The Relationship between Solid Content and Particle Size Ratio of Waterborne Polyurethane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Small-Particle Waterborne Polyurethane (WPU-S)
2.3. Preparation of Large-Particle Waterborne Polyurethane (WPU-L) and High Solid Content Waterborne Polyurethane (WPU-H)
2.4. Preparation of WPU Films
3. Characterizations
4. Results and Discussion
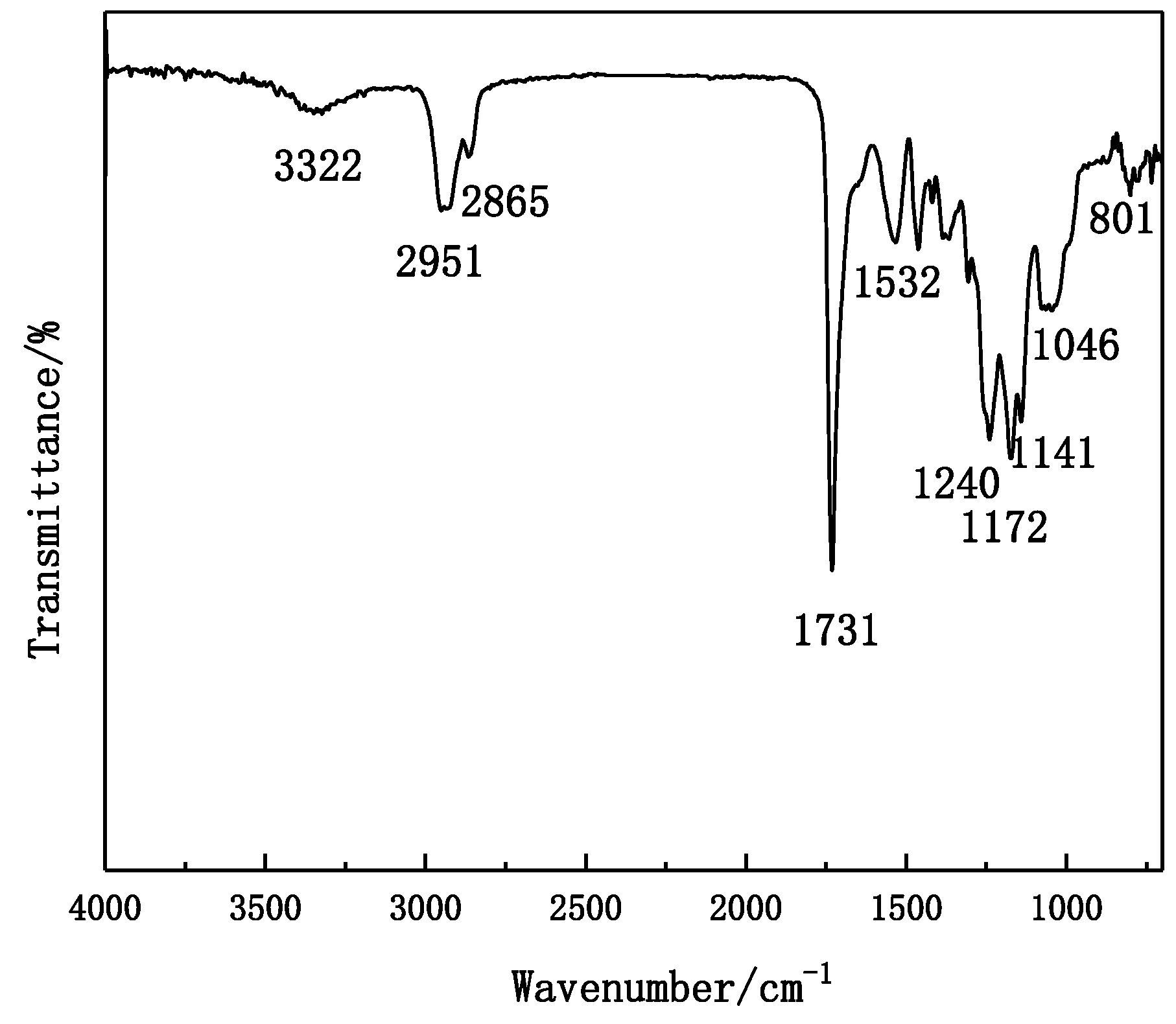
4.1. FTIR Spectra of WPU
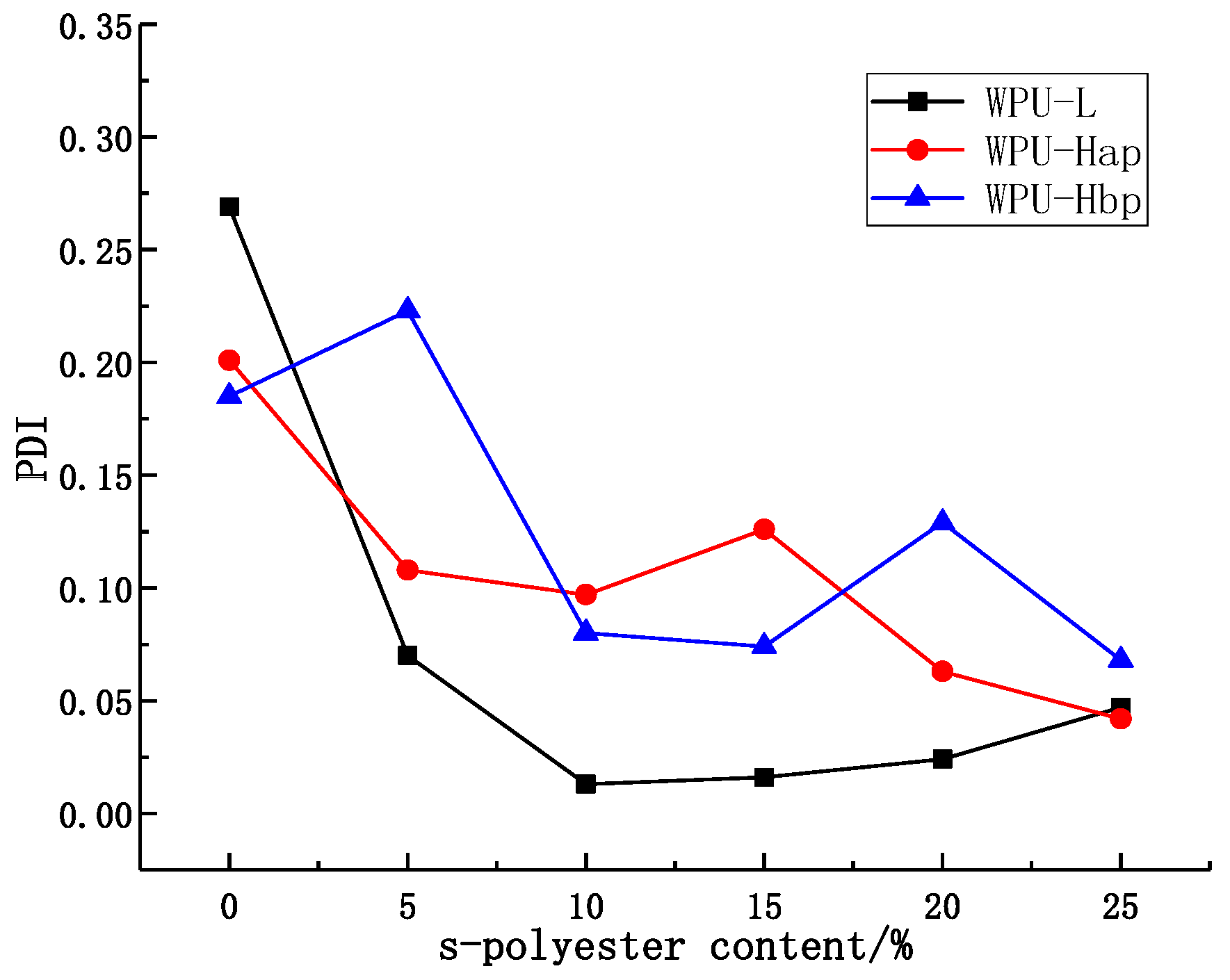
4.2. Particle Size of Emulsions
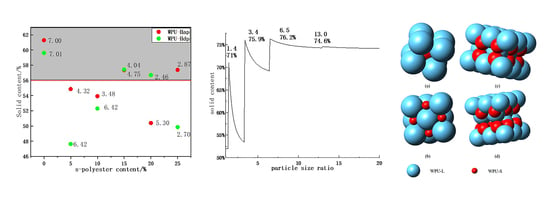
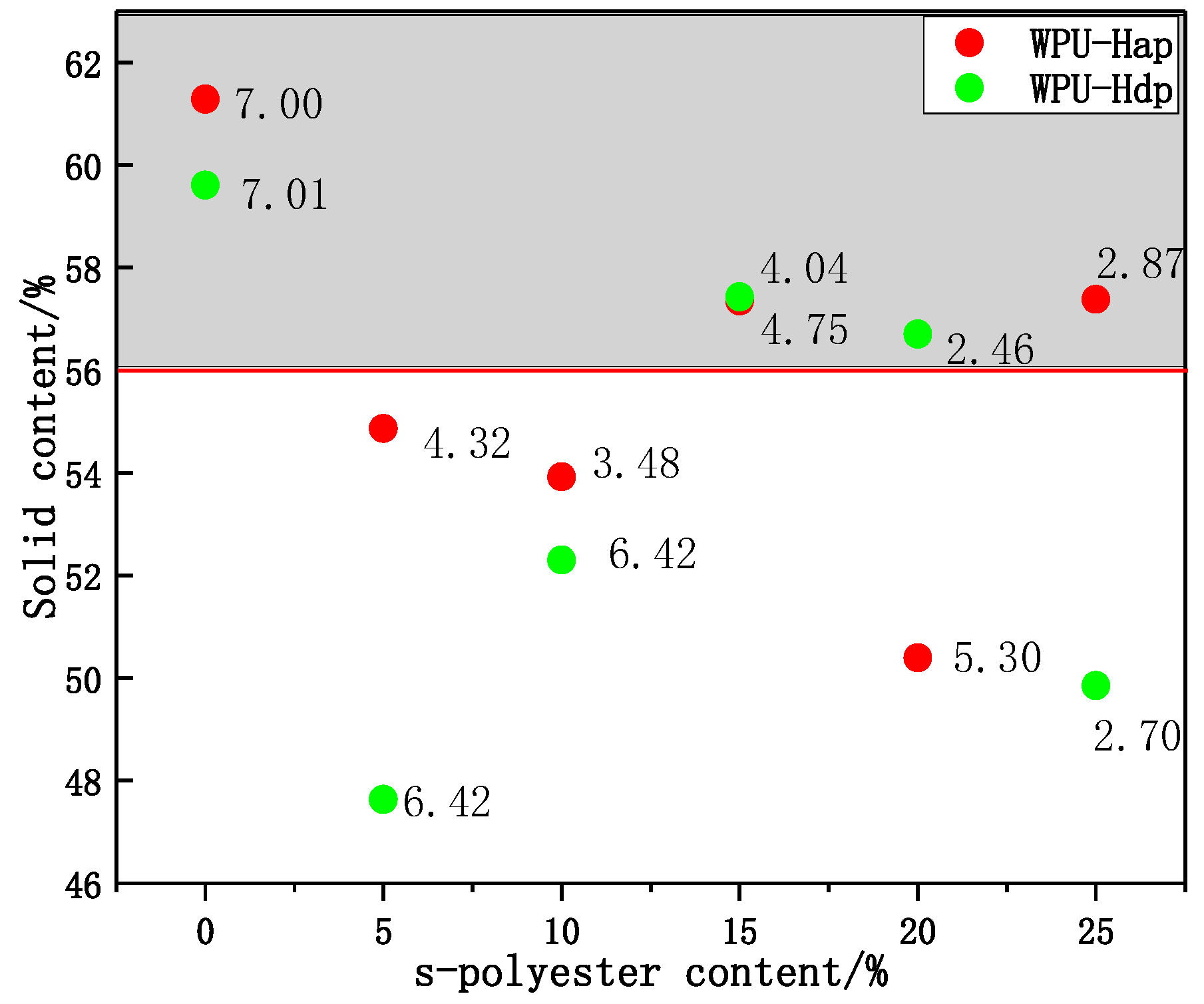
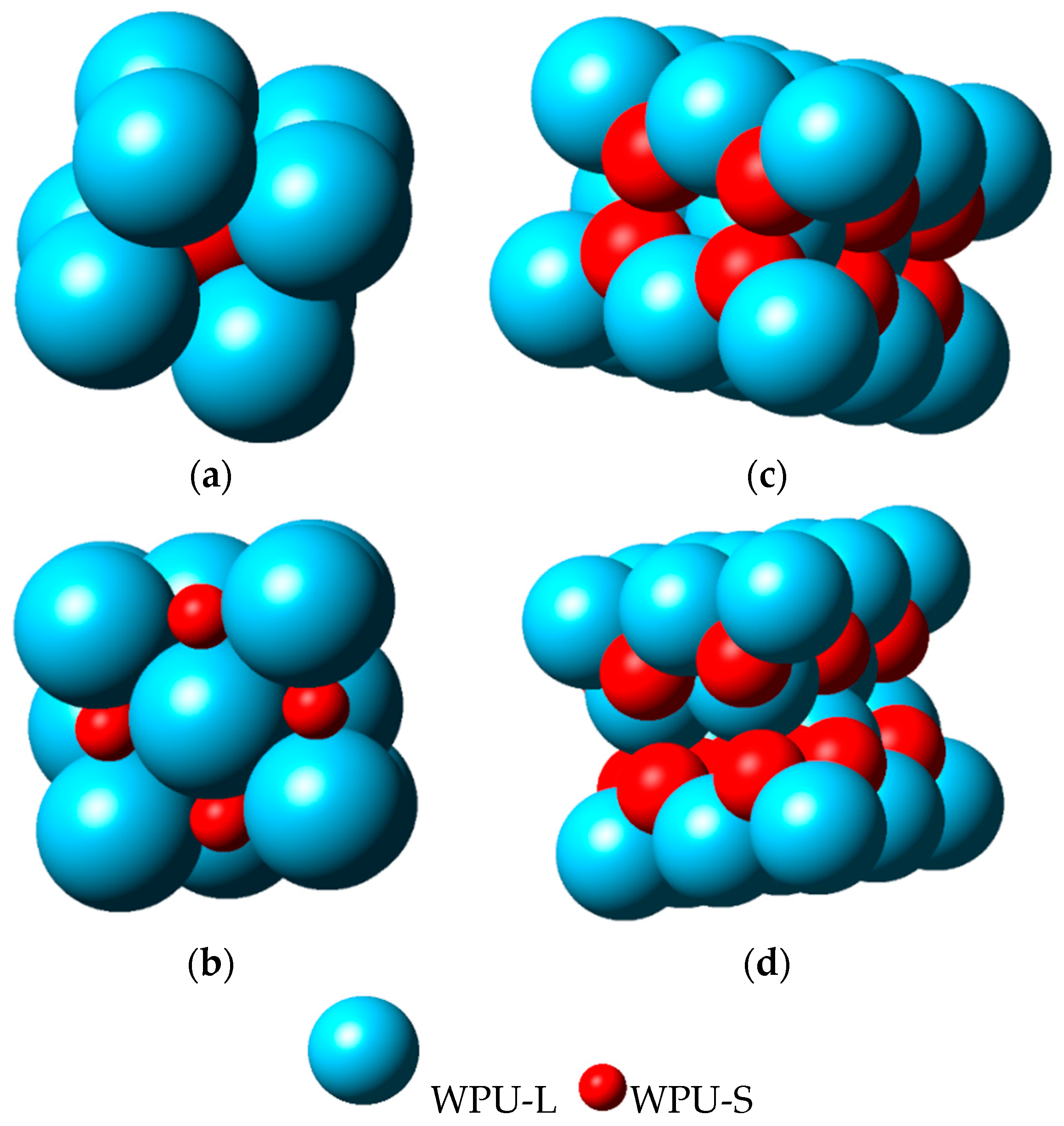
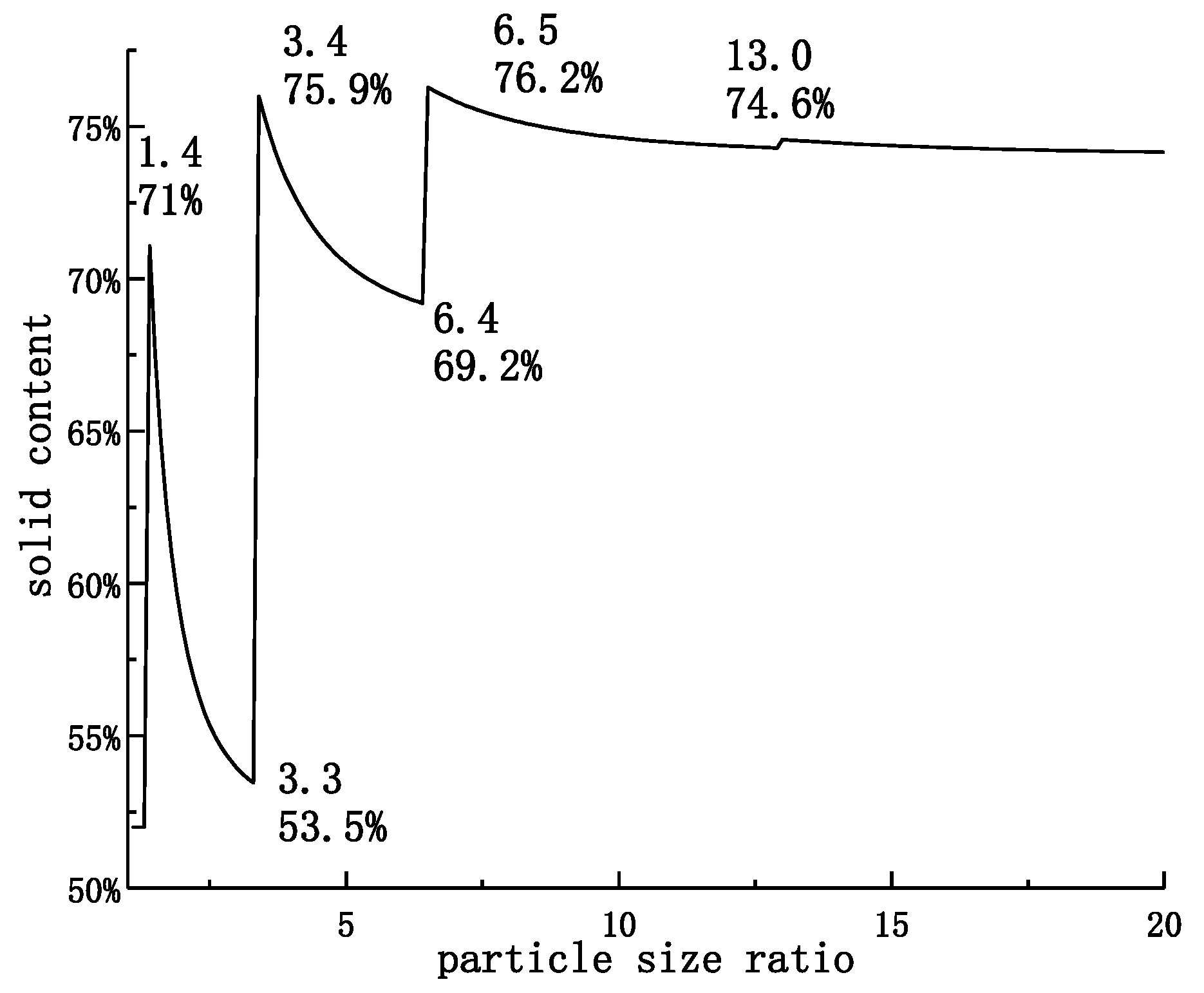
4.3. The Relationship between Solid Content and Particle Size Ratio
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; Vatanparast, R.; Lemmetyinen, H. Cross-linking kinetics and swelling behaviour of aliphatic polyurethane. Polymer 2000, 41, 5571–5576. [Google Scholar] [CrossRef]
- Lee, D.K.; Yang, Z.D.; Tsai, H.B.; Tsai, R.-S. Polyurethane dispersions derived from polycarbonatediols and m-di(2-isocyanatopropyl) benzene. Polym. Eng. Sci. 2009, 49, 2264–2268. [Google Scholar] [CrossRef]
- Yang, Z.; Wicks, D.A.; Hoyle, C.E.; Pu, H.; Yuan, J.; Wan, D.; Liu, Y. Newly UV-curable polyurethane coatings prepared by multifunctional thiol- and ene-terminated polyurethane aqueous dispersions mixtures: Preparation and characterization. Polymer 2009, 50, 1717–1722. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Otaigbe, J.U. Recent advances in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and films. Prog. Polym. Sci. 2009, 34, 1283–1332. [Google Scholar] [CrossRef]
- Puszka, A. Thermal and mechanical behavior of new transparent thermoplastic polyurethane elastomers derived from cycloaliphatic diisocyanate. Polymers 2018, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Puszka, A.; Kultys, A. New thermoplastic polyurethane elastomers based on aliphatic diisocyanate: Synthesis and characterization. J. Therm. Anal. Calorim. 2017, 128, 407. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, E.Y.; Kang, Y.S.; Kim, B.K. High solid and high performance UV cured waterborne polyurethanes. Coll. Surf. A 2010, 370, 58–63. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, B.K. High solid and high stability waterborne polyurethanes via ionic groups in soft segments and chain termini. J. Coll. Interface Sci. 2009, 336, 208–214. [Google Scholar] [CrossRef]
- Park, S.H.; Chung, I.D.; Hartwig, A.; Kim, B.K. Hydrolytic stability and physical properties of waterborne polyurethane based on hydrolytically stable polyol. Coll. Surf. A 2007, 305, 126–131. [Google Scholar] [CrossRef]
- Morsi, S.M.M.; Mohamed, H.A. A comparative study of new linear and hyperbranched polyurethanes built up from a synthesized isocyanate-terminated polyester/urethane. Polym. Bull. 2017, 74, 5011–5027. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Morsi, S.M.M.; Badran, B.M.; Rabie, A.M. Polyurethane/aromatic polyamide sulfone copolymer dispersions from transesterified castor oil. Polym. Bull. 2017, 74, 531–554. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Badran, B.M.; Rabie, A.M.; Morsi, S.M.M. Synthesis and characterization of aqueous (polyurethane/aromatic polyamide sulfone) copolymer dispersions from castor oil. Prog. Org. Coat. 2014, 77, 965–974. [Google Scholar] [CrossRef]
- Yang, J.-E.; Kong, J.-S.; Park, S.-W.; Lee, D.-J.; Kim, H.-D. Preparation and properties of waterborne polyurethane–urea anionomers. I. The influence of the degree of neutralization and counterion. J. Appl. Polym. Sci. 2002, 86, 9. [Google Scholar] [CrossRef]
- Athawale, V.D.; Nimbalkar, R.V. Emulsifyable air drying urethane alkyds. Prog. Org. Coat. 2010, 67, 66–71. [Google Scholar] [CrossRef]
- Li, Q.A.; Sun, D.C. Synthesis and characterization of high solid content aqueous polyurethane dispersion. J. Appl. Polym. Sci. 2007, 105, 2516–2524. [Google Scholar] [CrossRef]
- Cao, G.; Xia, Z.; Lei, L.; Zhang, Y.; Xing, J. Crystallinity evolution of soft segments during the synthesis of polyester-based waterborne polyurethane. J. Appl. Polym. Sci. 2014, 131, 245–253. [Google Scholar] [CrossRef]
- Chai, C.; Ma, Y. Preparation and performance of waterborne polyurethane with different particle sizes. Trans. Beijing Inst. Technol. 2018, 38, 417–422. (In Chinese) [Google Scholar]
- Peng, S.-J.; Jin, Y.; Cheng, X.-F.; Sun, T.-B.; Qi, R.; Fan, B.-Z. A new method to synthesize high solid content waterborne polyurethanes by strict control of bimodal particle size distribution. Prog. Org. Coat. 2015, 86, 1–10. [Google Scholar] [CrossRef]
- Furtado, C.A.; de Souza, P.P.; Goulart Silva, G.; Matencio, T.; Pernaut, J.M. Electrochemical behavior of polyurethane ether electrolytes/carbon black composites and application to double layer capacitor. Electrochim. Acta 2001, 46, 1629–1634. [Google Scholar] [CrossRef]
- Chai, C.; Ma, Y.; Li, G.; Ge, Z.; Ma, S.; Luo, Y. The preparation of high solid content waterborne polyurethane by special physical blending. Prog. Org. Coat. 2018, 115, 79–85. [Google Scholar] [CrossRef]


| Sample Designation | IPDI/g | DMPA/g | AAS/g | s-polyester/g | PHNA/g | TEA/g | BDO/g | Water/g |
|---|---|---|---|---|---|---|---|---|
| WPU-S1 | 12.45 | 0 | 4.52 | 4.0 | 36.0 | 0 | 1.80 | 25.19 |
| WPU-S2 | 18.69 | 2.58 | 0 | 0.9 | 39.1 | 1.94 | 1.20 | 27.6 |
| Sample Designation | IPDI/g | DMPA/g | PHNA/g | s-Polyester/g | TEA/g | BDO/g |
|---|---|---|---|---|---|---|
| WPU-L1 | 14.90 | 1.15 | 40.0 | 0 | 0.86 | 1.20 |
| WPU-L2 | 14.90 | 1.15 | 38.0 | 2.0 | 0.86 | 1.20 |
| WPU-L3 | 14.90 | 1.15 | 36.0 | 4.0 | 0.86 | 1.20 |
| WPU-L4 | 14.90 | 1.15 | 34.0 | 6.0 | 0.86 | 1.20 |
| WPU-L5 | 14.90 | 1.15 | 32.0 | 8.0 | 0.86 | 1.20 |
| WPU-L6 | 14.90 | 1.15 | 30.0 | 10.0 | 0.86 | 1.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Ma, Y.; Zhang, Z.; Yang, X.; Huang, M.; Chai, C. The Relationship between Solid Content and Particle Size Ratio of Waterborne Polyurethane. Coatings 2019, 9, 401. https://doi.org/10.3390/coatings9060401
Hou J, Ma Y, Zhang Z, Yang X, Huang M, Chai C. The Relationship between Solid Content and Particle Size Ratio of Waterborne Polyurethane. Coatings. 2019; 9(6):401. https://doi.org/10.3390/coatings9060401
Chicago/Turabian StyleHou, Jinghui, Yifei Ma, Zihan Zhang, Xuanhe Yang, Muhua Huang, and Chunpeng Chai. 2019. "The Relationship between Solid Content and Particle Size Ratio of Waterborne Polyurethane" Coatings 9, no. 6: 401. https://doi.org/10.3390/coatings9060401
APA StyleHou, J., Ma, Y., Zhang, Z., Yang, X., Huang, M., & Chai, C. (2019). The Relationship between Solid Content and Particle Size Ratio of Waterborne Polyurethane. Coatings, 9(6), 401. https://doi.org/10.3390/coatings9060401