Low-Pressure Plasma Sterilization for Test Specimens to be Worn on Splints in the Oral Cavity
Abstract
:1. Introduction
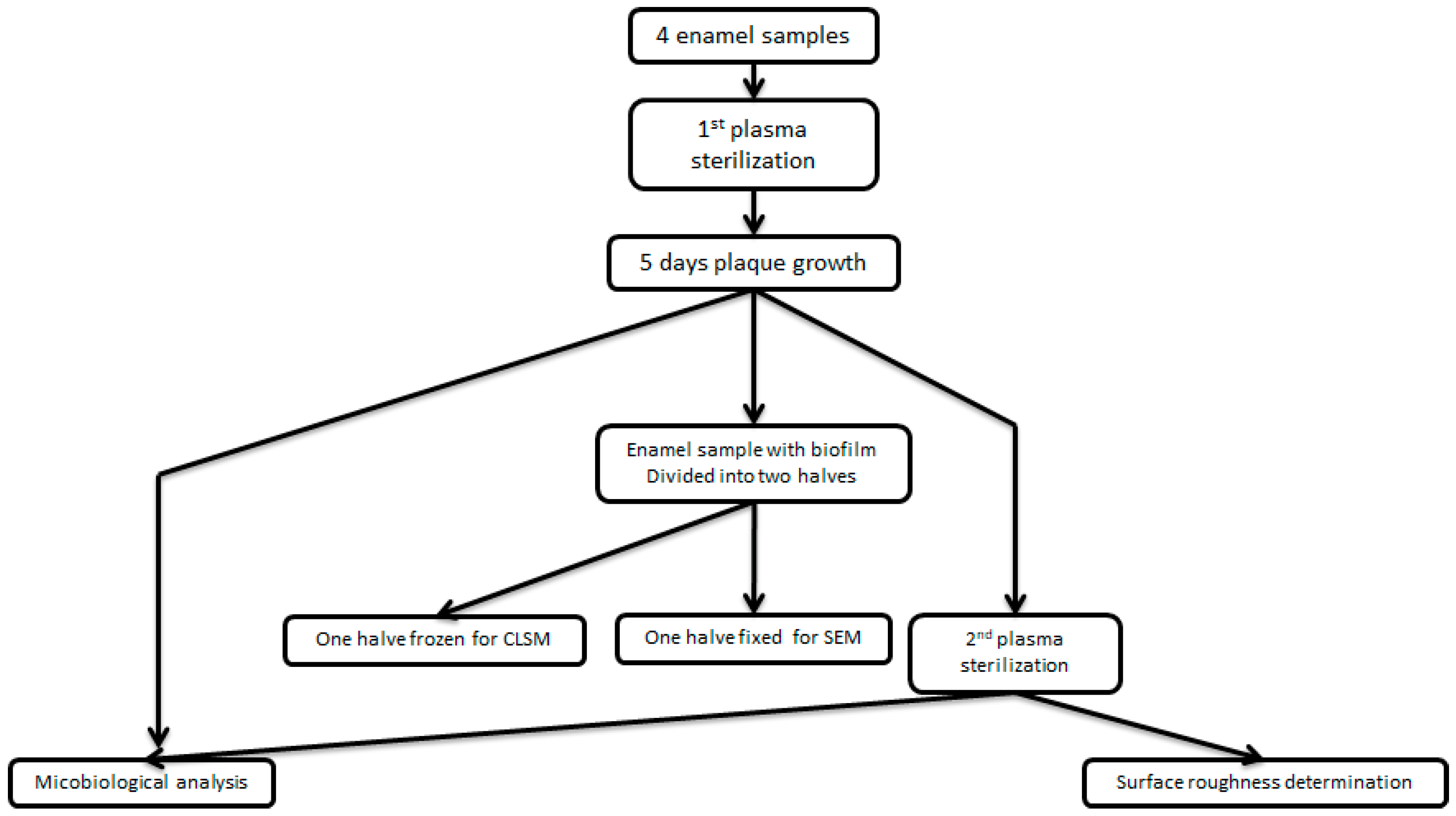
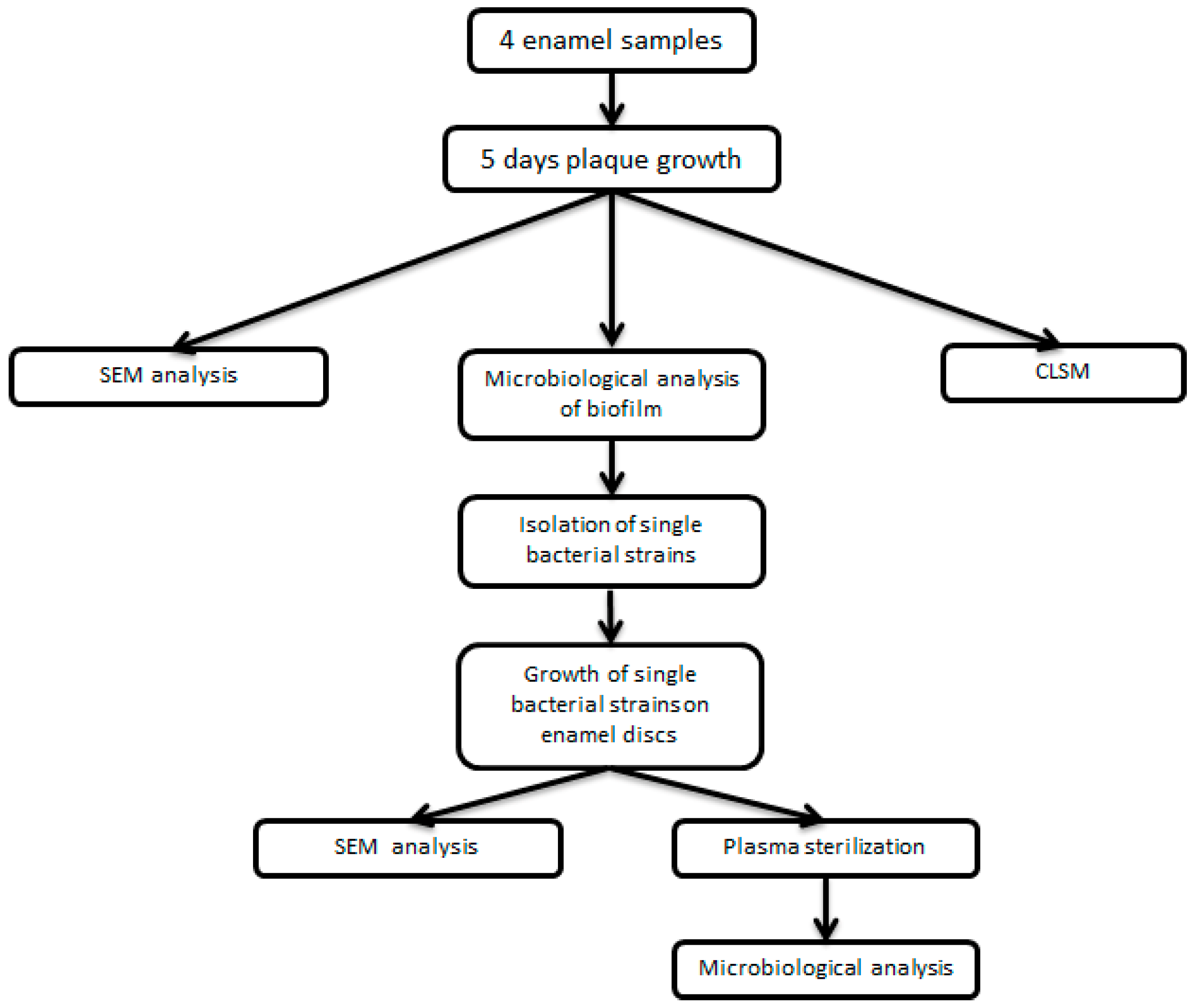
2. Material and Methods
2.1. Study Design
2.2. Electron Microscopy (SEM)
2.3. Laser Scanning Microscopy
2.4. Surface Roughness Determination
2.5. Plasma Sterilization
2.6. Microbiology
2.7. Statistical Analysis
3. Results
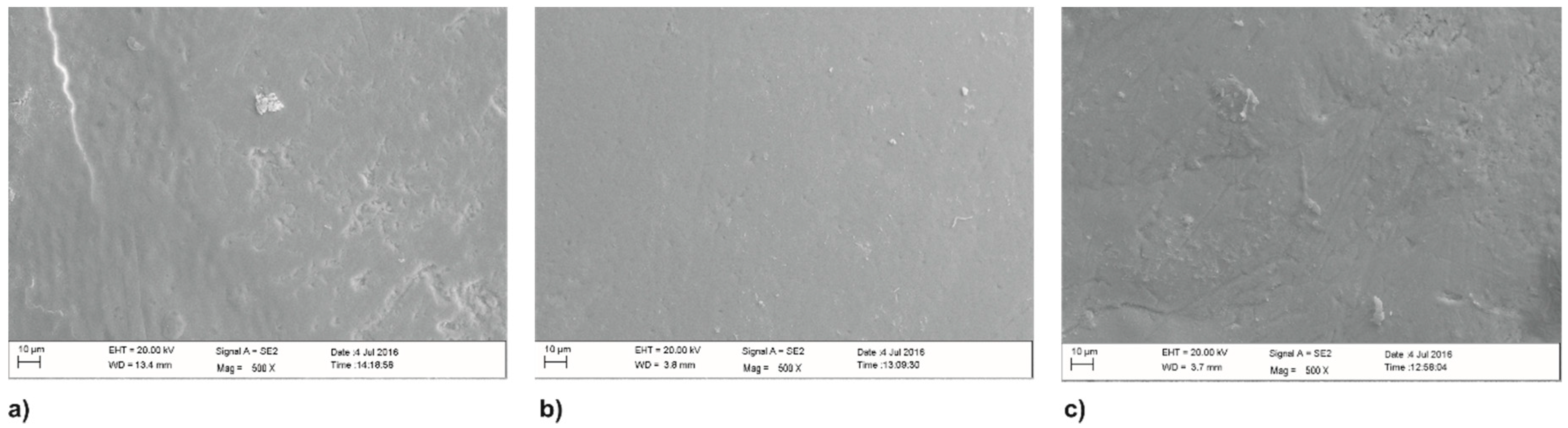
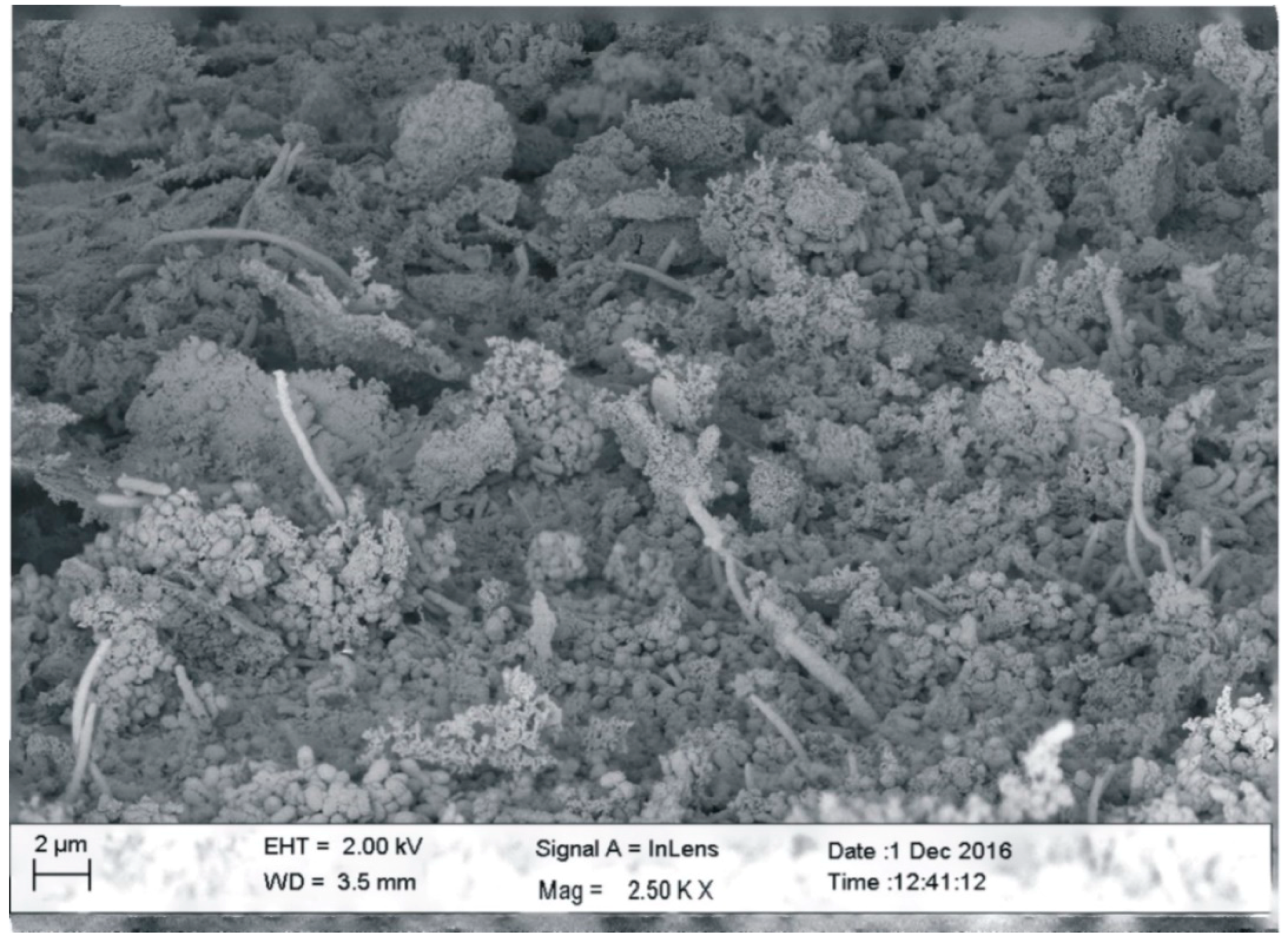
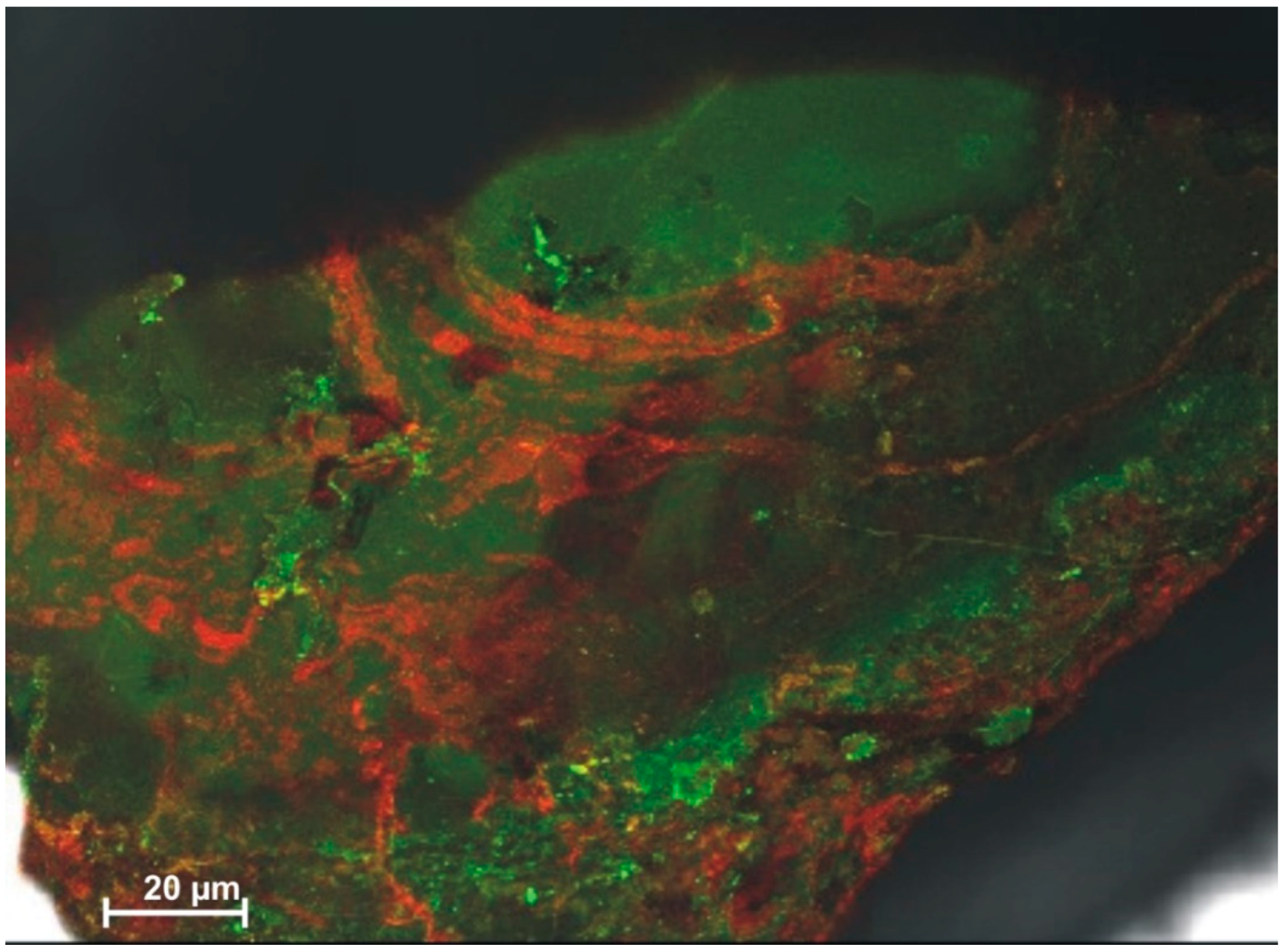
3.1. Scanning Electron Microscopy (SEM)
3.2. Laser Scanning Microscopy (CLSM)
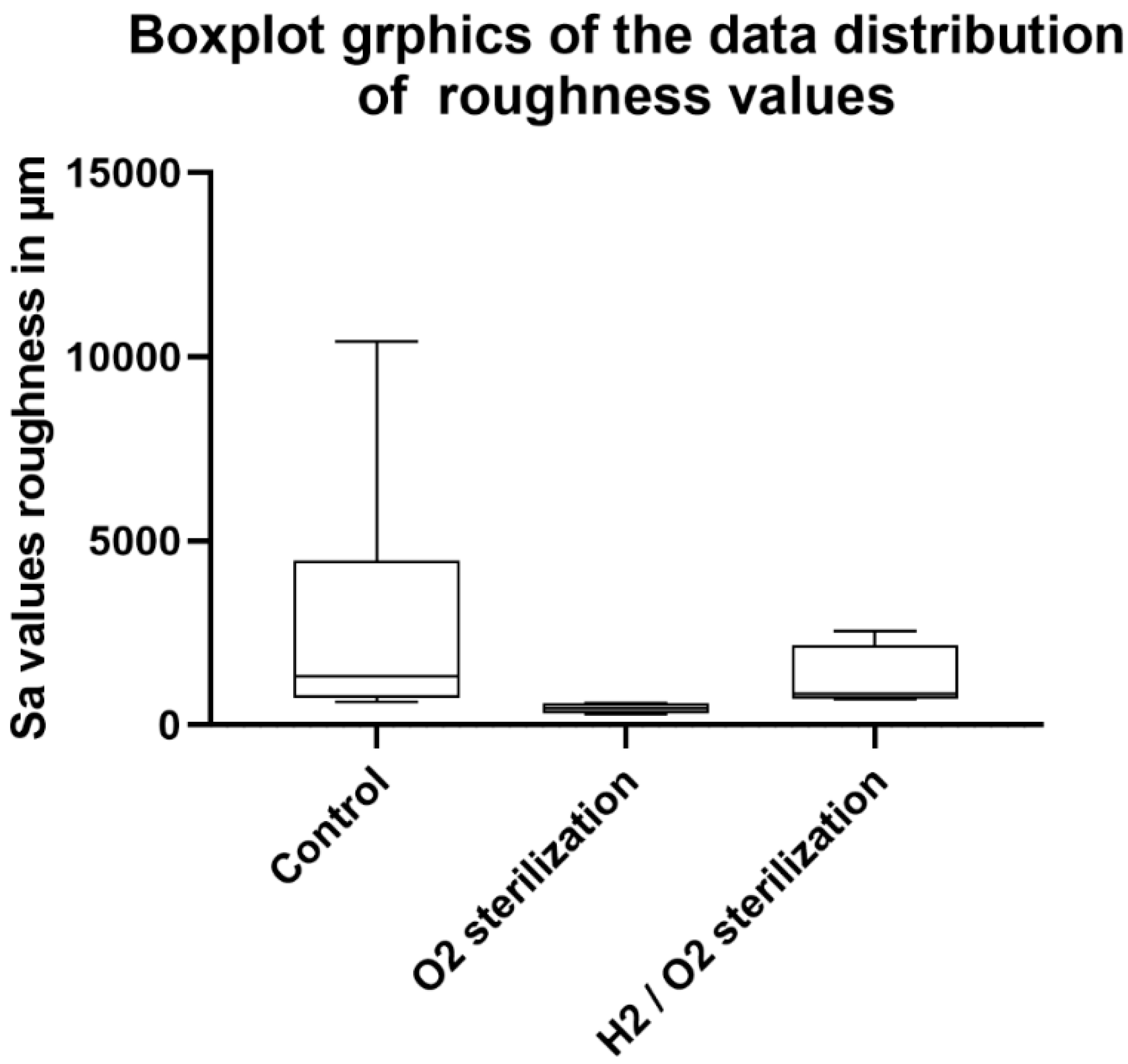
Surface Roughness Determination
3.3. Microbiology
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Marsh, P.D.; Devine, D.A. How is the development of dental biofilms influenced by the host? J. Clin. Periodontal. 2011, 11, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Auschill, T.M.; Arweiler, N.B.; Brecx, M.; Reich, E.; Sculean, A.; Netuschil, L. The effect of dental restorative materials on dental biofilm. Eur. J. Oral Sci. 2002, 110, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontal. 2005, 6 (Suppl. 32), 7–15. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Tam, A.; Aharoni, R.; Steinberg, D. Genetic adaptation of Streptococcus mutans during biofilm formation on different types of surfaces. BMC Microbiol. 2010, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015. [CrossRef] [PubMed]
- Souza, J.C.; Mota, R.R.; Sordi, M.B.; Passoni, B.B.; Benfatti, C.A.; Magini, R.S. Biofilm formation on different materials used in oral rehabilitation. Braz. Dent. J. 2016, 27, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Bowden, G.H. The effect of environmental pH and fluoride from the substratum on the development of biofilms of selected oral bacteria. J. Dent. Res. 1994, 73, 1615–1626. [Google Scholar] [CrossRef]
- Van Dijken, J.W.; Kalfas, S.; Litra, V.; Oliveby, A. Fluoride and mutans streptococci levels in plaque on aged restorations of resin-modified glass ionomer cement, compomer and resin composite. Caries Res. 1997, 31, 379–383. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection and sterilization: An overview. Am. J. Infect. Control 2013, 41, S2–S5. [Google Scholar] [CrossRef]
- Araujo, P.A.; Machado, I.; Meireles, A.; Leiknes, T.; Mergulhao, F.; Melo, L.F.; Simoes, M. Combination of selected enzymes with cetyltrimethylammonium bromide in biofilm inactivation, removal and regrowth. Food Res. Int. 2017, 95, 101–107. [Google Scholar] [CrossRef]
- Ercan, U.K.; Joshi, S.S.; Yost, A.; Gogotsi, N.; O’Toole, S.; Paff, M.; Melchior, E.; Joshi, S.G. Inhibition of biofilms by non-thermal plasma treated novel solutions. Adv. Microbiol. 2014, 4, 1188–1196. [Google Scholar] [CrossRef]
- Johansen, C.; Falholt, P.; Gram, L. Enzymatic removal and disinfection of bacterial biofilms. Appl. Environ. Microbiol. 1997, 63, 3724–3728. [Google Scholar] [PubMed]
- Bak, J.; Ladefoged, S.D.; Tvede, M.; Begovic, T.; Gregersen, A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling 2009, 25, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M.; David Woolfson, A. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J. Photochem. Photobiol. B Biol. 2007, 86, 59–69. [Google Scholar] [CrossRef]
- Kim, C.; Hung, Y.-C.; Brackett, R.E.; Frank, J.F. Inactivation of Listeria monocytogenes biofilms by electrolyzed oxidizing water. J. Food. Process Preserv. 2001, 25, 91–100. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Grillo-Puertas, M.; Cerioni, L.; Rapisarda, V.A.; Volentini, S.I. Removal of pathogenic bacterial biofilms by combinations of oxidizing compounds. Can. J. Microbiol. 2015, 61, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Fiebrandt, M.; Lackmann, J.-W.; Stapelmann, K. From patent to product? 50 years of low-pressure plasma sterilization. Plasma Process. Polym. 2018, 15, 1800139. [Google Scholar] [CrossRef]
- Keudell, A.v.; Awakowicz, P.; Benedikt, J.; Raballand, V.; Yanguas-Gil, A.; Opretzka, J.; Floetgen, C.; Reuter, R.; Byelykh, L.; Halfmann, H.; et al. Inactivation of bacteria and biomolecules by low-pressure plasma discharges. Plasma Processes Polym. 2010, 7, 327–352. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Processes Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Lerouge, S.; Wertheimer, M.R.; Yahia, L.H. Plasma sterilization: A Review of parameters, mechanisms, and limitations. Plasmas Polym. 2001, 6, 175–187. [Google Scholar] [CrossRef]
- Moisan, M.; Boudam, K.; Carignan, D.; Kéroack, D.; Levif, P.; Barbeau, J.; Séguin, J.; Kutasi, K.; Elmoualij, B.; Thellin, O.; et al. Sterilization/disinfection of medical devices using plasma: The flowing afterglow of the reduced-pressure N2-O2 discharge as the inactivating medium. Eur. Phys. J. Appl. Phys. 2013, 63, 10001. [Google Scholar] [CrossRef]
- Priehn, M.; Denis, B.; Aumeier, P.; Kirchner, W.H.; Awakowicz, P.; Leichert, L.I. Sterilization of beehive material with a double inductively coupled low pressure plasma. J. Phys. D Appl. Phys 2016, 49, 374002. [Google Scholar] [CrossRef]
- Jongebloed, W.L.; Stokroos, I.; Van der Want, J.J.; Kalicharan, D. Non-coating fixation techniques or redundancy of conductive coating, low kV FE-SEM operation and combined SEM/TEM of biological tissues. J. Microsc. 1999, 193, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Lackmann, J.-W.; Fiebrandt, M.; Raguse, M.; Kartaschew, K.; Havenith, M.; Bandow, J.E.; Moeller, R.; Awakowicz, P.; Stapelmann, K. A combined low-pressure hydrogen peroxide evaporation plus hydrogen plasma treatment method for sterilization—Part 2: An intercomparison study of different biological systems. Plasma Processes Polym. 2016, 14, 201600199. [Google Scholar] [CrossRef]
- Stapelmann, K.; Fiebrandt, M.; Raguse, M.; Awakowicz, P.; Reitz, G.; Moeller, R. Utilization of low-pressure plasma to inactivate bacterial spores on stainless steel screws. Astrobiology 2013, 13, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Stapelmann, K.; Lackmann, J.-W.; Buerger, I.; Bandow, J.E.; Awakowicz, P. A H2 very high frequency capacitively coupled plasma inactivates glyceraldehyde 3-phosphate dehydrogenase(GapDH) more efficiently than UV photons and heat combined. J. Phys. D Appl. Phys. 2014, 47, 085402. [Google Scholar] [CrossRef]
- ISO 11138-1:2017 Sterilization of Health Care Products-Biological Indicators-Part 1: General Requirements; Standards South Africa: Pretoria, South Africa, 2007.
- Kolenbrander, P.E.; London, J. Adhere today, here tomorrow: Oral bacterial adherence. J. Bacterial. 1993, 175, 3247–3252. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded human microbiome project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef]
- Naumova, E.A.; Weber, L.; Pankratz, V.; Czenskowski, V.; Arnold, W.H. Bacterial viability in oral biofilm after tooth brushing with amine fluoride or sodium fluoride. Arch. Oral. Biol. 2019, 97, 91–96. [Google Scholar] [CrossRef]
- Rashid, H. The effect of surface roughness on ceramics used in dentistry: A review of literature. Eur. J. Dent. 2014, 8, 571–579. [Google Scholar] [CrossRef]
- Amaecha, B.T.; Higham, S.M.; Edgar, W.M. Effect of sterilisation methods on the structural integrity of artificial enamel caries for intra-oral cariogenicity tests. J. Dent. 1999, 27, 313–316. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Higham, S.M. Dental erosion: Possible approaches to prevention and control. J. Dent. 2005, 33, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Chandler, N.P. Preparation of dental enamel for use in intraoral cariogenicity experiments. J. Dent. 1990, 18, 54–58. [Google Scholar] [CrossRef]
- Stapelmann, K.; Fiebrandt, M.; Raguse, M.; Lackmann, J.-W.; Postema, M.; Moeller, R.; Awakowicz, P. A combined low-pressure hydrogen peroxide evaporation plus hydrogen plasma treatment method for sterilization-part 1: Characterization of the condensation process and proof-of-concept. Plasma Process. Polym. 2017, 14, 1600198. [Google Scholar] [CrossRef]


| Identified Isolates | Pre-Sterilization | Post-Sterilization |
|---|---|---|
| Streptococcus oralis/mitis | +++ | − |
| Staphylococcus hominis | +++ | − |
| Neisseria sp. | + | − |
| Veillonella sp. | + | − |
| Porphyromonas sp. | + | − |
| Prevotella sp. | + | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naumova, E.A.; Engel, A.-S.; Kranz, H.T.; Schneider, M.; Tietze, J.; Dittmar, T.; Fiebrandt, M.; Stapelmann, K.; Piwowarczyk, A.; Kuczius, T.; et al. Low-Pressure Plasma Sterilization for Test Specimens to be Worn on Splints in the Oral Cavity. Coatings 2019, 9, 99. https://doi.org/10.3390/coatings9020099
Naumova EA, Engel A-S, Kranz HT, Schneider M, Tietze J, Dittmar T, Fiebrandt M, Stapelmann K, Piwowarczyk A, Kuczius T, et al. Low-Pressure Plasma Sterilization for Test Specimens to be Worn on Splints in the Oral Cavity. Coatings. 2019; 9(2):99. https://doi.org/10.3390/coatings9020099
Chicago/Turabian StyleNaumova, Ella A., Alexander-Simon Engel, Hagen Tizian Kranz, Marvin Schneider, Jan Tietze, Thomas Dittmar, Marcel Fiebrandt, Katharina Stapelmann, Andree Piwowarczyk, Thorsten Kuczius, and et al. 2019. "Low-Pressure Plasma Sterilization for Test Specimens to be Worn on Splints in the Oral Cavity" Coatings 9, no. 2: 99. https://doi.org/10.3390/coatings9020099
APA StyleNaumova, E. A., Engel, A.-S., Kranz, H. T., Schneider, M., Tietze, J., Dittmar, T., Fiebrandt, M., Stapelmann, K., Piwowarczyk, A., Kuczius, T., & Arnold, W. H. (2019). Low-Pressure Plasma Sterilization for Test Specimens to be Worn on Splints in the Oral Cavity. Coatings, 9(2), 99. https://doi.org/10.3390/coatings9020099







