Differentiating Generic versus Branded Pharmaceutical Tablets Using Ultra-High-Resolution Optical Coherence Tomography
Abstract
1. Introduction
2. Materials and Methods
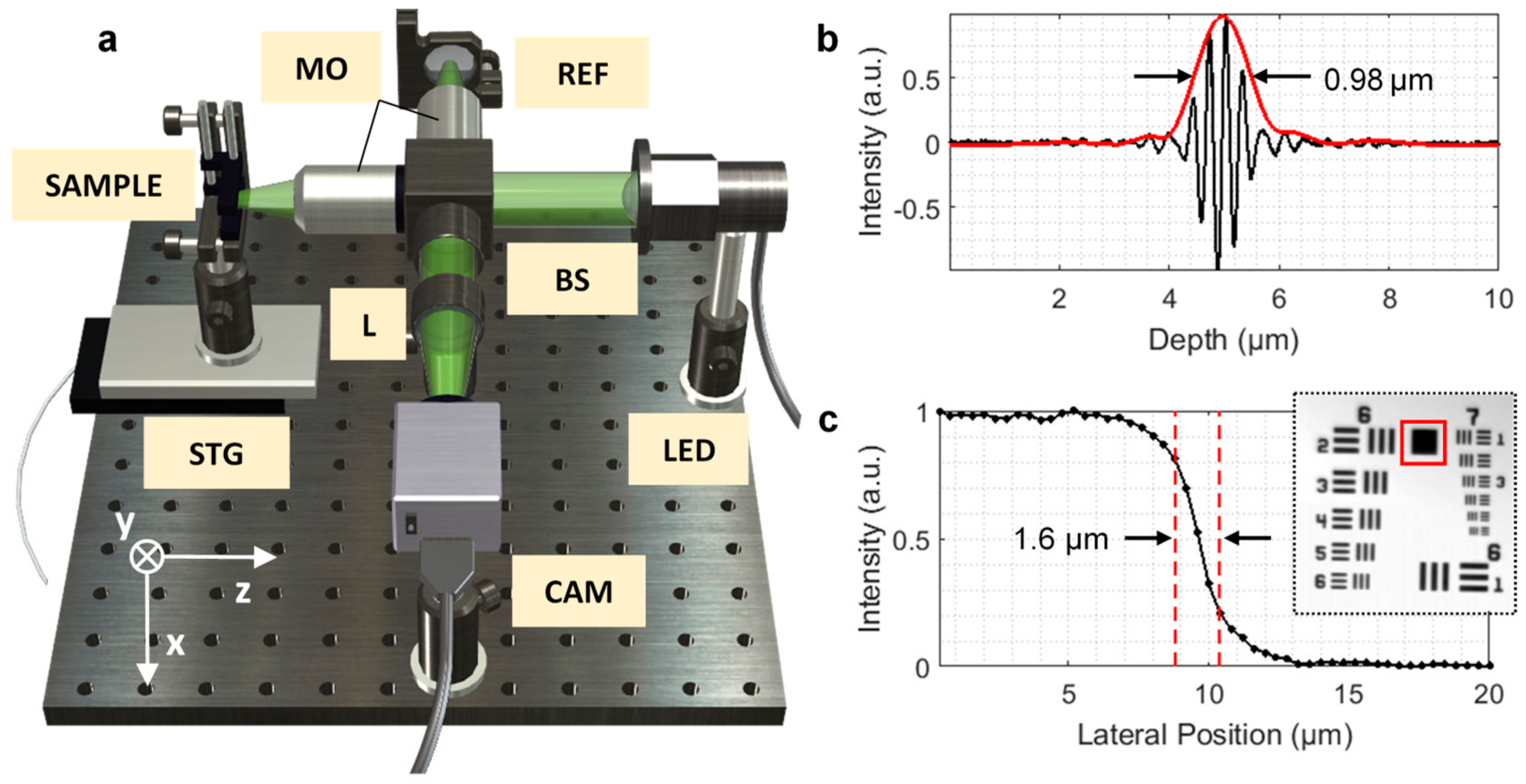
2.1. System Setup and Performance
2.2. Paracetamol Tablet Samples
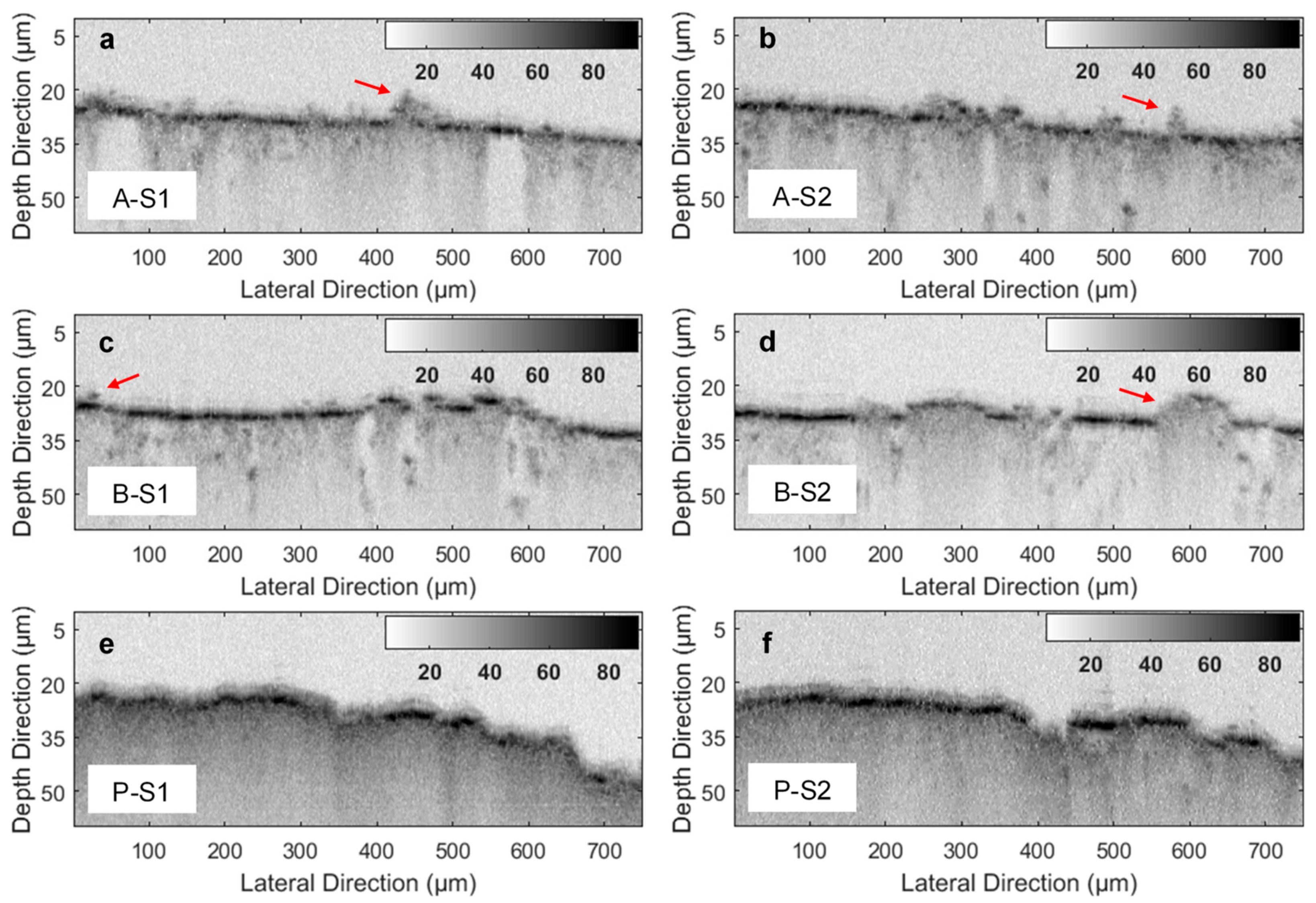
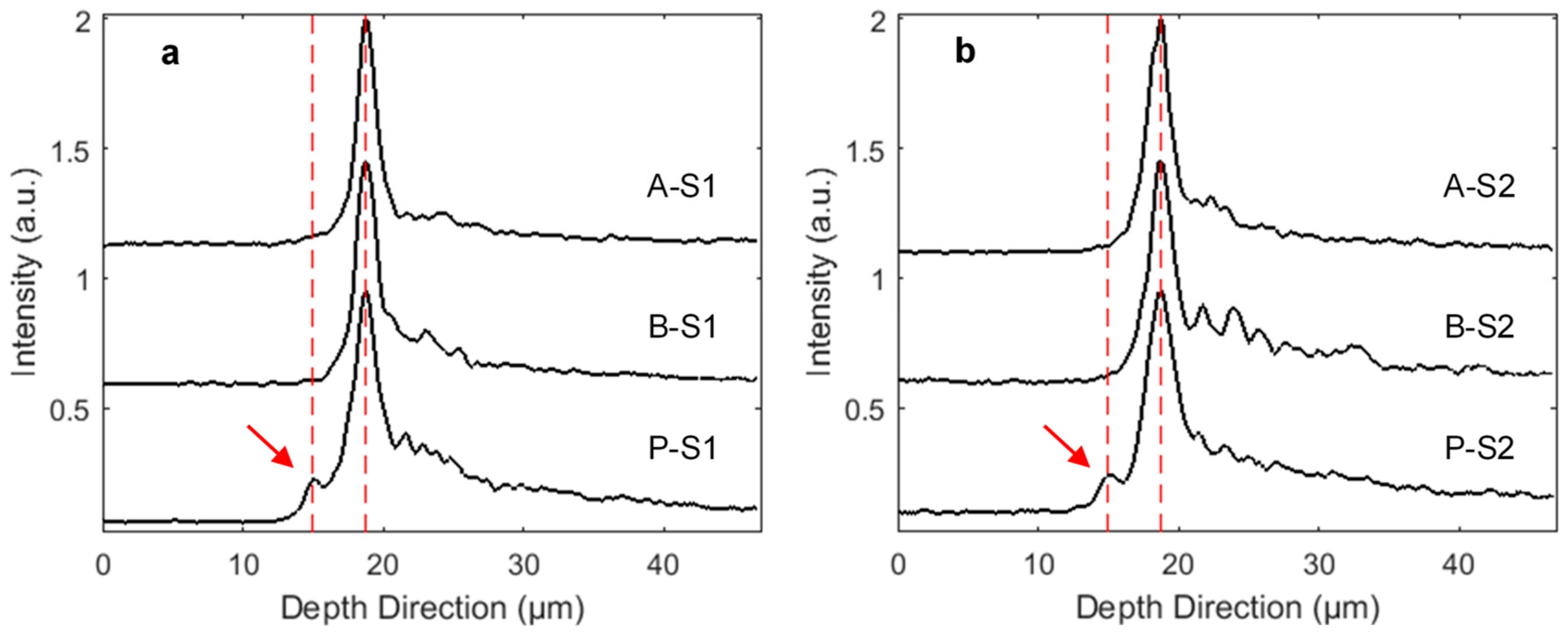
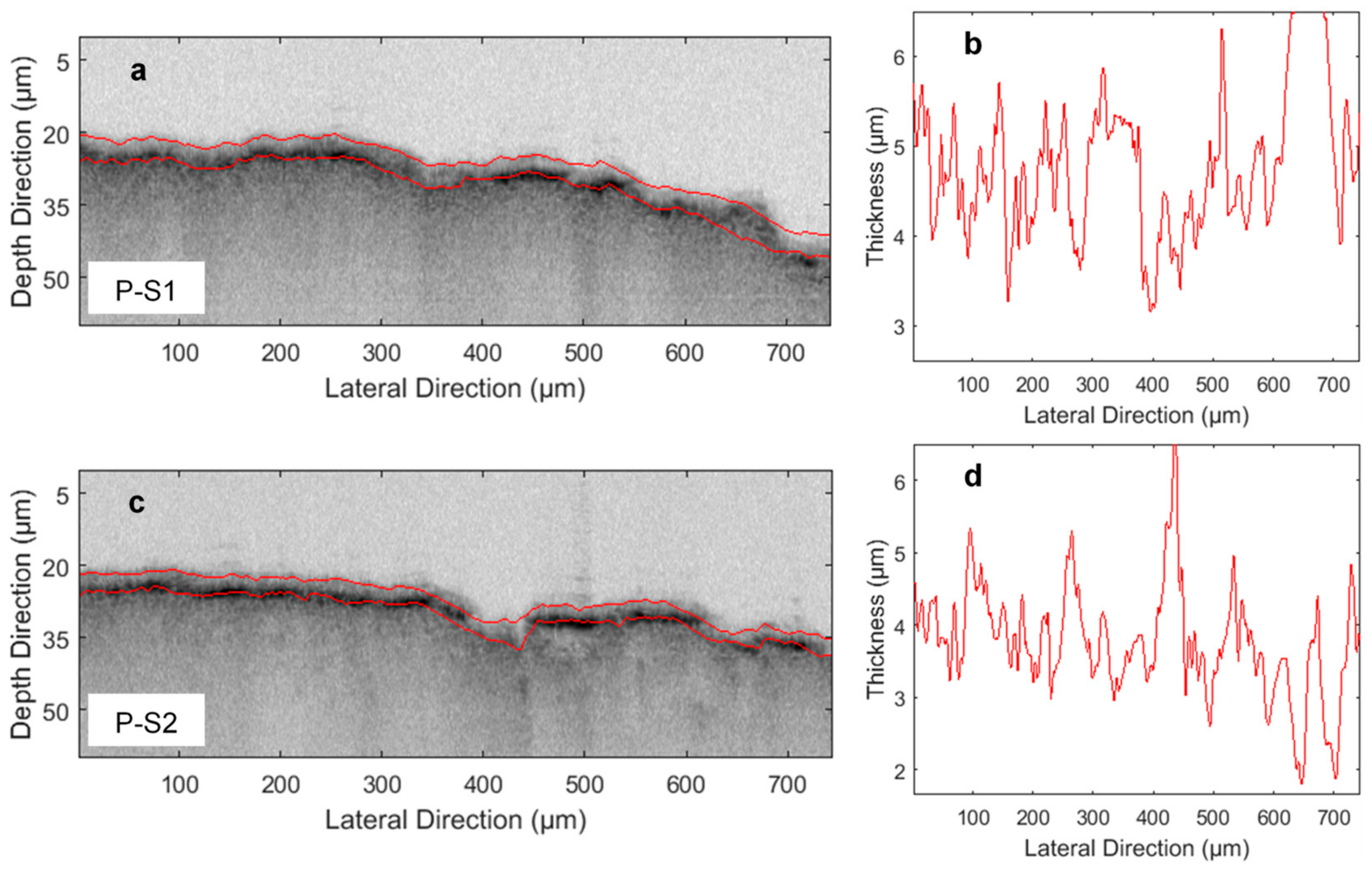
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jantzen, G.M.; Robinson, J.R. Sustained-and controlled-release drug delivery systems. In Modern Pharmaceutics, 4th ed.; Banker, G.S., Rhodes, C.T., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 501–528. [Google Scholar]
- Kirsch, J.D.; Drennen, J.K. Near-infrared spectroscopic monitoring of the film coating process. Pharm. Res. 1996, 13, 234–237. [Google Scholar] [CrossRef]
- Romero-Torres, S.; Pérez-Ramos, J.D.; Morris, K.R.; Grant, E.R. Raman spectroscopic measurement of tablet-to-tablet coating variability. J. Pharm. Biomed. Anal. 2005, 38, 270–274. [Google Scholar] [CrossRef]
- Djemai, A.; Sinka, I. NMR imaging of density distributions in tablets. Int. J. Pharm. 2006, 319, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wray, P.; Chan, K.L.; Kimber, J.; Kazarian, S.G. Compaction of pharmaceutical tablets with different polymer matrices studied by FTIR imaging and X-ray microtomography. J. Pharm. Sci. 2008, 97, 4269–4277. [Google Scholar] [CrossRef]
- Shen, Y.C.; Taday, P.F. Development and application of terahertz pulsed imaging for nondestructive inspection of pharmaceutical tablet. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 407–415. [Google Scholar] [CrossRef]
- Mauritz, J.M.; Morrisby, R.S.; Hutton, R.S.; Legge, C.H.; Kaminski, C.F. Imaging pharmaceutical tablets with optical coherence tomography. J. Pharm. Sci. 2010, 99, 385–391. [Google Scholar] [CrossRef]
- Ho, L.; Müller, R.; Gordon, K.C.; Kleinebudde, P.; Pepper, M.; Rades, T.; Shen, Y.C.; Taday, P.F.; Zeitler, J.A. Terahertz pulsed imaging as an analytical tool for sustained-release tablet film coating. Eur. J. Pharm. Biopharm. 2009, 71, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, J.A.; Shen, Y.C.; Baker, C.; Taday, P.F.; Pepper, M.; Rades, T. Analysis of coating structures and interfaces in solid oral dosage forms by three dimensional terahertz pulsed imaging. J. Pharm. Sci. 2007, 96, 330–340. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Dong, Y.; Shen, Y.C.; Zeitler, J.A. Quantifying pharmaceutical film coating with optical coherence tomography and terahertz pulsed imaging: an evaluation. J. Pharm. Sci. 2015, 104, 3377–3385. [Google Scholar] [CrossRef]
- Lin, H.; Dong, Y.; Markl, D.; Zhang, Z.J.; Shen, Y.C.; Zeitler, J.A. Pharmaceutical film coating catalog for spectral domain optical coherence tomography. J. Pharm. Sci. 2017, 106, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Dong, Y.; Markl, D.; Williams, B.M.; Zheng, Y.L.; Shen, Y.C.; Zeitler, J.A. Measurement of the intertablet coating uniformity of a pharmaceutical pan coating process with combined terahertz and optical coherence tomography in-line sensing. J. Pharm. Sci. 2017, 106, 1075–1084. [Google Scholar] [CrossRef]
- Markl, D.; Hannesschläger, G.; Sacher, S.; Leitner, M.; Khinast, J.G. Optical coherence tomography as a novel tool for in-line monitoring of a pharmaceutical film-coating process. Eur. J. Pharm. Sci. 2014, 55, 58–67. [Google Scholar] [CrossRef]
- Markl, D.; Zettl, M.; Hannesschläger, G.; Sacher, S.; Leitner, M.; Buchsbaum, A.; Khinast, J.G. Calibration-free in-line monitoring of pellet coating processes via optical coherence tomography. Chem. Eng. Sci. 2015, 125, 200–208. [Google Scholar] [CrossRef]
- Li, C.; Zeitler, J.A.; Dong, Y.; Shen, Y.C. Non-destructive evaluation of polymer coating structures on pharmaceutical pellets using full-field optical coherence tomography. J. Pharm. Sci. 2014, 103, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.C.; Shen, Y.C.; Ho, L.; May, R.K.; Zeitler, J.A.; Evans, M.; Taday, P.F.; Pepper, M.; Rades, T.; Gordon, K.C. Non-destructive quantification of pharmaceutical tablet coatings using terahertz pulsed imaging and optical coherence tomography. Opt. Lasers Eng. 2011, 49, 361–365. [Google Scholar] [CrossRef]
- Ogien, J.; Dubios, A. High-resolution full-field optical coherence microscopy using a broadband light-emitting diode. Opt. Express 2016, 24, 9922–9931. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Grieve, K.; Moneron, G.; Lecaque, R.; Vabre, L.; Boccara, A.C. Ultrahigh-resolution full-field optical coherence tomography. Appl. Opt. 2004, 43, 2874–2883. [Google Scholar] [CrossRef]
- Dubois, A.; Vabre, L.; Boccara, A.C.; Beaurepaire, E. High-resolution full-field optical coherence tomography with a Linnik microscope. Appl. Opt. 2002, 41, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Akiba, M.; Chan, K.; Tanno, N. Full-field optical coherence tomography by two-dimensional heterodyne detection with a pair of CCD cameras. Opt. Lett. 2003, 28, 816–818. [Google Scholar] [CrossRef]
- Sato, M.; Nagata, T.; Niizuma, T.; Neagu, L.; Dabu, R.; Watanabe, Y. Quadrature fringes wide-field optical coherence tomography and its applications to biological tissues. Opt. Commun. 2007, 271, 573–580. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Ikpatt, U.; Lawman, S.; Williams, B.; Zheng, Y.L.; Lin, H.; Shen, Y.C. Sub-surface imaging of soiled cotton fabric using full-field optical coherence tomography. Opt. Express 2019, 27, 13951–13964. [Google Scholar] [CrossRef]
- Tay, C.; Quan, C.; Li, M. Investigation of a dual-layer structure using vertical scanning interferometry. Opt. Lasers Eng. 2007, 45, 907–913. [Google Scholar] [CrossRef]
- Prescott, L.F. Paracetamol: Past, present, and future. Am. J. Ther. 2000, 7, 143–147. [Google Scholar] [CrossRef]
| Paracetamol Suppliers | Front Side | Back Side |
|---|---|---|
| ASPAR |  |  |
| Boots |  |  |
| GSK (branded) |  |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Williams, B.; Zheng, Y.; Lin, H.; Shen, Y. Differentiating Generic versus Branded Pharmaceutical Tablets Using Ultra-High-Resolution Optical Coherence Tomography. Coatings 2019, 9, 326. https://doi.org/10.3390/coatings9050326
Zhang Z, Williams B, Zheng Y, Lin H, Shen Y. Differentiating Generic versus Branded Pharmaceutical Tablets Using Ultra-High-Resolution Optical Coherence Tomography. Coatings. 2019; 9(5):326. https://doi.org/10.3390/coatings9050326
Chicago/Turabian StyleZhang, Zijian, Bryan Williams, Yalin Zheng, Hungyen Lin, and Yaochun Shen. 2019. "Differentiating Generic versus Branded Pharmaceutical Tablets Using Ultra-High-Resolution Optical Coherence Tomography" Coatings 9, no. 5: 326. https://doi.org/10.3390/coatings9050326
APA StyleZhang, Z., Williams, B., Zheng, Y., Lin, H., & Shen, Y. (2019). Differentiating Generic versus Branded Pharmaceutical Tablets Using Ultra-High-Resolution Optical Coherence Tomography. Coatings, 9(5), 326. https://doi.org/10.3390/coatings9050326







