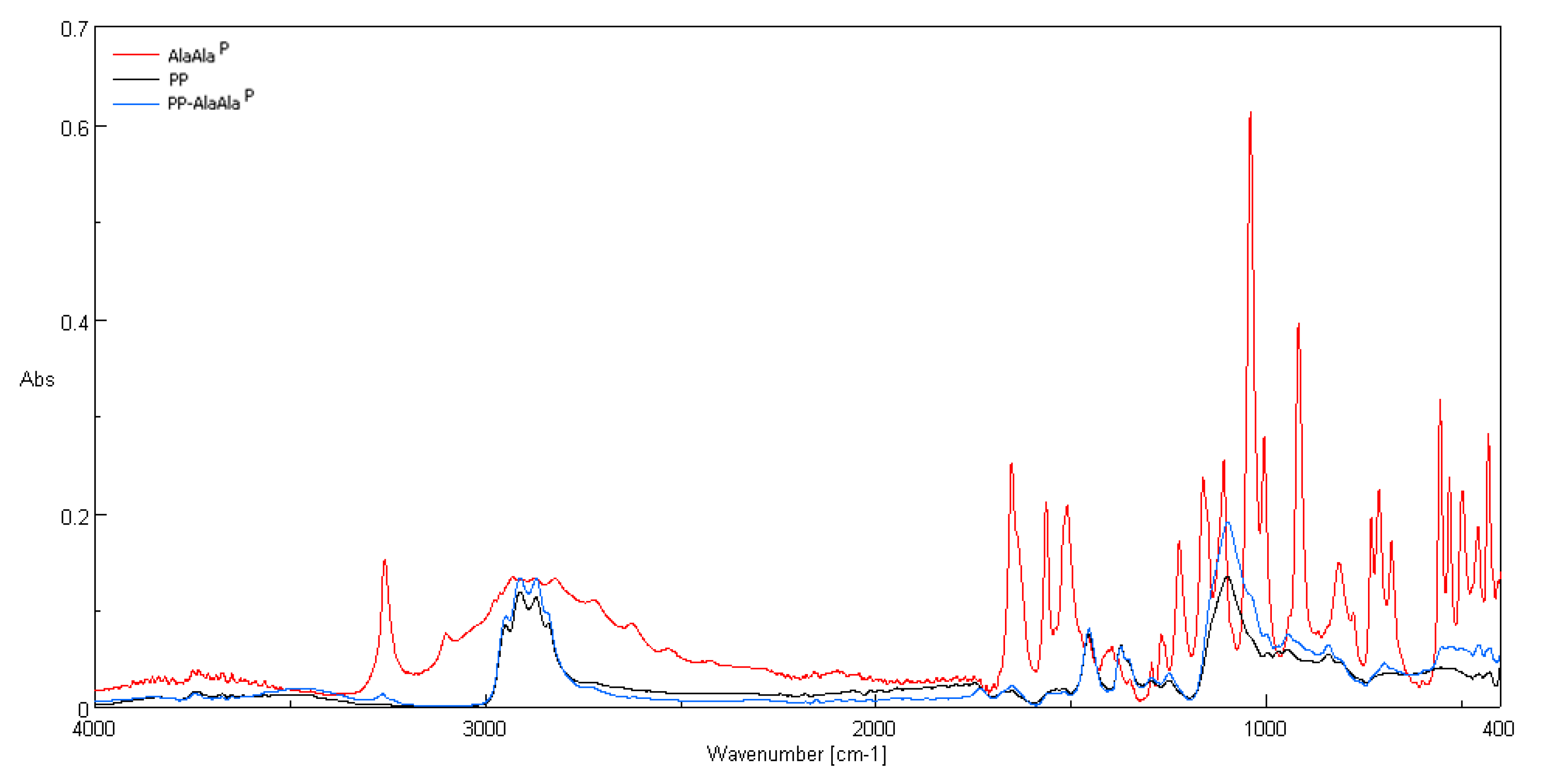
3.2. ATR-FTIR Spectra
3.3. UV/Vis Transmittance Spectra
3.4. Technical Parameters
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, K.S.; Lian, H.S.; Chen, J.B. Study on the modification of PP nonwoven fabric. Fibres Text. East. Eur. 2011, 3, 82–87. [Google Scholar]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Simoes, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Wang, C.C.; Su, C.H.; Chen, C.C. Water absorbing and antibacterial properties of N-isopropyl acrylamide grafted and collagen/chitosan immobilized polypropylene nonwoven fabric and its application on wound healing enhancement. J. Biomed. Mater. Res. A 2008, 84, 1006–1017. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, C.C.; Chen, F.L.; Lin, N.-S. An improvement on water absorbing and permeating properties: Heparin immobilizing on acrylic acid-grafted and collagen/chitosan-immobilized wound dressing. J. Appl. Polym. Sci. 2008, 109, 1431–1438. [Google Scholar] [CrossRef]
- Wang, C.C.; Su, C.H.; Chen, J.P.; Chen, C.C. An enhancement on healing effect of wound dressing: Acrylic acid grafted and gamma-polyglutamic acid/chitosan immobilized polypropylene non-woven. Mater. Sci. Eng. C 2009, 29, 1715–1724. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, J.P.; Chen, C.C. An enhancement on water absorbing and permeating abilities of acrylic acid grafted and chitosan/collagen immobilized polypropylene non-woven fabric: Chitosan obtained from Mucor. Mater. Sci. Eng. C 2009, 29, 1133–1139. [Google Scholar] [CrossRef]
- Xin, Z.; Du, S.; Zhao, C.; Chen, H.; Sun, M.; Yan, S.; Luan, S.; Yin, J. Antibacterial performance of polypropylene nonwoven fabric wounddressing surfaces containing passive and active components. Appl. Surf. Sci. 2016, 365, 99–107. [Google Scholar] [CrossRef]
- Chen, K.S.; Ku, T.Y.A.; Lee, C.H.; Lin, H.R.; Lin, F.H.; Chen, T.M. Immobilization of chitosan gel with cross-linking reagent on PNIPAAm gel/PP nonwoven composites surface. Mater. Sci. Eng. C 2005, 25, 472–478. [Google Scholar] [CrossRef]
- Simovic, L.M.; Skundric, P.D.; Kostic, M.M.; Tasic, G.M.; Kojic, Z.Z.; Milakovic, B.D.; Medovic, A.H. Efficiency and biocompatibility of antimicrobial textile material of broad spectrum activity. J. Appl. Polym. Sci. 2011, 120, 1459–1467. [Google Scholar] [CrossRef]
- Chen, J.P.; Lee, W.L. Collagen-grafted temperature-responsive nonwoven fabric for wound dressing. Appl. Surf. Sci. 2008, 255, 412–415. [Google Scholar] [CrossRef]
- Lin, F.H.; Tsai, J.C.; Chen, T.M.; Chen, K.S.; Yang, J.M.; Kang, P.L.; Wu, T.H. Fabrication and evaluation of auto-stripped tri-layer wound dressing for extensive burn injury. Mater. Chem. Phys. 2007, 102, 152–158. [Google Scholar] [CrossRef]
- Yang, J.M.; Lin, H.T. Properties of chitosan containing PP-g-AA-g-NIPAAmbigraft nonwoven fabric for wound dressing. J. Membr. Sci. 2004, 243, 1–7. [Google Scholar] [CrossRef]
- Tyan, Y.C.; Liao, J.D.; Lin, S.P. Surface properties and in vitro analyses of immobilized chitozan onto polepropylene non-wofen fabric surface using antenna-coupling microwave plasma. J. Mater. Sci. Mater. Med. 2003, 14, 775–781. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Baier, G.; Landfester, K.; Musin, E.; Al-bataineh, S.; Cameron, D.C.; Whittle, J.D.; Sillanpää, M. Attachment of poly(l-lactide) nanoparticles to plasma-treated non-woven polymer fabrics using inkjet printing. Macromol. Biosci. 2015, 15, 1274–1282. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Krumpolec, R.; Homola, T.; Musin, E.; Baier, G.; Landfester, K.; Cameron, D.C.; Černák, M. Ambient air plasma pre-treatment of non-woven fabrics for deposition of antibacterial poly(l-lactide) nanoparticles. Plasma Process. Polym. 2017, 14, 1600231. [Google Scholar] [CrossRef]
- Kukhar, V.P.; Hudson, H.R. Aminophosphonic and Aminophosphinic Acids. Chemistry and Biological Activity; Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Maier, L. Phosphoroorganic detergents. Chimia 1969, 23, 323–330. [Google Scholar]
- Petrov, K.A.; Chauzov, V.A.; Erokhina, T.S. Aminoalky lorganophosphorus compounds. Russ. Chem. Rev. 1974, 43, 984. [Google Scholar] [CrossRef]
- Rizkalla, E.N. Metal chelates of phosphonate-containing ligands. Rev. Inorg. Chem. 1983, 5, 223–304. [Google Scholar]
- Sikorski, J.A.; Logush, E.W. Aliphatic carbon-phosphorus compound as herbicides. In Handbook of Organophosphorus Chemistry, 1st ed.; Engel, R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1992; pp. 751–800. [Google Scholar]
- Neuzil, E.; Cassaigne, A. Natural compounds with biologic value containing a P-C bond and phosphonates. Expos. Annu. Biochim. Med. 1980, 34, 165–210. [Google Scholar]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable potential of the α-aminophosphonate/ phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Orsini, F.; Sello, G.; Sisti, M. Aminophosphonic acids and derivatives. Synthesis and biological applications. Curr. Med. Chem. 2010, 17, 264–289. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic acids-phosphorus analogues of natural amino acids. Part 1: Syntheses of α-aminophosphonic acids. Curr. Org. Chem. 2011, 15, 2015–2071. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Kudzin, Z.H.; Drabowicz, , J. Thioureidoalkylphosphonates in the synthesis of 1-aminoalkylphosphonic acids. The Ptc-aminophosphonate method. Arkivoc 2011, VI, 227–269. [Google Scholar] [CrossRef]
- Drabowicz, J.; Jakubowski, H.; Kudzin, M.H.; Kudzin, Z.H. Nomenclature of aminoalkylphosphonic acids and derivatives. Evolution of the code system. Acta Biochim. Polon. 2015, 62, 139–150. [Google Scholar] [CrossRef]
- Grandcoin, A.; Piel, S.; Baurès, E. AminoMethylPhosphonic acid (AMPA) in natural waters: Its sources, behavior and environmental fate. Water Res. 2017, 117, 187–197. [Google Scholar] [CrossRef]
- Aparicio, V.C.; De Gerónimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethylphosphonic acid and methoxyacetic acid induce apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2015, 16, 11750–11765. [Google Scholar] [CrossRef]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethylphosphonic acid inhibits growth and metastasis of human prostate cancer in an orthotopicxenograft mouse model. Oncotarget 2016, 7, 10616–10626. [Google Scholar] [CrossRef][Green Version]
- Gomes, M.P.; Smedbol, E.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef]
- Horiguchi, M.; Kandatsu, M. Isolation of 2-aminoethane phosphonic acid from rumen protozoa. Nature 1959, 184 (Suppl. 12), 901–902. [Google Scholar] [CrossRef]
- Alhadeff, J.A.; Van Bruggen, J.T.; Daves, D., Jr. Biosynthetic studies on 2-aminoethylphosphonic acid in a mammalian (rat) system. Biochim. Biophys. Acta 1972, 286, 103–106. [Google Scholar] [CrossRef]
- Tan, S.A.; Tan, L.G. Distribution of ciliatine (2-aminoethylphosphonic acid) and phosphonoalanine (2-amino-3-phosphonopropionic acid) in human tissues. Clin. Physiol. Biochem. 1989, 7, 303–309. [Google Scholar]
- Watts, M. Glufosinate-Ammonium Monograph. 2008. Available online: http://www.pananz.net/wp-content/uploads/2013/04/Glufosinate-monograph-12-Dec-2008.pdf (accessed on 18 June 2019).
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate: A Unique Global Herbicide; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Dill, G.M.; Sammons, R.D.; Feng, P.C.C.; Kohn, F.; Kretzmer, K.; Mehrsheikh, A.; Bleeke, M.; Honegger, J.L.; Farmer, D.; Wright, D.; et al. Glyphosate: discovery, development, applications, and properties. In Glyphosate Resistance in Crops and Weeds. History, Development and Management; Nandula, V.K., Ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassal, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature 1978, 272, 56–58. [Google Scholar] [CrossRef]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Alaphosphin and related phosphonopeptides. Antimicrob. Agents Chemother. 1979, 15, 684–695. [Google Scholar] [CrossRef]
- Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Lambert, R.W.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Rationale, chemistry and structure-activity relationships. Antimicrob. Agents Chemother. 1979, 15, 677–683. [Google Scholar] [CrossRef]
- Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Lambert, R.W.; Lloyd, W.J.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Mechanism of action of alaphosphin. Antimicrob. Agents Chemother. 1979, 15, 696–705. [Google Scholar] [CrossRef]
- Allen, J.G.; Lees, L.J. Pharmacokinetics of alafosfalin, alone and in combination with cephalexin, in humans. Antimicrob. Agents Chemother. 1980, 17, 973–979. [Google Scholar] [CrossRef]
- Atherton, F.R.; Hassall, C.H.; Lambert, R.W. Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonic acid and (aminomethyl)-phosphonic acid. J. Med. Chem. 1986, 29, 29–40. [Google Scholar] [CrossRef]
- Neuman, H.J. Recent developments in the field of phosphonic acid antibiotics. J. Antimicrob. Chemother. 1984, 14, 309–311. [Google Scholar] [CrossRef]
- Lejczak, B.; Kafarski, P.; Sztajer, H.; Mastalerz, P. Antibacterial activity of phosphono dipeptides related to alafosfalin. J. Med. Chem. 1986, 29, 2212–2217. [Google Scholar] [CrossRef]
- Traub, W.H. In vitro evaluation of alaphosphin (Ro 03-7008) against Serratiamarcescens. Chemotherapy 1980, 26, 103–110. [Google Scholar] [CrossRef]
- Arisawa, M.; Ohshima, J.; Ohsawa, E.; Maruyama, H.B. In vitro potentiation of cephalosporins by alafosfalin against urinary tract bacteria. Antimicrob. Agents. Chemother. 1982, 21, 706–710. [Google Scholar] [CrossRef]
- Blanchard, E.J.; Graves, E.E. Phosphorylation of cellulose with some phosphonic acid derivatives. Text. Res. J. 2003, 73, 22–26. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Depczyński, R.; Andrijewski, G.; Drabowicz, J.; Łuczak, J. 1-(N-Acylamino)alkanephosphonates. Part IV. N-Acylation of 1-Aminoalkanephosphonic Acids . Pol. J. Chem. 2005, 79, 499–513. [Google Scholar]
- Kudzin, Z.H.; Depczyński, R.; Kudzin, M.H.; Drabowicz, J. 1-(N-chloroacetylamino)alkylphosphonic acids-synthetic precursors of phosphonopeptides. Amino Acids 2008, 34, 163–168. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Depczynski, R.; Kudzin, M.H.; Luczak, J.; Drabowicz, J. 1-(N-Trifluoroacetylamino)alkylphosphonic acids: Synthesis and properties. Amino Acids 2007, 33, 663–667. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Urbaniak, P.; Drabowicz, J. Phosphorylation of cellulose. In Proceedings of the 20th International Symposium Advances in the Chemistry of Heteroorganic Compounds, Łódź, Poland, 23–24 November 2017; p. 130. [Google Scholar]
- EN ISO 9237:1998–Textiles–Determination of the Permeability of Fabrics to Air; International Organization for Standardization: Geneva, Switzerland, 1998.
- EN ISO 10319:2015-08–Geosynthetics-Wide-Width Tensile Test; International Organization for Standardization: Geneva, Switzerland, 2015.
- EN ISO 20645:2006–Textile Fabrics–Determination of Antibacterial Activity—Agar Diffusion Plate Test; International Organization for Standardization: Geneva, Switzerland, 2006.
- Urbaniak-Domagala, W. The use of the spectrometric technique FTIR-ATR to examine the polymers surface. In Advanced Aspects of Spectroscopy; Farrukh, M.A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 85–112. [Google Scholar]
- Podstawka, E.; Andrzejak, M.; Kafarski, P.; Proniewicz, L.M. Comparison of adsorption mechanism on colloidal silver surface of alafosfalin and its analogs. J. Raman Spectrosc. 2008, 39, 1238–1249. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, F.; Han, F.; Prinyawiwatkul, W.; No, H.K.; Ge, B. Evaluation of diffusion and dilution methods to determine the antimicrobial activity of water-soluble chitosan derivatives. J. Appl. Microbiol. 2012, 114, 956–963. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
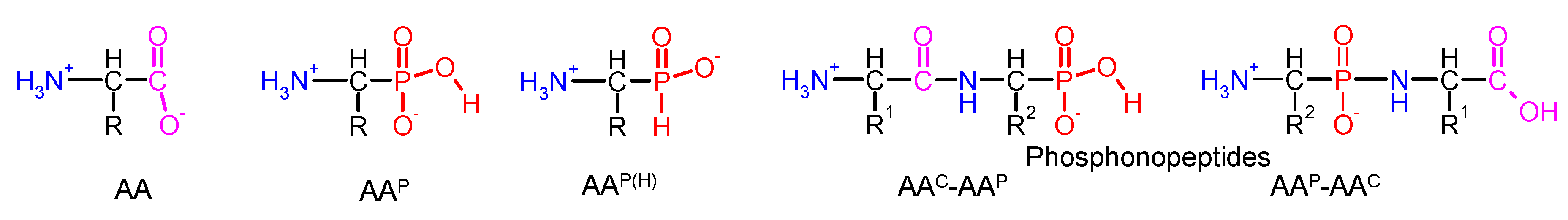

| Abbrev. | Action/Application | Tested Bacteria | Ref. |
|---|---|---|---|
| PP-NIPAAm-Cg-Cs | Membrane-wound healing enhancement | S. aureus | [5] |
| PP-g-AA-Cg-Cs | |||
| PP-g-AA-Cs-Cg | Various concentrations of heparin | S. aureus | [6] |
| PP-g-AA-Cs-Cg-Hi | |||
| PP-AAg-Cg-Cs | Anti-bacterial property and the effect on accelerating wound healing strong | S. aureus | [7,8] |
| PP-g-AA-PG-Cs | |||
| PP-g-PGMA | Antimicrobial activity; platelet and red blood cell adhesion | E. coli; S. aureus | [9] |
| PP-g-PHMG | |||
| PP-g-PNVP-PGMA | |||
| PP-g-PNVP-PHMG | |||
| PP-g-NIPAAm | Wound dressing applications; good biocompatibility to fibroblast cells | E. coli; S. aureus | [10] |
| PP-g-NIPAAm-Cs | |||
| (PP)-viscose-Cs-Gc(2%–2.5%) | Good biocompatibility; the correlation between efficiency in vitro and in vivo results, qualifies these antimicrobial biomaterials for clinical use | E. coli; S. aureus; Klebsiella aeruginosa; Candida albicans | [11] |
| PP-g-AA-Cg | Wound healing tests | Tests on rats | [12] |
| PP-g-AA-Cg-PNIPAAm | |||
| PP/PNIPAAm/Gelatin-hyaluronan-chodroitin-6-sulfate | Tri-layer membrane as the artificial skin for extensive burn injury | The wound dressing did not induce the tissue inflammatory or immune response. | [13] |
| PP-g-AA-NIPAAm | Potential wound dressing | Pseudomonas aeruginosa; S. aureus | [14] |
| PP-g-AA-NIPAAm-Cs | |||
| PP-g-AA-Cs | Potential wound dressing substitute for second-degree burns | Bioactivity assessments by aPTT; TT and fibrinogen concentration | [15] |
| PP-g-PLLA-Oct-HSA | Active dressings for the treatment of wounds | E. coli; S. aureus | [16] |
| PP-g-PLLA-Oct | Medical dressings specific to infected wounds | E. coli; S. aureus | [17] |
| Abbrev. (Name) | Structure | Action/Application | Ref. |
|---|---|---|---|
| GlyP (phosphoglycine) | 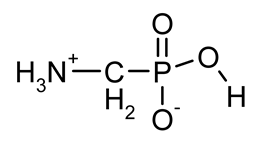 | Primary degradation product of glyphosate, inhibitor of prostate cancer cell growth in vitro, phytotoxin | [18,29,30,31,32,33] |
| β-AlaP (β-phosphono-alanine, 2-AEP, ciliatine) | 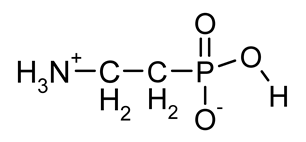 | The most widespread, first AAP isolated in natural sources | [18,34,35,36] |
| GluγP(Me) (phosphinothricin, PPT) |  | A naturally occurring broad-spectrum systemic herbicide produced by several species of Streptomyces; inhibiting glutamine synthetase | [18,37] |
| PMG (PhosphonoMethyl-Glycine; Glyphosate) | 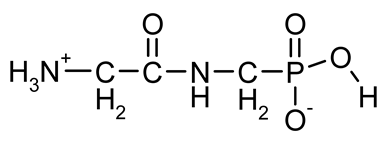 | A broad spectrum, non-selective systemic herbicide, inhibiting the activity of 5-enolpyruvyl-shikimic acid-3-phosphate synthase | [18,38,39] |
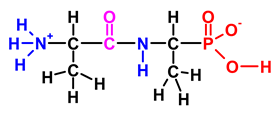
| Ala-AlaP (alaphosphin; alafosfalin) | 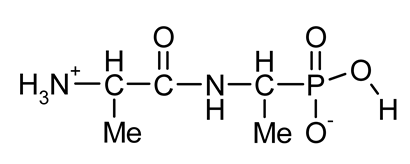 | Ala-AlaP selectively inhibited peptidoglycan biosynthesis in both Gram-negative and Gram-positive bacteria. The mechanism of action of Ala-AlaP involve three stages: (i) active transport by peptide permeases; (ii) intracellular peptidase cleavage; and (iii) action of Ala-AlaP on alanine racemase (EC 5.1.1.1) | [18,40,41,42,43,44,45,46,47,48,49] |
| Applied names are in accordance with the general rules elaborated by Kudzin et al. [24,25]. | |||
| Processing Parameters | |
|---|---|
| Temperature of the extruder in zone 1 | 240 °C |
| Temperature of the extruder in zone 2 | 285 °C |
| Temperature of the extruder in zone 3 | 290 °C |
| Head temperature | 240 °C |
| Air heater temperature | 270 °C |
| Air flow rate | 7–8 m3/h |
| Polymer yields | 4 g/min |
| Mass per unit area of nonwovens | 80 g/m2 |
| Styrene-Acrylic Resin | Wetting Agent | Thickening Agent | Water |
|---|---|---|---|
| 5 g | 5 g | 1 g | 89 g |
| 5% | 5% | 1% | 89% |
| Polypropylene-IR according to Urbaniak-Domagala, 2012 [58] | |||||||||
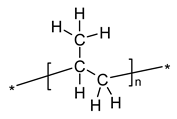 | |||||||||
| Vibrations | |||||||||
| Wave number (cm−1) | 2916 | 2959 | 2881 | 2841 | 1460 | 1376 | 1357 | 1170, 1153 | 975, 899 |
| Vibration type (group) | ν(CH2) | ν(CH3) | ν(CH3) | ν(CH2) | δ(CH3) | δ(CH3) | γ(CH2–CH) | γ(CH3), δ(CH2), δ(CH) | γ(CH3), ν(CH2), ν(CH) |
| Ala-AlaP-IR according toPodstawka et al., 2008 [59] | |||||||||
 | |||||||||
| Vibrations | |||||||||
| Wave number, (cm−1) | 3273 | 3100 | 2982 | 2940, 2885 | 1664 | 1081 | 930 | 700 | 558 |
| Vibration type (group) | ν(N–H) | ν(C–H) | ν(CS–H) | ν(C–H)CH3 | ν(C=O),ν(C–N)A,Δ(CCA(=O)NA) | ν(C–N),ν(C–C)Ala,δ(CCH3)Ala,ρb(C–N+H3) | δ(C–C)Ala,ν(C–N+H3) | ν(CS–P), ν(C–CA) | Δ(NACS(P) C) δ(NACS(P) C) |
| Ala-AlaP/PP | |||||||||
| Wave number, (cm−1) | 3273 | – | – | – | 1664 | 1081 | 930 | 700 | 558 |
| Vibration type (group) | ν(N–H) | – | – | – | ν(C=O), ν(C–N)A,Δ((CCA(=O)NA) | ν(C–N), ν(C–C)Ala,δ(CCH3),ρb(C–N+H3) | δ(C–C)Ala, ν(C–N+H3) | ν(CS–P), ν(C–CA) | Δ(NACS(P)C) δ(NACS(P)C) |
| Parameter | PP | PP-Ala-AlaP (%Ala-AlaP Paste Concentrations) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 5 | 10 | 15 | |||
| Average air permeability (mm/s), pressure decrease: | 100 Pa | 250 | 196 | 198 | 185 | 196 | 193 | 191 |
| 200 Pa | 482 | 369 | 355 | 322 | 351 | 360 | 355 | |
| Parameter | PP | PP-Ala-AlaP (%Ala-AlaP Paste Concentrations) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 5 | 10 | 15 | ||
| Tensile strength (kN/m) | 0.32 | 0.40 | 0.41 | 0.39 | 0.38 | 0.37 | 0.37 |
| Relative elongation at maximum load (%) | 35.0 | 38.7 | 38.1 | 38.2 | 37.5 | 37.3 | 37.2 |
| Sample No. | Ala-AlaP on Polypropylene Nonwovens [Ala-AlaP/PP] | Average Inhibition Zone (ZoI) of Bacterial Growth for Bacteria (mm) | |
|---|---|---|---|
| Ala-AlaP Coating Pastes Concentrations (%) | E. coli | S. aureus | |
| 1 | 0 | 0 | 0 |
| 2 | 0.005 | 0 | 0 |
| 3 | 0.01 | 12 | 0 |
| 4 | 0.05 | 12 | 12 |
| 5 | 0.1 | 14 | 14 |
| 6 | 0.15 | 14 | 14 |
| Surface of applied disks with 1 cm diameter equals 0.785 cm2 Concentration of inoculum (bacterial suspension) amount of live bacteria CFU/mL = 0.4 × 108 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudzin, M.H.; Mrozińska, Z.; Walawska, A.; Sójka-Ledakowicz, J. Biofunctionalization of Textile Materials.1. Biofunctionalization of Poly(Propylene) (PP) Nonwovens Fabrics by Alafosfalin. Coatings 2019, 9, 412. https://doi.org/10.3390/coatings9070412
Kudzin MH, Mrozińska Z, Walawska A, Sójka-Ledakowicz J. Biofunctionalization of Textile Materials.1. Biofunctionalization of Poly(Propylene) (PP) Nonwovens Fabrics by Alafosfalin. Coatings. 2019; 9(7):412. https://doi.org/10.3390/coatings9070412
Chicago/Turabian StyleKudzin, Marcin H., Zdzisława Mrozińska, Anetta Walawska, and Jadwiga Sójka-Ledakowicz. 2019. "Biofunctionalization of Textile Materials.1. Biofunctionalization of Poly(Propylene) (PP) Nonwovens Fabrics by Alafosfalin" Coatings 9, no. 7: 412. https://doi.org/10.3390/coatings9070412
APA StyleKudzin, M. H., Mrozińska, Z., Walawska, A., & Sójka-Ledakowicz, J. (2019). Biofunctionalization of Textile Materials.1. Biofunctionalization of Poly(Propylene) (PP) Nonwovens Fabrics by Alafosfalin. Coatings, 9(7), 412. https://doi.org/10.3390/coatings9070412





