TiO2 Nano Flowers Based EGFET Sensor for pH Sensing
Abstract
:1. Introduction
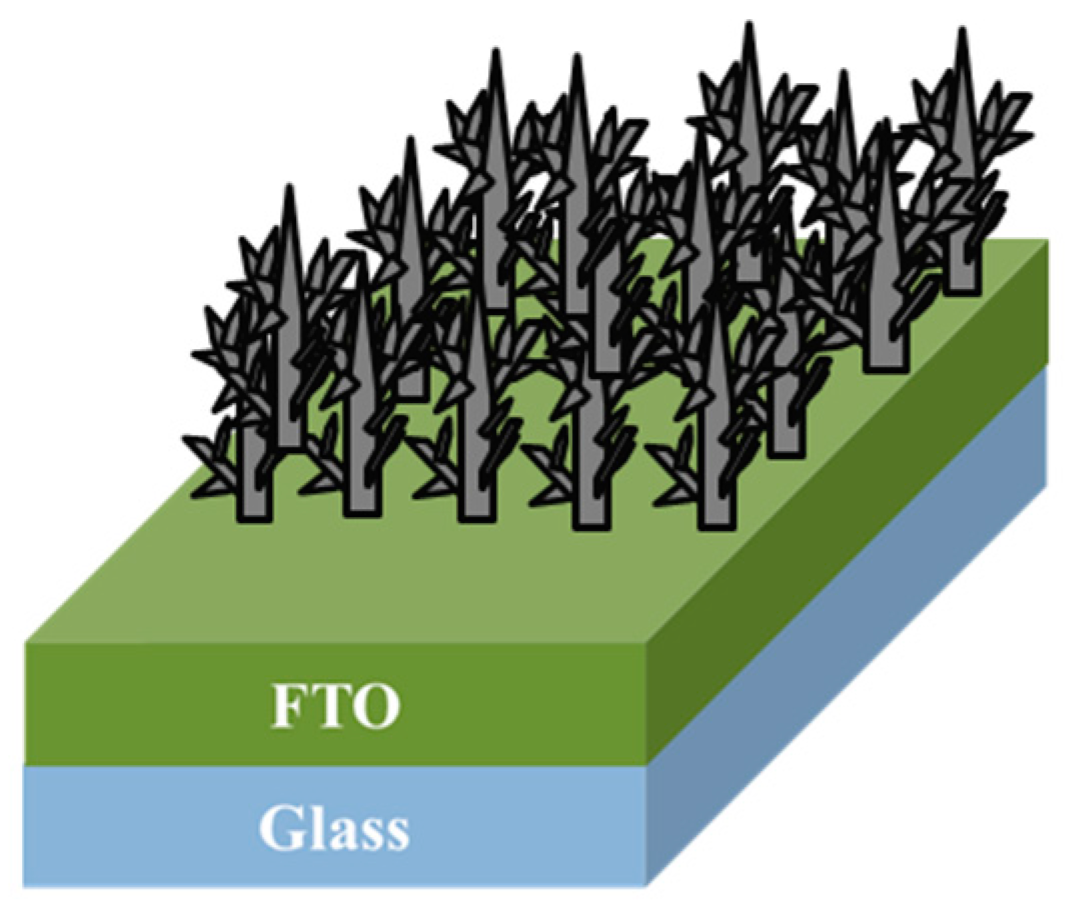
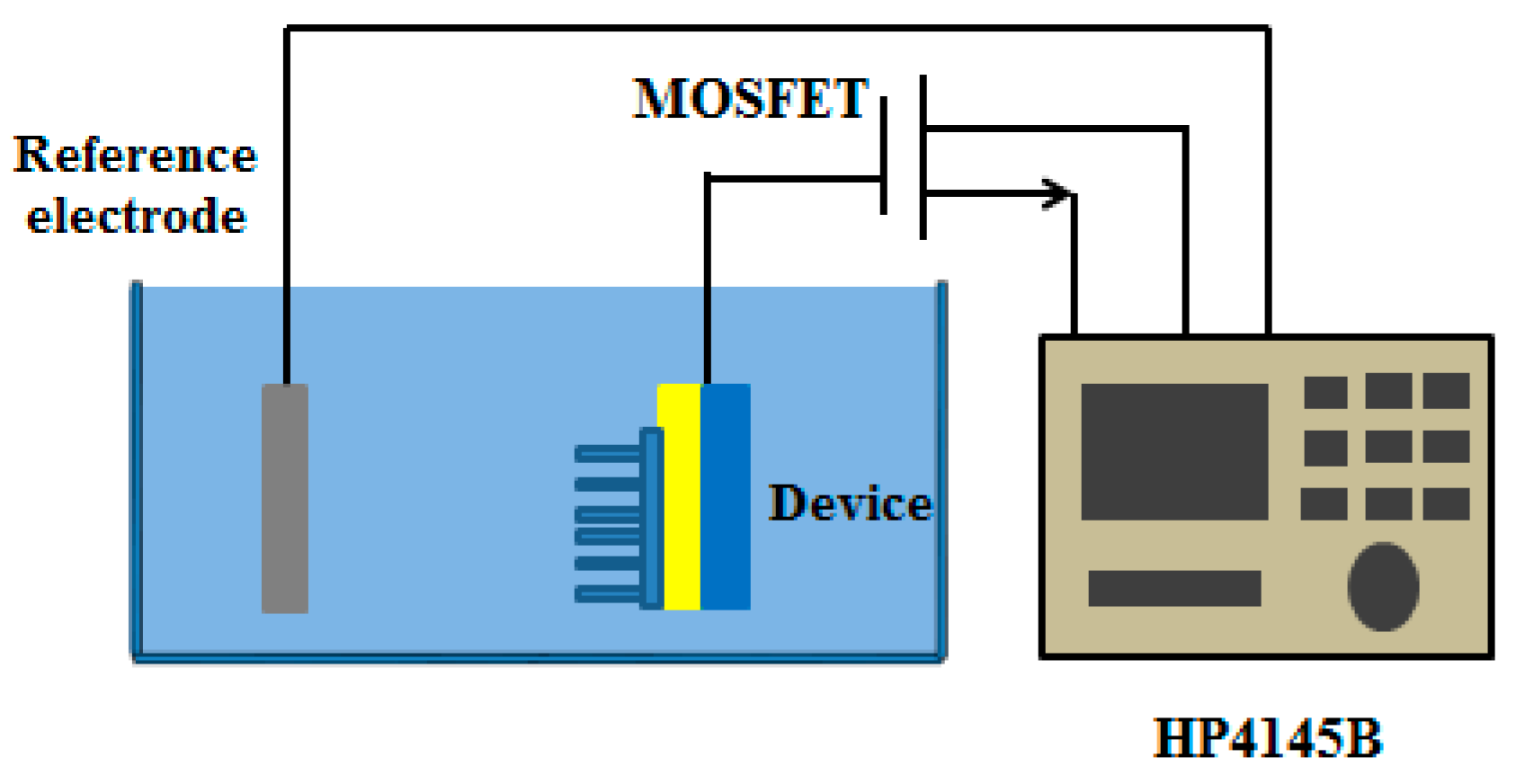
2. Experiment
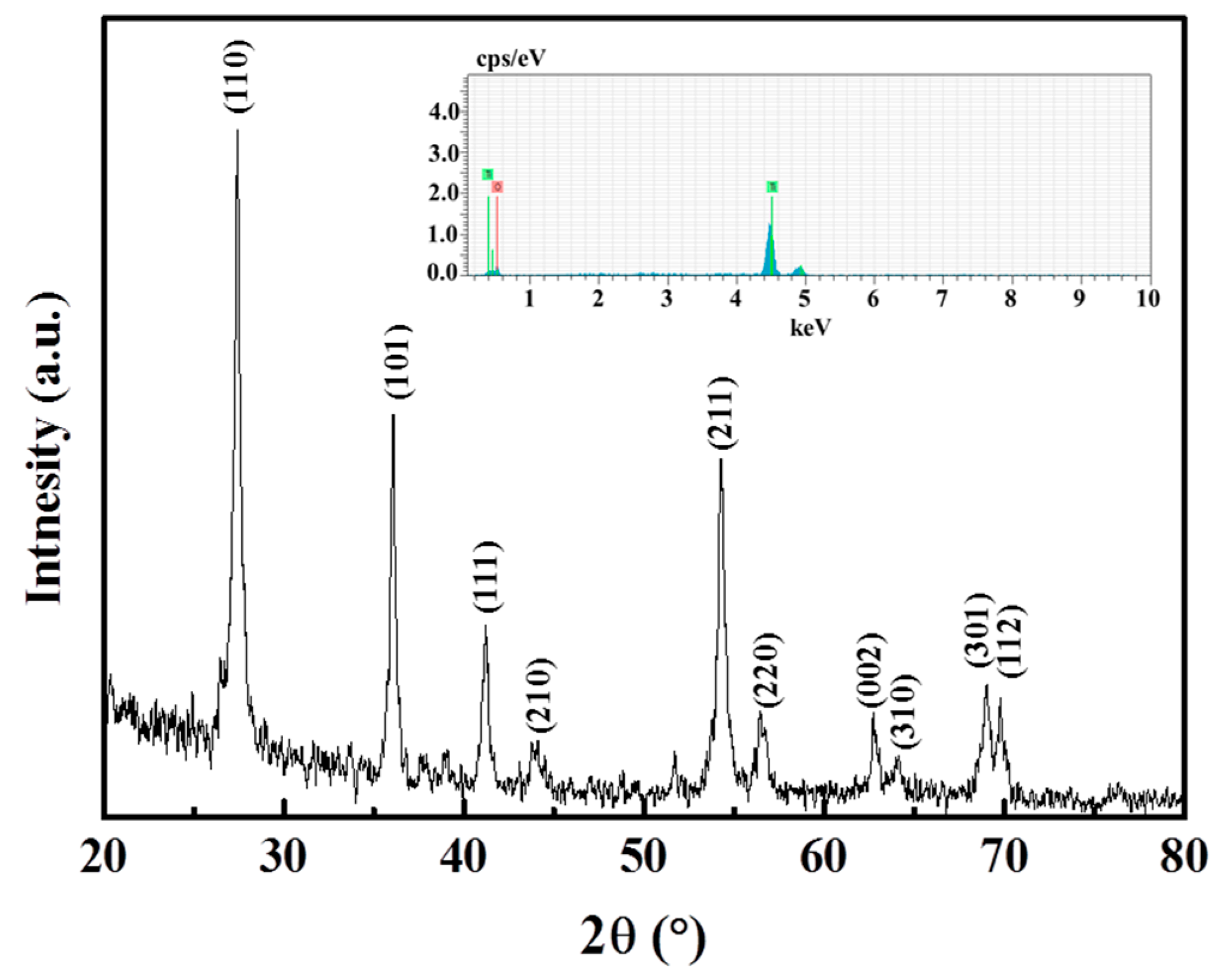
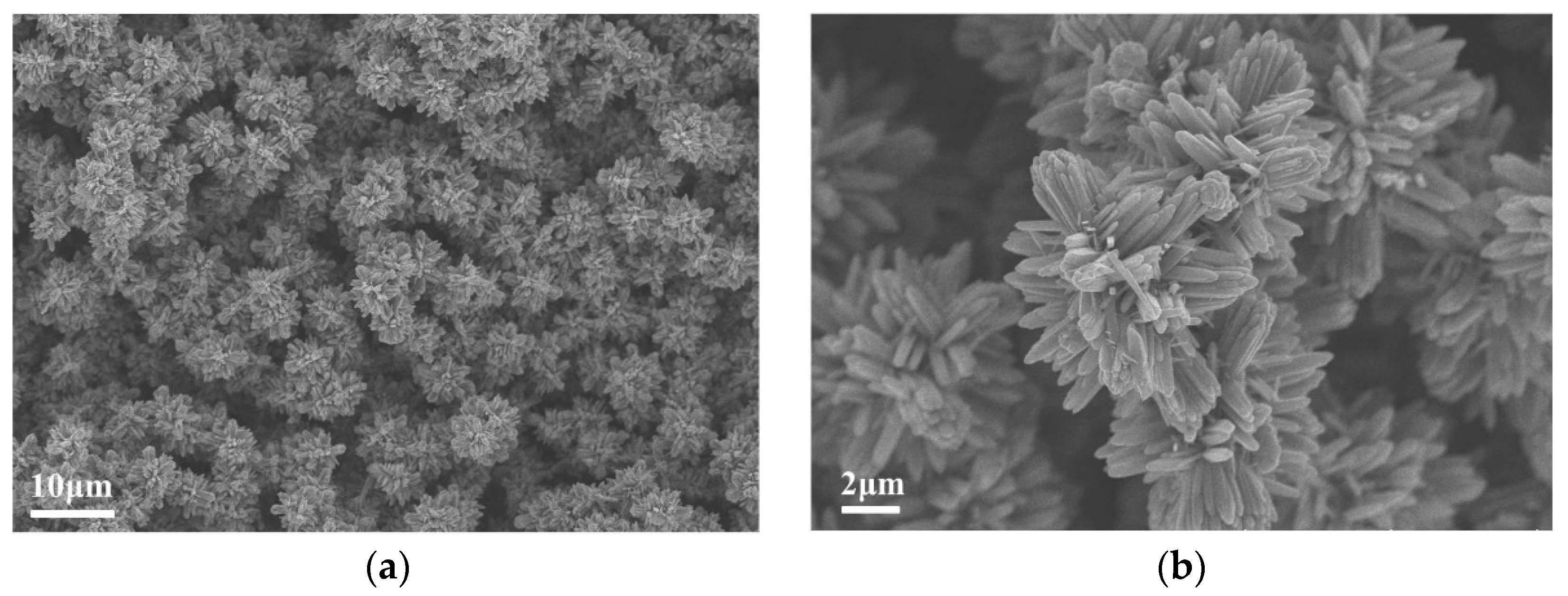
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Huang, T.; Liu, J.; Lin, Z.; Li, S.; Wang, R.; Ge, Y. Soil pH value, organic matter and macronutrients contents prediction using optical diffuse reflectance spectroscopy. Comput. Electron. Agric. 2015, 111, 69–77. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.P.; Schurgers, L.J.; Vervloet, M.G.; Neradova, A. Influence of pH and phosphate concentration on the phosphate binding capacity of five contemporary binders. An in vitro study. Nephrology 2019, 24, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Moser, N.; Lande, T.S.; Toumazou, C.; Georgiou, P. ISFETs in CMOS and emergent trends in instrumentation: A review. IEEE Sens. J. 2016, 16, 6496–6514. [Google Scholar] [CrossRef]
- Pullano, S.; Critello, C.; Mahbub, I.; Tasneem, N.; Shamsir, S.; Islam, S.; Greco, M.; Fiorillo, A.S. EGFET-based sensors for bioanalytical applications: A review. Sensors 2018, 18, 4042. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, H.; Ji, Z.; Xu, M.; Zhang, Y. ZnO nano-array-based EGFET biosensor for glucose detection. Appl. Phys. A 2015, 119, 807–811. [Google Scholar] [CrossRef]
- Chen, P.Y.; Yin, L.T.; Cho, T.H. Optical and impedance characteristics of EGFET based on SnO2/ITO sensing gate. Life Sci. J. 2014, 11, 871–875. [Google Scholar]
- Guidelli, E.J.; Guerra, E.M.; Mulato, M. V2O5/WO3 mixed oxide films as pH-EGFET sensor: Sequential re-usage and fabrication volume analysis. ECS J. Solid State Sci. Technol. 2012, 1, N39–N44. [Google Scholar] [CrossRef]
- Chang, S.P.; Yang, T.H. Sensing performance of EGFET pH sensors with CuO nanowires fabricated on glass substrate. Int. J. Electrochem. Sci. 2012, 7, 5020–5027. [Google Scholar]
- Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A. Novel photocatalytic PVDF/nano-TiO2 hollow fibers for environmental remediation. Polymers 2018, 10, 1134. [Google Scholar] [CrossRef]
- Galstyan, V. Porous TiO2-based gas sensors for cyber chemical systems to provide security and medical diagnosis. Sensors 2017, 17, 2947. [Google Scholar] [CrossRef]
- Liu, H.Y.; Sun, W.C.; Wei, S.Y.; Yu, S.M. Characterization of TiO2-based MISIM ultraviolet photodetectors by ultrasonic spray pyrolysis. IEEE Photonics Technol. Lett. 2016, 28, 637–640. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Yin, X.; Yerramilli, A.; Shen, Y.; Song, Y.; Bian, W.; Li, N.; Zhao, Z.; Qu, W.; et al. Resistive switching characteristics of flexible TiO2 thin film fabricated by deep ultraviolet photochemical solution method. IEEE Electron Device Lett. 2017, 38, 1528–1531. [Google Scholar] [CrossRef]
- Lazar, M.; Varghese, S.; Nair, S. Photocatalytic water treatment by titanium dioxide: Recent updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N., Jr.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Mikrochim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, W.; Sun, C.; Zhang, H.; Pang, W.; Zhang, D.; Duan, X. On-chip surface modified nanostructured ZnO as functional pH sensors. Nanotechnology 2015, 26, 355202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Chen, P.; Rehman, F.; Jiang, Y.; Cao, M.; Zhao, Y.; Jin, H. Hydrothermal growth of VO2 nanoplate thermochromic films on glass with high visible transmittance. Sci. Rep. 2016, 6, 27898. [Google Scholar] [CrossRef]
- Issar, S.; Poddar, P.; Mehra, N.C.; Mahapatro, A.K. Growth of flower-like patterns of TiO2 nanorods over FTO substrate. Integr. Ferroelectr. 2017, 184, 166–171. [Google Scholar] [CrossRef]
- Kumar, A.; Madaria, A.R.; Zhou, C. Growth of aligned single-crystalline rutile TiO2 nanowires on arbitrary substrates and their application in dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 7787–7792. [Google Scholar] [CrossRef]
- Das, A.; Ko, D.H.; Chen, C.H.; Chang, L.B.; Lai, C.S.; Chu, F.C.; Chow, L.; Lin, R.M. Highly sensitive palladium oxide thin film extended gate FETs as pH sensor. Sens. Actuators B 2014, 205, 199–205. [Google Scholar] [CrossRef]
- Bergveld, P. The impact of MOSFET-based sensors. Sens. Actuators 1985, 8, 109–127. [Google Scholar] [CrossRef] [Green Version]
- Yusof, K.A.; Abdul Rahman, R.; Zulkefle, M.A.; Herman, S.H.; Abdullah, W.F.H. EGFET pH sensor performance dependence on sputtered TiO2 sensing membrane deposition temperature. J. Sens. 2016, 2016, 7594531. [Google Scholar] [CrossRef]
- Dar, G.N.; Umar, A.; Zaidi, S.A.; Baskoutas, S.; Kim, S.H.; Abaker, M.; Al-Hajry, A.; Al-Sayari, S.A. Fabrication of highly sensitive non-enzymatic glucose biosensor based on ZnO nanorods. Sci. Adv. Mater. 2011, 3, 901–906. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Cai, Z.; Oyama, M.; Chen, X. Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim. Acta 2013, 181, 689–705. [Google Scholar] [CrossRef]
- Ali, G.M. Interdigitated extended gate field effect transistor without reference electrode. J. Electron. Mater. 2017, 46, 713–717. [Google Scholar] [CrossRef]
- Guerra, E.M.; Mulato, M. Synthesis and characterization of vanadium oxide/hexadecylamine membrane and its application as pH-EGFET sensor. J. Sol-Gel Sci. Technol. 2009, 52, 315–320. [Google Scholar] [CrossRef]
- Zulkefle, M.A.; Rahman, R.A.; Rusop, M.; Abdullah, W.F.H.; Herman, S.H. Porous TiO2 thin film for EGFET pH sensing application. Int. J. Eng. Technol. 2018, 7, 112–114. [Google Scholar]
- Li, J.Y.; Chang, S.P.; Chang, S.J.; Tsai, T.Y. Sensitivity of EGFET pH sensors with TiO2 nanowires. ECS Solid State Lett. 2014, 3, P123–P126. [Google Scholar] [CrossRef]
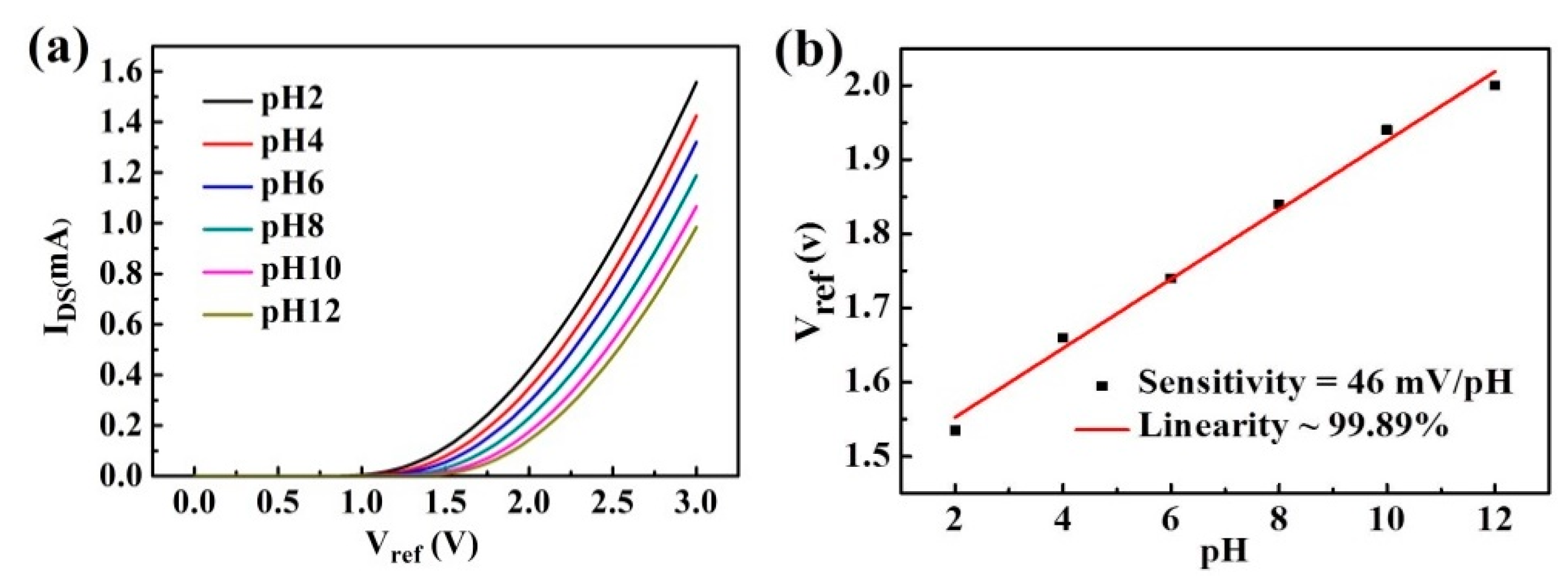
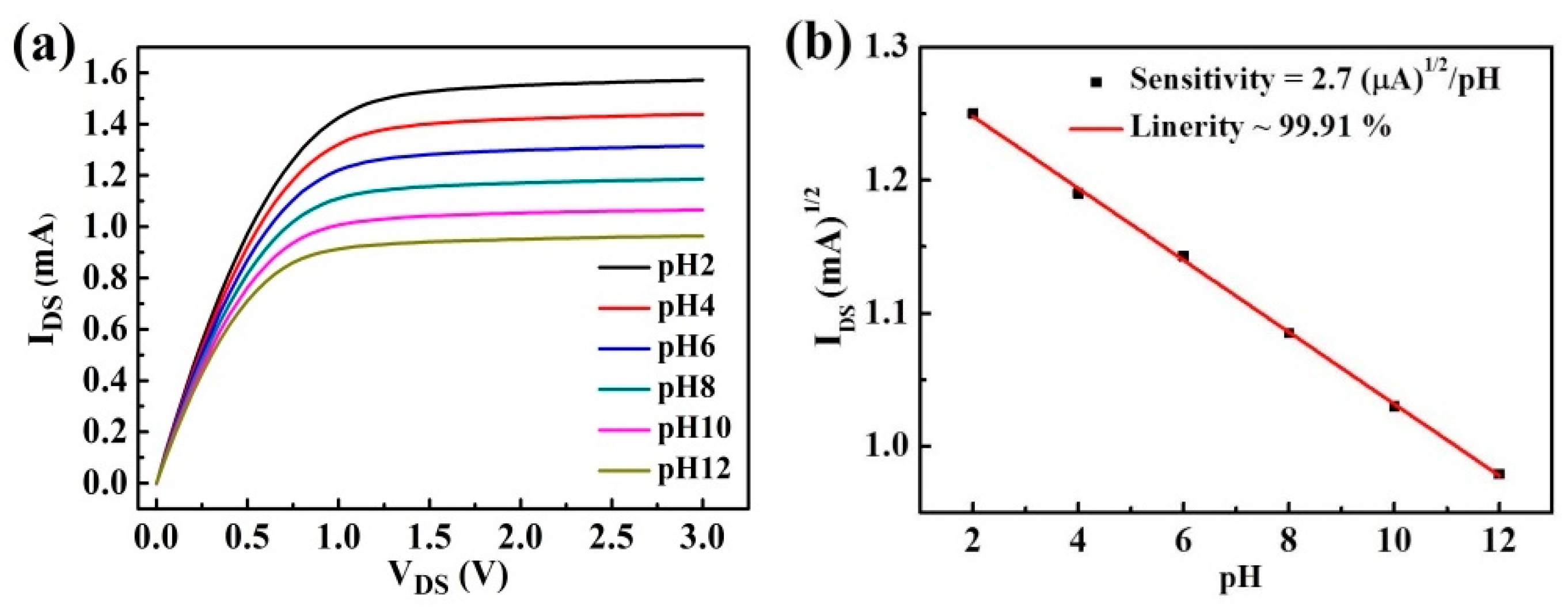
| Sample | Fabrication Method | Sensitivity (mV/pH) | Ref. |
|---|---|---|---|
| ZnO thin film | sol–gel | 26.5 | [27] |
| V2O5/had | Hydrothermal | 38.1 | [28] |
| Porous TiO2 | Hydrothermal | 19.3 | [29] |
| TiO2 nanowires | E-beam evaporator | 32.65 | [30] |
| TiO2 nanoflower | Hydrothermal | 46 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-C.; Chen, K.-Y.; Su, Y.-K. TiO2 Nano Flowers Based EGFET Sensor for pH Sensing. Coatings 2019, 9, 251. https://doi.org/10.3390/coatings9040251
Yang C-C, Chen K-Y, Su Y-K. TiO2 Nano Flowers Based EGFET Sensor for pH Sensing. Coatings. 2019; 9(4):251. https://doi.org/10.3390/coatings9040251
Chicago/Turabian StyleYang, Chih-Chiang, Kuan-Yu Chen, and Yan-Kuin Su. 2019. "TiO2 Nano Flowers Based EGFET Sensor for pH Sensing" Coatings 9, no. 4: 251. https://doi.org/10.3390/coatings9040251
APA StyleYang, C.-C., Chen, K.-Y., & Su, Y.-K. (2019). TiO2 Nano Flowers Based EGFET Sensor for pH Sensing. Coatings, 9(4), 251. https://doi.org/10.3390/coatings9040251






