Structure and Conductivity Studies of Scandia and Alumina Doped Zirconia Thin Films
Abstract
1. Introduction
2. Materials and Methods
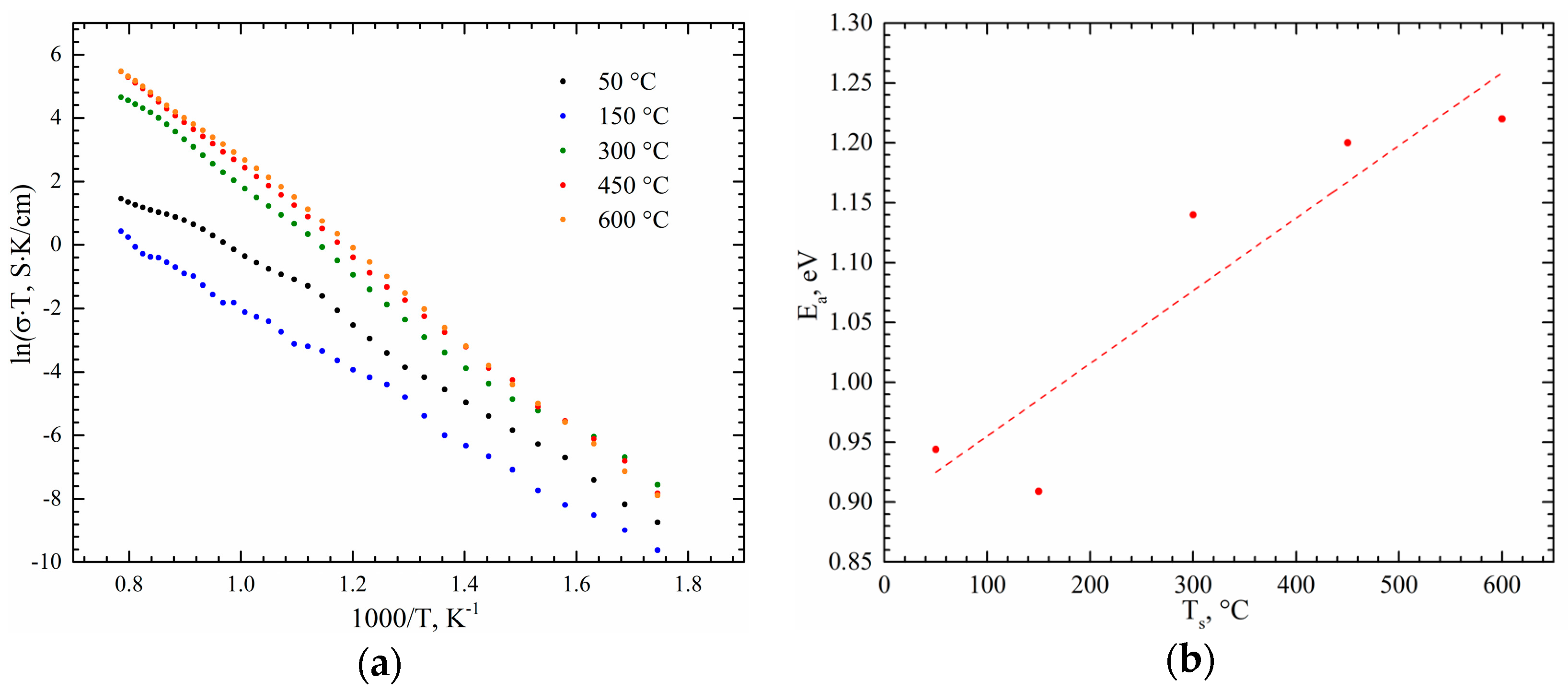
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawada, T.; Mizusaki, J. Current electrolytes and catalysts. In Handbook of Fuel Cells—Fundamentals, Technology and Applications; Vielstich, W., Lamm, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Jung, W.C.; Hertz, J.L.; Tuller, H.L. Enhanced ionic conductivity and phase meta-stability of nano-sized thin film yttria-doped zirconia (YDZ). Acta Mater. 2009, 57, 1399–1404. [Google Scholar] [CrossRef]
- Sternik, M.; Parlinski, K. Lattice vibrations in cubic, tetragonal, and monoclinic phases of ZrO2. J. Chem. Phys. 2005, 122, 064707. [Google Scholar] [CrossRef] [PubMed]
- Simeone, D.; Gosset, D.; Bechade, J.L.; Chevarier, A. Analysis of the monoclinic–tetragonal phase transition of zirconia under irradiation. J. Nucl. Mater. 2002, 300, 27–38. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Wang, X.; Yu, J.; Li, L. A review of zirconia-based solid electrolytes. Ionics 2016, 22, 2249–2262. [Google Scholar] [CrossRef]
- Xue, Q.N.; Wang, L.G.; Huang, X.W.; Zhang, J.X.; Zhang, H. Influence of codoping on the conductivity of Sc-doped zirconia by first-principles calculations and experiments. Mater. Des. 2018, 160, 131–137. [Google Scholar] [CrossRef]
- Agarkov, D.A.; Borik, M.A.; Bredikhin, S.I.; Burmistrov, I.N.; Eliseeva, G.M.; Kolotygin, V.A.; Kulebyakin, A.V.; Kuritsyna, I.E.; Lomonova, E.E.; Milovich, F.O.; et al. Structure and transport properties of zirconia crystals co-doped by scandia, ceria and yttria. J. Mater. 2019. [Google Scholar] [CrossRef]
- Taylor, M.A.; Argirusis, C.; Kilo, M.; Borchardt, G.; Luther, K.-D.; Assmus, W. Correlation between ionic radius and cation diffusion in stabilised zirconia. Solid State Ion. 2004, 173, 51–56. [Google Scholar] [CrossRef]
- Mago, S.; Sharma, C.; Mehra, R.; Pandey, O.P.; Singh, K.L.; Singh, A.P. Preparation of YZT a mixed conductor by microwave processing: A different mechanism in the solid state thermochemical reaction. Mater. Chem. Phys. 2018, 216, 372–379. [Google Scholar] [CrossRef]
- Mahato, N.; Gupta, A.; Balani, K. Doped zirconia and ceria-based electrolytes for solid oxide fuel cells: A review. Nanomater. Energy 2012, 1, 27–45. [Google Scholar] [CrossRef]
- Arachi, Y.; Sakai, H.; Yamamoto, O.; Takeda, Y.; Imanishai, N. Electrical conductivity of the ZrO2–Ln2O3 (Ln = lanthanides) system. Solid State Ion. 1999, 121, 133–139. [Google Scholar] [CrossRef]
- Dasari, H.P.; Ahn, J.S.; Ahn, K.; Park, S.-Y.; Hong, J.; Kim, H.; Yoon, K.J.; Son, J.-W.; Lee, H.-W.; Lee, J.-H. Synthesis, sintering and conductivity behavior of ceria-doped Scandia-stabilized zirconia. Solid State Ion. 2014, 263, 103–109. [Google Scholar] [CrossRef]
- Ishii, T. Structural phase transition and ionic conductivity in 0.88ZrO2–(0.12 − x)Sc2O3–xAl2O3. Solid State Ion. 1995, 78, 333–338. [Google Scholar] [CrossRef]
- Sarat, S.; Sammes, N.; Smirnova, A. Bismuth oxide doped scandia-stabilized zirconia electrolyte for the intermediate temperature solid oxide fuel cells. J. Power Sources 2006, 160, 892–896. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, M.; Bi, Z.; Dong, Y.; Zhang, H.; Zhang, J.; Feng, Z.; Li, C. Structure and impedance of ZrO2 doped with Sc2O3 and CeO2. Mater. Lett. 2005, 59, 2579–2582. [Google Scholar] [CrossRef]
- Ota, Y.; Ikeda, M.; Sakuragi, S.; Iwama, Y.; Sonoyama, N.; Ikeda, S.; Hirano, A.; Imanishi, N.; Takeda, Y.; Yamamoto, O. Crystal structure and oxygen ion conductivity of Ga3+ Co-doped scandia-stabilized zirconia. J. Electrochem. Soc. 2010, 157, B1707–B1712. [Google Scholar] [CrossRef]
- Yarmolenko, S.; Sankar, J.; Bernier, N.; Klimov, M.; Kapat, J.; Orlovskaya, N. Phase stability and sintering behavior of 10 mol % Sc2O3–1 mol % CeO2–ZrO2 ceramics. J. Fuel Cell Sci. Technol. 2009, 6, 21007–21008. [Google Scholar] [CrossRef]
- Hirata, T.; Asari, E.; Kitajima, M. Infrared and Raman spectroscopic studies of ZrO2 polymorphs doped with Y2O3 or CeO2. J. Solid State Chem. 1994, 110, 201–207. [Google Scholar] [CrossRef]
- Feinberg, A.; Perry, C.H. Structural disorder and phase transitions in ZrO2–Y2O3 system. J. Phys. Chem. Solids 1981, 42, 513–518. [Google Scholar] [CrossRef]
- Jawhari, T. Micro-Raman spectroscopy of the solid state: Applications to semiconductors and thin films. Analusis 2000, 28, 15–21. [Google Scholar] [CrossRef]
- Nomura, K.; Mizutani, Y.; Kawai, M.; Nakamura, Y.; Yamamoto, O. Aging and Raman scattering study of scandia and yttria doped zirconia. Solid State Ion. 2000, 132, 235–239. [Google Scholar] [CrossRef]
- Li, L.; Wang, W. Synthesis and characterization of monoclinic ZrO2 nanorods by a novel and simple precursor thermal decomposition approach. Solid State Commun. 2003, 127, 639–643. [Google Scholar] [CrossRef]
- Xue, Q.; Huang, X.; Zhang, H.; Xu, H.; Zhang, J.; Wang, L. Synthesis and characterization of high ionic conductivity ScSZ core/shell nanocomposites. J. Rare Earths 2017, 35, 567–573. [Google Scholar] [CrossRef]
- Kumar, A.; Jaiswal, A.; Sanbui, M.; Omar, S. Scandia stabilized zirconia-ceria solid electrolyte (xSc1CeSZ, 5 < x < 11) for IT-SOFCs: Structure and conductivity studies. Scr. Mater. 2016, 121, 10–13. [Google Scholar] [CrossRef]
- Jamil, Z.; Ruiz-Trejo, E.; Boldrin, P.; Brandon, N.P. Anode fabrication for solid oxide fuel cells: Electroless and electrodeposition of nickel and silver into doped ceria scaffolds. Int. J. Hydrogen Energy 2016, 41, 9627–9637. [Google Scholar] [CrossRef]
- Shi, H.; Ran, R.; Shao, Z. Wet powder spraying fabrication and performance optimization of IT-SOFCs with thin-film ScSZ electrolyte. Int. J. Hydrogen Energy 2012, 37, 1125–1132. [Google Scholar] [CrossRef]
- Ksapabutr, B.; Chalermkiti, T.; Wongkasemjit, S.; Panapoy, M. Fabrication of scandium stabilized zirconia thin film by electrostatic spray deposition technique for solid oxide fuel cell electrolyte. Thin Solid Films 2010, 518, 6518–6521. [Google Scholar] [CrossRef]
- Pshyk, A.V.; Coy, E.; Kempiński, M.; Scheibe, B.; Jurga, S. Low-temperature growth of epitaxial Ti2AlC MAX phase thin films by low-rate layer-by-layer PVD. Mater. Res. Lett. 2019, 7, 244–250. [Google Scholar] [CrossRef]
- Xu, Z.; He, S.; He, L.; Mu, R.; Huang, G.; Cao, X. Novel thermal barrier coatings based on La2(Zr0.7Ce0.3)2O7/8YSZ double-ceramic-layer systems deposited by electron beam physical vapor deposition. J. Alloy. Compd. 2011, 509, 4273–4283. [Google Scholar] [CrossRef]
- Lopatin, S.I.; Shugurov, S.M. Thermodynamic properties of the Lu2O3–ZrO2 solid solutions by Knudsen effusion mass spectrometry at high temperature. J. Chem. Thermodyn. 2014, 72, 85–88. [Google Scholar] [CrossRef]
- Jacobson, N.S. Thermodynamic properties of some metal oxide-zirconia systems (No. NASA-E-5060). In NASA Technical Memorandum 102351; NASA: Cleveland, OH, USA, 1989. [Google Scholar]
- Fujimori, H.; Yashima, M.; Kakihana, M.; Yoshimura, M. Structural changes of Scandia-doped zirconia solid solutions: rietveld analysis and Raman scattering. J. Am. Ceram. Soc. 2005, 81, 2885–2893. [Google Scholar] [CrossRef]
- Le Bail, A. The profile of a Bragg reflection for extracting intensities. In Powder Diffraction: Theory and Practice; Dinnebier, R.E., Billinge, S.J.L., Eds.; The Royal Society of Chemistry: London, UK, 2008; pp. 134–165. [Google Scholar]
- Siu, G.G.; Stokes, M.J.; Liu, Y. Variation of fundamental and higher-order Raman spectra of ZrO2 nanograins with annealing temperature. Phys. Rev. B 1999, 59, 3173–3179. [Google Scholar] [CrossRef]
- Garvie, R.C. The occurrence of metastable tetragonal zirconia as a crystallite size effect. J. Phys. Chem. 1965, 69, 1238–1243. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. A 2003, 21, S117–S128. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Madden, P.A.; Norberg, S.T.; Hull, S. Structural disorder in doped zirconias, Part II: Vacancy ordering effects and the conductivity maximum. Chem. Mater. 2011, 23, 1365–1373. [Google Scholar] [CrossRef]
- Kosacki, I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nanocrystalline CeO2 thin films. Solid State Ion. 2002, 149, 99–105. [Google Scholar] [CrossRef]
- Spirin, A.; Ivanov, V.; Nikonov, A.; Lipilin, A.; Paranin, S.; Khrustov, V.; Spirina, A. Scandia-stabilized zirconia doped with yttria: Synthesis, properties, and ageing behavior. Solid State Ion. 2012, 225, 448–452. [Google Scholar] [CrossRef]
- Maier, J. On the conductivity of polycrystalline materials. Berichte der Bunsengesellschaft für Physikalische Chemie 1986, 90, 26–33. [Google Scholar] [CrossRef]
- Tschöpe, A.; Sommer, E.; Birringer, R. Grain size-dependent electrical conductivity of polycrystalline cerium oxide: I. Experiments. Solid State Ion. 2001, 139, 255–265. [Google Scholar] [CrossRef]
- Tao, J.; Dong, A.; Wang, J. The influence of microstructure and grain boundary on the electrical properties of scandia stabilized zirconia. Mater. Trans. 2013, 54, 825–832. [Google Scholar] [CrossRef]
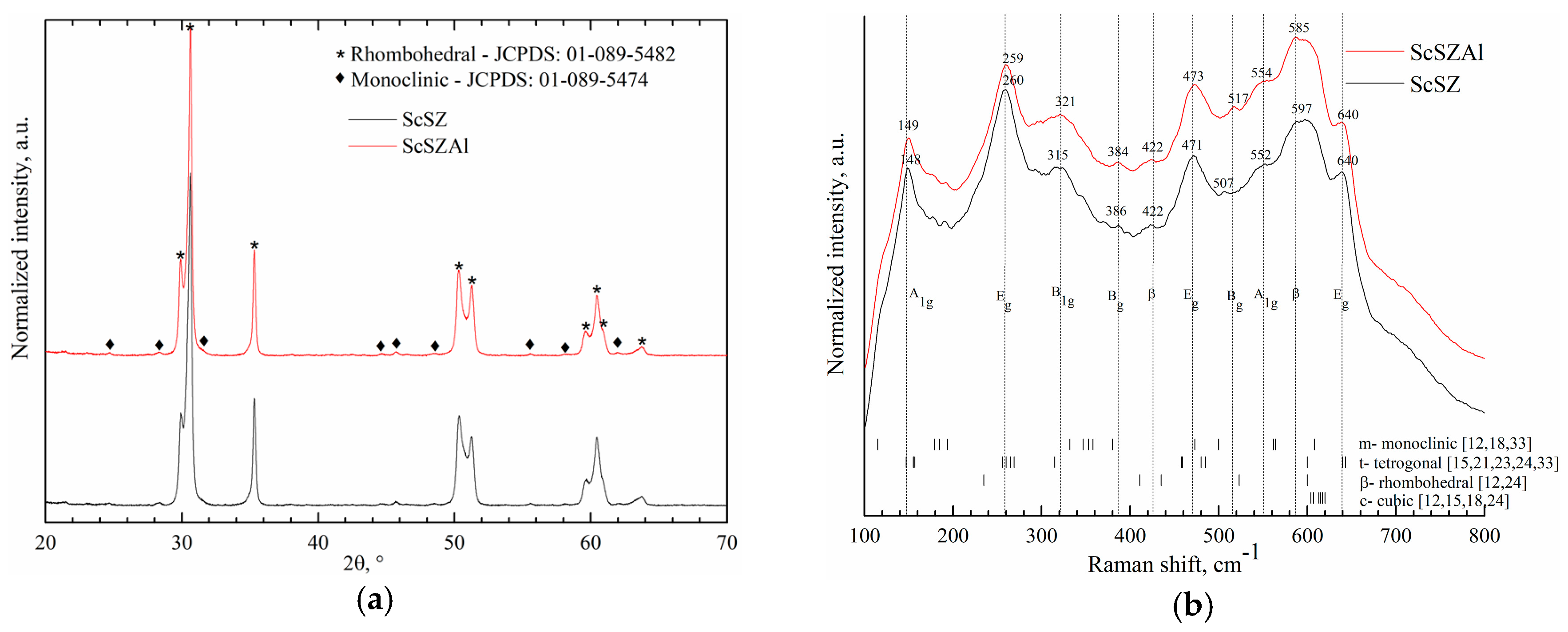
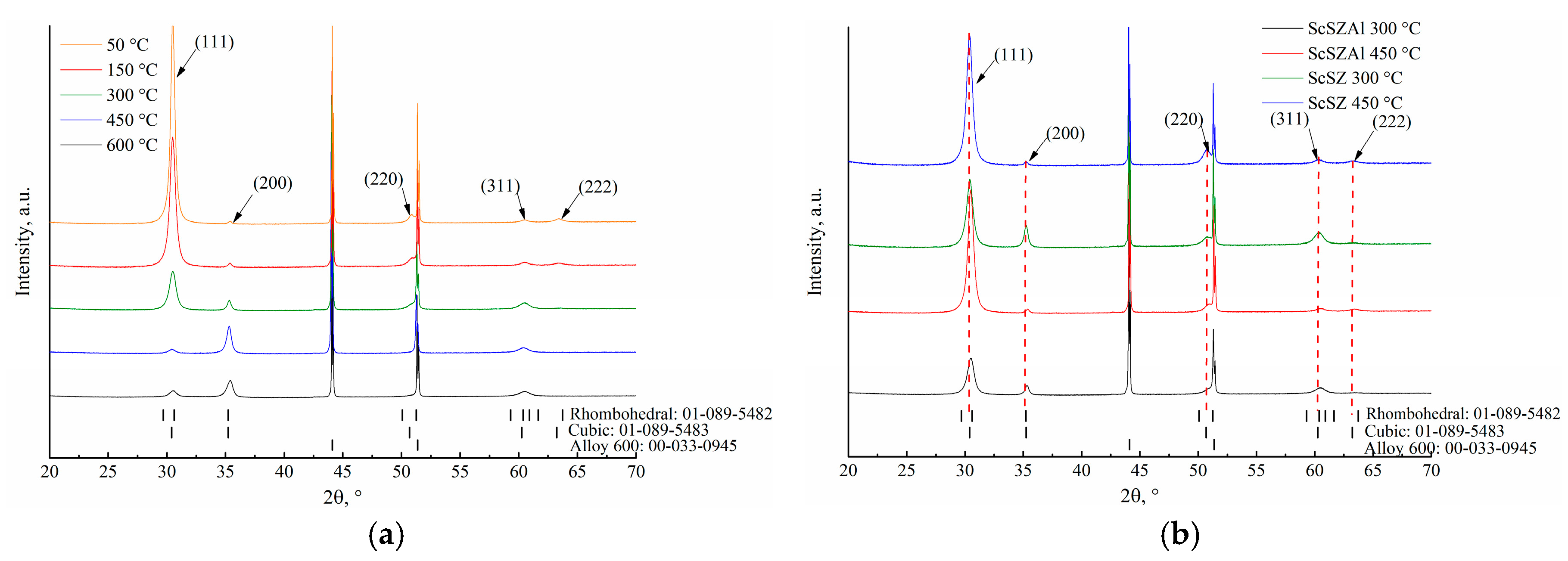

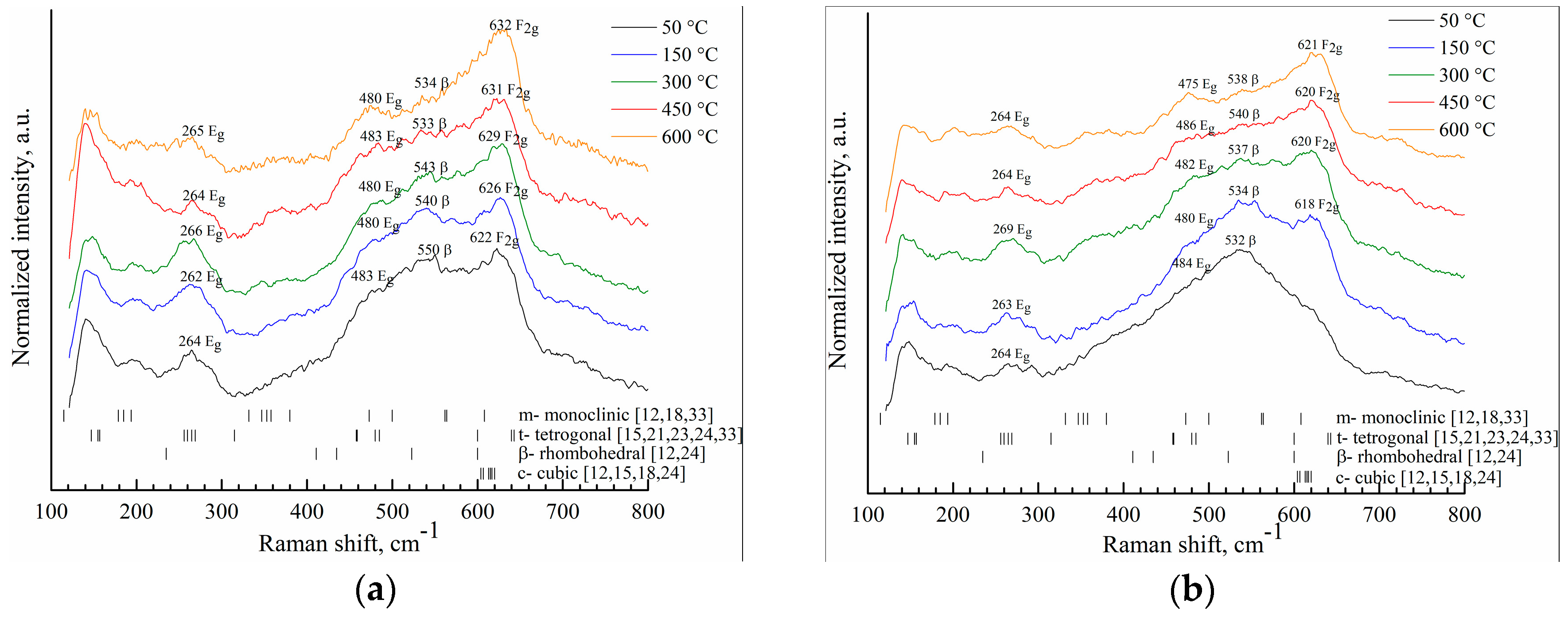
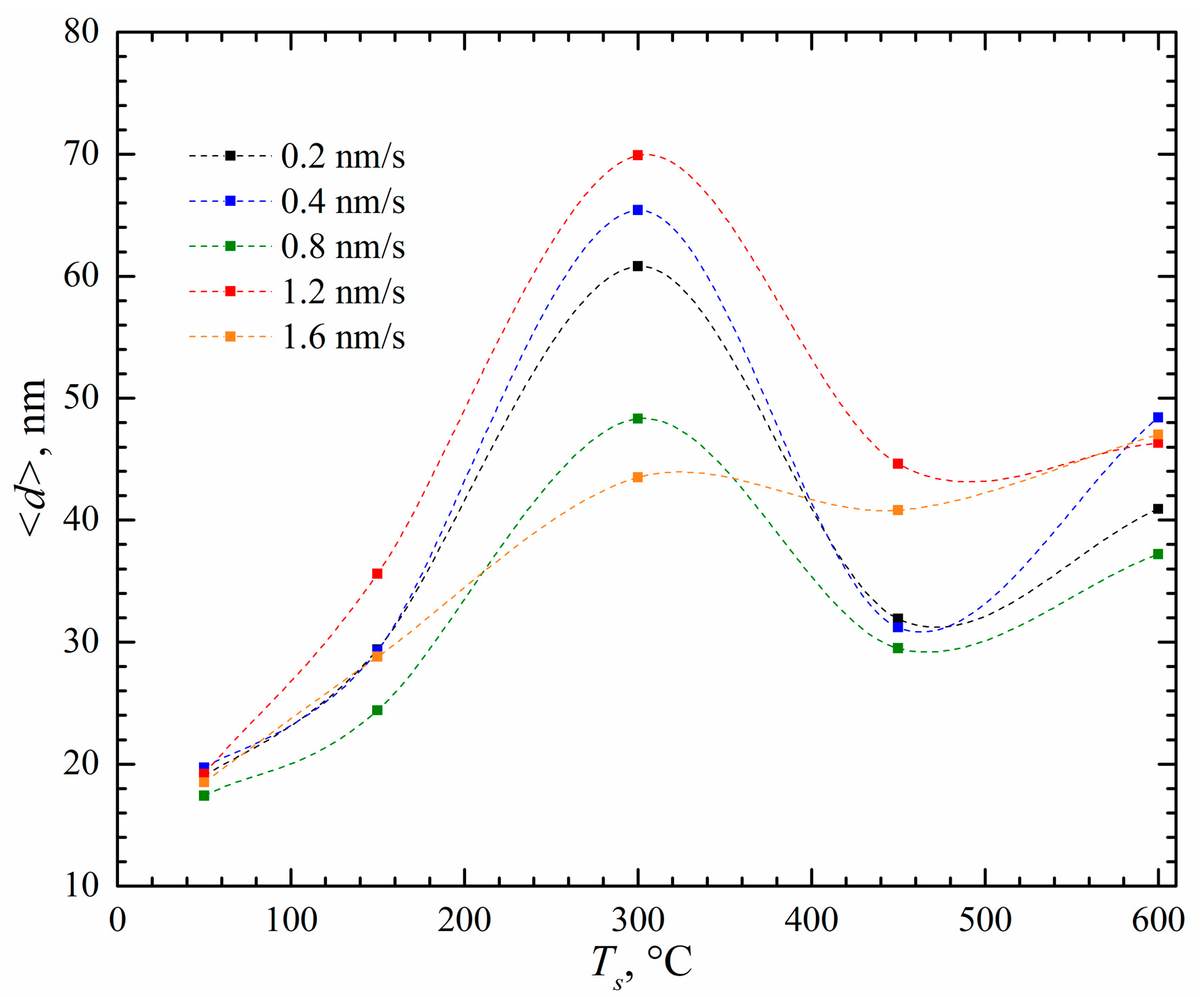
| Initial Powders | cSc, % | cAl, % | cZr, % |
|---|---|---|---|
| ScSZ | 0.19 | – | 0.81 |
| ScAlSZ | 0.19 | 0.02 | 0.79 |
| Substrate Temperature (°C) | Cubic | Tetragonal | Rhombohedral |
|---|---|---|---|
| ScSZ | |||
| 50 | 43% | 15% | 42% |
| 150 | 42% | 19% | 39% |
| 300 | 45% | 18% | 37% |
| 450 | 46% | 17% | 37% |
| 600 | 53% | 17% | 30% |
| ScAlSZ | |||
| 50 | – | 22% | 78% |
| 150 | 41% | 13% | 46% |
| 300 | 42% | 19% | 39% |
| 450 | 43% | 20% | 37% |
| 600 | 43% | 23% | 34% |
| Thin Films | Substrate Temperature | ||||
|---|---|---|---|---|---|
| 50 °C | 150 °C | 300 °C | 450 °C | 600 °C | |
| ScSZ | 1.7 × 10−5 | 2.5 × 10−5 | 7.4 × 10−4 | 3.6 × 10−3 | 4.2 × 10−3 |
| ScAlSZ | 4.1 × 10−5 | 2.3 × 10−4 | 1.1 × 10−3 | 1.9 × 10−3 | 2.4 × 10−3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriubas, M.; Kainbayev, N.; Virbukas, D.; Bočkutė, K.; Rutkūnienė, Ž.; Laukaitis, G. Structure and Conductivity Studies of Scandia and Alumina Doped Zirconia Thin Films. Coatings 2019, 9, 317. https://doi.org/10.3390/coatings9050317
Sriubas M, Kainbayev N, Virbukas D, Bočkutė K, Rutkūnienė Ž, Laukaitis G. Structure and Conductivity Studies of Scandia and Alumina Doped Zirconia Thin Films. Coatings. 2019; 9(5):317. https://doi.org/10.3390/coatings9050317
Chicago/Turabian StyleSriubas, Mantas, Nursultan Kainbayev, Darius Virbukas, Kristina Bočkutė, Živilė Rutkūnienė, and Giedrius Laukaitis. 2019. "Structure and Conductivity Studies of Scandia and Alumina Doped Zirconia Thin Films" Coatings 9, no. 5: 317. https://doi.org/10.3390/coatings9050317
APA StyleSriubas, M., Kainbayev, N., Virbukas, D., Bočkutė, K., Rutkūnienė, Ž., & Laukaitis, G. (2019). Structure and Conductivity Studies of Scandia and Alumina Doped Zirconia Thin Films. Coatings, 9(5), 317. https://doi.org/10.3390/coatings9050317




