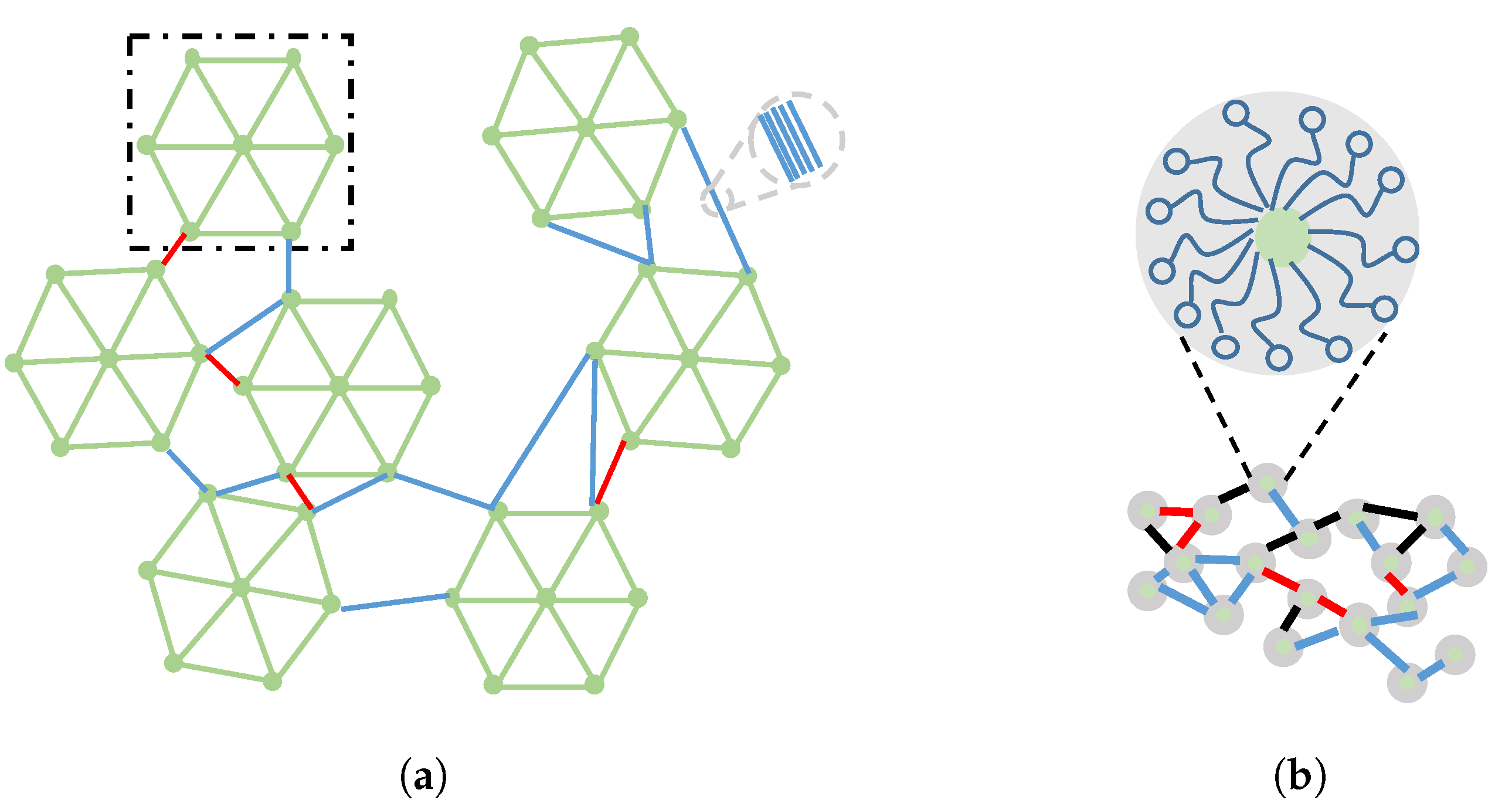
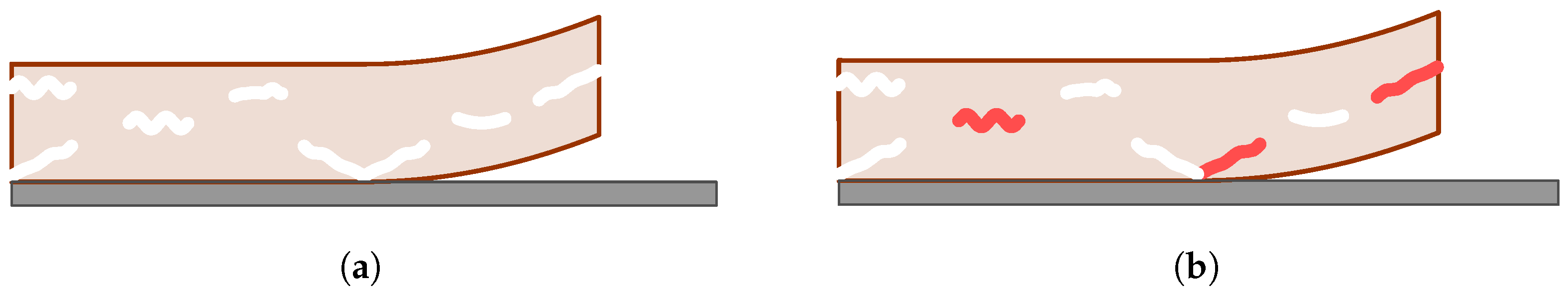
1. Introduction
Self-healing materials are smart materials that present the inherent (intrinsic) or built in (extrinsic) ability of damage detection and repair. The development of these types of materials has attracted much attention from both industrial and academic forums in the last two decades, and progress has been made in a wide range of materials [
1,
2]. Coatings are not an exception, and different self-healing mechanisms have been proposed. In the most general context, a coating shall be seen as a sequence of layers, each one optimized with respect to a certain functionality (for instance, adhesion, corrosion protection, mechanical or thermal protection, etc.). The available self-healing mechanisms are adjusted to the specific functionality they aim at recovering. This work focuses on the mechanisms promoting the recovery of the barrier function. However, due to their nature, certain mechanisms leading to the reestablishment of the corrosion protection will also be taken into consideration.
Barrier recovery can be achieved by means of the enhancement of polymer chain mobility, the production of continuous phases at the crack gap or by the expansion of dispersed phases. The increase of polymer mobility has been pursued by the implementation of reversible chemistries, such as those based on (retro) Diels-Alder reactions [
3,
4,
5] and disulfide [
6,
7,
8,
9,
10], hydrogen [
11] and trithiocarbonate [
12] bond reactions. Most of these approaches require the supply of an external trigger (such as an increase of temperature or photostimulation) to activate the healing response, but the self healing mechanism is repeatable. Shape memory polymers can also be used to force the sealing of an open crack [
13,
14], provided the adequate thermal stimulus. The production of continuous phases has been promoted by means of the polymerization of encapsulated liquid agents [
15,
16,
17] in polymeric coatings and the precipitation of solid compounds [
18,
19] in thermal barrier coatings. In the former case, the encapsulation of the healing agent is necessary to prevent its reaction with the matrix, and the healing response is autonomous (i.e., the progress of damage breaks the capsule and activates the healing response) but limited by the depletion of healing resources. Furthermore, this healing route to perform properly requires that the polymerization of the liquid agent is sufficiently fast to arrest the progression of damage. In the case of thermal barrier coatings, the precipitation of solid phases (TiO
, but also Mo
Si
and ZrSiO
as secondary phases) is governed by oxidative reactions at high temperatures, and thus the presence of external stimulus (such as oxygen) is required. The volumetric expansion of the coating layer(s), sealing the crack, can be obtained by the growth of hydraulic inorganic crystals [
20], clay layers [
21] and superabsorbent polymers [
22]. The extraordinary swelling properties of hydrogels combined with reversible polymer chemistry [
12] present as well the possibility to recover the barrier function. As in previous self healing mechanisms, these expansive processes require the presence of external compounds such as moisture and/or carbon dioxide.
While the experimental development of the self-healing materials (and self-healing coatings in particular) follows a growing trend, the progress of mathematical and computational frameworks to help understand and broaden the experimental observations is still far from its true potential. This occurs despite the fact that systematic computational simulation, based on physically sound and validated mathematical models, provides an additional tool from which to seek material optimization and cost reduction. A clear example of this symbiotic relation between experimental and computational frameworks is given in [
23,
24,
25]. In this group of works, the authors investigate the self-replenishing behavior of damaged films by the rearrangement of low surface energy dangling chains. Their computational framework, based on the dissipative particle dynamics method, replicates the considered experimental set ups and it is used to refine the knowledge derived from them. For instance, the computational model allowed the authors to optimize the concentration and length of the dangling chains to attain the maximum surface recovery.
The aim of this work is to revisit different modeling frameworks that can be used to further understand and optimize a range of self-healing routes. The modeling frameworks have been chosen bearing in mind the basic elements of the desired self-healing response (activation, mobilization and reaction of healing resources) and the damage length-scale. In particular, this paper addresses the modeling aspects of the dynamic adaptation of the polymeric network (
Section 2), the degradation and recovery of the mechanical properties of the coating (
Section 3 and
Section 4) and the transport and reaction of species within the coating matrix (
Section 5). Each section is self-contained and the notation is adapted accordingly. The paper concludes with a critical discussion of the observed advantages and limitations of the discussed models, and proposes some lines of future work.
3. Models Based on Continuum Damage-Healing Mechanics
Continuum damage-healing mechanics incorporate the healing effects into the continuum damage mechanics framework. In that context, the presence of cracks is homogenized through the computational domain and damage and healing variables are introduced to account for the loss and recovery of mechanical integrity, respectively, of the material at the macroscopic scale. Barbero et al. [
38] introduced this framework to study the damage and healing behavior in self-healing polymer composites, as an attempt to model the healing mechanism based on encapsulated healing agents [
15].
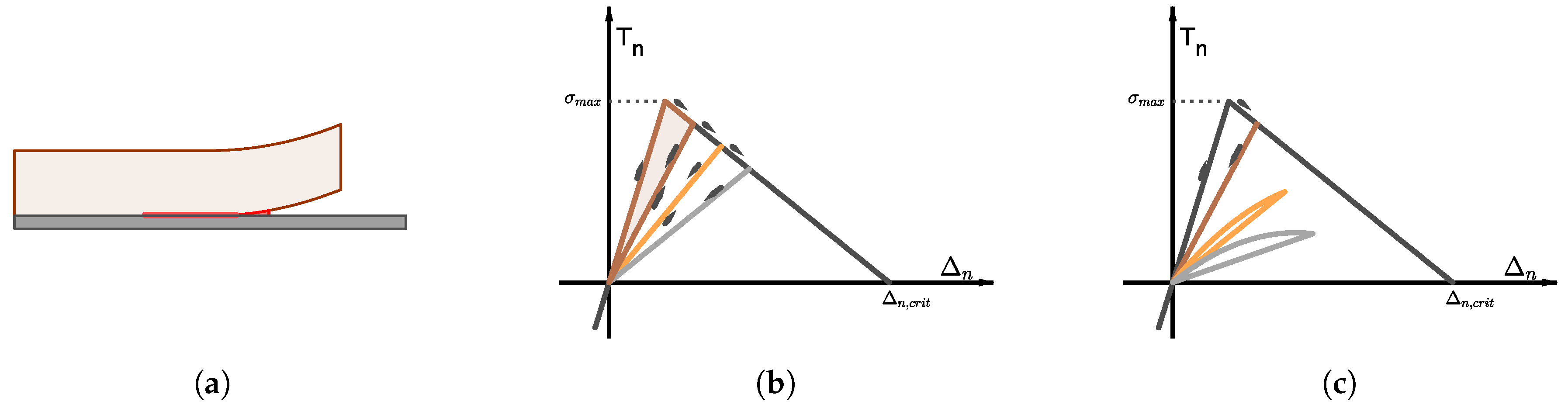
In the case of isotropic damage (and healing), the presence of microcracks in the material can be incorporated through a scalar damage variable
. The case
represents the undamaged state, whereas
represents the fully damaged state (see
Figure 2a). The damage variable is used to relate the nominal stress tensor of the damaged material,
, with the effective stress tensor of the undamaged material,
, through the relation:
Equation (
14) also establishes the reduction of the elastic modulus in the presence of damage. If healing is taken into account, then damage is reversible and can be partly recovered by the considered healing mechanisms. A scalar healing variable
h shall be defined to account for the level of damage recovery. If one considers
with
meaning no healing and
meaning full damage recovery, then Equation (
14) can be further refined through:
Under these definitions of
h and
, the the mechanical properties of the original material are recovered for
. In the case of anisotropic damage and healing, as for in the case of composite materials [
39], the damage and healing variables are fourth rank tensors
d and
h, and the relation between the nominal
and the effective
is given by
, where
M denotes the fourth rank damage effect tensor that is constructed from
d and
h. The interested reader is referred to [
40], and references therein, for a detailed description of anisotropic continuum damage-healing models.
The core of the continuum damage-healing mechanics framework rests on the definition of the damage and healing variables, and on their temporal evolution. The early definitions of the damage and healing variables based on the loss and recovery of the cross-sectional area were soon replaced by definitions based on the experimentally measurable material parameters [
41,
42] such as the induced changes in the elastic modulus, shear modulus, etc. Regarding the temporal evolution of damage and healing variables, although some phenomenological laws have been proposed [
40], they are usually derived from thermodynamic principles [
38,
39,
42,
43,
44]. In general terms, the Helmholtz free energy
may take into account contributions from the elastic, plastic and/or viscoplastic deformations as well as from inelastic variables such as hardening. The damage and healing variables is usually set out as the zero-value surfaces of the damage and healing thermodynamic conjugate forces,
Y and
H, respectively:
Darabi et al. [
43] consider isothermal damage and healing processes, however, they introduce the endogenous nature of the healing process. Assuming that the rate of healing
is proportional to the rate of temperature supply
, then Equation (
16) is replaced by:
where
K denotes the above-mentioned proportionality constant. The rate of energy dissipation can then be written as:
where
denotes the material density, and
,
denote the temporal derivatives of the damage and healing variables respectively. Taking in mind that damage and healing do not occur simultaneously, the rate of energy dissipation can be rewritten as:
Darabi et al. [
43] argue that the damage and healing thermodynamic forces account for both energetic and dissipative contributions, which can be written as:
After mathematical manipulations, that includes the search of the local extrema of
and the use of Lagrange multipliers, Darabi et al. establish that the dissipation components are obtained from:
Thus, according to the framework derived by Darabi et al. [
43], the rate equations for the damage and healing variables can be readily deduced once the mathematical expressions of the Helmholz free energy
and of the energy dissipation rate
are given. It is worth mentioning here that derivation of this framework is independent of the equivalence hypothesis used to relate the nominal (damaged) and the effective (undamaged) or the nominal and the healing configurations. The different hypothesis (for instance, the frequently used strain equivalence hypothesis, assumes that the strains in the damage and the effective, or healing, configurations are the same) are used to obtain the relations between the stiffness modulus at the different configurations. The framework presented in [
43] can be particularized to obtain different damage-healing kinetics in the literature, and has been validated against experimental results of fatigue damage in asphalt mixtures [
45].
One modeling aspect that remains to be tackled in continuum damage-healing mechanics approaches is the coupling of the healing variable with the healing process at the microscopic scale. Note that the healing rate
is frequently formulated only in mechanical terms. To alleviate this drawback, and keeping in mind the system based on dispersed microcapsules [
15], Voyiadjis et al. [
42] introduce a phenomenological law that relates the damage and healing variables through the amount of healing agent that has been released during damage. To this end, the following phenomenological relations were used:
where
and
are material parameters that serve to correlate the physical characteristics and dispersion of the microcapsules though the matrix. The function
gives an estimate of the amount of healing agents that has been made available by the damage variable
d (as the microcapsules are more disperse,
increases and
decreases). If the amount of healing material made available is large enough, the damage is fully recovered (
), whereas if it is not sufficient, then only the equivalent amount of damage can be restored (i.e., some residual damage remains,
). Note that the definitions of the damage and healing variables in Voyiadjis et al. [
42] are such that
represents the complete restoration of the damage. Shojeaei et al. [
46] extended the ideas of [
42,
44] to propose a multiscale model in which the healing variable, or recovery function in their work, is decomposed as the additive contribution of the wetting and diffusion processes at molecular level in shape memory polymer based self healing polymers.
4. Cohesive Models of Crack Growth and Healing
Cohesive models bridge the gap between fracture mechanics and continuum damage mechanics. The fracture is treated explicitly but as a segment of small width embedded in the intact material (see
Figure 3a). The evolution of the fracture is governed by the relation between the traction
T and displacement jump
vectors at the surfaces of the fracture, given by the softening function (see
Figure 3b,c) [
47].
To the author’s knowledge, the first attempt to use cohesive models in the context of self-healing materials is due to Maiti and co-workers [
48,
49,
50]. Maiti and Geubelle [
48] first adapted a cohesive damage model to include fatigue fracture. A bilinear, rate independent and damage dependent softening function was considered for Mode I loading (see
Figure 3b):
where
and
denote the normal components of the traction and displacement jump vectors at the surface of the cohesive fracture,
denotes the tensile strength of the material and
denotes the critical displacement jump. Equation (
24) incorporates a scalar damage variable
d that accounts for the monotonic degradation of the loading capacity of the cohesive fracture:
represents no damage and corresponds with
, whereas
represents complete failure and corresponds with
. The value
is used to define the slope in the first section of the
–
curve. The effects of fatigue were incorporated as an exponential decay of the instantaneous cohesive stiffness,
, with the number of loading cycles:
where
denotes the number of loading cycles after damage initiation and
is power law. The evolution law Equation (
25) establishes that the cohesive stiffness is degraded during loading, and remains unchanged during unloading (see
Figure 3c). Healing effects were incorporated by the inclusion of a rigid wedge in the rear of the crack tip [
49]. The thickness and mechanical strength of the wedge shall be interpreted—at this point—as the outcome of the healing events taking place during the resting period between consecutive loading steps. The interaction between the wedge and the host material was formulated through the Hertz-Signorini-Moreau law for contact, that imposes no tension and no interpenetration between the contacting surfaces. The presence of the wedge means that the closure of the crack cannot be fully attained during unloading, and reduces the effective stress intensity factor. This results in the retardation of crack growth when loading is resumed. Computational results show that the retardation effect is increased with the thickness of the wedge, but also when the wedge is formed next to the crack tip or at shorter crack lengths. These results clearly indicate the competition between healing and crack growth dynamics, and hence only fast enough healing mechanisms can be successful.
The healing kinetics (given by the polymerization of dicyclopentadiene (DCPD)) were considered in [
50]. Molecular dynamics simulation of DCPD structures were used to fit a phenomenological evolution law of for degree of curing of the wedge,
, based on the Prout-Tompkins reaction kinetics:
where
is a temperature dependent Arrhenius-type multiplicative factor, and
n,
m are the exponents fitted to molecular dynamics simulations. The mechanical properties of the polymerized DCPD structure were also obtained from the molecular dynamics simulation and correlated to the curing degree
. Finally, a phenomenological law relating the wedge thickness and the curing degree was proposed to further investigate the competition between the healing and crack growth kinetics. The simulations in [
50] supplement those in [
49] in the sense that the rest periods can be included in the discussion. Thus, crack growth is inhibited only at fast healing kinetics, and for a prescribed healing kinetics, better results are obtained for longer rest periods.
Ponnusami et al. [
51] apply a cohesive damage model to analyze the effects of repeated healing events in the mechanical performance of the material. Following the same notation as in [
51], we have that the first loading cycle induces a damage
in the original material that can be estimated as the ratio of the dissipated energy
and the cohesive energy
,
. Recall that
is computed as the area enclosed by the loading-unloading cycle, whereas
is the area enclosed by the
–
curve (see
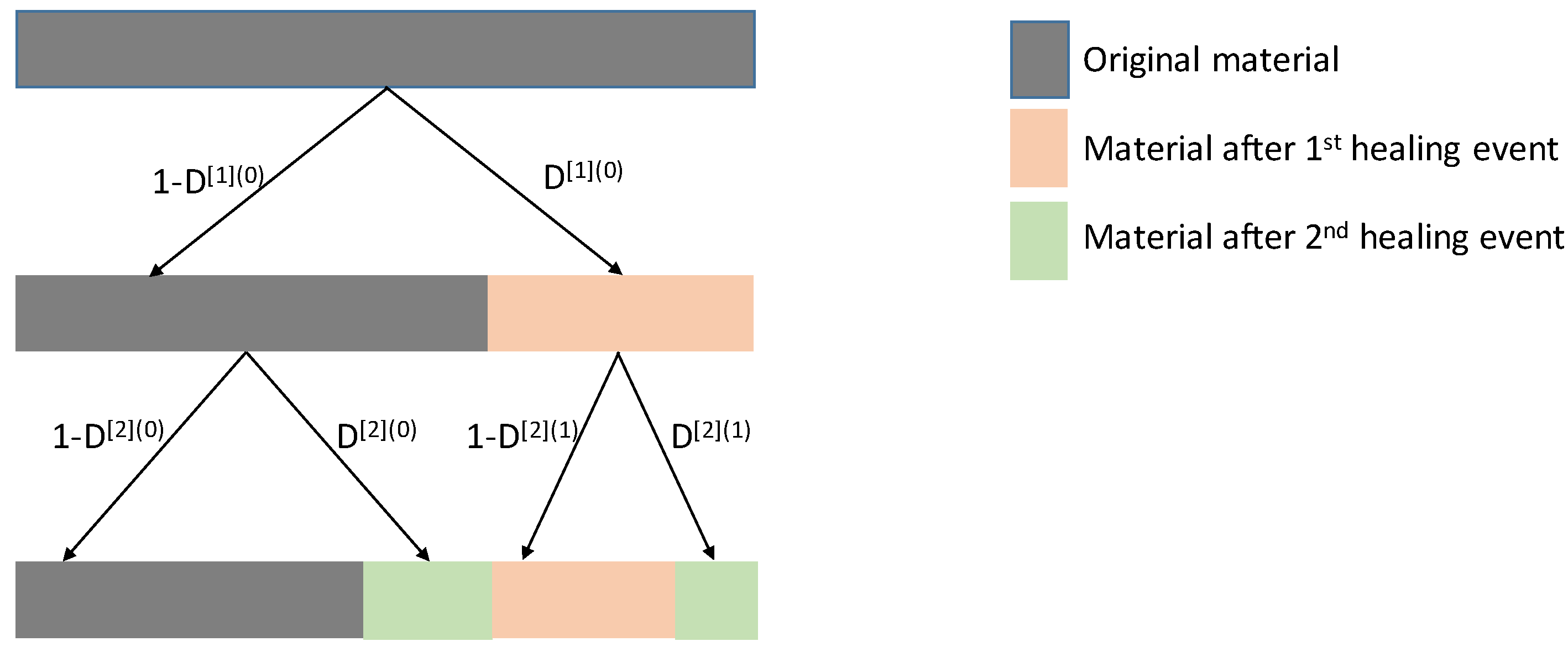
Figure 3b). If one assumes that all the damage produced in this first loading-unloading cycle is healed in the subsequent resting period, the outcome of the first damage-healing cycle is a composite of two materials: the original one (in a weighted ratio of
) and the healed one (in a weighted ratio of
) (see
Figure 4). The traction-separation curve of the constituents is constructed as a shifted version of the traction-separation curve of the original material, bearing in mind the following considerations. The crack opening is partly reduced by the healing event in the original material, whereas the healed material is created free of tension. A new loading-unloading cycle on this composite material will introduce two new damage parameters (
relative to the original material,
relative to the healed material). The resting period after the second loading step will heal the damages and introduce a new constituent to the composite material: the material that is healed in the second period. The weighted fractions of the constituents after two healing events are, respectively,
,
and
(see
Figure 4). The fracture energy of the composite material is computed as the weighted average of the fracture energies of its constituents. This idea can be reproduced for as many loading-unloading cycles as desired, keeping in mind that each rest period introduces a new constituent material. The model furthermore allows the definition of specific fracture strength for each constituent of the composite material, and therefore the incorporation of the healing history in the performance of the material. Results clearly demonstrate the dependence of the load bearing capacity of the material on the mechanical properties of the healed material. The load carrying capacity decreases with the decrease in the strength and fracture energy of the healed material(s). Furthermore, results also indicate a deterioration of the load carrying capacity when the degree of crack filling reduces. Ponnusami et al. [
51] interpret the filling efficiency as a geometrical factor (i.e., the ratio of the crack volume filled with healing agent). However, in a fully coupled healing model, the filling efficiency should be deduced from the the mobilization and reaction of the healing agents.
5. Mobilization of Healing Agents
A critical point in extrinsic self-healing mechanisms is the activation of the healing agents. This can occur by the rupture (or dissolution) of the encapsulation systems or by the ingress of external species (such as oxygen or moisture) into the coating matrix. Computational calculations of the stress distribution around the capsules reveal that an evolving crack will not be attracted to capsules with a higher stiffness than that of the host matrix [
15]. This observation has been further refined by means of computational approaches based on cohesive formulations of the capsule-matrix interface [
52,
53]. Using intensive parametric analyses, these works demarcate the basic material parameters (such as capsule to matrix stiffness or fracture strength ratios [
52] and capsule thickness to radio ratio [
53]) that allow to identify suitable matrix-capsule systems. The dissolution of encapsulated systems has been modeled as a phase transformation under equilibrium conditions [
54].
On the other hand, the activation of healing agents by the ingress of external species—and the subsequent transport of the healing agents to the crack site—is strongly influenced by the interaction between the polymeric matrix and the diffusing species [
55,
56,
57,
58,
59,
60]. In the remainder of this section, the activation and transport of corrosion inhibitors will be used as an illustrative example. The interest of this mechanism rests on the variability of leaching behaviors reported in the literature [
57,
58,
59,
60,
61,
62]. Although the discussed models will focus on the basic steps leading to the recovery of the corrosion protection functionality, the principles upon which they are built can be easily translated to extrinsic self-healing mechanisms of coating barrier restoration. Note that differences will arise on the reaction mechanism that the activated and/or mobilized healing agents undergo at or in the vicinity of the scratch (such as the precipitation of solid phases [
18] or the volumetric expansion of hydrophilic phases [
12,
21,
22]). In the remainder of this section, the terms corrosion inhibitor and healing agent are interchangeable.
Organic coatings loaded with carriers of corrosion inhibitors prevent the onset of electrochemical corrosion after the barrier functionality of the coating is lost (see
Figure 5). The polymeric matrix interacts with the transport of both the release-triggering and the inhibitor species [
55,
56]. Moisture uptake in neat vynil ester resins has been proved to follow a Fickian behavior (i.e., the mass of absorbed water is proportional with
until saturation, where
t denotes the immersion time). However, Drozdov et al. [
56] extended this observation with the inclusion of montmorillonite clay nanoparticles in the vynil ester matrix, and obtained uptake curves that deviate from those of Fickian diffusion. The clay fraction was found to be a critical parameter, having a clear effect on the moisture diffusivity and rate of adsorption. These observations were modelled by means of a system of differential equations that coupled the evolution of two different forms of water concentrations: the concentration of mobile (penetrant) water
n and the concentration of immobilized water (water molecules bounded at the clay surface)
, based on mass conservation principles:
D denotes the diffusion rate of mobile water,
k the rate of adsorption of water molecules and
the number of sites on the clay particle surface where the moisture molecules can bind (i.e.,
denotes the number of unoccupied sites where a water molecule can be immobilized). Drozdove et al. [
56] use this basic model to correlate the dependence of
D and
k on the content of clay nanoparticles within the matrix, obtaining a decrease on both rates with the content of clay nanoparticles.
Accurate modeling of corrosion reactions at the exposed substrate shall involve the electrochemical reactions at the cathode and the anode, the hydrolysis reactions of the substrate and the release and transport of inhibitors. The former chemical reactions are formulated in terms of mass conservation of the chemical species, where the transport terms take into account the contribution of both random dispersal (i.e., diffusion) and bias transport induced by the potential, and the charge neutrality of the solution [
61,
63]. The release of inhibitors, on the other hand, remains not fully understood and is frequently disregarded. Wang et al. [
61] adopted an intermediate approach and considered the presence of inhibitors though a phenomenological law formulated in terms of the pH in the solution, which can be deduced from the evolution of the chemical species.
The gap between the previously discussed class of models rests on the actual transport and release of the corrosion inhibitors from the coating. Recently, two different mathematical models [
54,
64] have been proposed to address the reasons behind the observed non-Fickian release of inhibitors in different coating-inhibitor systems [
59,
60]. The main difference in these works rests on how the inhibitors within the matrix are idealized: Javierre et al. [
54] consider the role that a certain distribution of encapsulated inhibitor particles may have on the release kinetics (see
Figure 5), whereas Tabor and Warszynski [
64] consider the inhibitors to be homogeneously distributed throughout the coating.
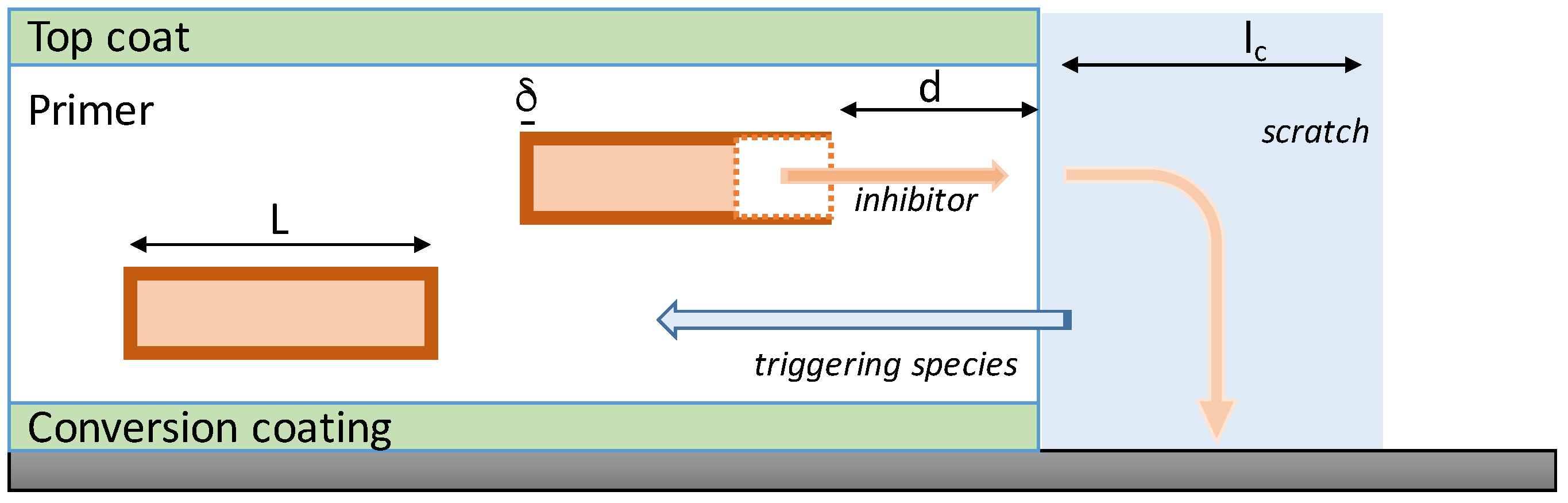
In the case of a distributed configuration of particles, the release of the inhibitors requires (1) the ingress of a release-triggering substance (for instance moisture), (2) the rupture or dissolution of the capsule, and (3) the dissolution of the inhibitor particle and transport of the inhibitor compounds to the scratch (see
Figure 5). Javierre et al. [
54] model the transport steps by Fick’s second law, and the dissolution steps as binary Stefan problems [
65]. The mathematical formulation of the Stefan problems combines Fick’s second law in a time-dependent domain with a mass conservation argument to obtain the velocity of the moving interface (i.e., the moving boundary of the dissolving particle). If we assume planar axial symmetry, the evolution of the inhibitor concentration
c released from the dissolution of an isolated particle is given by:
where the velocity of the particle-coating interface is given by:
For the sake of the comparison with [
64], it should be noted here that the model assumes that the coating-scratch interface is in equilibrium (i.e., the same solubility is imposed at both sides of the coating-scratch interface). Note furthermore that with the current notation, the origin of coordinates has been located at the left side of the particle, and then the coating-scratch surface is located at
(see
Figure 5). Then, the mass of the inhibitor released into the scratch space can be computed from the concentration
c, taking into account the scratch width (
) and its distance to the origin of coordinates:
The plot of
for different distances
is presented in
Figure 6a,b. Both the amount of mass leached into the scratch and the rate at which the leaching occurs is affected by the size of the particle and its distance to the scratch. Note furthermore that the time lapse required for the mass of a certain particle to reach the scratch is also affected by the specific values of the distances.
The central idea of the model presented in [
54] is to analyze how the mass
leached from particle
i combines with the mass leached from other particles, and how their combination can lead to an overall mass
that presents a behavior in time different from
(i.e., different to Fickian). The overall mass
is computed from:
where the times
account for the activation time of particle
i. Javierre et al. [
54] record the mass
of each particle in different configurations, and use the activation times
to fit
to different leaching kinetics. They observe that leaching kinetics proportional to
—with
—can be obtained by this procedure. They furthermore derive self-similar solutions allowing to relate the activation times
with the transport of the release-triggering species and the dissolution of the capsule. Thus, a tool is provided to evaluate a desired leaching kinetics against the diffusion coefficients and saturation levels of the release-triggering species and the capsule compounds.
Tabor and Warszynski [
64] also considered the interaction between a release-triggering species and the inhibitor species itself. The evolution of theses species was formulated in terms of diffusion equations for each species at each domain (scratch and coating). Different solubilities are considered for each species in each domain, and hence the boundary conditions on the scratch-coating interface are formulated in terms of the corresponding partition coefficients. Continuity of the normal fluxes across the scratch-coating interface are imposed, and hence the total mass of the system is conserved. Tabor and Warszynski also consider the existence of reservoirs of inhibitors within the matrix, but do not treat them explicitly (as in [
54]). Instead, they introduce a new variable that represent the homogenized version of the dispersed corrosion inhibitor particles. At the scratch domain, the triggering species is assumed to remain at a constant level, and the inhibitor species is transported by standard diffusion. Of interest to the present discussion is the coupling of the different species within the coating domain, and hence the governing equations will be reproduced here. Without loss of generality, we denote by
,
and
the concentration of the triggering species, the (active) inhibitor and the (inactive) inhibitor. The inactive inhibitor refers to the inhibitors stored at the reservoirs. Again under planar symmetry, the coupling of these species within the coating domain is given by:
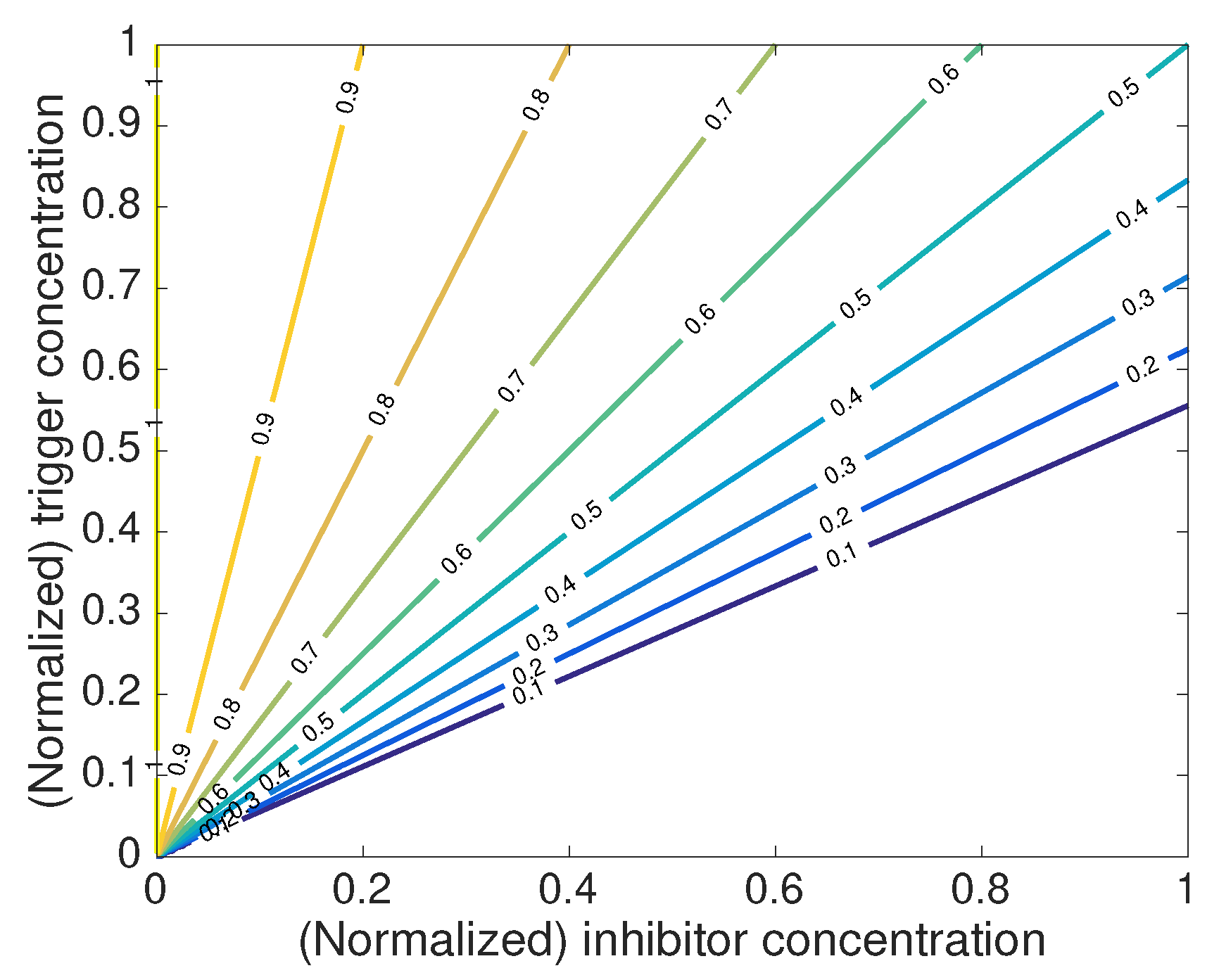
where the function
P establishes the nonlinear coupling between the activation of inhibitors from the reservoirs:
The function
P establishes the activation rate of the stored inhibitors (see
Figure 7). This activation depends on actual levels of both the inhibitor and the triggering species, and consequently, on their transport within the coating (Equations (
34) and (
35)) and within the scratch. The reservoirs are not extinguished and for a fixed concentration of the inhibitor, the activation of more inhibitors only occurs when the concentration of the trigger exceeds the threshold level
. On the other hand, as inhibitors are been transported towards the scratch, i.e., removed from the coating, the activation rate gradually decreases.
Under these basic principles, Tabor and Warszynski [
64] report release kinetics proportional to
with
. By means of parametric analyses, the authors demonstrate that the exponent
depends on the rate parameter
b in Equation (
37) and the partition coefficient of the inhibitor (i.e., the ratio between the solubilities of the inhibitor at the solution in the scratch and within the coating matrix).
6. Discussion
A self-healing mechanism, to be successful, requires the timely activation, mobilization and reaction of the healing agents. In the course of these stages, both mechanical stress and external environment may play an active role. This paper reviews four modeling frameworks that address the healing mechanisms from different perspectives: the dynamic adaptation of the polymeric network (
Section 2), the degradation and recovery of the mechanical properties of the coating (
Section 3 and
Section 4) and the transport and reaction of species within the matrix (
Section 5).
Dynamic polymeric networks based on reversible chemistries lead to coating systems that actively react to externally applied stimulus such as changes in temperature [
5,
10] and exposition to UV light [
12]. The incorporation of reversible bonds enhances polymer flow, but at the expense of its strength. Moreover, the external stimulus that triggers the self-healing response depends on the specific bond chemistry. Thus, approaches using retro Diels-Alder reactions require higher activation temperatures than systems based on disulfide bonds [
6]. The Bell model has been generalized to incorporate the contributions of both temperature and stress on the process of bond formation and rupture [
26], which allows the evaluation of specific bond kinetics provided an accurate knowledge of the involved parameters. While these are not made available, the best option to calibrate the model continues to be its fit to measurable macroscopic features [
33]. The incorporation of dynamic bond kinetics into complex network configurations allows the evaluation of the macroscopic deformation [
26,
29] and the load bearing capacity [
33] for each particular bond chemistry. If polymer diffusion is also taken into account, the capabilities of the predictive model include the adhesion of interfaces [
33] and, therefore, a full characterization of the self healing response in terms of bond chemistry, but also cross-linker density [
32] and polymer chain length [
33].
Models based on the continuum damage healing mechanics offer the possibility to couple the healing mechanisms at the molecular scale with the (homogenized) macroscopic description of the material. The complexity of this approach arises from the need for an accurate formulation of the evolution laws for the damage and healing internal variables [
43]. Phenomenological laws in terms of the mass of healing agents [
42] and the experimentally measured healing efficiencies [
39] have been used to reproduce the response to mechanical load in polymeric and composite materials. Recently, a more elaborated model that couples the closure of the scratch by shape memory polymers with the diffusion of the self-healing agent across the interface has been formulated in [
46]. Extrinsic self-healing mechanisms, however, seem to remain elusive from these approaches. On the other hand, cohesive formulations of the evolving crack ease the treatment of damage and healing. Relatively straight forward formulations of the traction-displacement function allow to capture changes in the mechanical performance of materials due to fatigue and healing [
48,
50] and repeated healing events [
51], though the actual healing step is not taken into account (a phenomenological law for the curing degree of the polymerization of liquid healing agents was used in [
50] to establish the dimensions and mechanical properties of the healed material). Therefore, it seems clear that the path to improve the predictive power of these models demands the incorporation of the transport and reaction of healing agents, either from the capillary flow of liquid agents [
66] or the diffusive transport and reaction of dissolved species [
54]. On the other hand, cohesive models have been proved to be very useful in the evaluation of encapsulation systems [
52,
53].
In the case of extrinsic self-healing mechanisms activated by the ingress of external species, such as those in thermal barrier coating [
18,
19] or expandable systems [
21,
22], the models discussed in
Section 5 focus on the interaction between the coating matrix and both the external triggering species (oxygen, moisture) and the internal healing agents through systems of diffusion-reaction reactions. This interaction leads to diffusion profiles that depart from Fickian diffusion, for both the ingress of moisture [
22,
56] and the transport of the healing agent [
59,
60], that are not fully understood. The models do predict an anomalous diffusion behavior and provide a framework to analyze transport kinetics faster than Fickian. The observed anomalous diffusion behavior can be traced back to the distribution of the encapsulated healing agents, the capsule dissolution kinetics and the moisture diffusion within the coating matrix [
54] and to the rate of healing agent activation and differences in the solubilities across the crack surface [
64]. Despite the potential that these models present to explain part of the spectrum of leaching kinetics of corrosion inhibitors, and to evaluate the potential performance of specific inhibitor-coating matrix systems, a thorough calibration with experimental data is necessary to surpass the present qualitative descriptions. Additionally, a possible extension that could be implemented in the presented diffusion-reaction models is the incorporation of differential equations of fractional order [
67]. This would introduce the anomalous diffusion of the external triggering species and/or the healing species and possibly broaden the range of leaching kinetics that can be predicted. Another consideration that could deeper the knowledge of the interactions between the matrix and the diffusing species may be to take into account the porous nature of the coating matrix [
68,
69]. Such a modification would allow to treat the different compounds in the material as different phases of the porous material [
70], but also to incorporate the effects of pore fluid (and/or gas) pressure and flow and of the evolving porosity on the transport of dissolved chemical compounds. At least from a conceptual point of view, such phenomena could have an important outcome in the performance of corrosion protection coatings and expanding hydrogels.