Fabrication of Cu2ZnSnS4 Thin Films Based on Facile Nanocrystals-Printing Approach with Rapid Thermal Annealing (RTA) Process
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis of CZTS NCs by Thermal Solution Route
2.2. Deposition of CZTS Thin Film by NCs-Printing Approach
2.3. Characterization Methods for CZTS Material
3. Results and Discussion
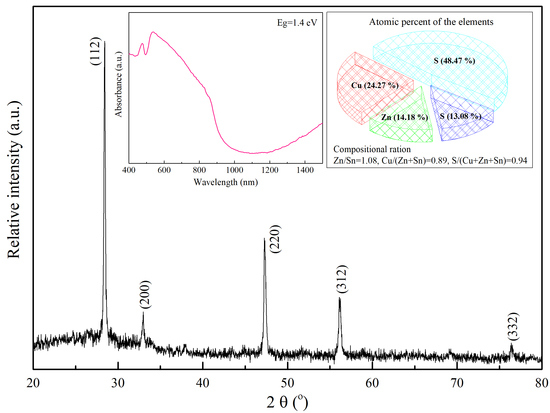
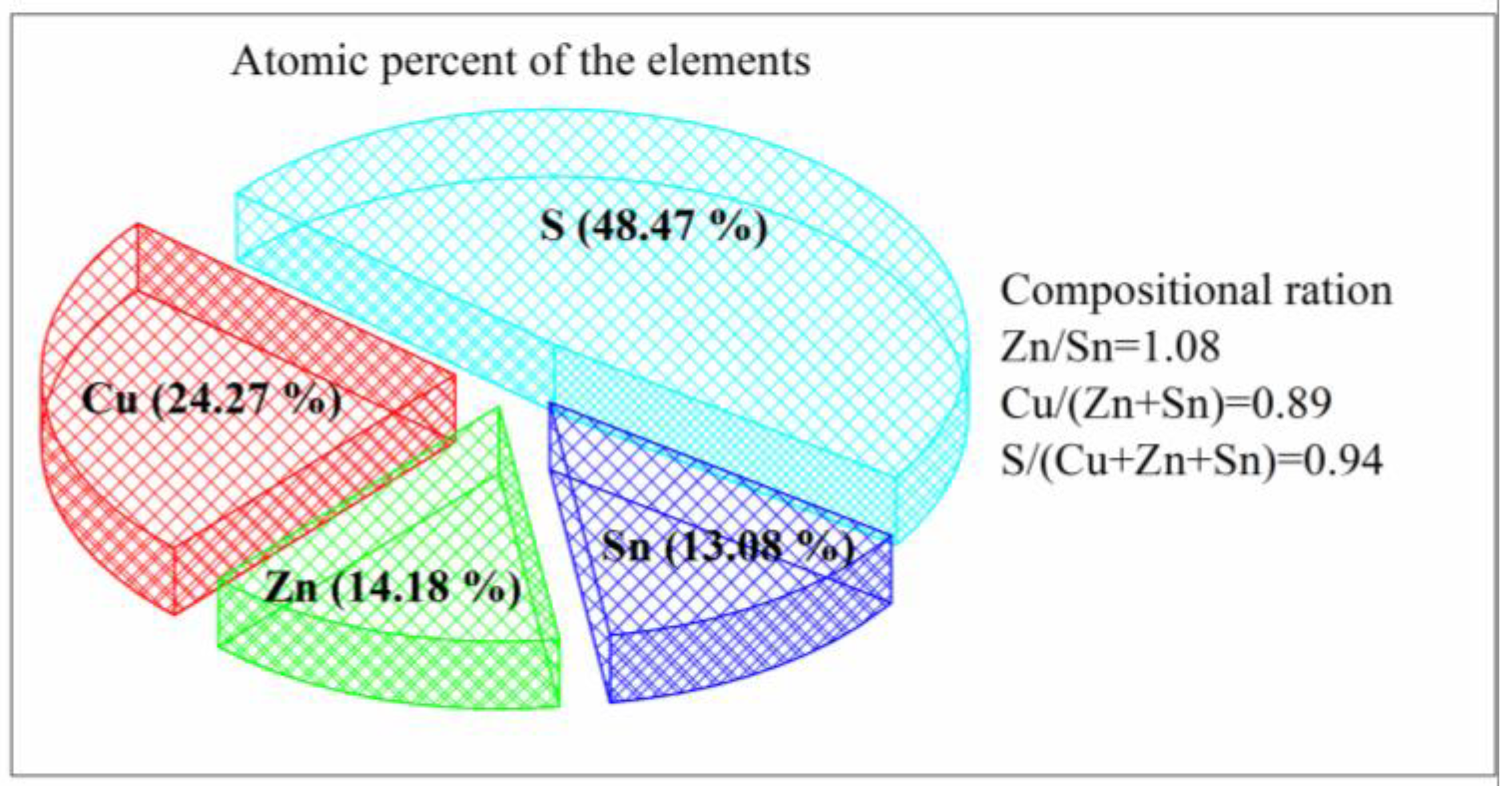
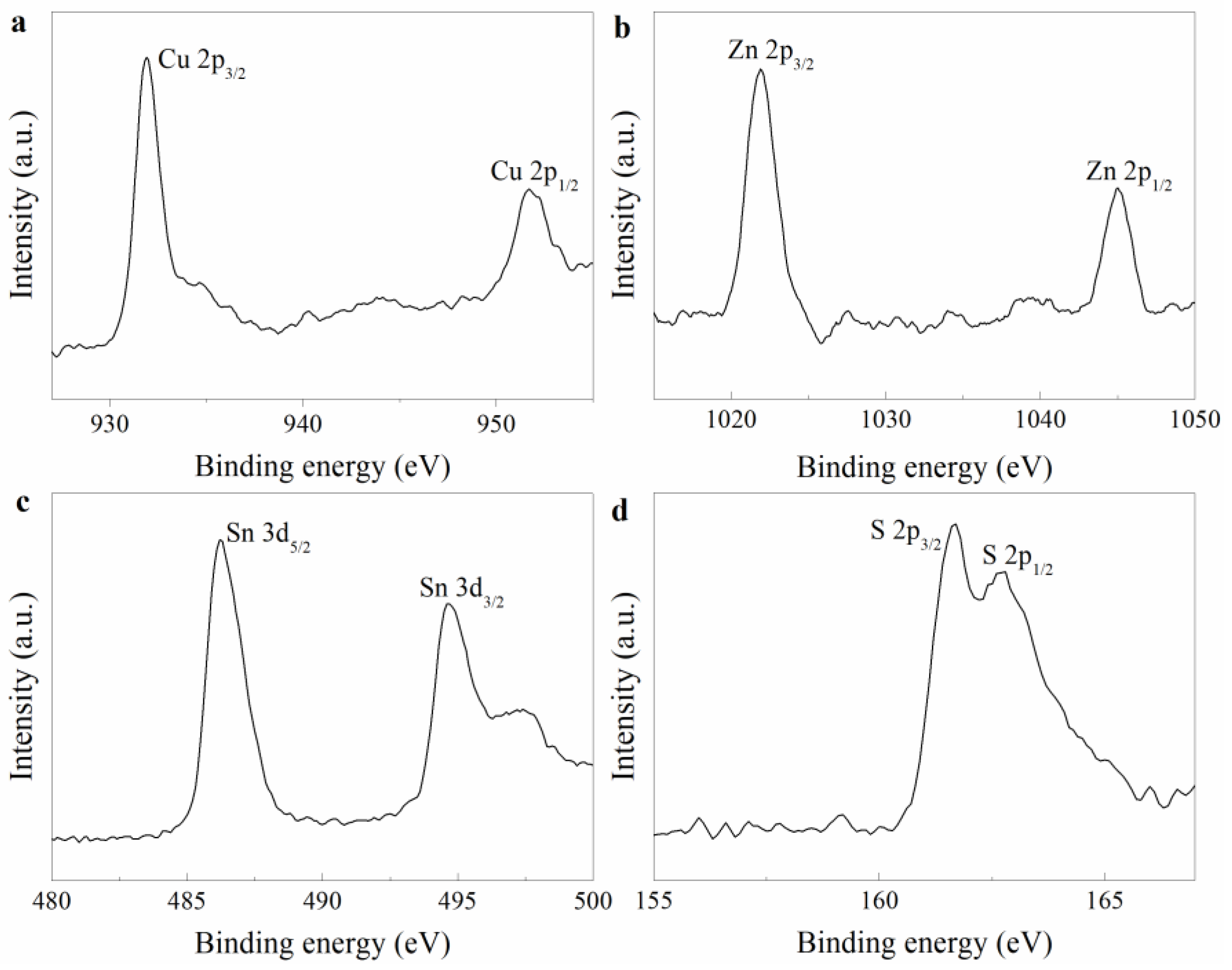
3.1. The Analysis of CZTS NCs Synthesis
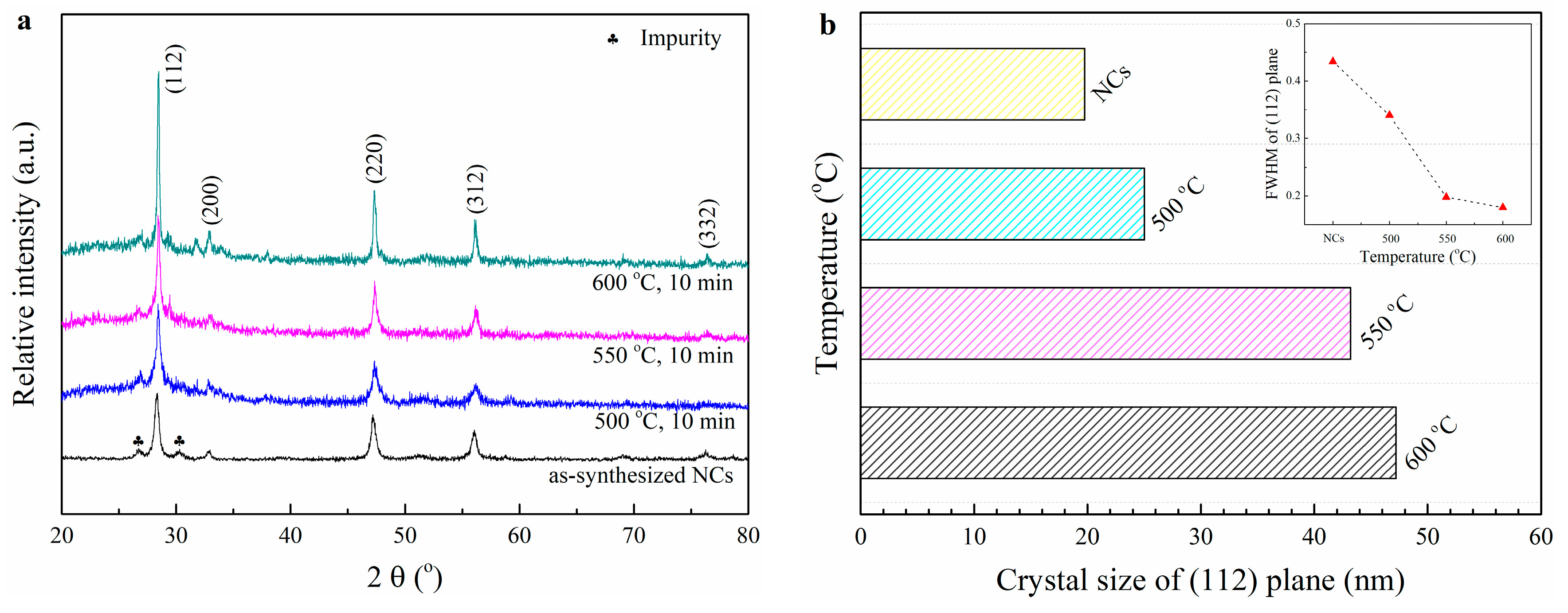
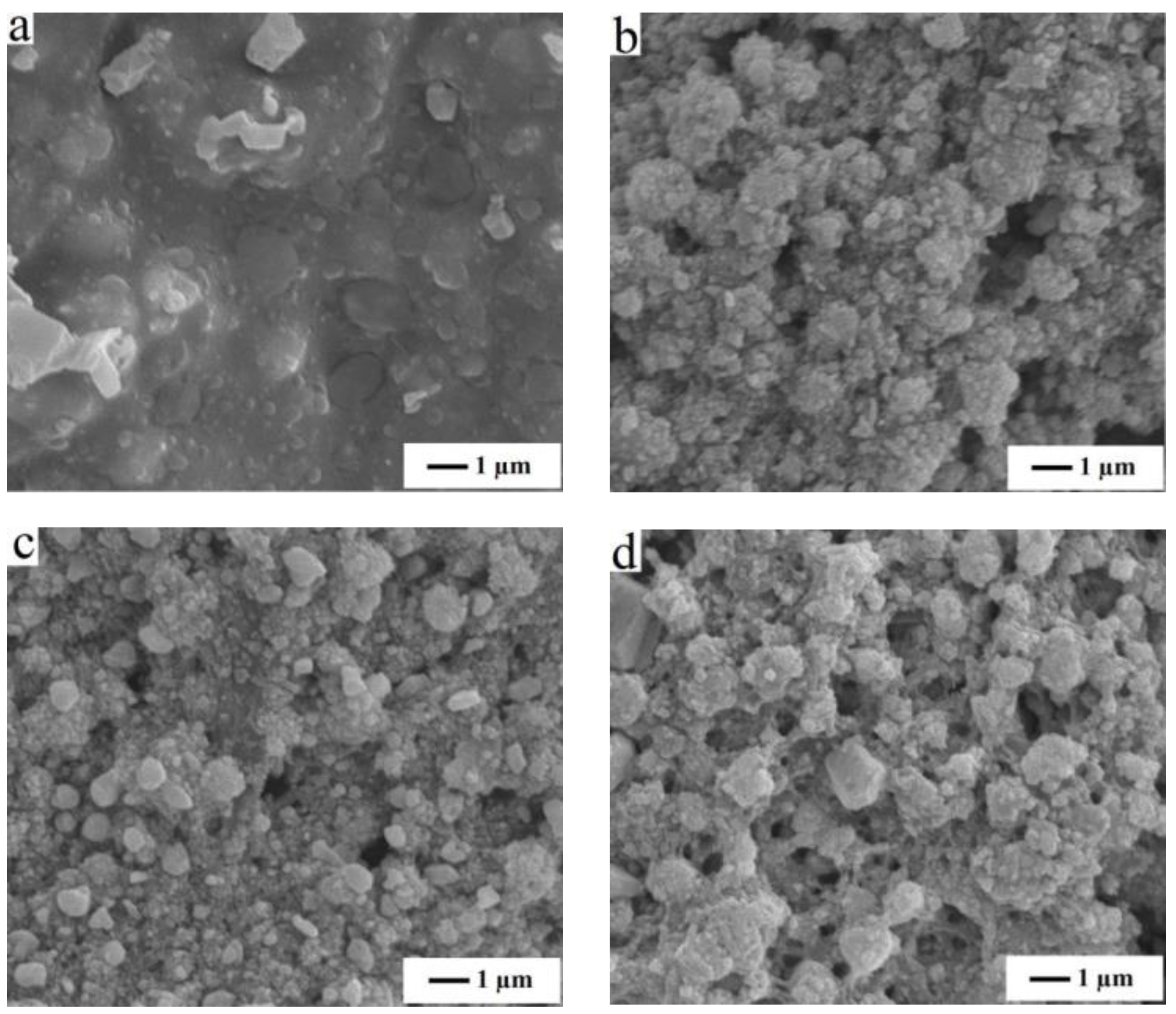
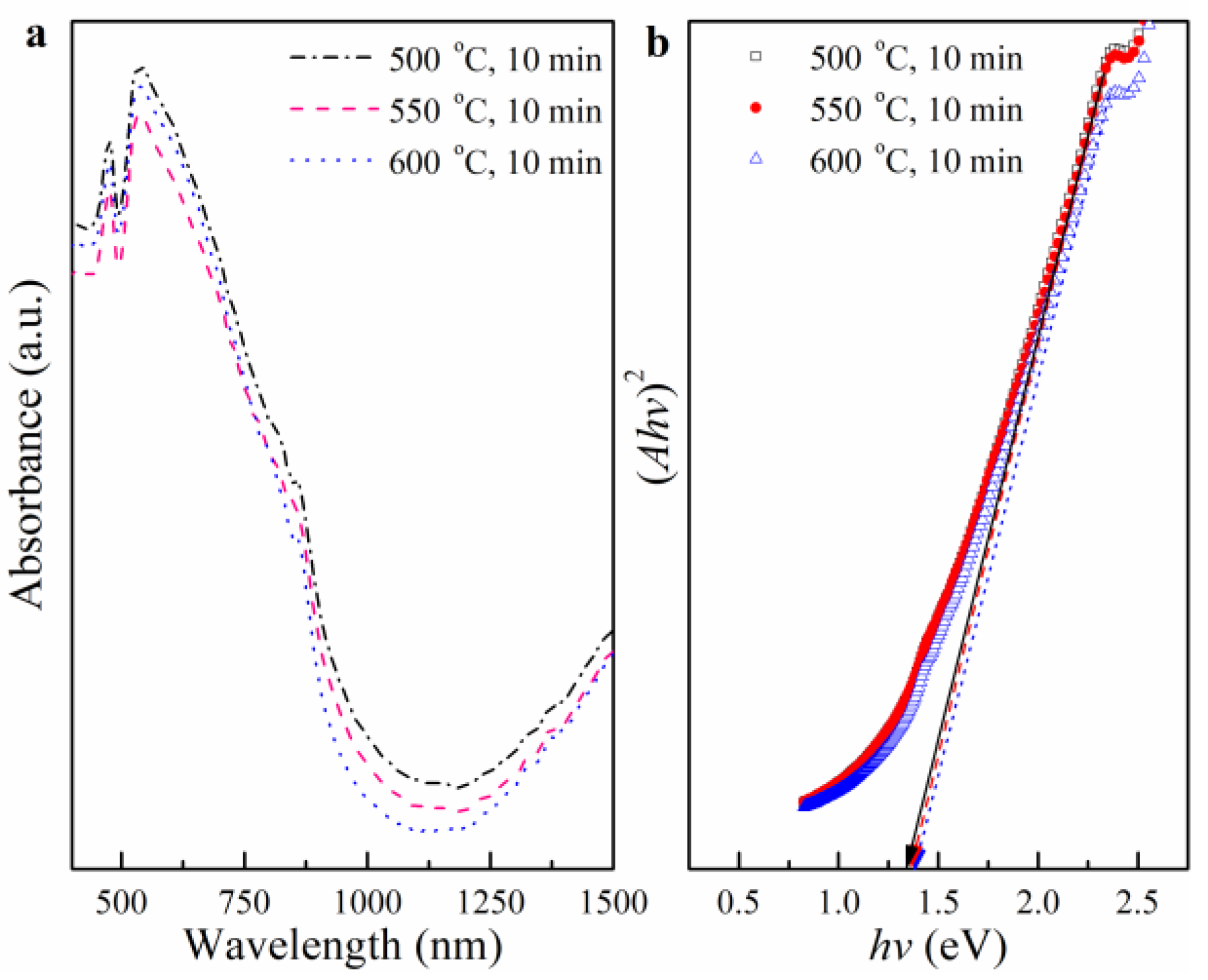
3.2. The Effects of RTA Toleration Temperature
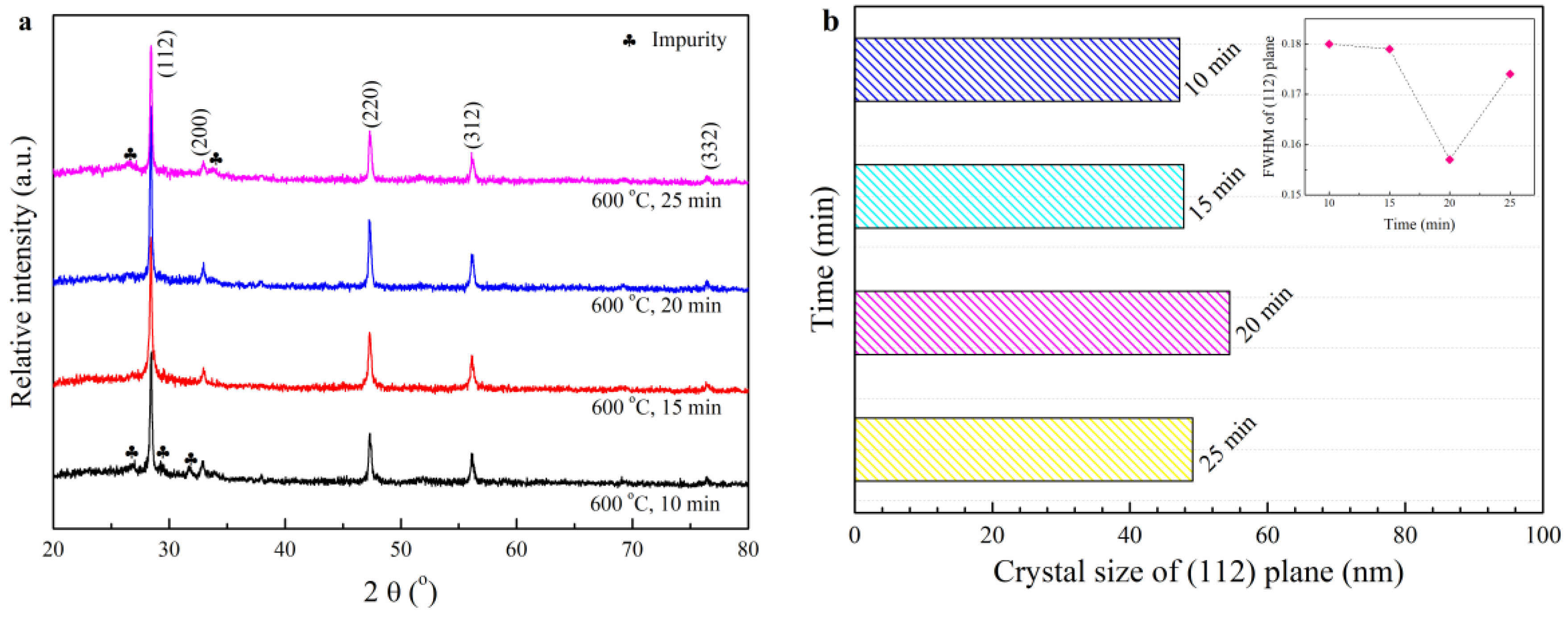
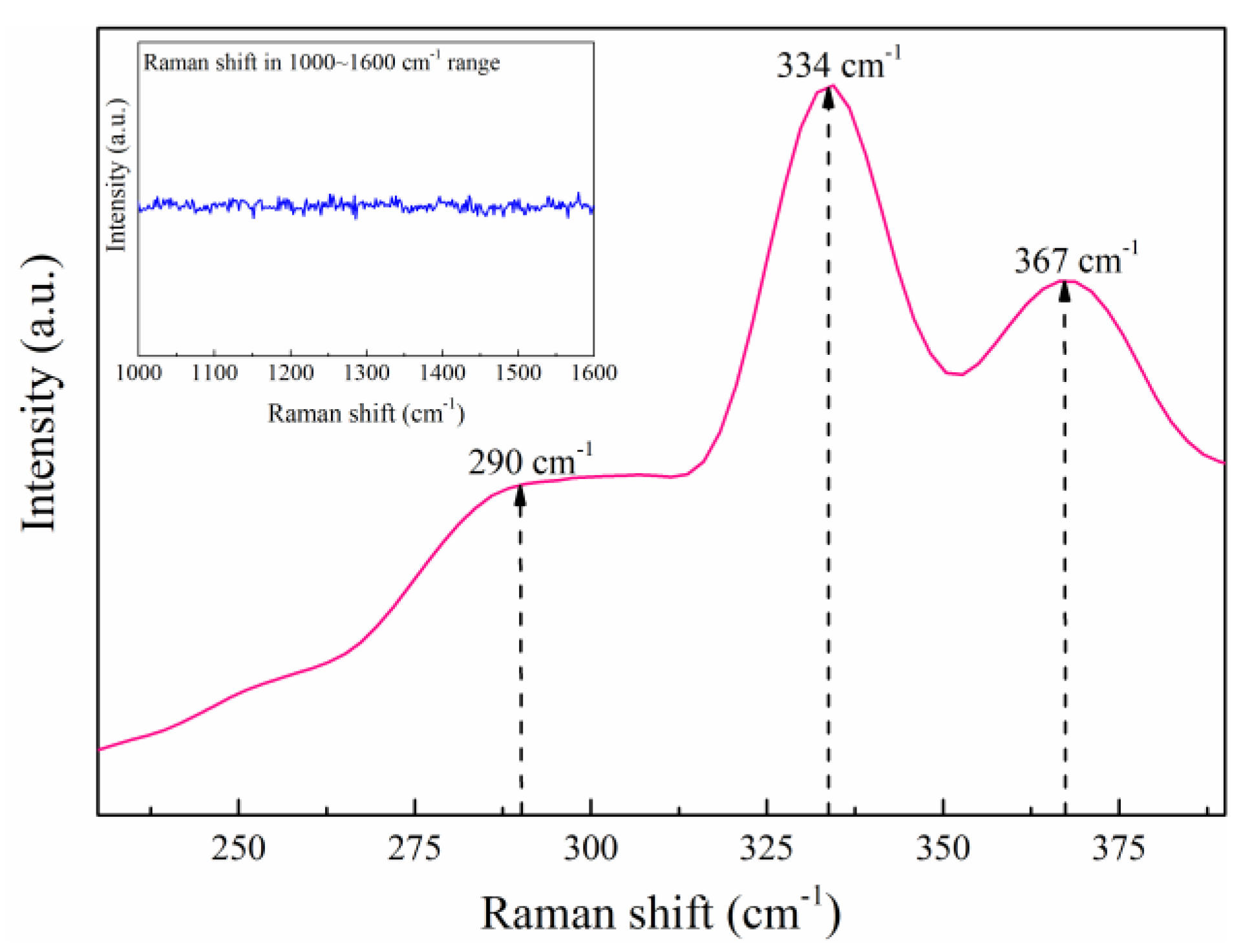
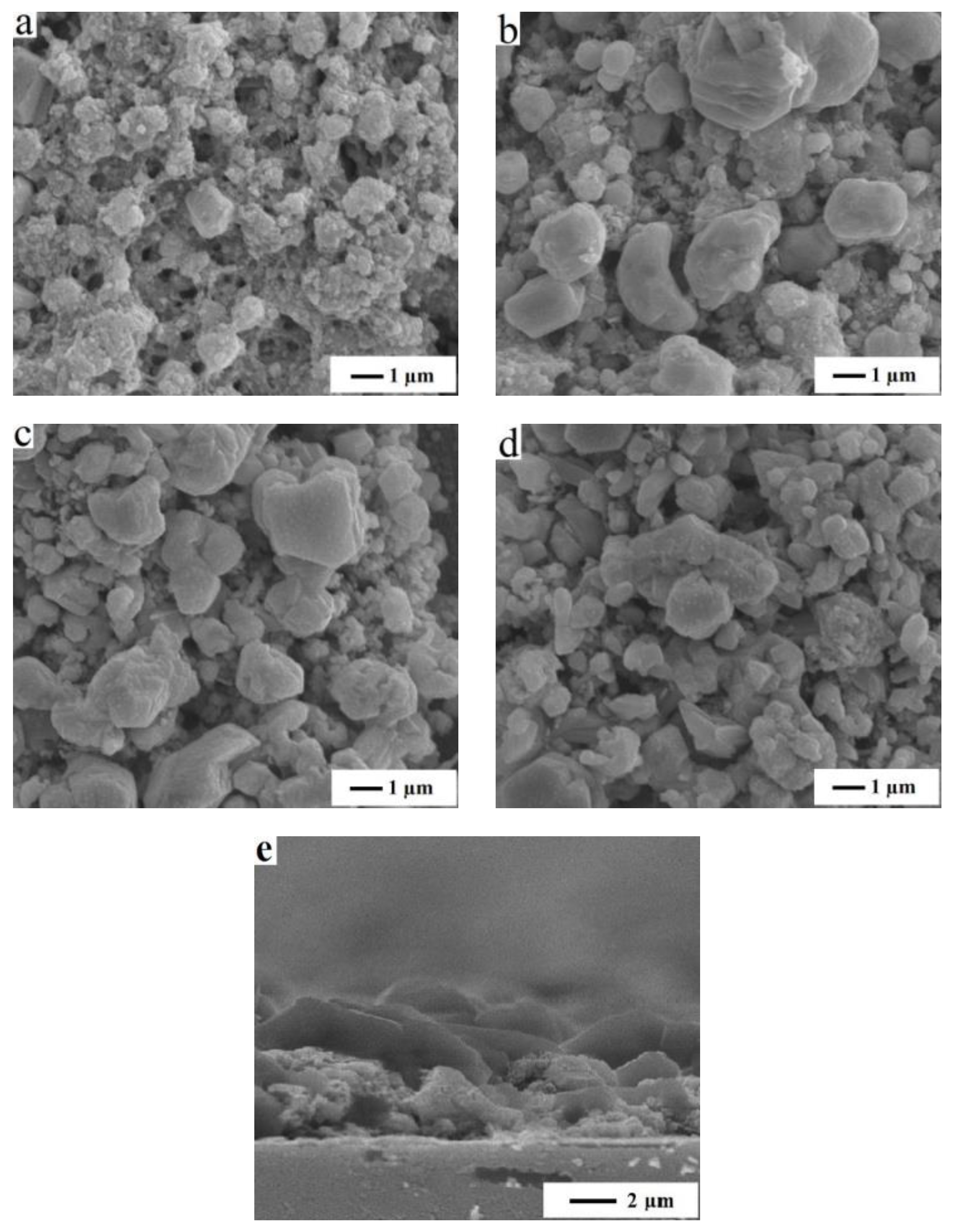
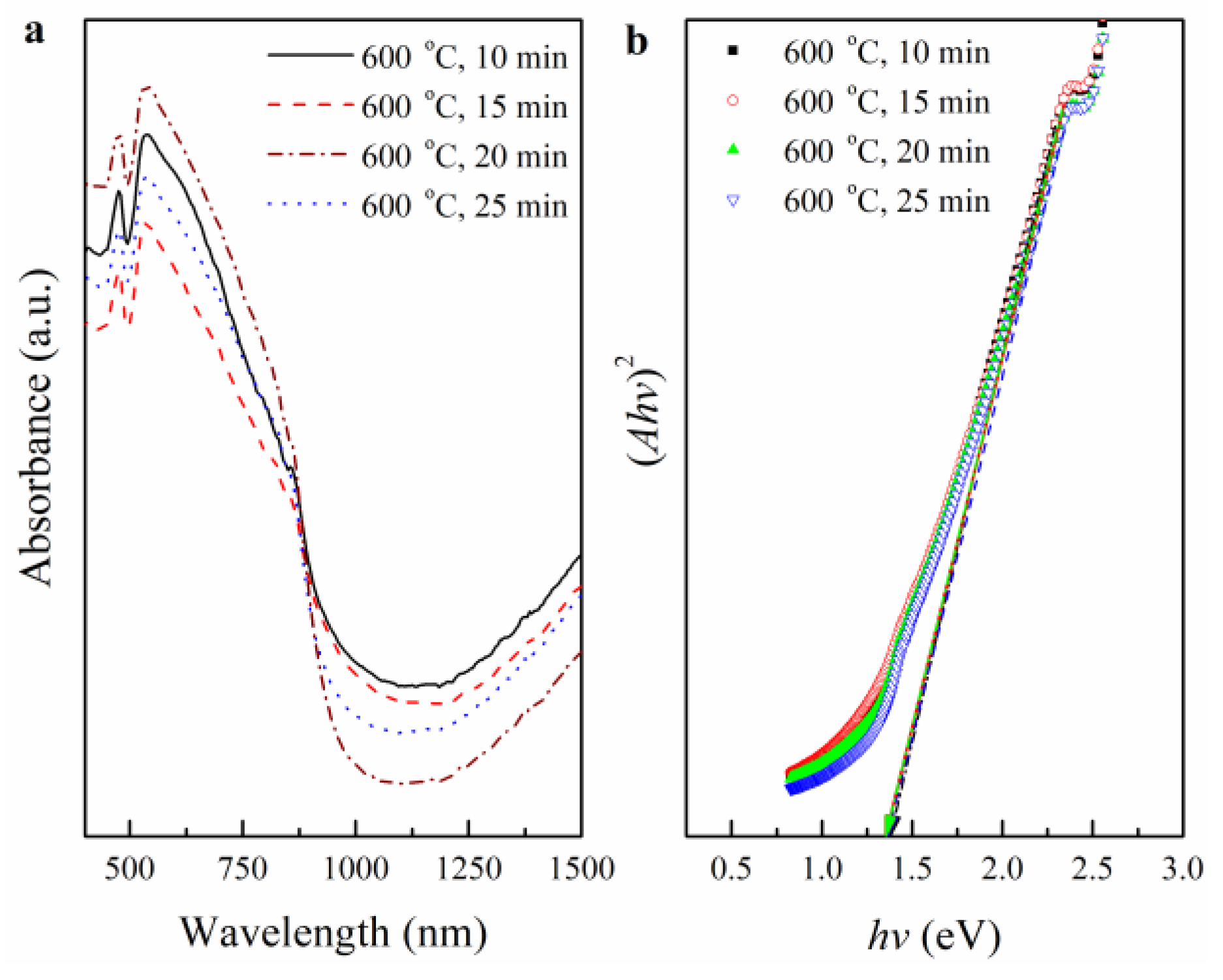
3.3. The Effects of RTA Duration Time
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, L.; Pei, C.; Xu, Y.; Li, Q. Template-directed synthesis of ordered single-crystalline nanowires arrays of Cu2ZnSnS4 and Cu2ZnSnSe4. J. Am. Chem. Soc. 2011, 133, 10328–10331. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Q.; Ni, Y.; Wang, T. Facile fabrication of Cu2ZnSnS4 thin film based on a novel metal-salt precursor printing route. Mater. Lett. 2015, 143, 185–187. [Google Scholar] [CrossRef]
- Cui, H.; Liu, X.; Sun, L.; Liu, F. Fabrication of efficient Cu2ZnSnS4 solar cells by sputtering single stoichiometric target. Coatings 2017, 7, 19. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, N.; Kumar, M. Strategic review of interface carrier recombination in earth abundant Cu-Zn-Sn-S-Se solar cells: Current challenges and future prospects. J. Mater. Chem. A 2017, 5, 3069–3090. [Google Scholar] [CrossRef]
- Shin, D.; Saparov, B.; Mitzi, D.B. Photovoltaic materials: Defect engineering in multinary earth bundant chalcogenide photovoltaic materials. Adv. Energy Mater. 2017, 7, 1602366. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Wang, T.; Zhou, F. The synthesis of Cu2ZnSnS4 nanocrystals prepared by a facile calcination route in the atmosphere. Mater. Lett. 2014, 125, 206–208. [Google Scholar] [CrossRef]
- Katagiri, H.; Jimbo, K.; Maw, W.S.; Oishi, K. Development of CZTS-based thin film solar cells. Thin Solid Films 2009, 517, 2455–2460. [Google Scholar] [CrossRef]
- Kahraman, S.; Çetinkaya, S.; Podlogar, M.; Bernik, S. Effects of the sulfurization temperature on sol gel-processed Cu2ZnSnS4 thin films. Ceram. Int. 2013, 39, 9285–9292. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Li, X.; Zeng, Y. Cation substitution in earth-abundant kesterite photovoltaic materials. Adv. Sci. 2018, 5, 1700744. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T. Device characteristics of CZTSSe thin-film solar cells with 12.6% efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Jimbo, K.; Kimura, R.; Kamimura, T.; Yamada, S. Cu2ZnSnS4-type thin film solar cells using abundant materials. Thin Solid Films 2007, 515, 5997–5999. [Google Scholar] [CrossRef]
- Liu, F.; Li, Y.; Zhang, K.; Wang, B. In situ growth of Cu2ZnSnS4 thin films by reactive magnetron co-sputtering. Sol. Energy Mater. Sol. C 2010, 94, 2431–2434. [Google Scholar] [CrossRef]
- Repins, I.; Beall, C.; Nirav, V.; DeHart, C. Co-evaporated Cu2ZnSnSe4 films and devices. Sol. Energy Mater. Sol. C 2012, 101, 154–159. [Google Scholar] [CrossRef]
- Schubert, B.A.; Marsen, B.; Cinque, S.; Unold, T. Cu2ZnSnS4 thin film solar cells by fast coevaporation. Prog. Photovolt. 2011, 19, 93–96. [Google Scholar] [CrossRef]
- Moriya, K.; Tanaka, K.; Uchiki, H. Fabrication of Cu2ZnSnS4 thin-film solar cell prepared by pulsed laser deposition. Jpn. J. Appl. Phys. 2007, 46, 5780–5781. [Google Scholar] [CrossRef]
- Sun, L.; He, J.; Kong, H.; Yue, F. Structure, composition and optical properties of Cu2ZnSnS4 thin films deposited by pulsed laser deposition method. Sol. Energy Mater. Sol. C 2011, 95, 2907–2913. [Google Scholar] [CrossRef]
- Todorov, T.K.; Reuter, K.B.; Mitzi, D.B. High-efficiency solar cell with earth-abundant liquid-processed absorber. Adv. Mater. 2010, 22, E156–E159. [Google Scholar] [CrossRef]
- Ahmed, S.; Reuter, K.B.; Gunawan, O.; Guo, L. A high efficiency electrodeposited Cu2ZnSnS4 solar cell. Adv. Energy Mater. 2012, 2, 253–259. [Google Scholar] [CrossRef]
- Ennaoui, A.; Lux-Steiner, M.; Weber, A.; Abou-Ras, D. Cu2ZnSnS4 thin film solar cells from electroplated precursors: Novel low-cost perspective. Thin Solid Films 2009, 517, 2511–2514. [Google Scholar] [CrossRef]
- Tanaka, K.; Fukui, Y.; Moritake, N.; Uchiki, H. Chemical composition dependence of morphological and optical properties of Cu2ZnSnS4 thin films deposited by sol-gel sulfurization and Cu2ZnSnS4 thin film solar cell efficiency. Sol. Energy Mater. Sol. C 2011, 95, 838–842. [Google Scholar] [CrossRef]
- Tanaka, K.; Oonuki, M.; Moritake, N.; Uchiki, H. Cu2ZnSnS4 thin film solar cells prepared by non-vacuum processing. Sol. Energy Mater. Sol. C 2012, 93, 583–587. [Google Scholar] [CrossRef]
- Vigil-Galán, O.; Espíndola-Rodríguez, M.; Courel, M.; Fontané, X. Secondary phases dependence on composition ratio in sprayed Cu2ZnSnS4 thin films and its impact on the high power conversion efficiency. Sol. Energy Mater. Sol. C 2013, 117, 246–250. [Google Scholar] [CrossRef]
- Kamoun, N.; Bouzouita, H.; Rezig, B. Fabrication and characterization of Cu2ZnSnS4 thin films deposited by spray pyrolysis technique. Thin Solid Films 2007, 515, 5949–5952. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Xu, D.; Zhang, Y. Fabrication of Cu2ZnSnS4 screen printed layers for solar cells. Sol. Energy Mater. Sol. C 2010, 94, 2042–2045. [Google Scholar] [CrossRef]
- Chen, Q.M.; Dou, X.M.; Li, Z.Q.; Cheng, S.Y. Preparation of Cu2ZnSnS4 film by printing process for low-cost solar cell. Adv. Mater. Res. 2011, 335, 1406–1411. [Google Scholar] [CrossRef]
- Guo, Q.; Ford, G.M.; Yang, W.C.; Walker, B.C. Fabrication of 7.2% efficient CZTSSe solar cells using CZTS nanocrystals. J. Am. Chem. Soc. 2010, 132, 17384. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.P.; Tsai, H.A.; Huang, A.N.; Pan, W.C. CIGS absorber preparation by non-vacuum particle-based screen printing and RTA densification. Appl. Energy 2016, 164, 1003–1011. [Google Scholar] [CrossRef]
- Chen, Q.; Dou, X.; Li, Z.; Cheng, S. Printed ethyl cellulose/CuInSe2 composite light absorber layer and its photovoltaic effect. J. Phys. D Appl. Phys. 2011, 44, 455401–455406. [Google Scholar] [CrossRef]
- Todorov, T.; Kita, M.; Carda, J.; Escribano, P. Cu2ZnSnS4 films deposited by a soft-chemistry method. Thin Solid Films 2009, 517, 2541–2544. [Google Scholar] [CrossRef]
- Moholkar, A.V.; Shinde, S.S.; Agawane, G.L. Studies of compositional dependent CZTS thin film solar cells by pulsed laser deposition technique: An attempt to improve the efficiency. J. Alloy. Compd. 2012, 544, 145–151. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Ni, Y.; Yamaguchi, Y. The synthesis of Cu2ZnSnS4 nanoparticles via an open-air solution route: Influences of Zn precursor content. J. Sol-Gel Sci. Technol. 2015, 75, 25–30. [Google Scholar] [CrossRef]
- Guo, Q.; Hillhouse, H.W.; Agrawal, R. Synthesis of Cu2ZnSnS4 nanocrystal ink and its use for solar cells. J. Am. Chem. Soc. 2009, 131, 11672–11673. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, H.; Jiang, F.; He, X. Low-cost chemical fabrication of Cu2ZnSnS4 microparticles and film. J. Mater. Sci. Mater. Electron. 2013, 24, 1813–1817. [Google Scholar] [CrossRef]
- Basha, S.S.; Rao, M.C. Effect of annealing temperature on structural and morphological studies of electrodeposited CZTS thin films. Ceram. Int. 2018, 44, 648–656. [Google Scholar] [CrossRef]
- Vanalakar, S.A.; Shin, S.W.; Agawane, G.L.; Suryawanshi, M.P. Effect of post-annealing atmosphere on the grain-size and surface morphological properties of pulsed laser deposited CZTS thin films. Ceram. Int. 2014, 40, 15097–15103. [Google Scholar] [CrossRef]
- Paier, J.; Asahi, R.; Nagoya, A.; Kresse, G. Cu2ZnSnS4 as a potential photovoltaic material: A hybrid Hartree-Fock density functional theory study. Phys. Rev. B 2009, 79, 115126. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Salomé, P.M.P.; Cunha, A.F.D. Study of polycrystalline Cu2ZnSnS4 films by Raman scattering. J. Alloy. Compd. 2011, 509, 7600–7606. [Google Scholar] [CrossRef]
- Gao, C.; Shen, H.; Jiang, F.; Guan, H. Preparation of Cu2ZnSnS4 film by sulfurizing solution deposited precursors. Appl. Surf. Sci. 2012, 261, 189–192. [Google Scholar] [CrossRef]
- Wei, M.; Du, Q.; Wang, D.; Liu, W. Synthesis of spindle-like kesterite Cu2ZnSnS4 nanoparticles using thiorea as sulfur source. Mater. Lett. 2012, 79, 177–179. [Google Scholar] [CrossRef]
- Li, J.; Shen, J.; Li, Z.; Li, X. Wet chemical route to the synthesis of kesterite Cu2ZnSnS4 nanocrystals and their applications in lithium ion batteries. Mater. Lett. 2013, 92, 330–333. [Google Scholar] [CrossRef]
- Wangperawong, A.; King, J.S.; Herron, S.M. Aqueous bath process for deposition of Cu2ZnSnS4 photovoltaic absorbers. Thin Solid Films 2011, 519, 2488–2492. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.H. Defect physics of the kesterite thin-film solar cell absorber Cu2ZnSnS4. Appl. Phys. Lett. 2010, 96, 021902. [Google Scholar] [CrossRef]
- Tang, D.; Wang, Q.; Liu, F.; Zhao, L. An alternative route towards low-cost Cu2ZnSnS4 thin film solar cells. Surf. Coat. Technol. 2013, 232, 53–59. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, F.; Yang, B.; Peng, X.; Chen, Q.; Zou, J.; Dou, X. Fabrication of Cu2ZnSnS4 Thin Films Based on Facile Nanocrystals-Printing Approach with Rapid Thermal Annealing (RTA) Process. Coatings 2019, 9, 130. https://doi.org/10.3390/coatings9020130
Chen J, Wang F, Yang B, Peng X, Chen Q, Zou J, Dou X. Fabrication of Cu2ZnSnS4 Thin Films Based on Facile Nanocrystals-Printing Approach with Rapid Thermal Annealing (RTA) Process. Coatings. 2019; 9(2):130. https://doi.org/10.3390/coatings9020130
Chicago/Turabian StyleChen, Jin, Fengchao Wang, Bobo Yang, Xiaogai Peng, Qinmiao Chen, Jun Zou, and Xiaoming Dou. 2019. "Fabrication of Cu2ZnSnS4 Thin Films Based on Facile Nanocrystals-Printing Approach with Rapid Thermal Annealing (RTA) Process" Coatings 9, no. 2: 130. https://doi.org/10.3390/coatings9020130
APA StyleChen, J., Wang, F., Yang, B., Peng, X., Chen, Q., Zou, J., & Dou, X. (2019). Fabrication of Cu2ZnSnS4 Thin Films Based on Facile Nanocrystals-Printing Approach with Rapid Thermal Annealing (RTA) Process. Coatings, 9(2), 130. https://doi.org/10.3390/coatings9020130