Numerical Simulation of Thermal Evolution and Solidification Behavior of Laser Cladding AlSiTiNi Composite Coatings
Abstract
:1. Introduction
2. Materials and Experiment
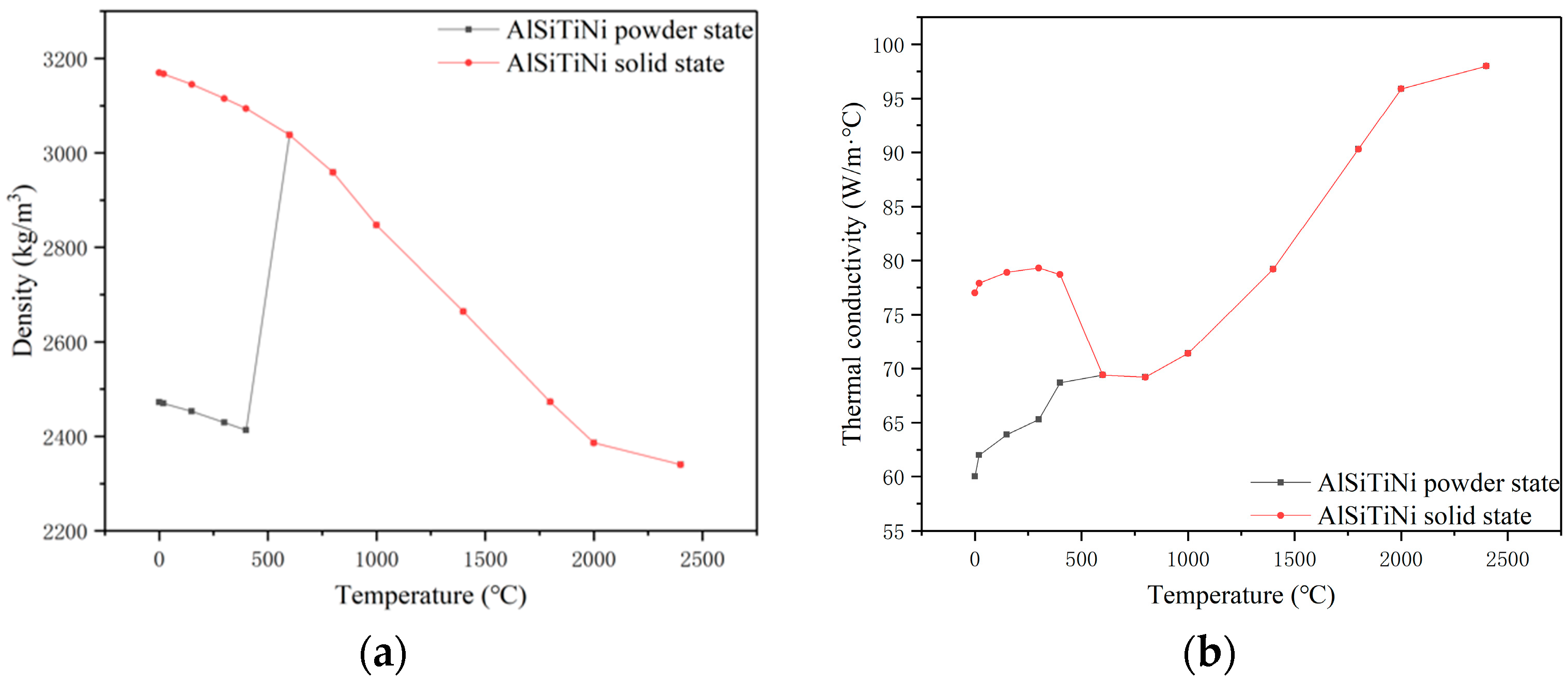
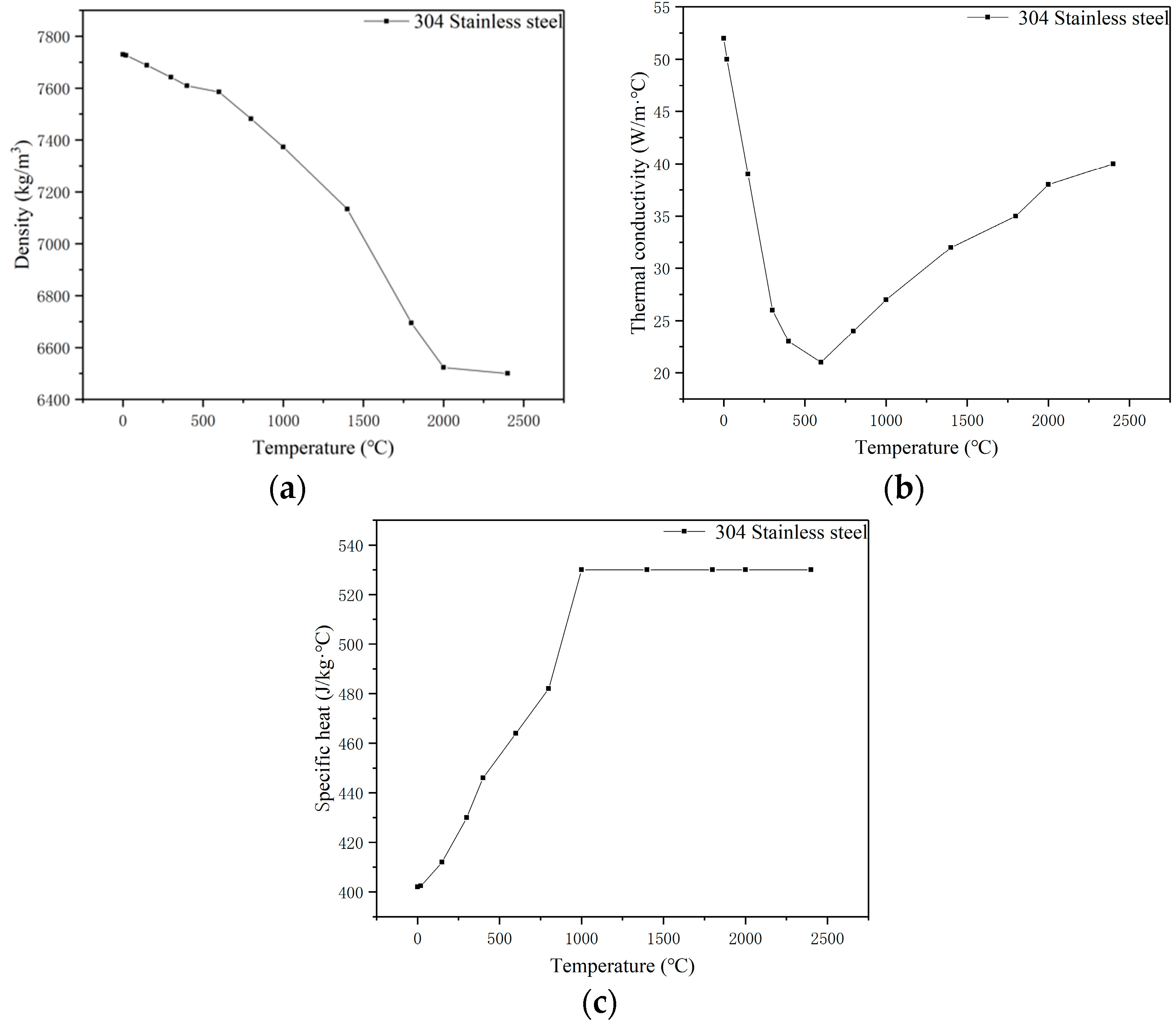
2.1. Material Properties
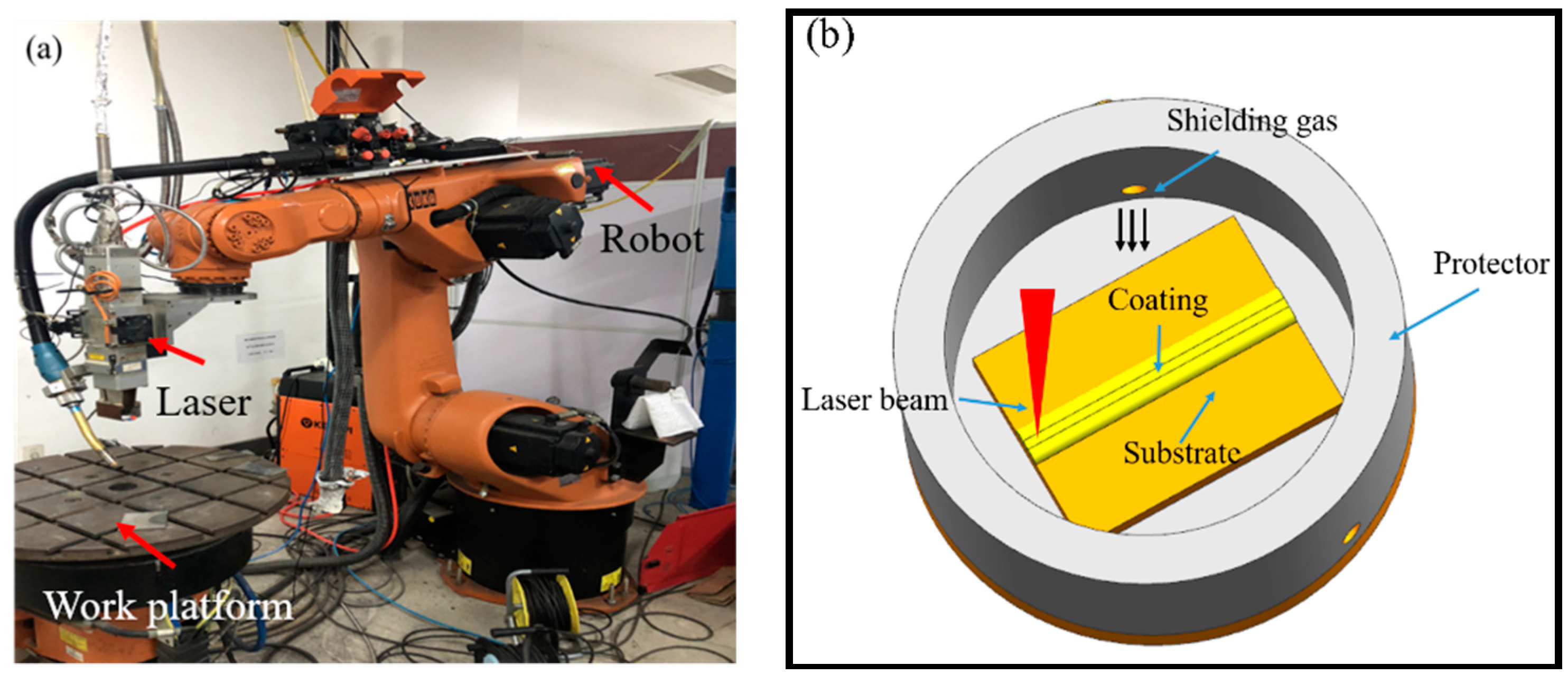
2.2. Experimental
3. FEM Model
3.1. Thermal Analysis
3.2. Heat Source Model
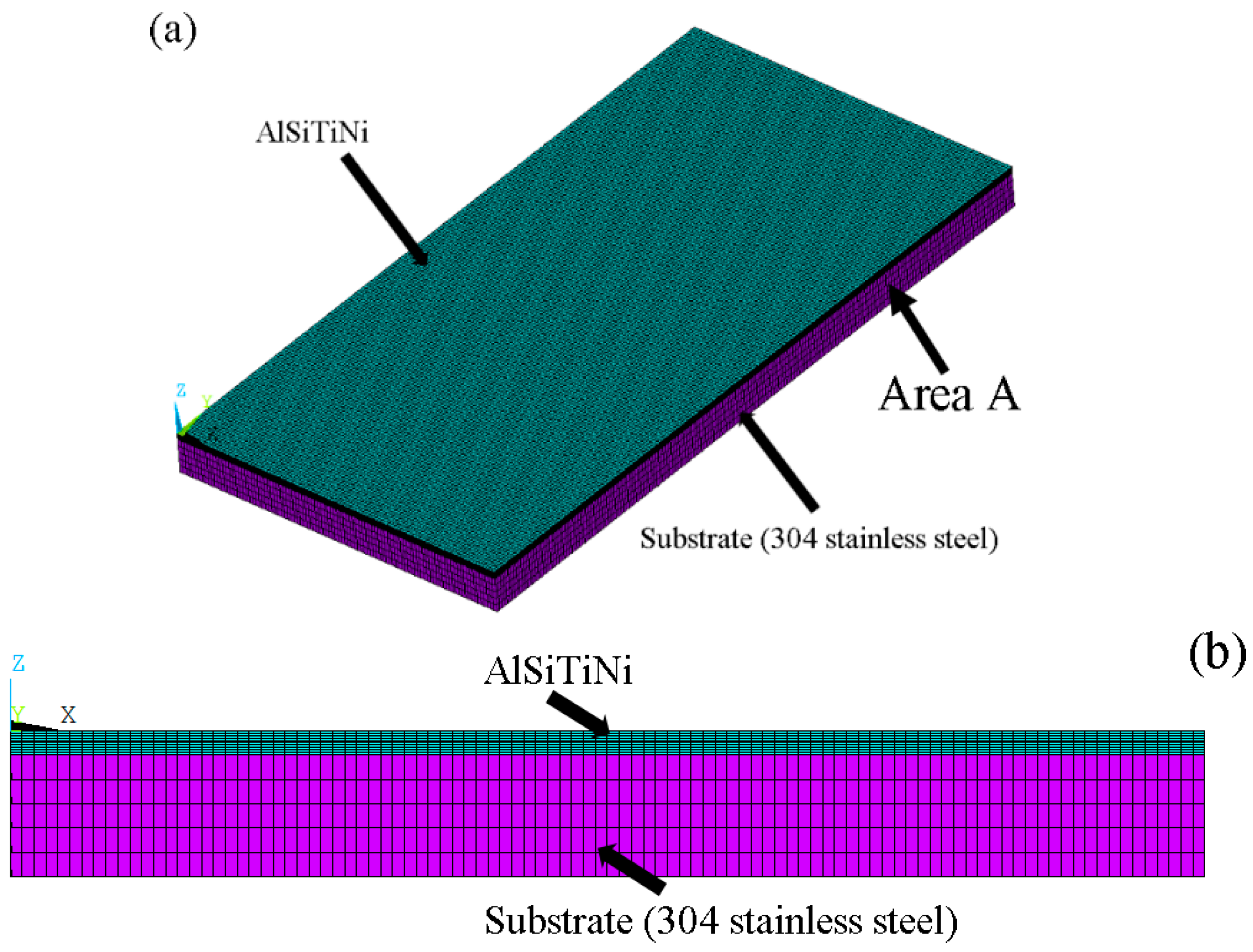
3.3. Setup of Model
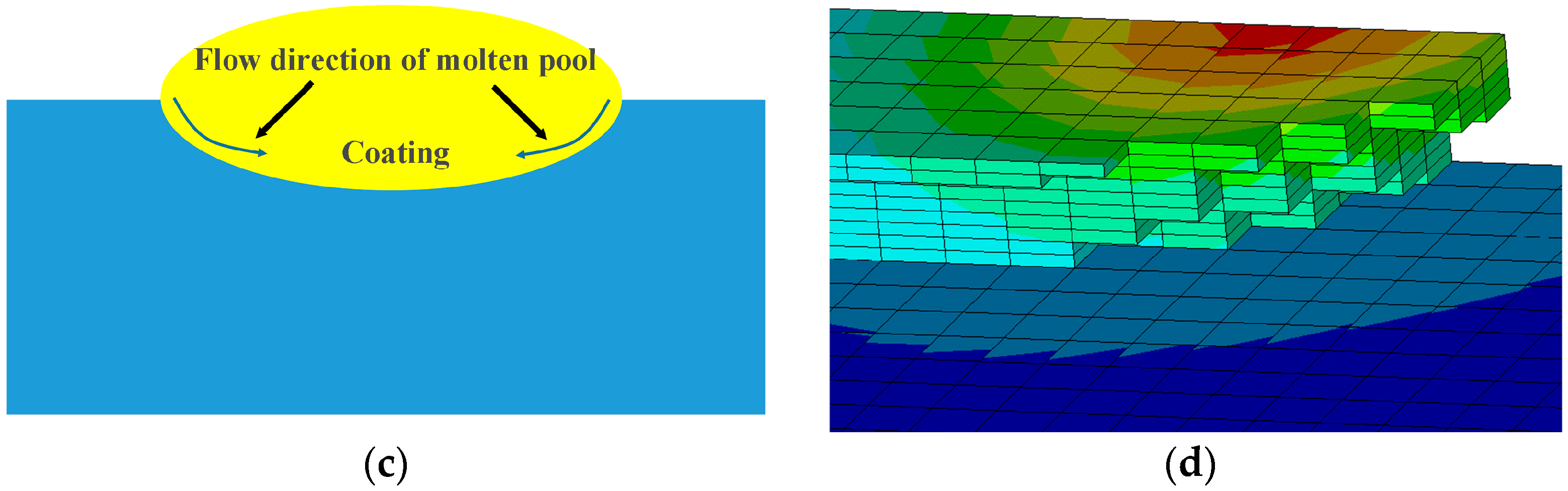
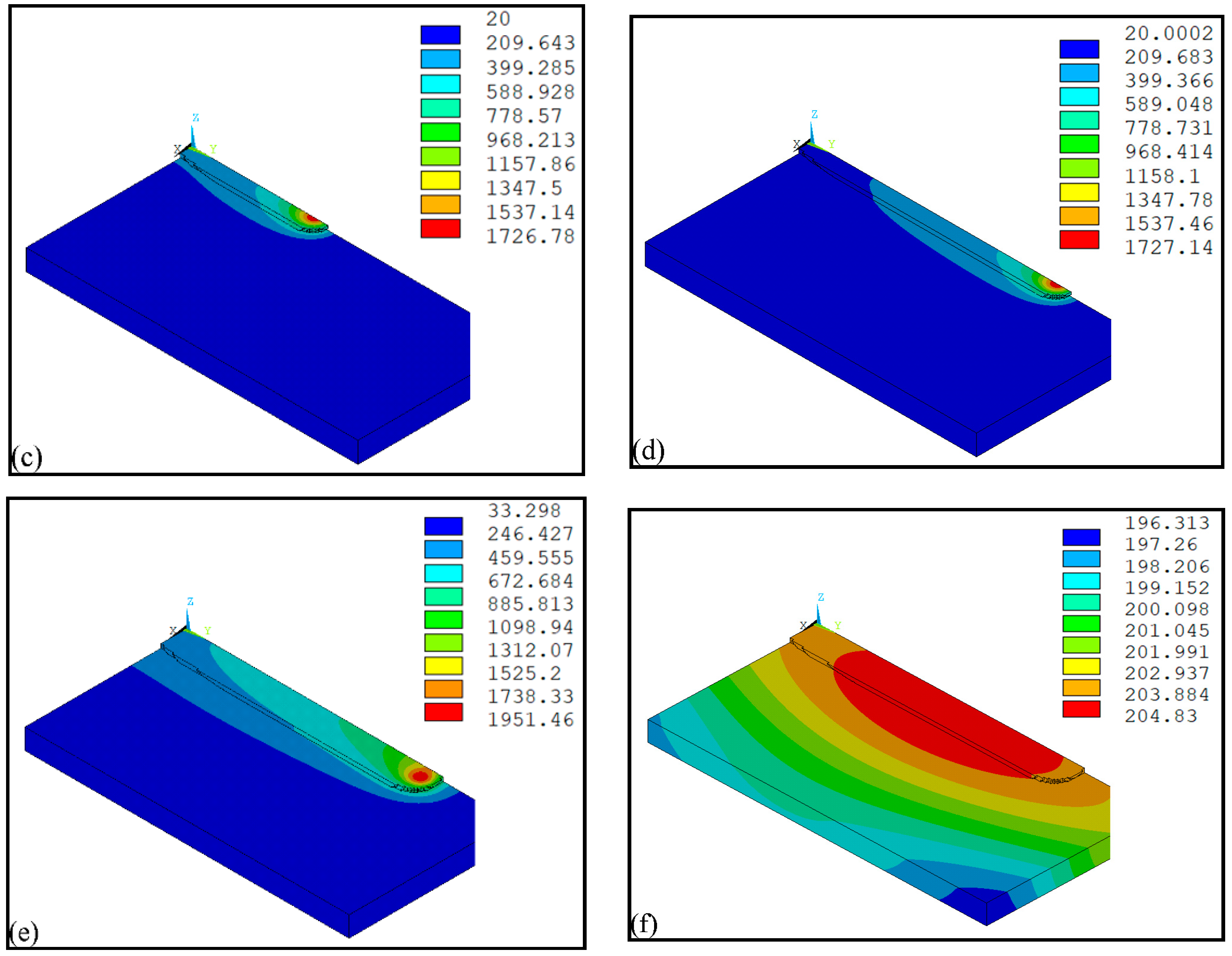
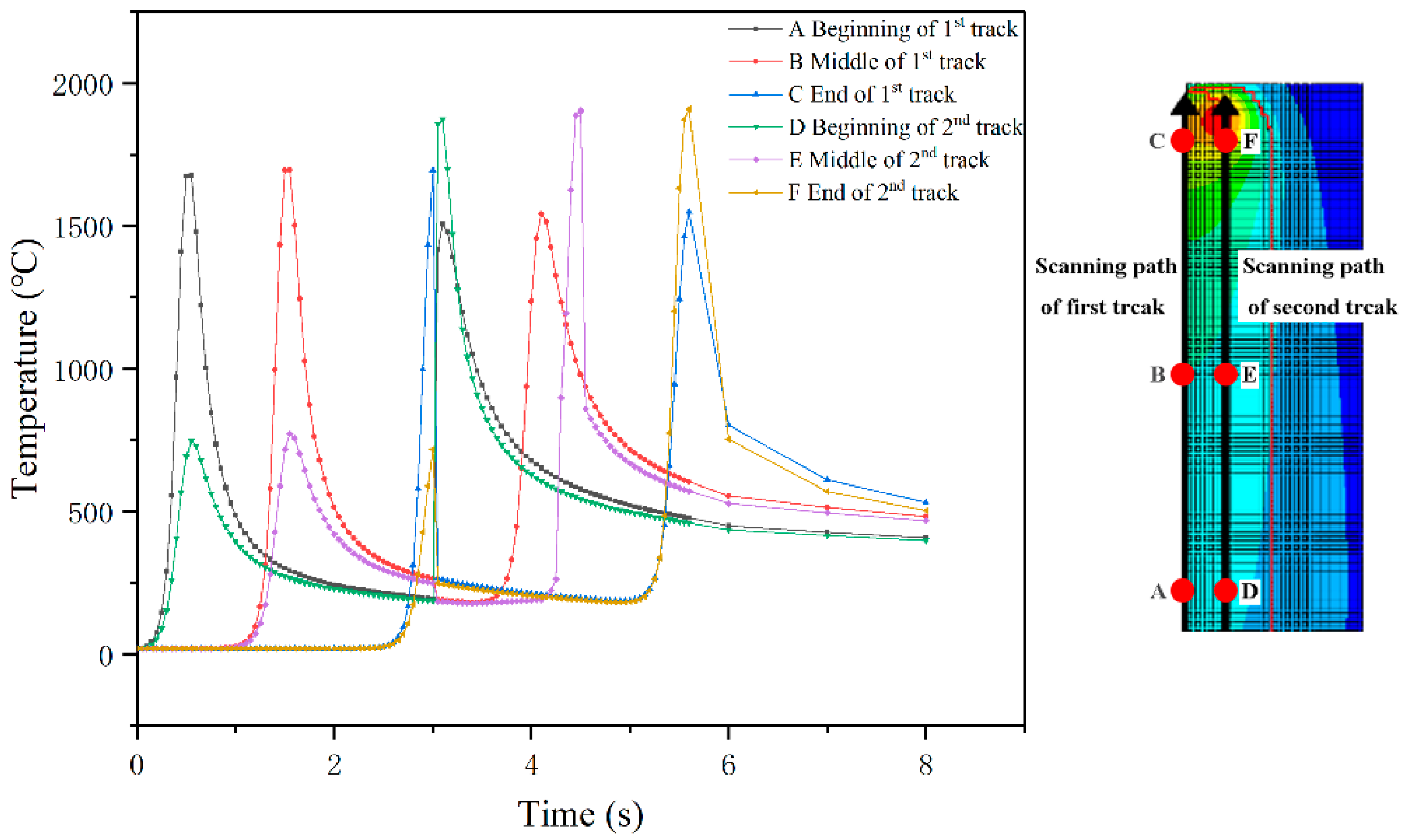
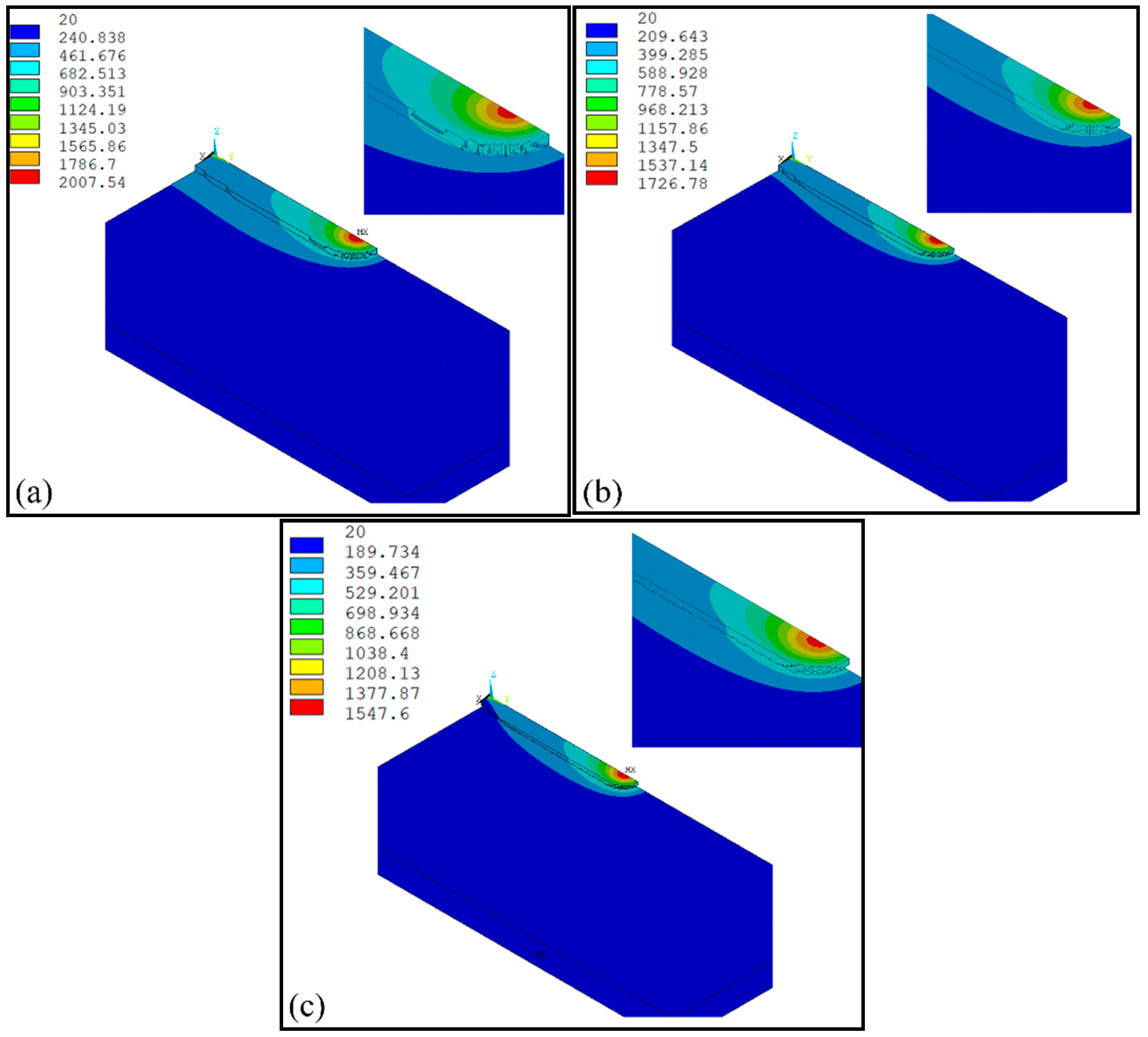
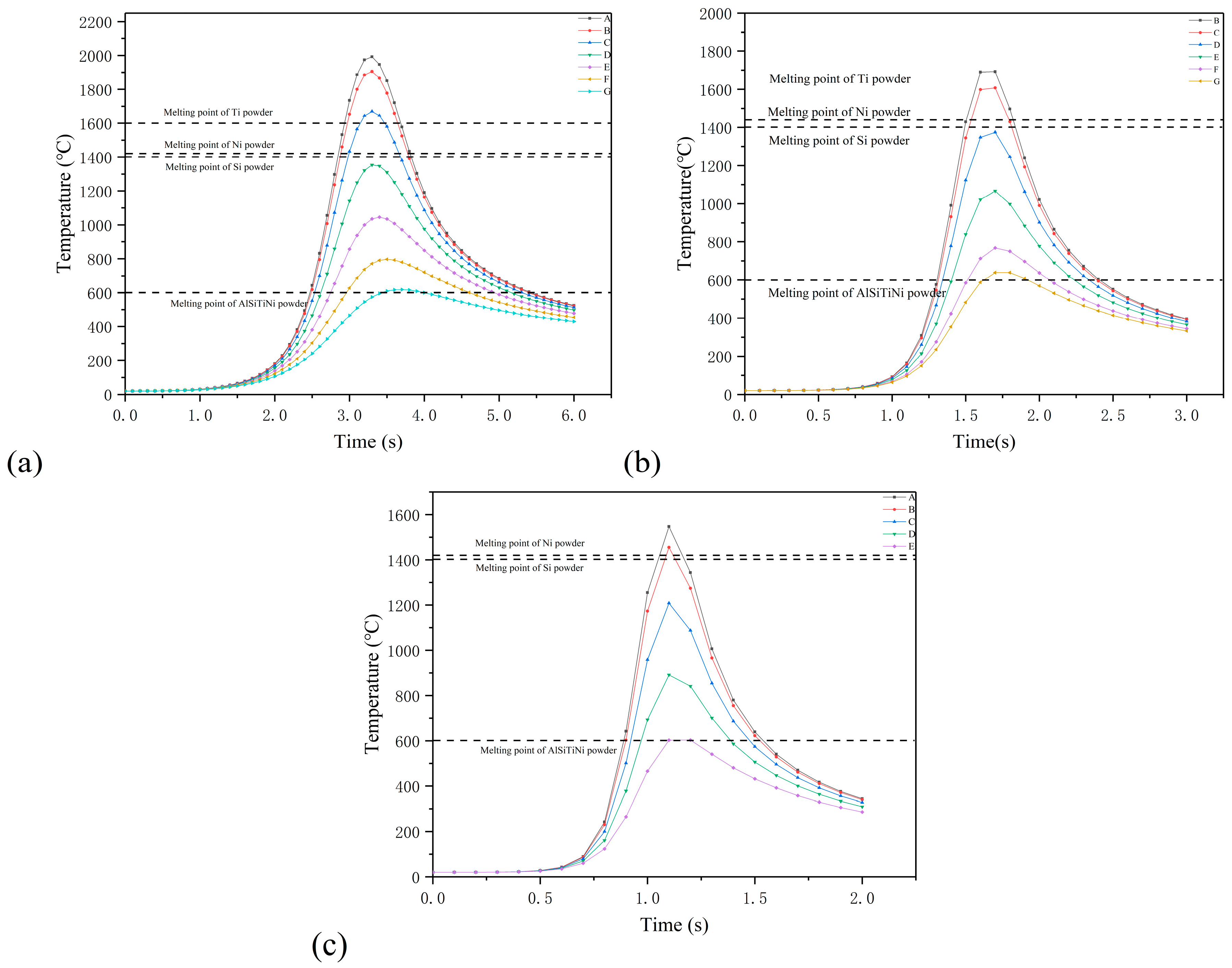
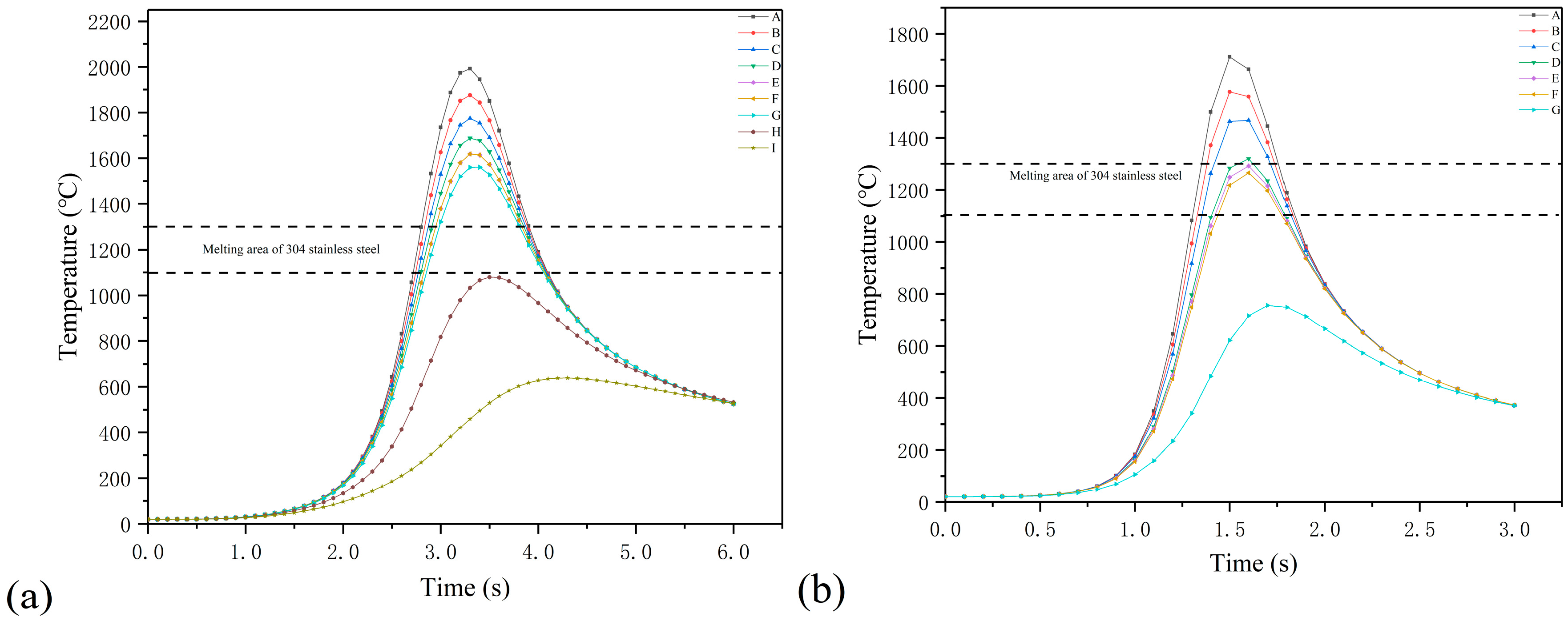
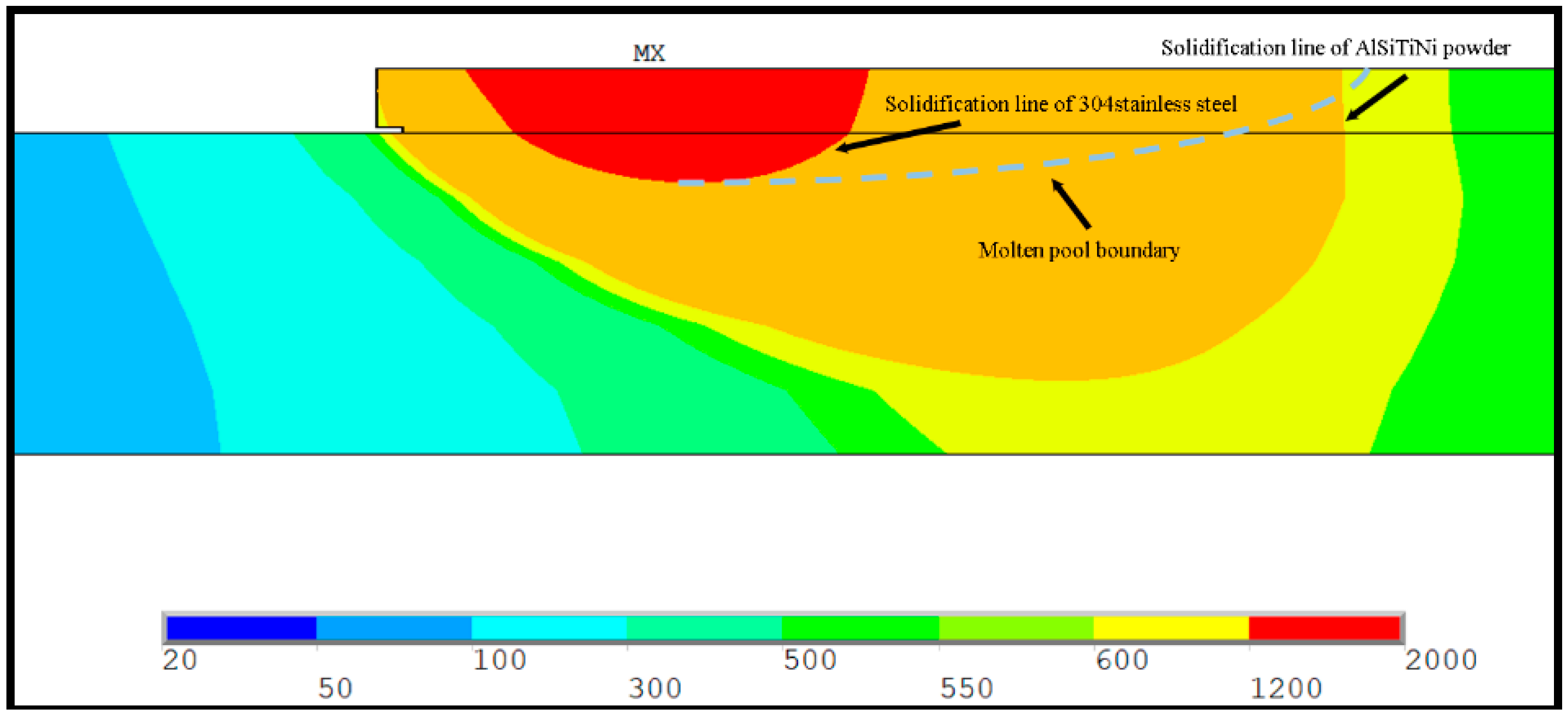
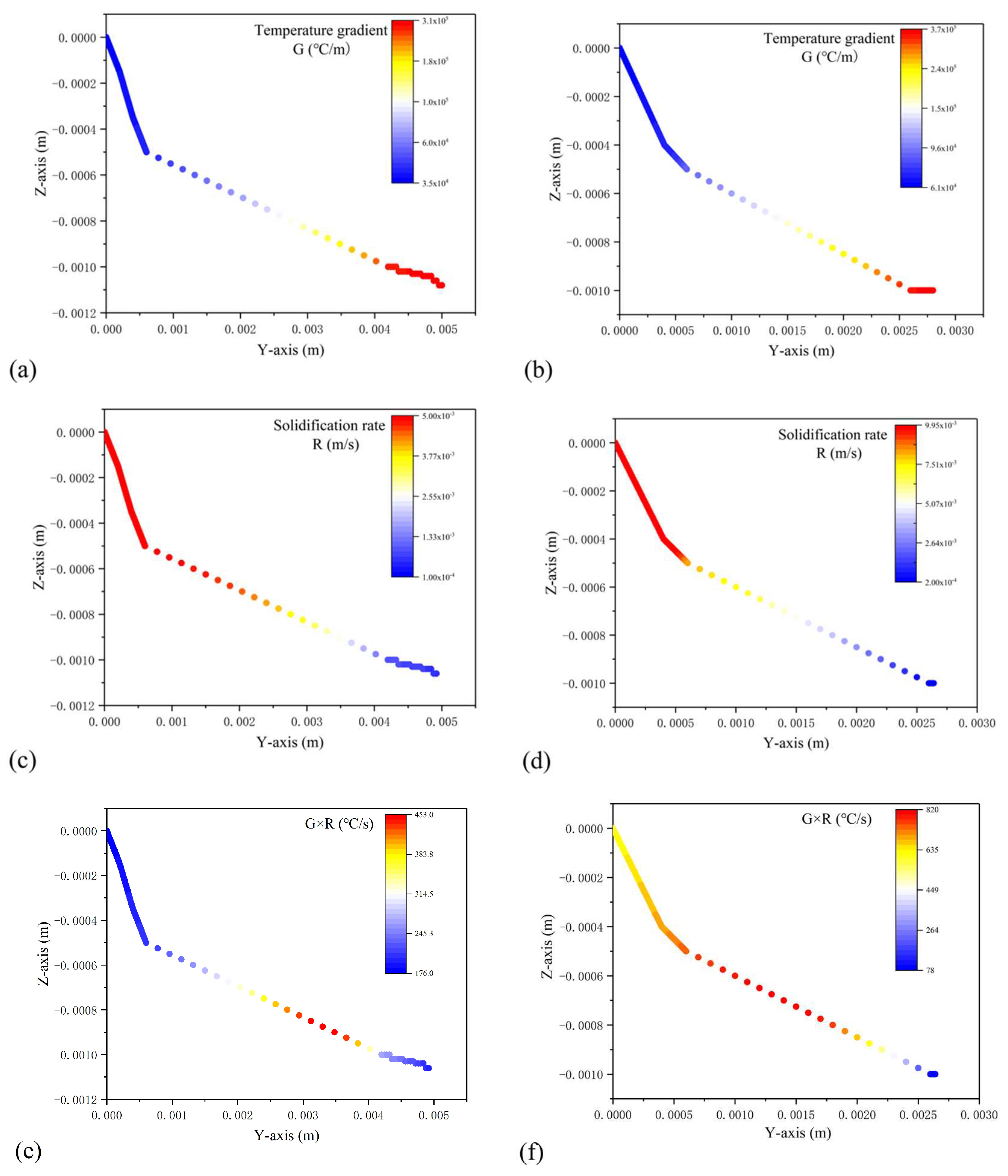
4. Result and Discussion
5. Conclusions
- By combining the planar Gauss heat source and the temperature selection judgment mechanism, the numerical model built in this paper was used to simulate the melting and solidification process and geometry of the AlSiTiNi coatings during the laser cladding process. The predicted geometry of the cladding coatings basically agrees with the experimental observations.
- The temperature evolution of both single-track and multi-track cladding was simulated using this model. The simulation results show that there is a 0.4 mm variation in width between the front and rear of the cladding coatings. For the multi-track cladding, a 0.2 mm increase in the width of the second track was observed compared with the previous track.
- The differences between the initial boundary conditions of the first and second cladding tracks were considered using the temperature selection judgment mechanism. During the laser cladding process, the temperature distribution of the remelting zone of the multi-track cladding was found to be asymmetric.
- The maximum temperature gradient G was found at the bottom of the molten pool, while the minimum value was located at the surface of the cladding coating. The maximum solidification rate R was found at the surface of the cladding coating, and the minimum value was located at the bottom of the molten pool. Both G and R were found to increase as the scanning speed increased.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liverani, E.; Toschi, S.; Ceschini, L.; Fortunato, A. Effect of selective laser melting (SLM) process parameters on microstructure and mechanical properties of 316L austenitic stainless steel. J. Mater. Process. Technol. 2017, 249, 255–263. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Guo, Z. Wear resistance of machine tools’ bionic linear rolling guides by laser cladding. Opt. Laser Technol. 2017, 91, 55–62. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Z.; Zeng, X. A comparison on metallurgical behaviors of 316L stainless steel by selective laser melting and laser cladding deposition. Mater. Sci. Eng. A-Struct. 2017, 685, 265–273. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Wang, J. Effects of Ti addition on microstructure homogenization and wear resistance of wide-band laser clad Ni60/WC composite coatings. Int. J. Refract. Met. Hard Mater. 2017, 64, 225–233. [Google Scholar] [CrossRef]
- Huebner, J.; Kata, D.; Kusiński, J.; Rutkowski, P.; Lisa, J. Microstructure of laser cladded carbide reinforced Inconel 625 alloy for turbine blade application. Ceram. Int. 2017, 43, 8677–8684. [Google Scholar] [CrossRef]
- Zhai, L.; Ban, C.; Zhang, J. Microstructure, microhardness and corrosion resistance of NiCrBSi coatings under electromagnetic field auxiliary laser cladding. Surf. Coat. Technol. 2019, 358, 531–538. [Google Scholar] [CrossRef]
- Heer, B.; Bandyopadhyay, A. Silica coated titanium using laser engineered net shaping for enhanced wear resistance. Addit. Manuf. 2018, 23, 303–311. [Google Scholar] [CrossRef]
- Farahmand, P.; Kovacevic, R. Corrosion and wear behavior of laser cladded Ni–WC coatings. Surf. Coat. Technol. 2015, 276, 121–135. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Tao, Y.; Hu, L. High-temperature wear and oxidation behaviors of TiNi/Ti2Ni matrix composite coatings with TaC addition prepared on Ti6Al4V by laser cladding. Appl. Surf. Sci. 2017, 402, 478–494. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Zeng, Z.; Fu, Y. Effect of rare-earth on friction and wear properties of laser cladding Ni-based coatings on 6063Al. J. Alloy. Compd. 2017, 727, 278–285. [Google Scholar] [CrossRef]
- Tseng, W.C.; Aoh, J.N. Simulation study on laser cladding on preplaced powder layer with a tailored laser heat source. Opt. Laser. Technol. 2013, 48, 141–152. [Google Scholar] [CrossRef]
- Gecu, R.; Yurekturk, Y.; Tekoglu, E.; Muhaffel, F.; Karaaslan, A. Improving wear resistance of 304 stainless steel reinforced AA7075 aluminum matrix composite by micro-arc oxidation. Surf. Coat. Technol. 2019, 368, 15–24. [Google Scholar] [CrossRef]
- Riveiro, A.; Mejías, A.; Lusquiños, F.; Val, J.D.; Comesaña, R.; Pardo, J.; Pou, J. Laser cladding of aluminium on AISI 304 stainless steel with high-power diode lasers. Surf. Coat. Technol. 2014, 253, 214–220. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Deng, P.; Zhang, Y.; Zhang, Q.; Sun, S.; Yan, H.; Ma, P.; Wang, Y. Microstructure and properties of laser-cladded bimodal composite coatings derived by composition design. J. Alloy. Compd. 2018, 745, 483–489. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Peng, G.; Yin, J.; Zeng, X. Microstructure prediction of selective laser melting AlSi10Mg using finite element analysis. Mater. Des. 2018, 142, 319–328. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, J.; Huo, W.; Cao, X.; Zhang, R.; Zhao, W. Characterization of high-current pulsed electron beam interaction with AISI 1045 Steel and the microstructure evolution. Procedia CIRP 2018, 68, 196–199. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, W.; Deng, D.; Tang, Z.; Liu, X.; Yan, Z.; Zhang, H. Effect of electric-magnetic compound field on the pore distribution in laser cladding process. Opt. Laser Technol. 2018, 108, 247–254. [Google Scholar] [CrossRef]
- He, X.; Song, R.; Kong, D. Effects of TiC on the microstructure and properties of TiC/TiAl composite coating prepared by laser cladding. Opt. Laser Technol. 2019, 112, 339–348. [Google Scholar] [CrossRef]
- Ren, K.; Chew, Y.; Zhang, Y.; Bi, G.; Fuh, J.Y.H. Thermal analyses for optimal scanning pattern evaluation in laser aided additive manufacturing. J. Mater. Process. Technol. 2019, 271, 178–188. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Yan, S.; Liu, X.; Li, E.; He, P.; Xu, B. Elimination of voids by laser remelting during laser cladding Ni based alloy on gray cast iron. Opt. Laser Technol. 2019, 112, 30–38. [Google Scholar] [CrossRef]
- Weingarten, C.; Buchbinder, D.; Pirch, N.; Meiners, W.; Wissenbach, K.; Poprawe, R. Formation and reduction of hydrogen porosity during selective laser melting of AlSi10Mg. J. Mater. Process. Technol. 2015, 221, 112–120. [Google Scholar] [CrossRef]
- Li, R.; Jin, Y.; Li, Z.; Zhu, Y.; Wu, M. Effect of the remelting scanning speed on the amorphous forming ability of Ni-based alloy using laser cladding plus a laser remelting process. Surf. Coat. Technol. 2014, 259, 725–731. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Qin, X.; Huang, S.; Hong, F. Numerical simulation and experimental analysis of wide-beam laser cladding. Int. J. Adv. Manuf. Technol. 2019, 100, 237–249. [Google Scholar] [CrossRef]
- Chen, T.; Wu, W.; Li, W.; Liu, D. Laser cladding of nanoparticle TiC ceramic powder: Effects of process parameters on the quality characteristics of the coatings and its prediction model. Opt. Laser Technol. 2019, 116, 345–355. [Google Scholar] [CrossRef]
- Du, L.; Gu, D.; Dai, D.; Shi, Q.; Ma, C.; Xia, M. Relation of thermal behavior and microstructure evolution during multi-track laser melting deposition of Ni-based material. Opt. Laser Technol. 2018, 108, 207–217. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Xu, M.; Ma, C.; Zhang, Q.; Wang, L.; Yao, J.; Li, Z. Study on the element segregation and Laves phase formation in the laser metal deposited IN718 superalloy by flat top laser and gaussian distribution laser. Mater. Sci. Eng. A-Struct. 2014, 759, 339–347. [Google Scholar] [CrossRef]
- Hofman, J.T.; Lange, D.F.; Pathiraj, B.; Meijer, J. FEM modeling and experimental verification for dilution control in laser cladding. J. Mater. Process. Technol. 2011, 211, 187–196. [Google Scholar] [CrossRef]
- Hao, M.; Sun, Y. A FEM model for simulating temperature field in coaxial laser cladding of Ti6Al4V alloy using an inverse modeling approach. Int. J. Heat Mass Transf. 2013, 64, 352–360. [Google Scholar] [CrossRef]
- Parekh, R.; Buddu, R.K.; Patel, R.I. Multiphysics simulation of laser cladding process to study the effect of process parameters on clad geometry. Procedia Technol. 2016, 23, 529–536. [Google Scholar] [CrossRef]
- Suárez, A.; Amado, J.M.; Tobar, M.J.; Yáñez, A.; Fraga, E.; Peel, M.J. Study of residual stresses generated inside laser cladded plates using FEM and diffraction of synchrotron radiation. Surf. Coat. Technol. 2010, 204, 1983–1988. [Google Scholar] [CrossRef]
- Gedda, H.; Kaplan, A.; Powell, J. Melt-solid interactions in laser cladding and laser casting. Metall. Mater. Trans. B 2005, 36, 683–689. [Google Scholar] [CrossRef]
- Contuzzi, N.; Campanelli, S.L.; Ludovico, A.D. 3D finite element analysis in the selective laser melting process. Int. J. Simul. Model. 2011, 3, 113–121. [Google Scholar] [CrossRef]
- Gusarov, A.V.; Laoui, T.; Froyen, L.; Titov, V.I. Contact thermal conductivity of a powder bed in selective laser sintering. Int. J. Heat Mass Transf. 2003, 46, 1103–1109. [Google Scholar] [CrossRef]
- Velaga, S.K.; Ravisankar, A. Finite element based parametric study on the characterization of weld process moving heat source parameters in austenitic stainless steel. Int. J. Press. Vessel. Pip. 2017, 157, 63–73. [Google Scholar] [CrossRef]
- Li, C.; Li, S.; Liu, C.; Zhang, Y.; Deng, P.; Guo, Y.; Wang, J.; Wang, Y. Effect of WC addition on microstructure and tribological properties of bimodal aluminum composite coatings fabricated by laser surface alloying. Mater. Chem. Phys. 2019, 234, 9–15. [Google Scholar] [CrossRef]
- Goldak, J.; Chakravarti, A.; Bibby, M. A new finite element model for welding heat sources. Metall. Mater. Trans. B 1984, 15, 299–305. [Google Scholar] [CrossRef]
- Amine, T.; Newkirk, J.W.; Liou, F. Numerical simulation of the thermal history multiple laser deposited layers. Int. J. Adv. Manuf. Technol. 2014, 73, 1625–1631. [Google Scholar] [CrossRef]
- Zhang, Z.; Farahmand, P.; Kovacevic, R. Laser cladding of 420 stainless steel with molybdenum on mild steel A36 by a high power direct diode laser. Mater. Des. 2016, 109, 686–699. [Google Scholar] [CrossRef] [Green Version]







| Material | Element (wt %) | |||
|---|---|---|---|---|
| Al | Si | Ti | Ni | |
| AlSiTiNi | 60 | 20 | 10 | 10 |
| Material | Element (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Cr | Ni | S | P | Fe | |
| 304 stainless steel | ≤0.08 | ≤1.00 | ≤2.00 | 18–20 | 8–10 | ≤0.03 | ≤0.045 | Balance |
| Sample Number | Laser Power (W) | Scanning Speed (m/s) | Laser Spot Diameter (mm) | Defocus of Laser Beam (mm) |
|---|---|---|---|---|
| 1 | 1500 | 0.005 | 3 | 40 |
| 2 | 1500 | 0.01 | 3 | 40 |
| 3 | 1500 | 0.015 | 3 | 40 |
| Scanning Speed (m/s) | Width of Simulation Result (mm) | Width of Experiment Result (mm) |
|---|---|---|
| 0.005 | 4.6 | 4.82 |
| 0.01 | 3.7 | 3.93 |
| 0.015 | 2.8 | 2.42 |
| Scanning Speed (m/s) | Simulation Result (mm) | Experiment Result (mm) |
|---|---|---|
| 0.005 | 1.4 | 1.35 |
| 0.01 | 0.75 | 1.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Liu, C.; Li, S.; Zhang, Z.; Zeng, M.; Wang, F.; Wang, J.; Guo, Y. Numerical Simulation of Thermal Evolution and Solidification Behavior of Laser Cladding AlSiTiNi Composite Coatings. Coatings 2019, 9, 391. https://doi.org/10.3390/coatings9060391
Li C, Liu C, Li S, Zhang Z, Zeng M, Wang F, Wang J, Guo Y. Numerical Simulation of Thermal Evolution and Solidification Behavior of Laser Cladding AlSiTiNi Composite Coatings. Coatings. 2019; 9(6):391. https://doi.org/10.3390/coatings9060391
Chicago/Turabian StyleLi, Chonggui, Chuanming Liu, Shuai Li, Zhe Zhang, Ming Zeng, Feifei Wang, Jinqian Wang, and Yajun Guo. 2019. "Numerical Simulation of Thermal Evolution and Solidification Behavior of Laser Cladding AlSiTiNi Composite Coatings" Coatings 9, no. 6: 391. https://doi.org/10.3390/coatings9060391
APA StyleLi, C., Liu, C., Li, S., Zhang, Z., Zeng, M., Wang, F., Wang, J., & Guo, Y. (2019). Numerical Simulation of Thermal Evolution and Solidification Behavior of Laser Cladding AlSiTiNi Composite Coatings. Coatings, 9(6), 391. https://doi.org/10.3390/coatings9060391





