Effects of Strontium-Hydroxyapatite Mediated Active Compounds from Hippocampus Kuda Bleeler (HKB) on Osteogenesis
Abstract
1. Introduction
2. Materials and Methods
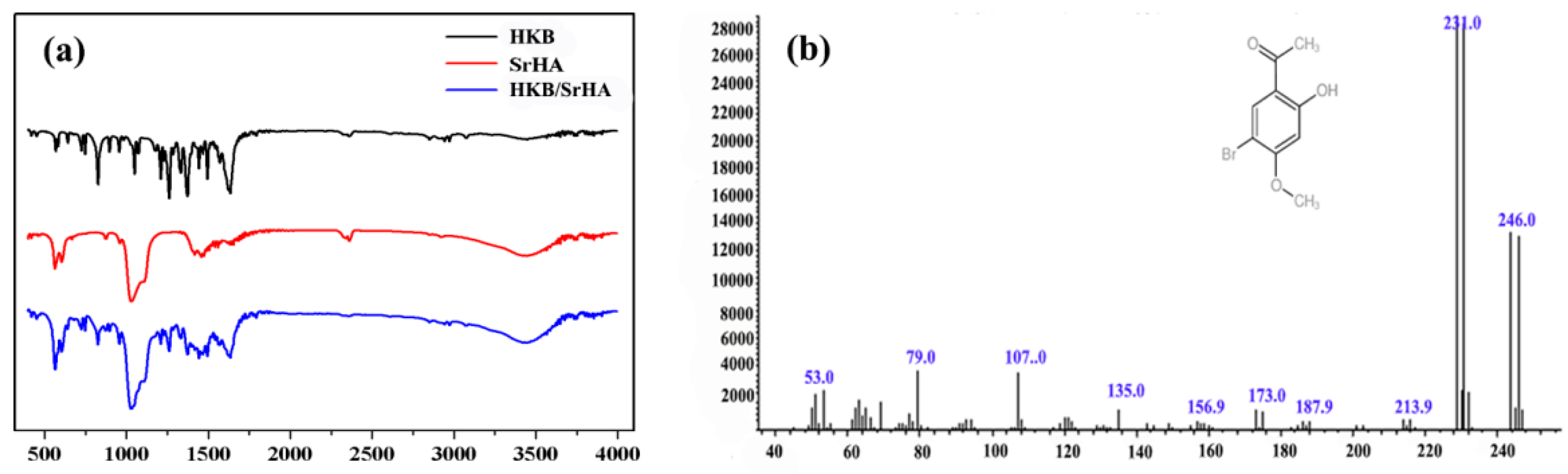
2.1. Extraction of 1-(5-Bromo-2-hydroxy-4-methoxyphenyl) Ethanone (HKB)
2.2. Synthesis and Characterization of Strontium-Doped Hydroxyapatite Microspheres
2.3. Preparation of HKB/SrHA Composite Microspheres
2.4. Culture of MC3T3-E1
2.5. Cell Attachment Experiment (Fluorescence Staining)
2.6. Examination of Cell Proliferation (MTT Method)
2.7. Determination of Alkaline Phosphatase (ALP)
2.8. Statistics
3. Results
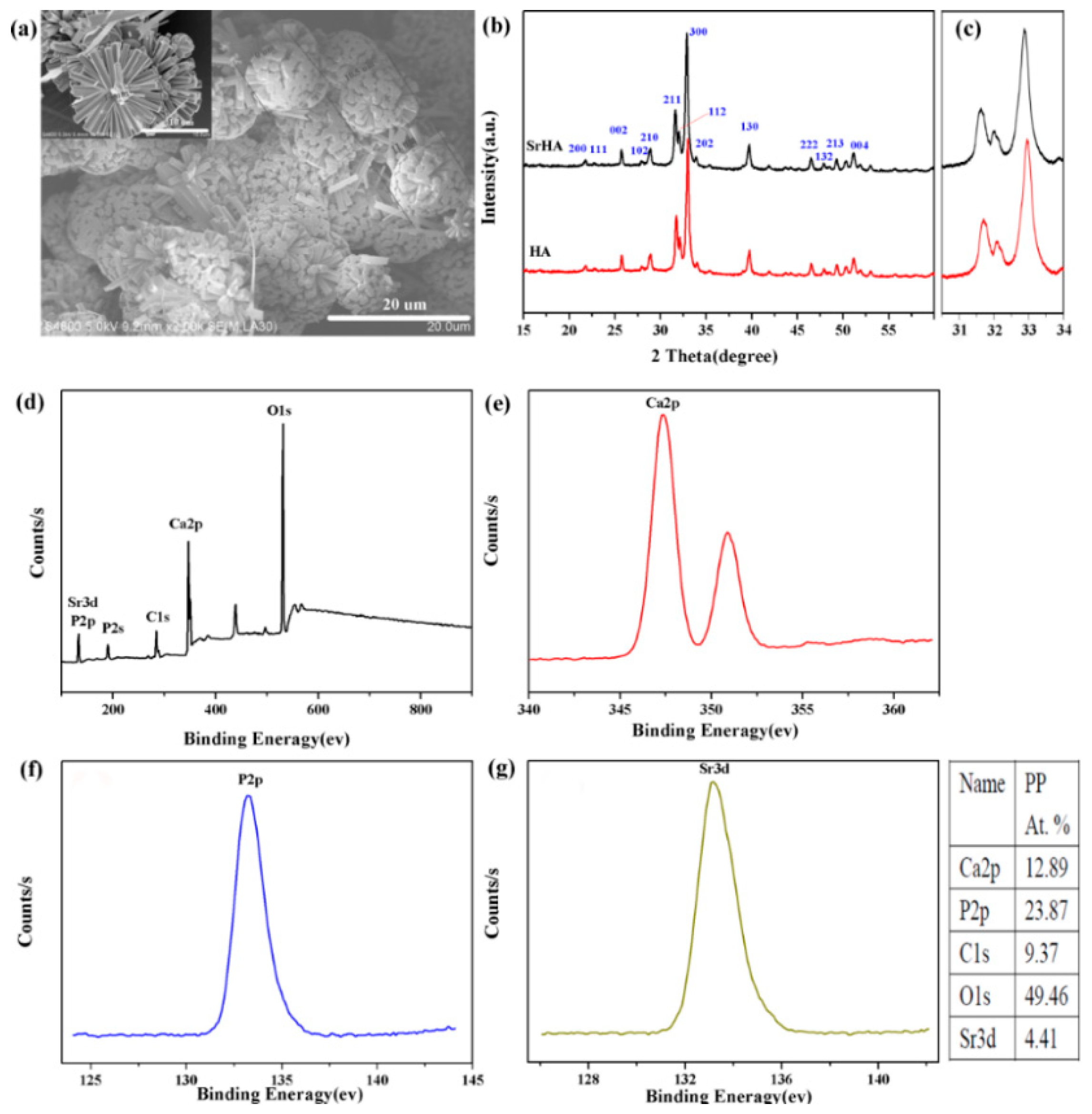
3.1. Characterization of Strontium-Doped Hydroxyapatite Microspheres
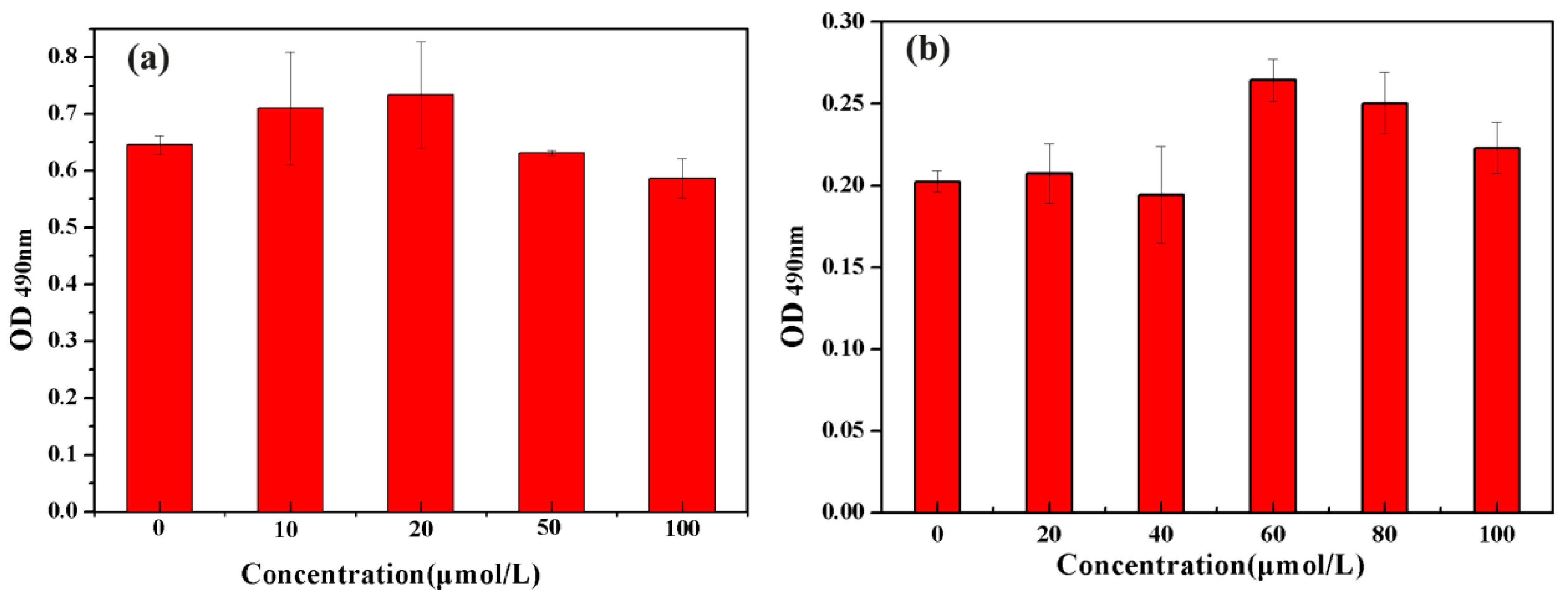
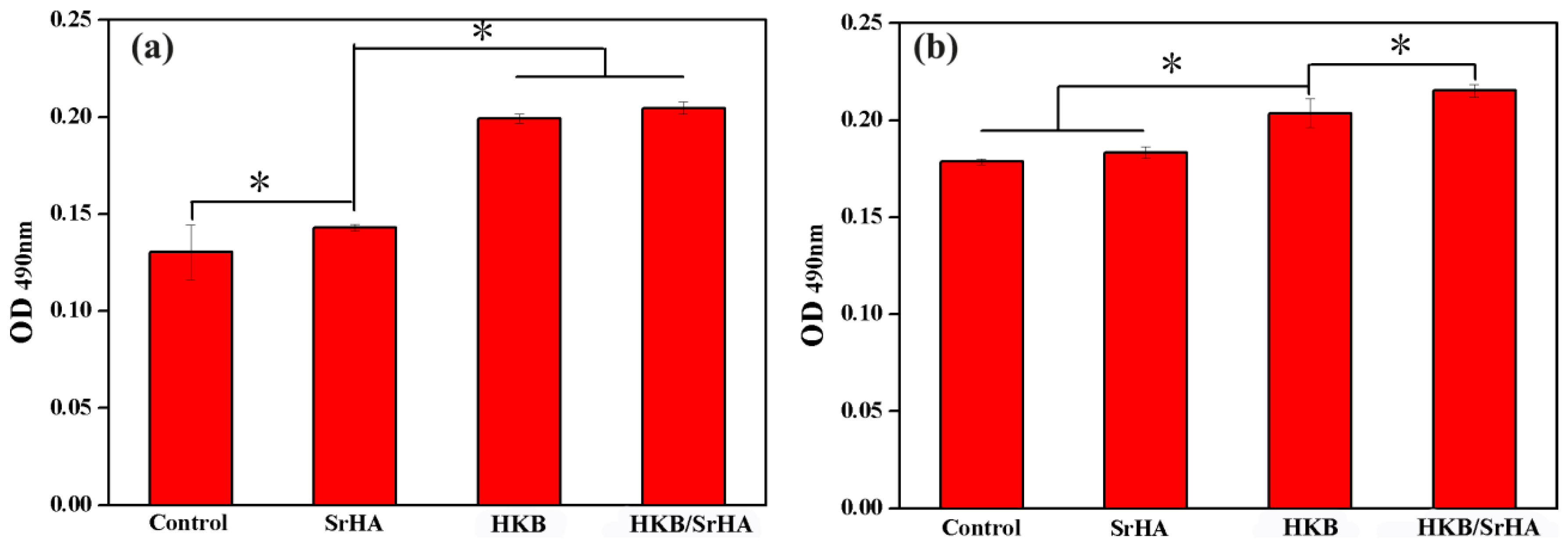
3.2. Screening of Optimal Concentrations of HKB and SrHA Co-Cultured with MC3T3-E1 Cells
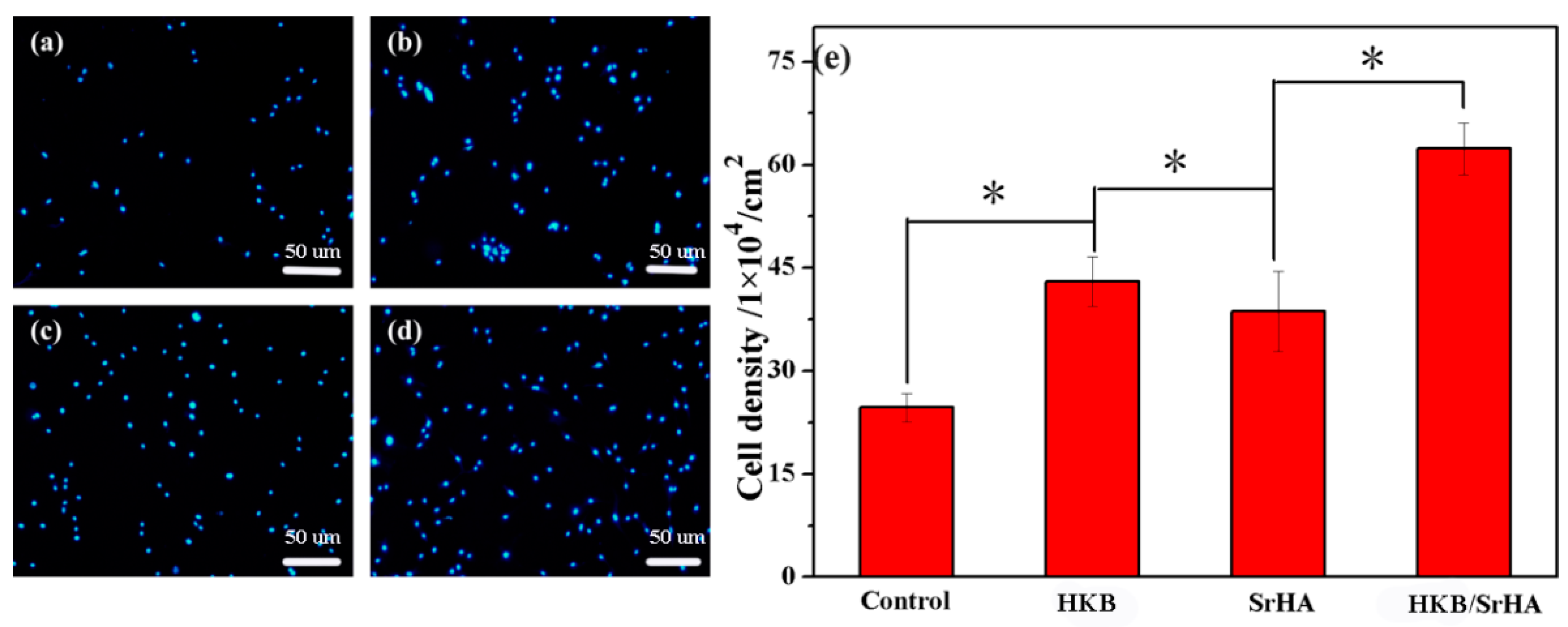
3.3. Evaluation of Adhesion Ability of Pre-Osteoblasts under Different Conditions
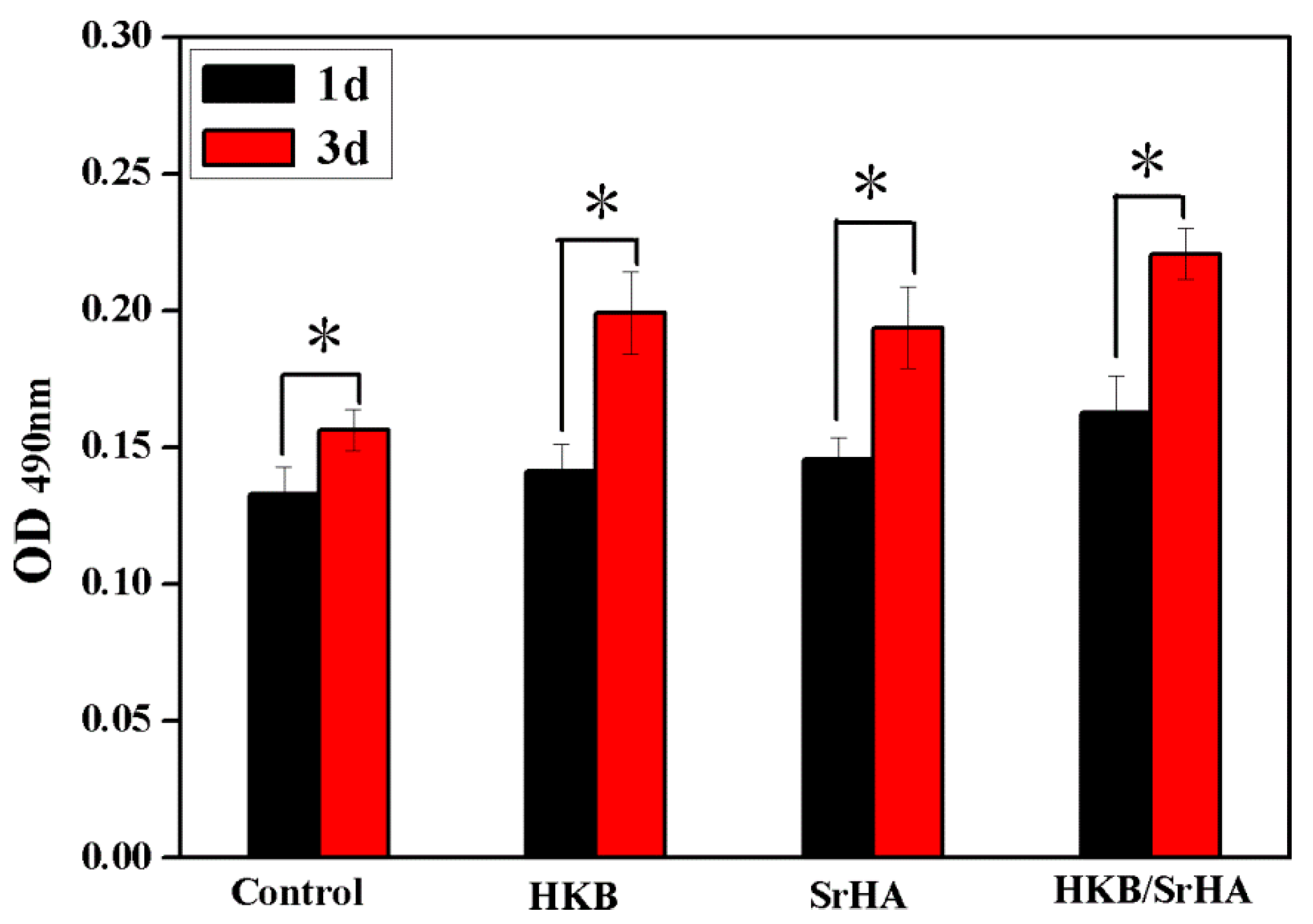
3.4. Effect of HKB/SrHA on Proliferation
3.5. Effect of HKB/SrHA on ALP Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, H.J.; Minashima, T.; Mccarthy, E.F.; Winkles, J.A.; Kirsch, T. Progressive ankylosis protein (ANK) in osteoblasts and osteoclasts controls bone formation and bone remodeling. J. Bone Miner. Res. 2010, 25, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Li, Y.; Wang, Y.; Cui, J.; Feng, K.; Kong, X.; Chen, L. Psoralidin, a prenylated coumestan, as a novel anti-osteoporosis candidate to enhance bone formation of osteoblasts and decrease bone resorption of osteoclasts. Eur. J. Pharmacol. 2017, 801, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Pro-Risquez, A.; Harris, S.S.; Song, L.; Rudicel, S.; Barnewolt, B.; Dawson-Hughes, B. Calcium supplement and osteoporosis medication use in women and men with recent fractures. Osteoporos. Int. 2004, 15, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, W.; Hai, G.; Zhang, Y. Effect of calcium supplement on superoxide dismutase and malonaldehyde of disuse osteoporosis in young rats. J. Hyg. Res. 2003, 32, 49–50. [Google Scholar]
- Sanfelix-Genovés, J.; Gil-Guillén, V.F.; Orozco-Beltran, D.; Giner-Ruiz, V.; Pertusa-Martínez, S.; Reig-Moya, B.; Carratalá, C. Determinant Factors of Osteoporosis Patients’ Reported Therapeutic Adherence to Calcium and/or Vitamin D Supplements. Drugs Aging 2009, 26, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Aobulikasimu, A.; Piao, J.; Aibibula, Z.; Koga, D.; Sato, S.; Ochi, H.; Tsuji, K.; Nakabayashi, T.; Miyata, T. A small-moleculePAI-1 inhibitor prevents bone loss by stimulating bone formation in a murine estrogen deficiency-induced osteoporosis model. Febs. Open Biol. 2018, 8, 523–532. [Google Scholar] [CrossRef] [PubMed]
- García, P.V.; Robinson, L.J.; Borysenko, C.W.; Lehmann, T.; Kalla, S.E.; Blair, H.C. Negative regulation of RANKL-induced osteoclastic differentiation in RAW264.7 Cells by estrogen and phytoestrogens. J. Biol. Chem. 2005, 280, 13720–13727. [Google Scholar] [CrossRef]
- Carano, A.; Teitelbaum, S.L.; Konsek, J.D.; Schlesinger, P.H.; Blair, H.C. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J. Clin. Investig. 1990, 85, 456–461. [Google Scholar] [CrossRef]
- Yin, X.X.; Chen, Z.Q.; Dang, G.T.; Ma, Q.J.; Liu, Z.J. Effects of Epimedium pubescens icariine on proliferation and differentiation of human osteoblasts. China J. Chin. Mater. Med. 2005, 30, 289–291. [Google Scholar]
- Zong, S.; Zeng, G.; Zou, B.; Li, K.; Fang, Y.; Lu, L.; Xiao, D.; Zhang, Z. Effects of Polygonatum sibiricum polysaccharide on the osteogenic differentiation of bone mesenchymal stem cells in mice. Int. J. Clin. Exp. Pathol. 2015, 8, 6169–6180. [Google Scholar]
- Kocabey, S.; Ceylan, H.; Tekinay, A.B.; Guler, M.O. Glycosaminoglycan mimetic peptide nanofibers promote mineralization by osteogenic cells. Acta Biomater. 2013, 9, 9075–9085. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.M.; Linnehan, S.K. Hydroxyapatite formed from Coral Skeletal Carbonate by Hydrothermal Exchange. Nature 1974, 247, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, Y.; Du, Z.; Weng, Z.; Yao, W.; Zhang, X.; Huang, X.; Yao, X.; Crawford, R.; Hang, R. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018, 76, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Sandeman, S.R.; Jeffery, H.; Howell, C.A.; Smith, M.; Mikhalovsky, S.V.; Lloyd, A.W. The in vitro corneal biocompatibility of hydroxyapatite coated carbon mesh. Biomaterials 2009, 30, 3143–3149. [Google Scholar] [CrossRef]
- Milovac, D.; Gamboa-Martínez, T.C.; Ivankovic, M.; Ferrer, G.G.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: In vitro cell culture studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ko, I.H.; Jeon, S.-H.; Chae, J.-H.; Chang, J.H. Micro-structured hydroxyapatite microspheres for local delivery of alendronate and BMP-2 carriers. Mater. Lett. 2013, 105, 136–139. [Google Scholar] [CrossRef]
- Alessandro, P.; Daniela, I.; Silvia, P.; Monica, M.; Anna, T.; Signorino, G. Hydroxyapatite-magnetite-MWCNT nanocomposite as a biocompatible multifunctional drug delivery system for bone tissue engineering. Nanotechnology 2014, 25, 425701. [Google Scholar]
- Guo, Y.J.; Long, T.; Chen, W.; Ning, C.Q.; Zhu, Z.A.; Guo, Y.P. Bactericidal property and biocompatibility of gentamicin-loaded mesoporous carbonated hydroxyapatite microspheres. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3583–3591. [Google Scholar] [CrossRef]
- Palazzo, B.; Iafisco, M.; Laforgia, M.; Margiotta, N.; Natile, G.; Bianchi, C.L.; Walsh, D.; Mann, S.; Roveri, N. Biomimetic Hydroxyapatite–Drug Nanocrystals as Potential Bone Substitutes with Antitumor Drug Delivery Properties. Adv. Funct. Mater. 2010, 17, 2180–2188. [Google Scholar] [CrossRef]
- Prodan, D.; Moldovan, M.; Prejmerean, C.; Silaghi-Dumitrescu, L.; Boboia, S.; Popescu, V.; Pascalau, V.; Molea, A.; Diana, L.; Perhaiå£A, I. Synthesis and Characterization of an Experimental Zn-Hydroxyapatite Powders with Application in Dentistry. Key Eng. Mater. 2013, 587, 43–51. [Google Scholar] [CrossRef]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-substituted hydroxyapatite: From synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef]
- Atkins, G.; Welldon, K.P.; Findlay, D. Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos. Int. 2009, 20, 653–664. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Z.; Yang, P.; Xu, Z.; Peng, C.; Li, G.; Lin, J. Architectures of strontium hydroxyapatite microspheres: Solvothermal synthesis and luminescence properties. Langmuir 2009, 25, 13591–13598. [Google Scholar] [CrossRef]
- Lin, K.; Liu, P.; Wei, L.; Zou, Z.; Zhang, W.; Qian, Y.; Shen, Y.; Chang, J. Strontium substituted hydroxyapatite porous microspheres: Surfactant-free hydrothermal synthesis, enhanced biological response and sustained drug release. Chem. Eng. J. 2013, 222, 49–59. [Google Scholar] [CrossRef]
- Sanaye, S.V.; Pise, N.M.; Pawar, A.P.; Parab, P.P.; Sreepada, R.A.; Pawar, H.B.; Revankar, A.D. Evaluation of antioxidant activities in captive-bred cultured yellow seahorse, Hippocampus kuda (Bleeker, 1852). Aquaculture 2014, 434, 100–107. [Google Scholar] [CrossRef]
- Sun, D.; Wu, S.; Jing, C.; Zhang, N.; Liang, D.; Xu, A. Identification, synthesis and characterization of a novel antimicrobial peptide HKPLP derived from Hippocampus kuda Bleeker. J. Antibiot. 2012, 65, 117–121. [Google Scholar] [CrossRef]
- Kang, N.; Kim, S.Y.; Rho, S.; Ko, J.Y.; Jeon, Y.J. Anti-fatigue activity of a mixture of seahorse (Hippocampus abdominalis ) hydrolysate and red ginseng. Fish. Aquat. Sci. 2017, 20, 3–10. [Google Scholar] [CrossRef]
- Nordberg, A.; Antoni, P.; Montañez, M.I.; Hult, A.; Von, H.H.; Malkoch, M. Highly adhesive phenolic compounds as interfacial primers for bone fracture fixations. ACS Appl. Mater. Interfaces 2010, 2, 654–657. [Google Scholar] [CrossRef]
- Manna, C.; Della, R.F.; Cucciolla, V.; Borriello, A.; D’Angelo, S.; Galletti, P.; Zappia, V. Biological effects of hydroxytyrosol, a polyphenol from olive oil endowed with antioxidant activity. Adv. Exp. Med. Biol. 1999, 472, 115–130. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yuan, Q.; He, L.; Qian, Z.-J.; Zhou, C.; Hong, P. Effects of Strontium-Hydroxyapatite Mediated Active Compounds from Hippocampus Kuda Bleeler (HKB) on Osteogenesis. Coatings 2019, 9, 141. https://doi.org/10.3390/coatings9020141
Li C, Yuan Q, He L, Qian Z-J, Zhou C, Hong P. Effects of Strontium-Hydroxyapatite Mediated Active Compounds from Hippocampus Kuda Bleeler (HKB) on Osteogenesis. Coatings. 2019; 9(2):141. https://doi.org/10.3390/coatings9020141
Chicago/Turabian StyleLi, Chengyong, Qiong Yuan, Lei He, Zhong-Ji Qian, Chunxia Zhou, and Pengzhi Hong. 2019. "Effects of Strontium-Hydroxyapatite Mediated Active Compounds from Hippocampus Kuda Bleeler (HKB) on Osteogenesis" Coatings 9, no. 2: 141. https://doi.org/10.3390/coatings9020141
APA StyleLi, C., Yuan, Q., He, L., Qian, Z.-J., Zhou, C., & Hong, P. (2019). Effects of Strontium-Hydroxyapatite Mediated Active Compounds from Hippocampus Kuda Bleeler (HKB) on Osteogenesis. Coatings, 9(2), 141. https://doi.org/10.3390/coatings9020141




