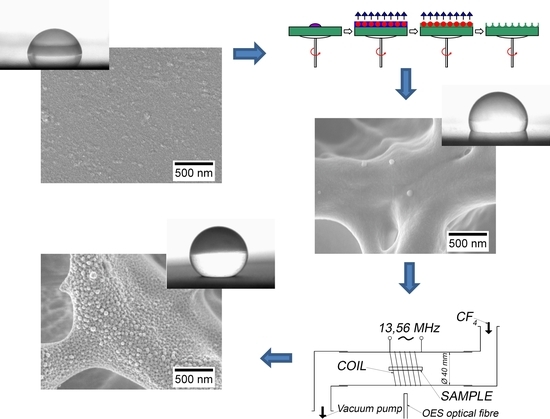
Preparation of Hierarchically Structured Polystyrene Surfaces with Superhydrophobic Properties by Plasma-Assisted Fluorination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
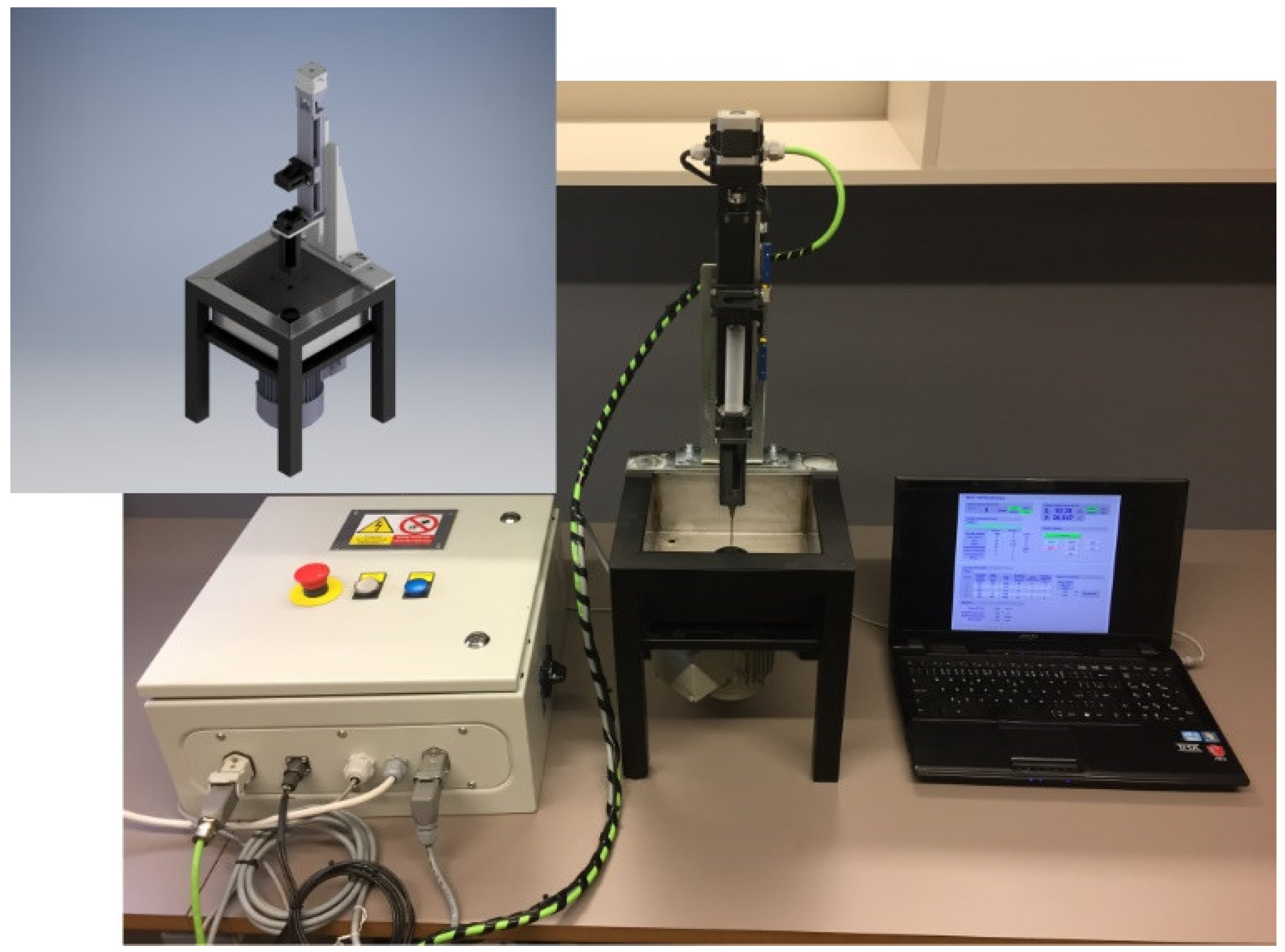
2.2. Plasma Surface Modification
2.3. Scanning Electron Microscopy (SEM)
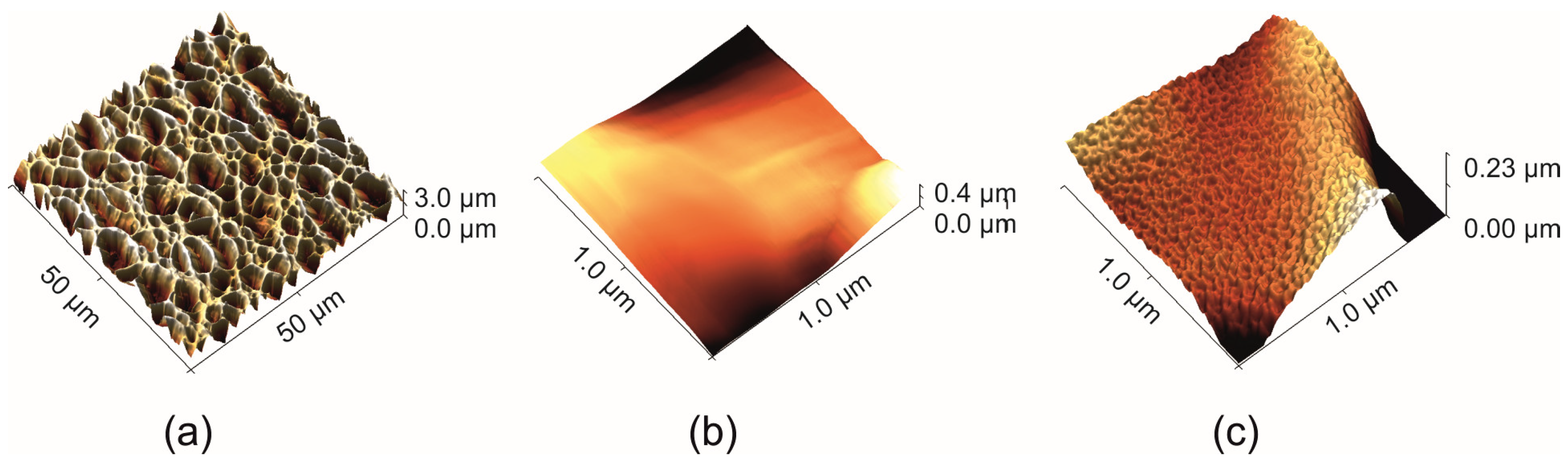
2.4. Atomic Force Microscopy (AFM)
2.5. Profilometry
2.6. Distribution of Micropores
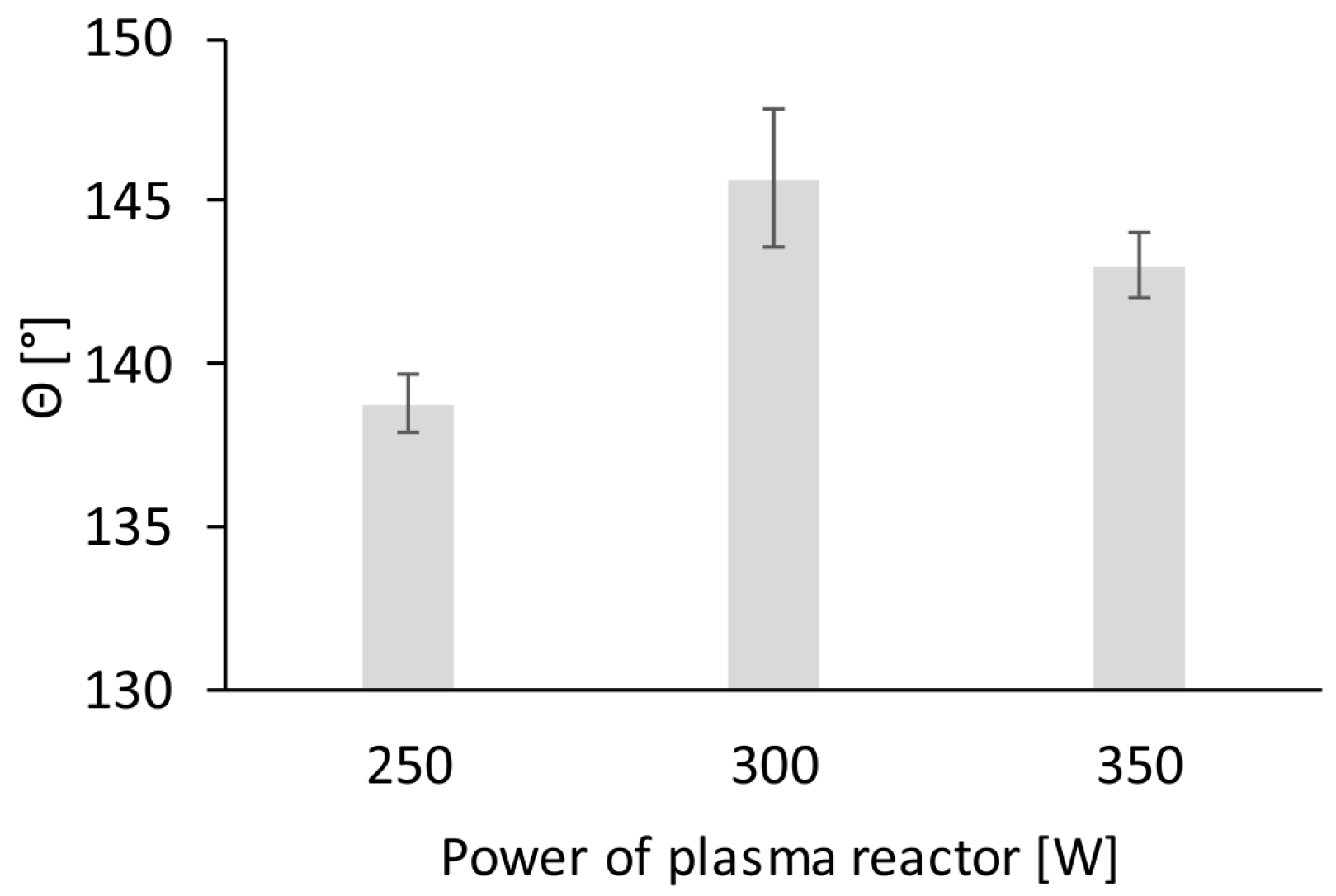
2.7. Contact Angle Measurement
2.8. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perez, J.M.; O’Loughin, T.; Simeone, F.J.; Weissleder, R.; Josephson, L. DNA-based magnetic nanoparticle assembly acts as a magnetic relaxation nanoswitch allowing screening of DNA-cleaving agents. J. Am. Chem. Soc. 2002, 124, 2856–2857. [Google Scholar] [CrossRef]
- Imada, M.; Noda, S.; Chutinan, A.; Tokuda, T.; Murata, M.; Sasaki, G. Coherent two-dimensional lasing action in surface-emitting laser with triangular-lattice photonic crystal structure. Appl. Phys. Lett. 1999, 75, 316–318. [Google Scholar] [CrossRef]
- Shastri, V.P.; Martin, I.; Langer, R. Macroporous polymer foams by hydrocarbon templating. Proc. Natl. Acad. Sci. USA 2000, 97, 1970–1975. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.S.; Talbot, E.L.; Wood, T.J.; Bain, C.D.; Badyal, J.P.S. Superhydrophobic Hierarchical Honeycomb Surfaces. Langmuir 2012, 28, 13712–13719. [Google Scholar] [CrossRef]
- Wasser, L.; Vacche, D.S.; Karasu, F.; Müller, L.; Castellino, M.; Vitale, A.; Bongiovanni, R.; Leterrier, Y. Bio-inspired fluorine-free self-cleaning polymer coatings. Coatings 2018, 8, 436. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, W.; Wang, Y.; Wang, H.; Zhang, R.; Song, X.; Li, J. One-step preparation of durable super-hydrophobic MSR/SiO2 coatings by suspension air spraying. Micromachines 2018, 9, 677. [Google Scholar] [CrossRef]
- Jia, S.; Deng, S.; Luo, S.; Qing, Y.; Yan, N.; Wu, Y. Texturing commercial epoxy with hierarchical and porous structure for robust superhydrophobic coatings. Appl. Surf. Sci. 2019, 466, 84–91. [Google Scholar] [CrossRef]
- Ishizaki, T.; Kumagai, S.; Tsunakawa, M.; Furukawa, T.; Nakamura, K. Ultrafast fabrication of superhydrophobic surfaces on engineering light metals by single-step immersion process. Mater. Lett. 2017, 193, 42–45. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Not simply repel water: The diversified nature of corrosion protection by superhydrophobic coatings. Mendeleev Commun. 2017, 27, 254–256. [Google Scholar] [CrossRef]
- Kadlečková, M.; Minařík, A.; Smolka, P.; Mráček, A.; Wrzecionko, E.; Novák, L.; Musilová, L.; Gajdošík, R. Preparation of textured surfaces on aluminum-alloy substrates. Materials 2018, 12, 109. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired surfaces with superwettability: New insight on theory, design, and applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired interfaces with superwettability: From materials to chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Widawski, G.; Rawiso, M.; François, B. Self-organized honeycomb morphology of star-polymer polystyrene film. Nature 1994, 369, 387–389. [Google Scholar] [CrossRef]
- Beysens, D.; Knobler, C.M. Growth of breath figures. Phys. Rev. Lett. 1986, 57, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, K.M.T.; Mafi, R.; Kietzig, A.M. Colored poly (vinyl chloride) by femtosecond laser machining. Ind. Eng. Chem. Res. 2018, 57, 6161–6170. [Google Scholar] [CrossRef]
- Ahmmed, K.M.T.; Patience, C.; Kietzig, A.M. Internal and external flow over laser-textured superhydrophobic polytetrafluoroethylene (PTFE). ACS Appl. Mater. Interfaces 2016, 8, 27411–27419. [Google Scholar] [CrossRef] [PubMed]
- Aitken, J. Breath figures. Nature 1911, 86, 516–517. [Google Scholar] [CrossRef]
- Rayleigh, L. Breath figures. Nature 1911, 86, 416–417. [Google Scholar] [CrossRef]
- Rayleigh, L. Breath figures. Nature 1912, 90, 436–438. [Google Scholar] [CrossRef]
- Bormashenko, E.; Balter, S.; Pogreb, R.; Bormashenko, Y.; Gendelman, O.; Aurbach, D. On the mechanism of patterning in rapidly evaporated polymer solutions: Is temperature-gradient-driven marangoni instability responsible for the large-scale patterning? J. Colloid Interface Sci. 2010, 343, 602–607. [Google Scholar] [CrossRef]
- Stenzel-Rosenbaum, M.H.; Davis, T.P.; Fane, A.G.; Chen, V. Porous polymer films and honeycomb structures made by the selforganization of well-defined macromolecular structures created by living radical polymerization techniques. Angew. Chem. Int. Ed. 2001, 40, 3428–3432. [Google Scholar] [CrossRef]
- Tian, Y.; Jiao, Q.; Ding, H.; Shi, Y.; Liu, B. The formation of honeycomb structure in polyphenylene oxide films. Polymer 2006, 47, 3866. [Google Scholar] [CrossRef]
- Wrzecionko, E.; Minařík, A.; Smolka, P.; Minařík, M.; Humpolíček, P.; Rejmontová, P.; Mráček, A.; Minaříková, M.; Gřundělová, L. Variations of polymer porous surface structures via the time-sequenced dosing of mixed solvents. ACS Appl. Mater. Inter. 2017, 9, 6472–6481. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, M.; Hong, Y.; Faris, R.; Rao, H. Microtextured polystyrene surfaces for three- dimensional cell culture made by a simple solvent treatment method. J. Appl. Polym. Sci. 2014, 131, 40181–40190. [Google Scholar] [CrossRef]
- Samuel, A.; Umapathy, S.; Ramakrishnan, S. Functionalized and postfunctionalizable porous polymeric films through evaporation-induced phase separation using mixed solvents. ACS Appl. Mater. Inter. 2011, 3, 3293–3299. [Google Scholar] [CrossRef]
- Li, W.; Ryan, A.J.; Meier, I.K. Morphology development via reaction-induced phase separation in flexible polyurethane foam. Macromolecules 2002, 35, 5034–5042. [Google Scholar] [CrossRef]
- Matsuzaka, K.; Jinnai, H.; Koga, T.; Hashimoto, T. Effect of oscillatory shear deformation on demixing processes of polymer blends. Macromolecules 1997, 30, 1146–1152. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Xiu, Y.; Zhu, L.; Hess, D.W.; Wong, C.P. Hierarchical silicon etched structures for controlled hydrophobicity/superhydrophobicity. Nano Lett. 2007, 7, 3388–3393. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Baxter, S.; Cassie, A.B.D. The water repellency of fabrics and a new water repellency test. J. Text. Inst. Trans. 1945, 36, T67–T90. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Large contact angles of plant and animal surfaces. Nature 1945, 155, 21–22. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Roughness optimization for biomimetic superhydrophobic surfaces. Microsyst. Technol. 2005, 11, 535–549. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Contact angle, adhesion, and friction properties of micro- and nanopatterned polymers for superhydrophobicity. Nanotechnology 2006, 17, 4970–4980. [Google Scholar] [CrossRef]
- Milne, A.J.B.; Amirfazli, A. The Cassie equation: How it is mean to be used. Adv. Colloid Interface Sci. 2012, 170, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Colloid Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Mozetič, M.; Zalar, A.; Panjan, P.; Bele, M.; Pejovnik, S.; Grmek, R. A method of studying carbon particle distribution in paint films. Thin Solid Films 2000, 376, 5–8. [Google Scholar] [CrossRef]
- Gunde, M.K.; Kunaver, M.; Mozetič, M.; Pelicon, P.; Simčič, J.; Budnar, M.; Bele, M. Microstructure analysis of metal-effect coatings. Surf. Coat. Int. B Coat. Trans. 2002, 85, 115–121. [Google Scholar] [CrossRef]
- Kunaver, M.; Gunde, M.K.; Mozetič, M.; Hrovat, A. The degree of dispersion of pigments in powder coatings. Dyes Pigment. 2003, 57, 235–243. [Google Scholar] [CrossRef]
- Mozetič, M. Controlled oxidation of organic compounds in oxygen plasma. Vacuum 2003, 71, 237–240. [Google Scholar] [CrossRef]
- Kunaver, M.; Mozetič, M.; Gunde, M.K. Selective plasma etching of powder coatings. Thin Solid Films 2004, 459, 115–117. [Google Scholar] [CrossRef]
- Drenik, A.; Vesel, A.; Mozetič, M. Controlled carbon deposit removal by oxygen radicals. J. Nucl. Mater. 2009, 386–388, 893–895. [Google Scholar] [CrossRef]
- Mozetič, M. Surface modification of materials using an extremely non-equilibrium oxygen plasma. Mater. Tehnol. 2010, 44, 165–171. [Google Scholar]
- Drenik, U.; Cvelbar, K.; Ostrikov, M.; Mozetič, M. Catalytic probes with nanostructured surface for gas/discharge diagnostics: A study of a probe signal behaviour. J. Phys. D Appl. Phys. 2008, 41, 115201. [Google Scholar] [CrossRef]
- Ricard, A.; Gaillard, M.; Monna, V.; Vesel, A.; Mozetič, M. Excited species in H2, N2, O2 microwave flowing discharges and post-discharges. Surf. Coat. Technol. 2001, 142–144, 333–336. [Google Scholar] [CrossRef]
- Babič, D.; Poberaj, I.; Mozetič, M. Fiber optic catalytic probe for weakly ionized oxygen plasma characterization. Rev. Sci. Instrum. 2001, 72, 4110–4114. [Google Scholar] [CrossRef]
- Vasiljević, J.; Gorjanc, M.; Tomšič, B.; Orel, B.; Jerman, I.; Mozetič, M.; Vesel, A.; Simončič, B. The surface modification of cellulose fibres to create super-hydrophobic, oleophobic and self-cleaning properties. Cellulose 2013, 20, 277–289. [Google Scholar] [CrossRef]
- Ji, H.; Yang, J.; Wu, Z.; Hu, J.; Song, H.; Li, L.; Chen, G. A simple approach to fabricate sticky superhydrophobic polystyrene surfaces. J. Adhes. Sci. Technol. 2013, 27, 2296–2303. [Google Scholar] [CrossRef]
- Vesel, A. Hydrophobization of polymer polystyrene in fluorine plasma. Mater. Tehnol. 2011, 45, 217–220. [Google Scholar]
- Chvátalová, L.; Čermák, R.; Mráček, A.; Grulich, O.; Vesel, A.; Ponížil, P.; Minařík, A.; Cvelbar, U.; Beníček, L.; Sajdl, P. The effect of plasma treatment on structure and properties of poly (1-butene) surface. Eur. Polym. J. 2012, 48, 866–874. [Google Scholar] [CrossRef]
- Grulich, O.; Kregar, Y.; Modic, M.; Vesel, A.; Cvelbar, U.; Mracek, A.; Ponizil, P. Treatment and stability of sodium hyaluronate films in low temperature inductively coupled ammonia plasma. Plasma Chem. Plasma Process. 2012, 32, 1075–1091. [Google Scholar] [CrossRef]
- ASME B46.1-2009 Surface Texture (Surface Roughness, Waviness, and Lay); ASME: New York, NY, USA, 2009.
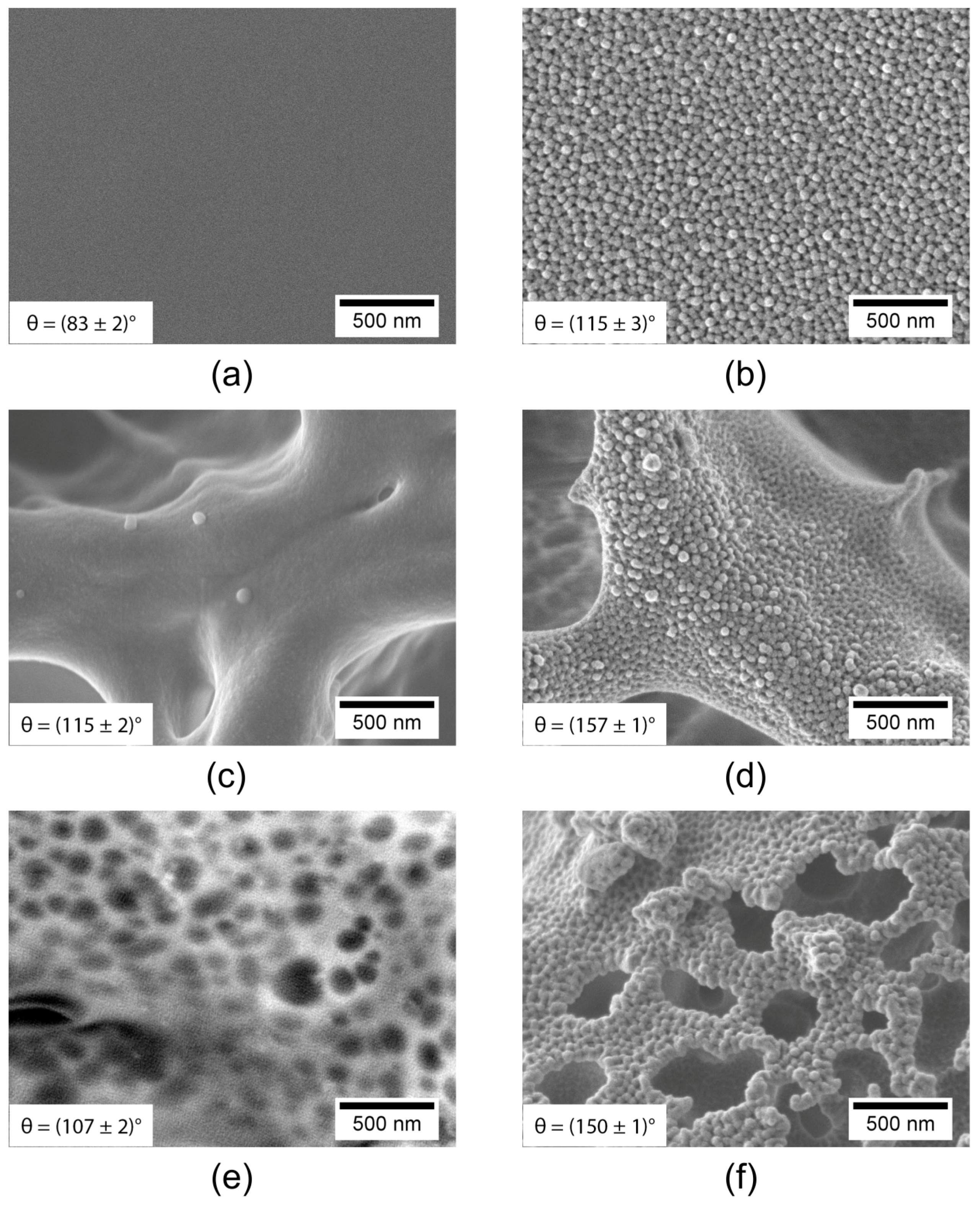
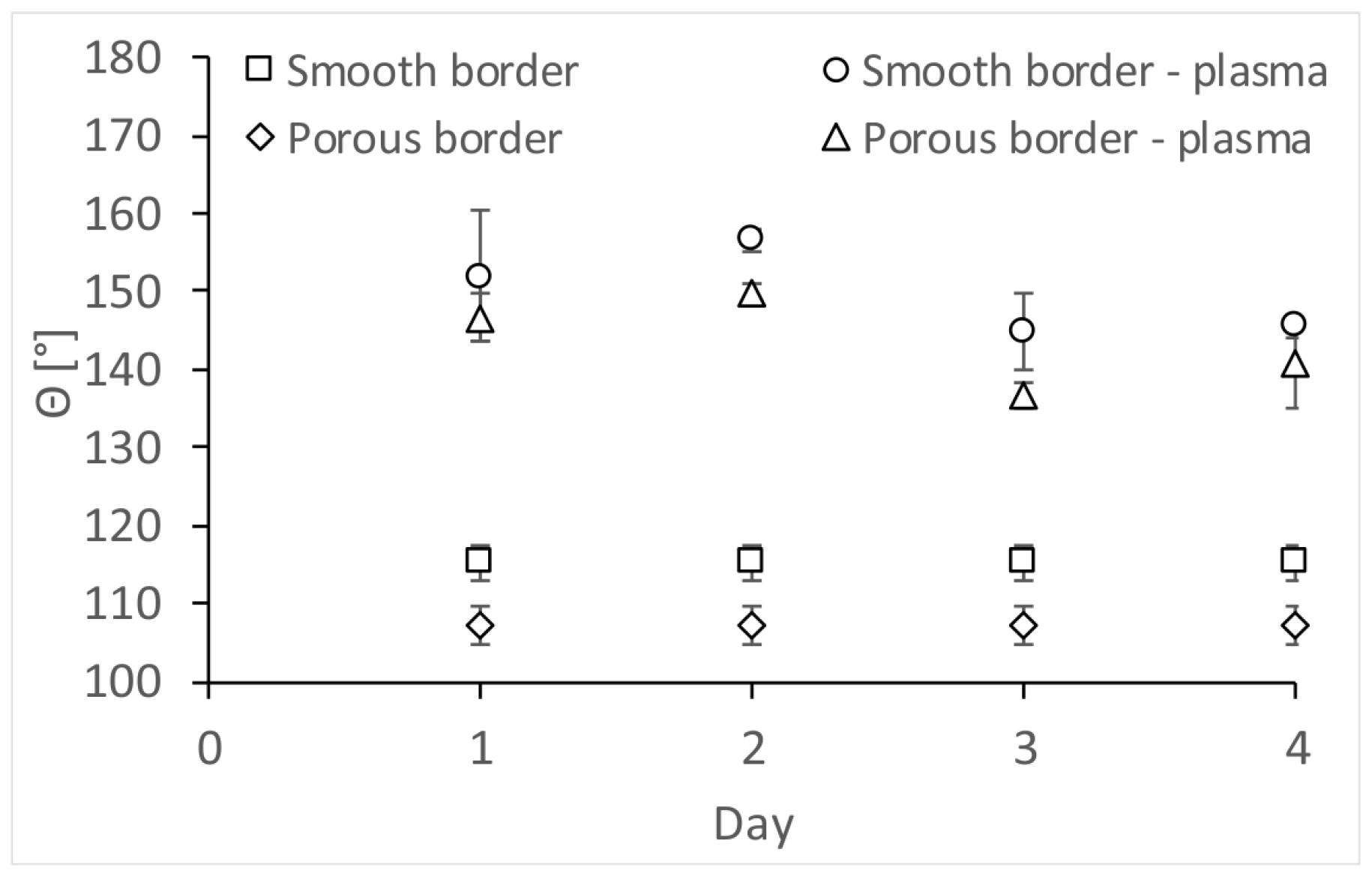
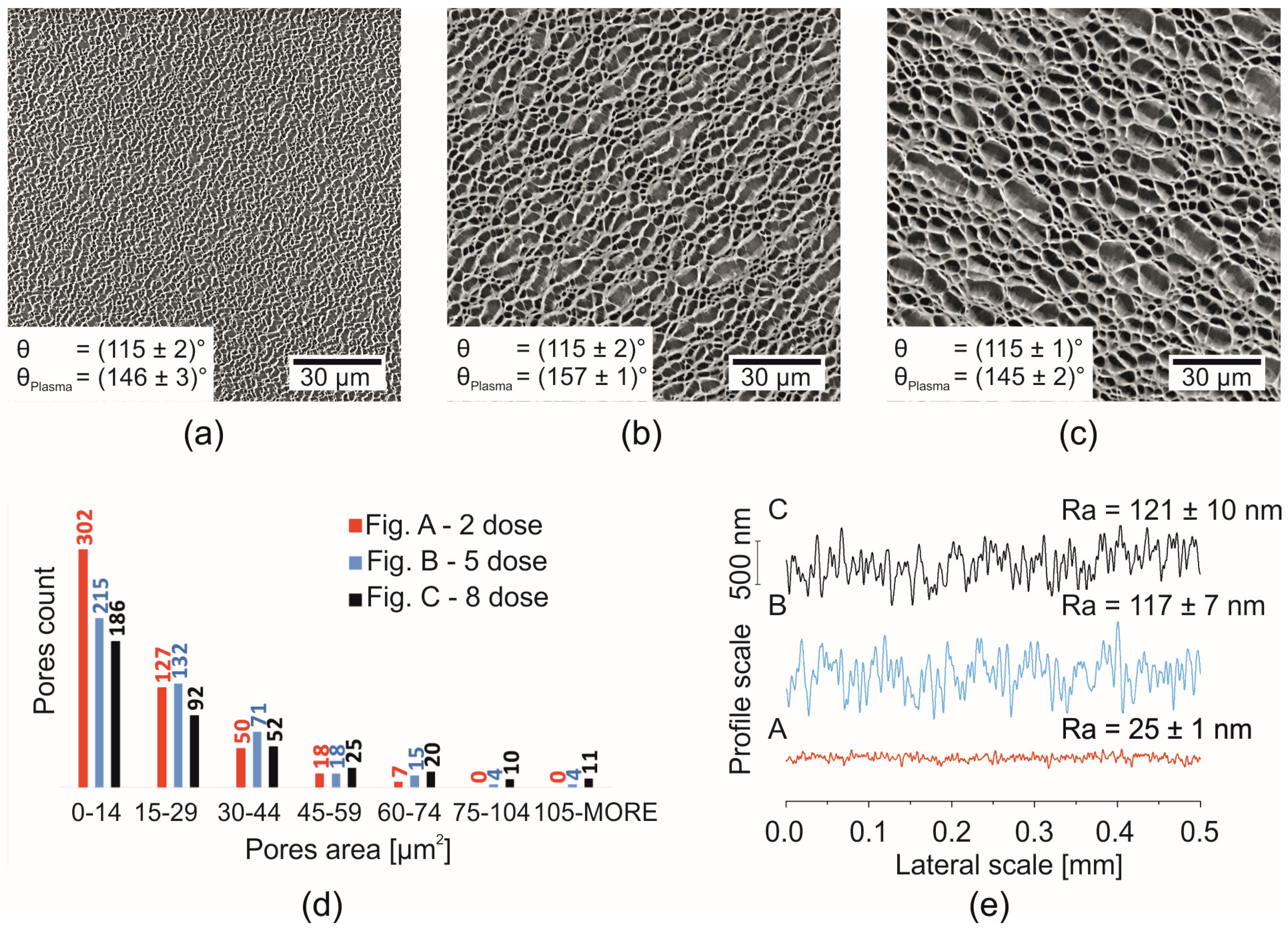
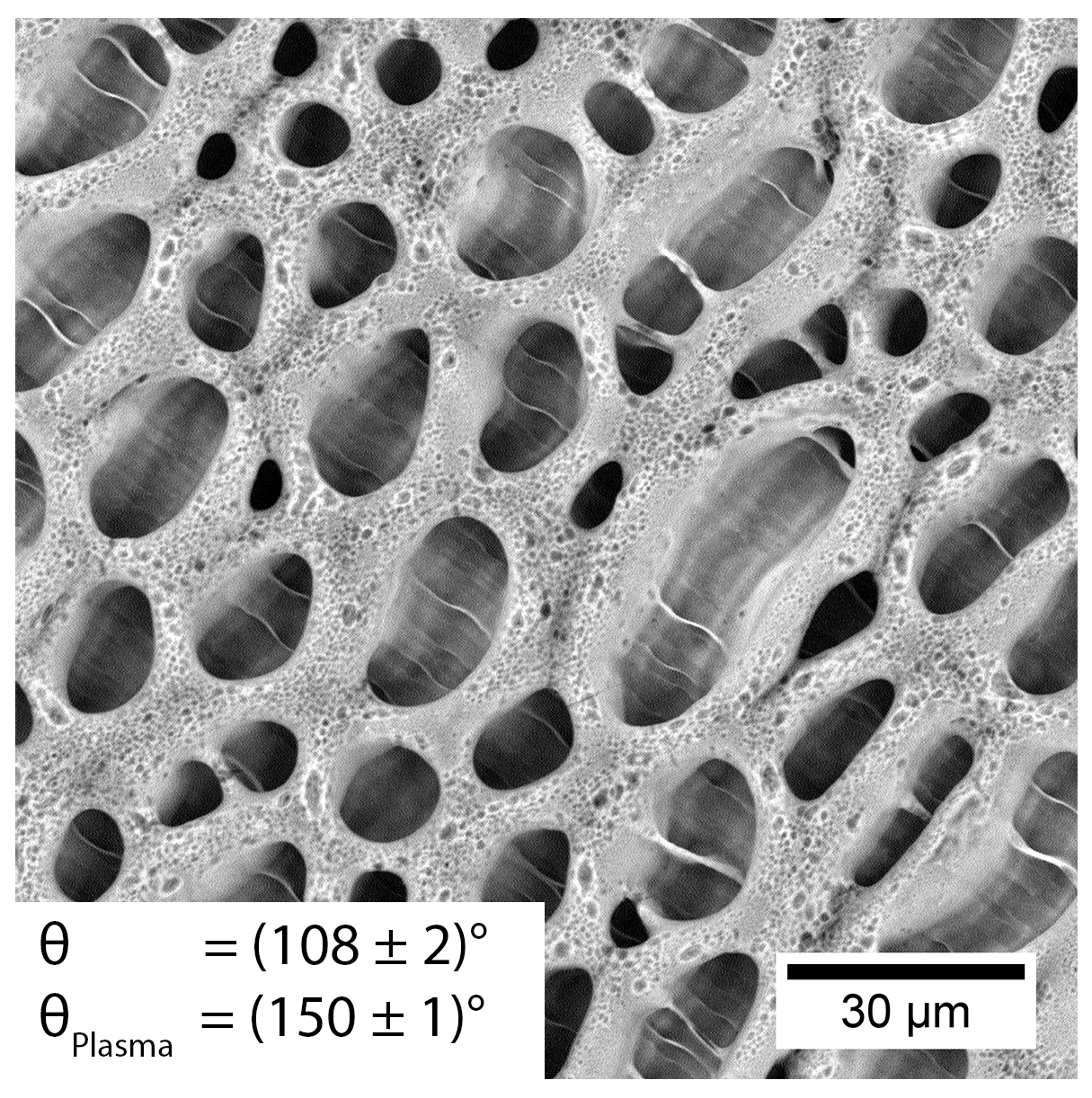
| Porous PS (Smooth Border) (XPS Characterization Immediately after Processing) | XPS Elemental Composition (± 0.5%) | θ [°] | ||
|---|---|---|---|---|
| C (%) | F (%) | O (%) | ||
| First plasma treatment | 38.4 | 56.7 | 5.0 | 142 ± 3 |
| Second plasma treatment | 43.0 | 56.3 | 0.7 | 157 ± 1 |
| Sample Type (XPS Characterization 14 Days after Processing) | XPS Elemental Composition (± 0.5%) | Type of Chemical Bonds | ||||||
|---|---|---|---|---|---|---|---|---|
| C (%) | F (%) | O (%) | C–C (%) | C–CF (%) | C–F (%) | CF2 (%) | CF3 (%) | |
| Flat PS | 96.6 | – | 2.6 | 100.0 | – | – | – | – |
| Plasma—Flat PS | 44.8 | 54.6 | 0.6 | 23.7 | 12.1 | 14.1 | 37.2 | 13.0 |
| Porous PS (smooth border) | 94.6 | – | 3.5 | 100.0 | – | – | – | – |
| Plasma—Porous PS (smooth border) | 42.9 | 56.6 | 0.5 | 15.6 | 15.0 | 22.2 | 38.2 | 9.1 |
| Porous PS (porous border) | 97.0 | – | 2.5 | 100.0 | – | – | – | – |
| Plasma—Porous PS (porous border) | 41.7 | 58.3 | 1.0 | 14.2 | 11.6 | 24.3 | 37.7 | 12.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minařík, M.; Wrzecionko, E.; Minařík, A.; Grulich, O.; Smolka, P.; Musilová, L.; Junkar, I.; Primc, G.; Ptošková, B.; Mozetič, M.; et al. Preparation of Hierarchically Structured Polystyrene Surfaces with Superhydrophobic Properties by Plasma-Assisted Fluorination. Coatings 2019, 9, 201. https://doi.org/10.3390/coatings9030201
Minařík M, Wrzecionko E, Minařík A, Grulich O, Smolka P, Musilová L, Junkar I, Primc G, Ptošková B, Mozetič M, et al. Preparation of Hierarchically Structured Polystyrene Surfaces with Superhydrophobic Properties by Plasma-Assisted Fluorination. Coatings. 2019; 9(3):201. https://doi.org/10.3390/coatings9030201
Chicago/Turabian StyleMinařík, Martin, Erik Wrzecionko, Antonín Minařík, Ondřej Grulich, Petr Smolka, Lenka Musilová, Ita Junkar, Gregor Primc, Barbora Ptošková, Miran Mozetič, and et al. 2019. "Preparation of Hierarchically Structured Polystyrene Surfaces with Superhydrophobic Properties by Plasma-Assisted Fluorination" Coatings 9, no. 3: 201. https://doi.org/10.3390/coatings9030201
APA StyleMinařík, M., Wrzecionko, E., Minařík, A., Grulich, O., Smolka, P., Musilová, L., Junkar, I., Primc, G., Ptošková, B., Mozetič, M., & Mráček, A. (2019). Preparation of Hierarchically Structured Polystyrene Surfaces with Superhydrophobic Properties by Plasma-Assisted Fluorination. Coatings, 9(3), 201. https://doi.org/10.3390/coatings9030201