Corrosion Behavior of Hydrotalcite Film on AZ31 Alloy in Simulated Body Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Films
2.2. Characterization
3. Results and Discussion
3.1. Morphology of the HT Film
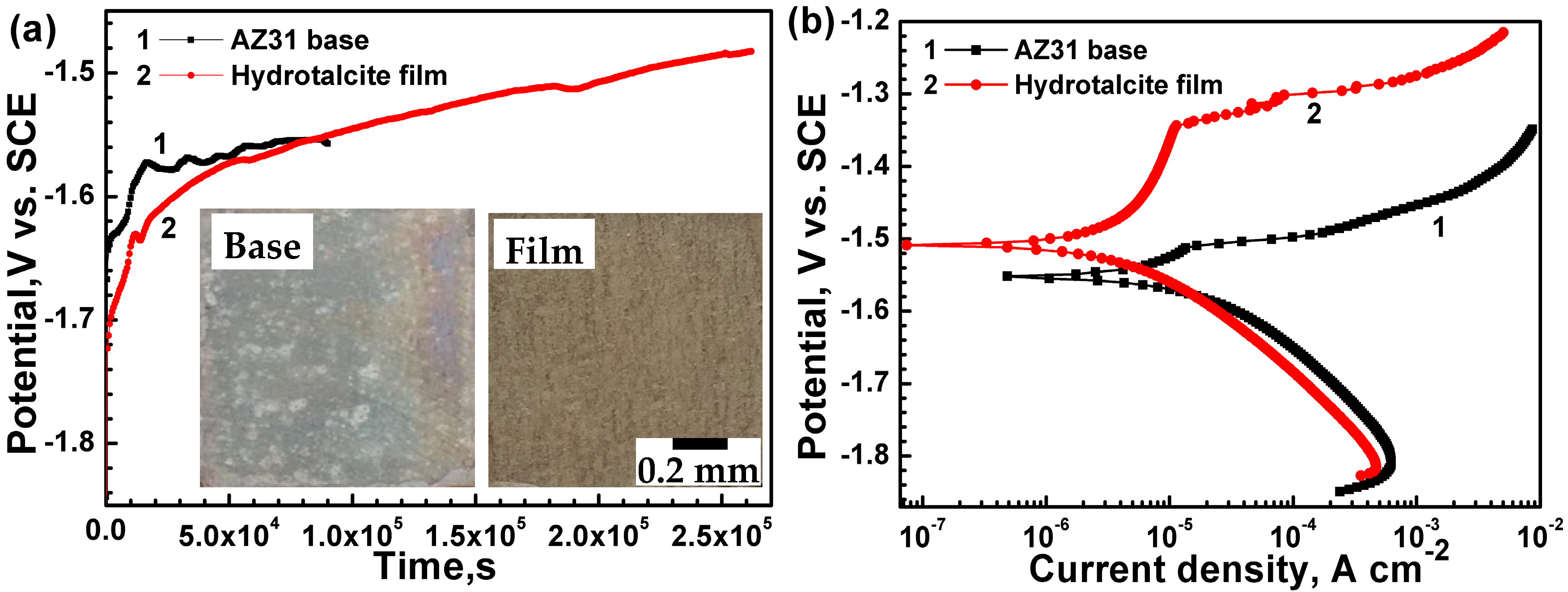
3.2. Electrochemical Corrosion Test
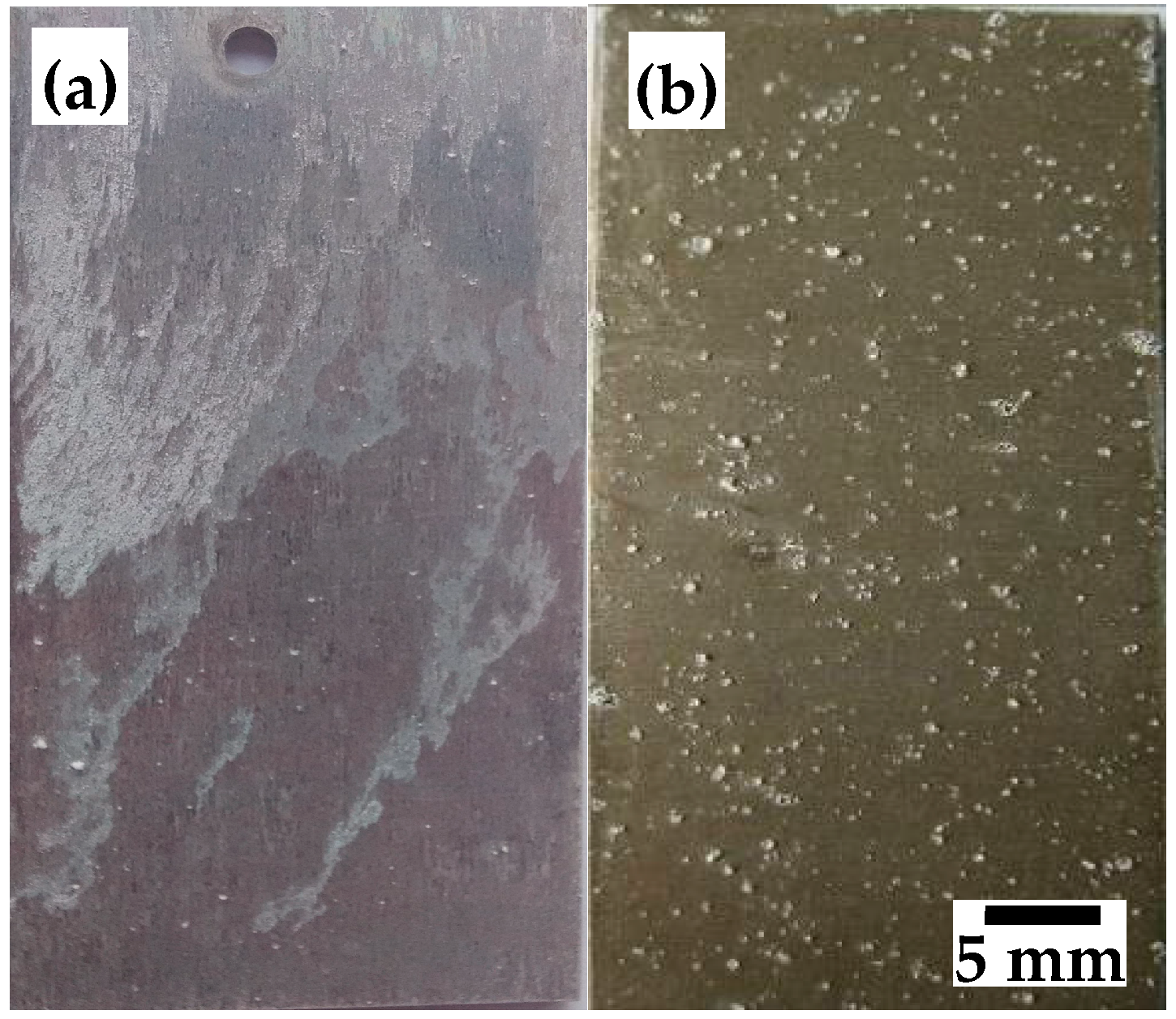
3.3. Corrosion Morphology of the HT Film
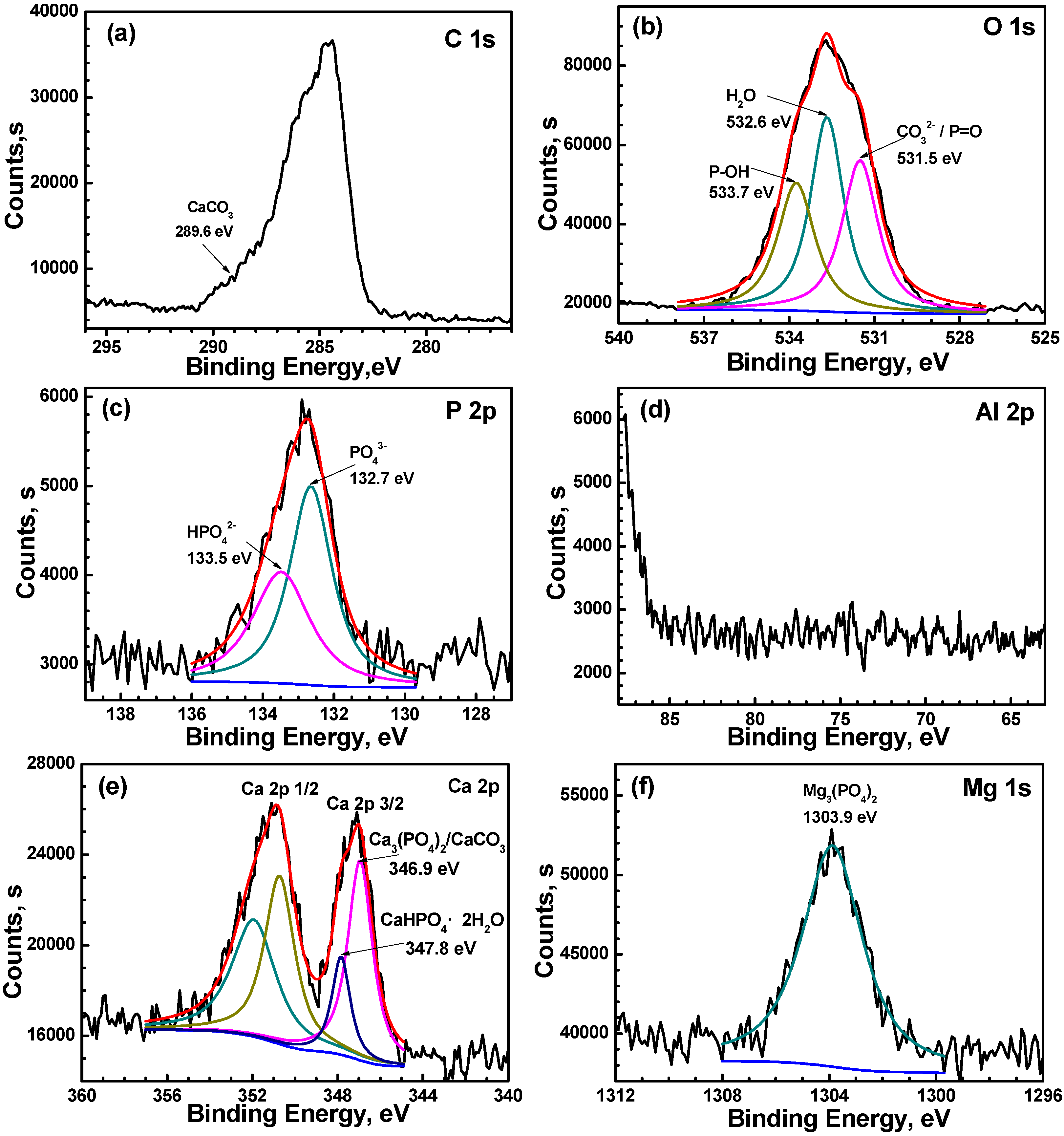
3.4. Composition Analysis of the Film
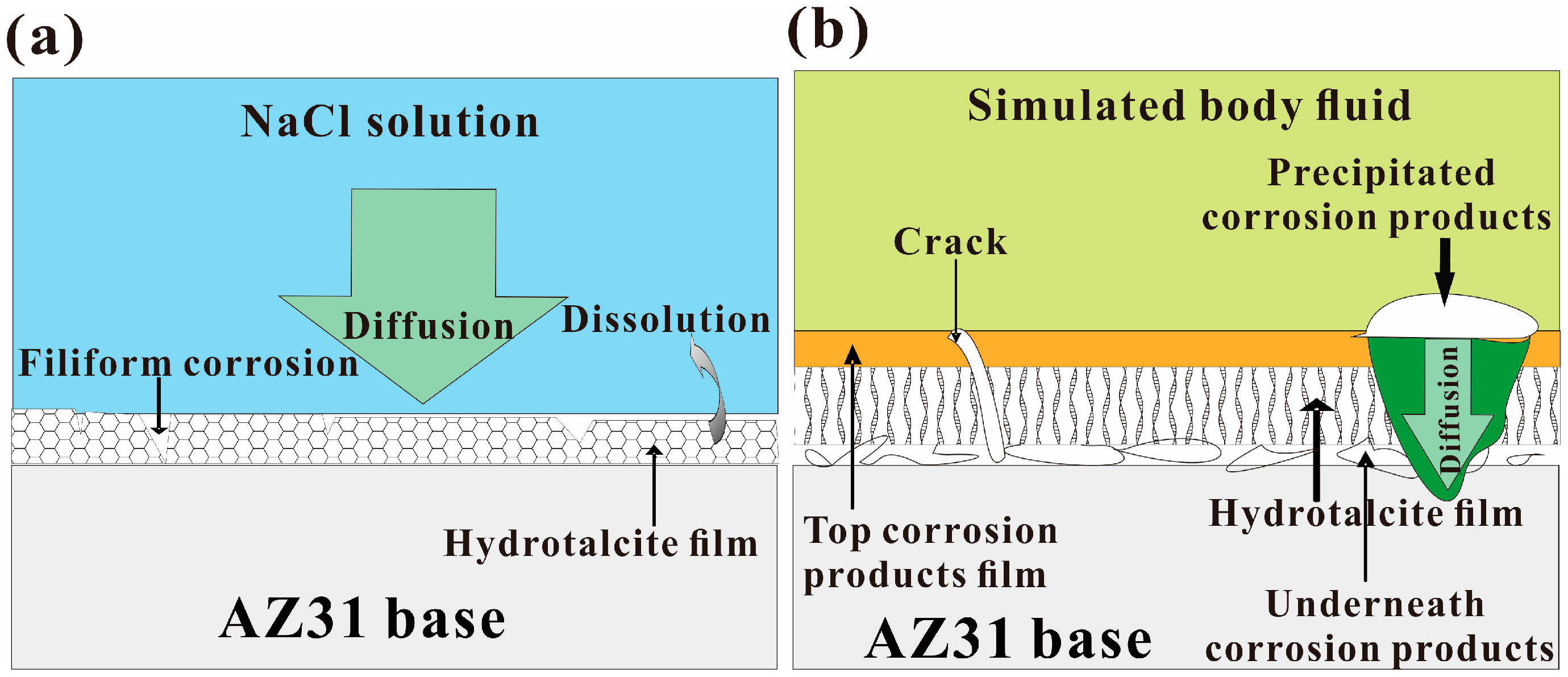
3.5. A Comparison of the Corrosion Behavior of AZ31 with HT Film in NaCl Solution and Hank’s Solution
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Monsees, T.K.; Azem, F.A.; Cotrut, C.M.; Braic, M.; Abdulgader, R.; Pana, I.; Birlik, I.; Kiss, A.; Booysen, R.; Vladescu, A. Biodegradable ceramics consisting of hydroxyapatite for orthopaedic implants. Coatings 2017, 7, 184. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Y.; Sun, L.; Zeng, R.C.; Zheng, Y.F.; Li, S.Q. In vitro degradation and biocompatibility of Mg-Li-Ca alloys-the influence of Li content. Sci. China Mater. 2018, 61, 607–618. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative in vitro study on pure metals (Fe, Mn, Mg, Zn and W) as biodegradable metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhao, D.L.; Ohodnicki, J.M.; Lee, B.; Roy, A.; Yao, R.; Chen, S.; Dong, Z.Y.; Heineman, W.R.; Kumta, P.N. In vitro and in vivo evaluation of multiphase ultrahigh ductility Mg-Li-Zn alloys for cardiovascular stent application. ACS Biomater. Sci. Eng. 2018, 4, 919–932. [Google Scholar] [CrossRef]
- He, M.F.; Wang, H.; Zhou, K.G.; Pan, D.; Liu, F. Effects of Li addition on the corrosion behaviour and biocompatibility of Mg(Li)-Zn-Ca metallic glasses. J. Mater. Sci. 2018, 53, 9928–9942. [Google Scholar] [CrossRef]
- Xu, L.P.; Yu, G.N.; Zhang, E.L.; Pan, F.; Yang, K. In vivo corrosion behavior of Mg-Mn-Zn alloy for bone implant application. J. Biomed. Mater. Res. A 2007, 83, 703–711. [Google Scholar] [CrossRef]
- Tan, L.L.; Yu, X.M.; Wan, P.; Yang, K. Biodegradable materials for bone repairs: A review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Degner, J.; Singer, F.; Cordero, L.; Boccaccini, A.R.; Virtanen, S. Electrochemical investigations of magnesium in DMEM with biodegradable polycaprolactone coating as corrosion barrier. Appl. Surf. Sci. 2013, 282, 264–270. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Huang, W.; Yang, W.Z.; Yin, X.S.; Liu, Y. In vitro degradation, cytocompatibility and hemolysis tests of CaF2 doped TiO2-SiO2 composite coating on AZ31 alloy. Appl. Surf. Sci. 2016, 382, 268–279. [Google Scholar] [CrossRef]
- Ding, J.D. A composite strategy to fabricate high-performance biodegradable stents for tissue regeneration. Sci. China Mater. 2018, 61, 1132–1134. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Dong, Z.Y.; Yang, D.E.; Schulz, M.J.; Shanov, V.N.; Yarmolenko, S.; Xu, Z.G.; Kumta, P.; Sfeir, C. Biodegradable Mg corrosion and osteoblast cell culture studies. Mater. Sci. Eng. C: Mater. Biol. Appl. 2009, 29, 1814–1821. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.Y.; Kriven, W.M.; Wallig, M.A.; Choy, J.H. Inorganic delivery vector for intravenous injection. Biomaterials 2004, 25, 5995–6001. [Google Scholar] [CrossRef]
- Tsukanov, A.A.; Psakhie, S.G. Energy and structure of bonds in the interaction of organic anions with layered double hydroxide nanosheets: A molecular dynamics study. Sci. Rep. 2016, 6, 19986. [Google Scholar] [CrossRef]
- Rojas, R.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Giacomelli, C.E. Release kinetics from LDH-drug hybrids: Effect of layers stacking and drug solubility and polarity. Colloids Surf. A-Physicochem. Eng. Aspects 2014, 463, 37–43. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M. Double layered hydroxides as potential anti-cancer drug delivery agents. Mini-Rev. Med. Chem. 2013, 13, 522–529. [Google Scholar] [CrossRef]
- Perioli, L.; Mutascio, P.; Pagano, C. Influence of the nanocomposite MgAl-HTlc on gastric absorption of drugs: In vitro and ex vivo studies. Pharmcol. Res. 2013, 30, 156–166. [Google Scholar] [CrossRef]
- Bellezza, F.; Alberani, A.; Posati, T.; Tarpani, L.; Latterini, L.; Cipiciani, A. Protein interactions with nanosized hydrotalcites of different composition. J. Inorg. Biochem. 2012, 106, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Hatakeyama, A.; Tsuhako, M. Encapsulation of nucleotides and DNA into Mg-Al layered double hydroxide. Int. J. Pharm. 2010, 393, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Pang, X.L.; Wei, L.; Gao, K.W. Insitu grown superhydrophobic Zn-Al layered double hydroxides films on magnesium alloy to improve corrosion properties. Appl. Surf. Sci. 2015, 337, 172–177. [Google Scholar] [CrossRef]
- Lin, J.K.; Uan, J.Y. Formation of Mg, Al-hydrotalcite conversion coating on Mg alloy in aqueous HCO3-/CO32- and corresponding protection against corrosion by the coating. Corros. Sci. 2009, 51, 1181–1188. [Google Scholar] [CrossRef]
- Yu, B.Y.; Lin, J.K.; Uan, J.Y. Applications of carbonic acid solution for developing conversion coating on Mg alloy. Trans. Nonferrous Met. Soc. China 2010, 20, 1331–1339. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y.; Han, E.H. In situ growth of Mg-Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2011, 53, 3281–3288. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y.; Han, E.H. Study of the corrosion mechanism of the in situ grown Mg-Al-CO32− hydrotalcite film on AZ31 alloy. Corros. Sci. 2012, 65, 268–277. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y.; Han, E.H. Modifications of the hydrotalcite film on AZ31 Mg alloy by phytic acid: The effects on morphology, composition and corrosion resistance. Corros. Sci. 2013, 74, 130–138. [Google Scholar] [CrossRef]
- Jobbágy, M.; Regazzoni, A.E. Dissolution of nano-size Mg-Al-Cl hydrotalcite in aqueous media. Appl. Clay Sci. 2011, 51, 366–369. [Google Scholar] [CrossRef]
- GB 10124-88 Standard Practice for Laboratory Immersion Corrosion Testing of Metals; Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- Wang, B.J.; Xu, D.K.; Dong, J.H.; Ke, W. Effect of texture on biodegradable behavior of an as-extruded Mg-3%Al-1%Zn alloy in phosphate buffer saline medium. J. Mater. Sci. Technol. 2016, 32, 646–652. [Google Scholar] [CrossRef]
- Wu, L.P.; Zhao, L.; Dong, J.H.; Ke, W.; Chen, N. Potentiostatic conversion of phosphate mineral coating on AZ31 magnesium alloy in 0.1 M K2HPO4 solution. Electrochim. Acta 2014, 145, 71–80. [Google Scholar] [CrossRef]
- Chen, Y.G.; Luan, B.L.; Song, G.L.; Yang, Q.; Kingston, D.M.; Bensebaa, F. An investigation of new barium phosphate chemical conversion coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2012, 210, 156–165. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Chen, R.S.; Zhang, F.; Han, E.H. A novel phosphate conversion film on Mg-8.8Li alloy. Surf. Coat. Technol. 2009, 203, 1107–1113. [Google Scholar] [CrossRef]
- Cui, X.F.; Li, Q.F.; Li, Y.; Wang, F.H.; Jin, G.; Ding, M.H. Microstructure and corrosion resistance of phytic acid conversion coatings for magnesium alloy. Appl. Surf. Sci. 2008, 255, 2098–2103. [Google Scholar] [CrossRef]
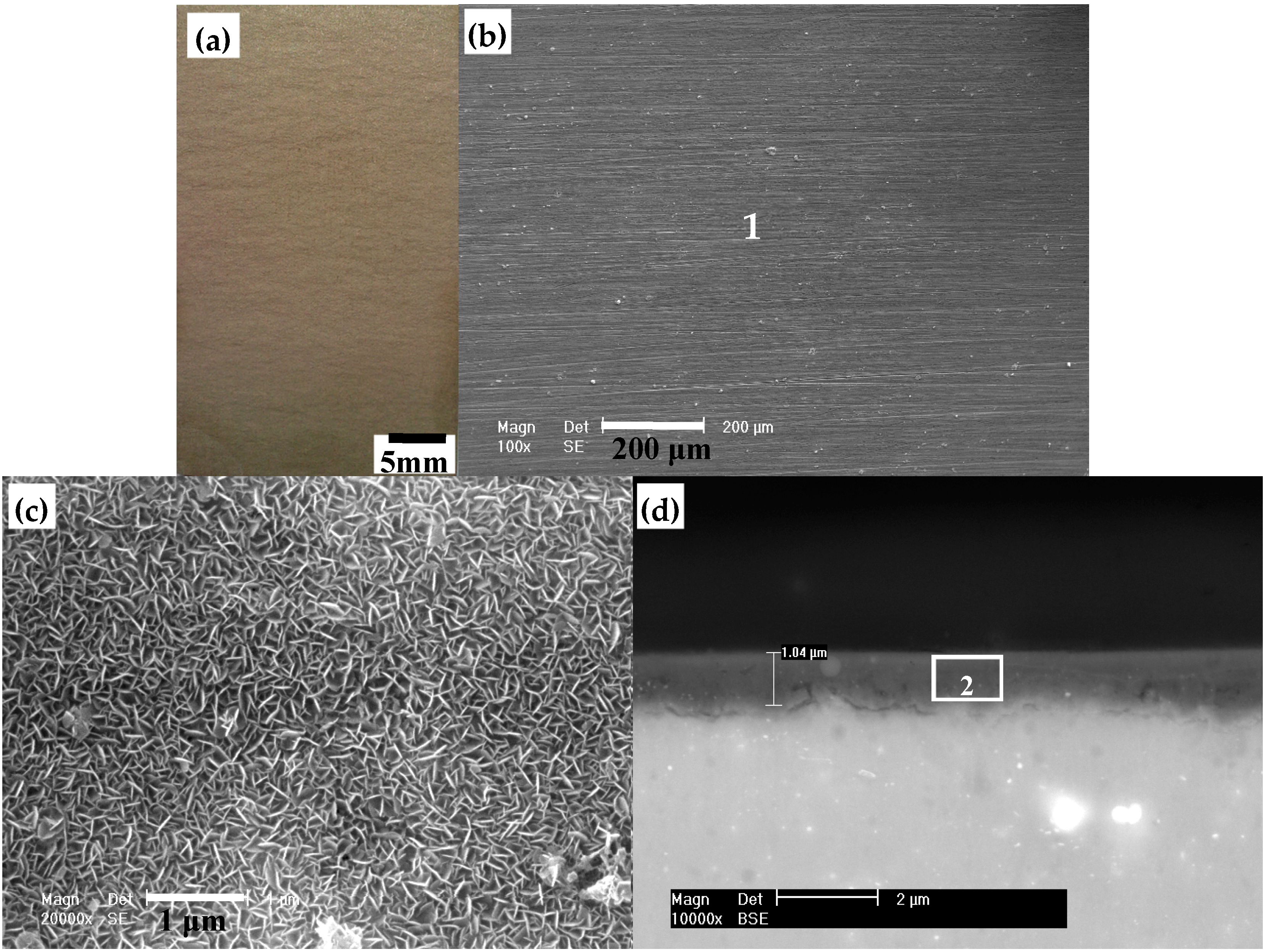
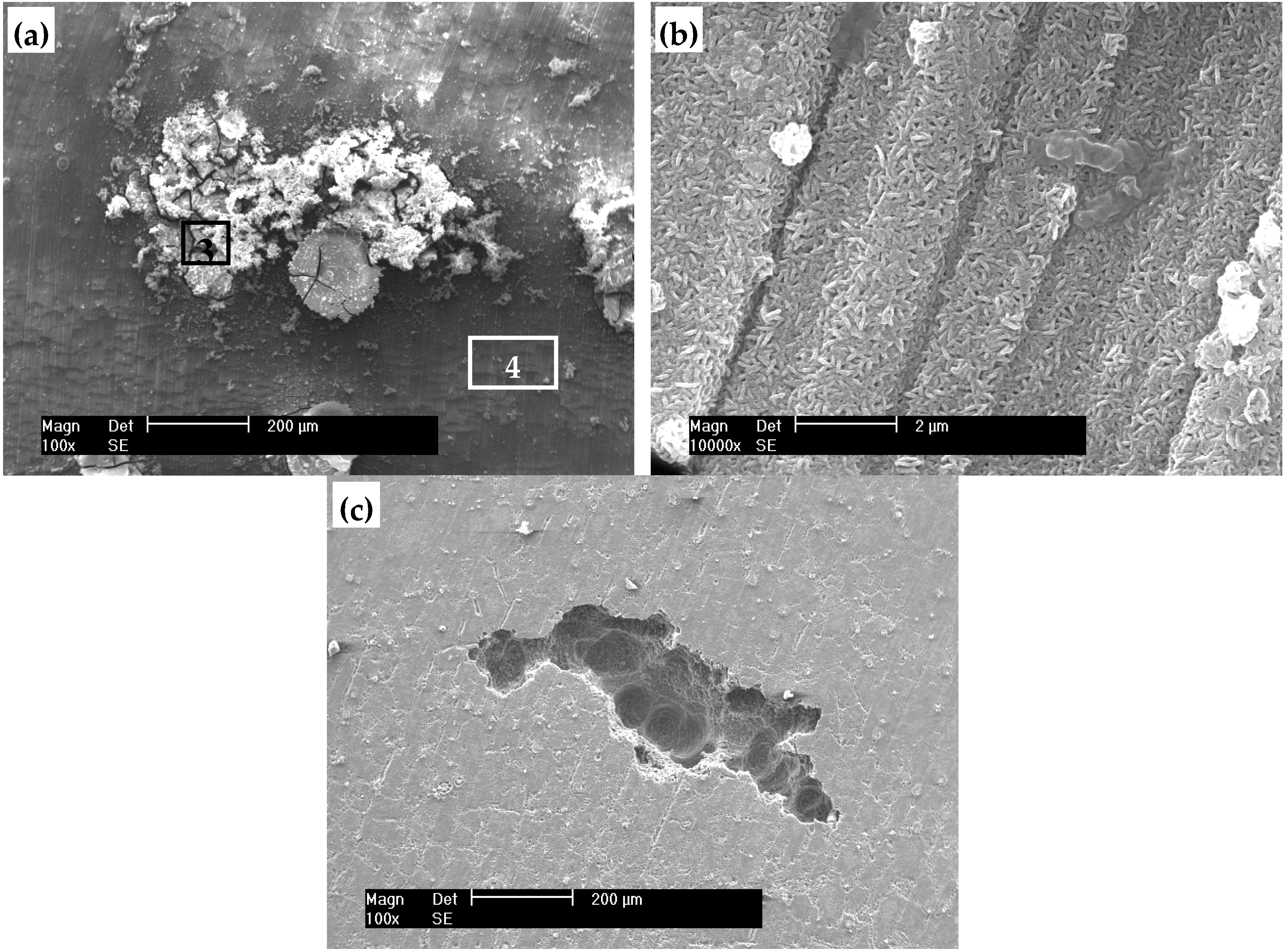
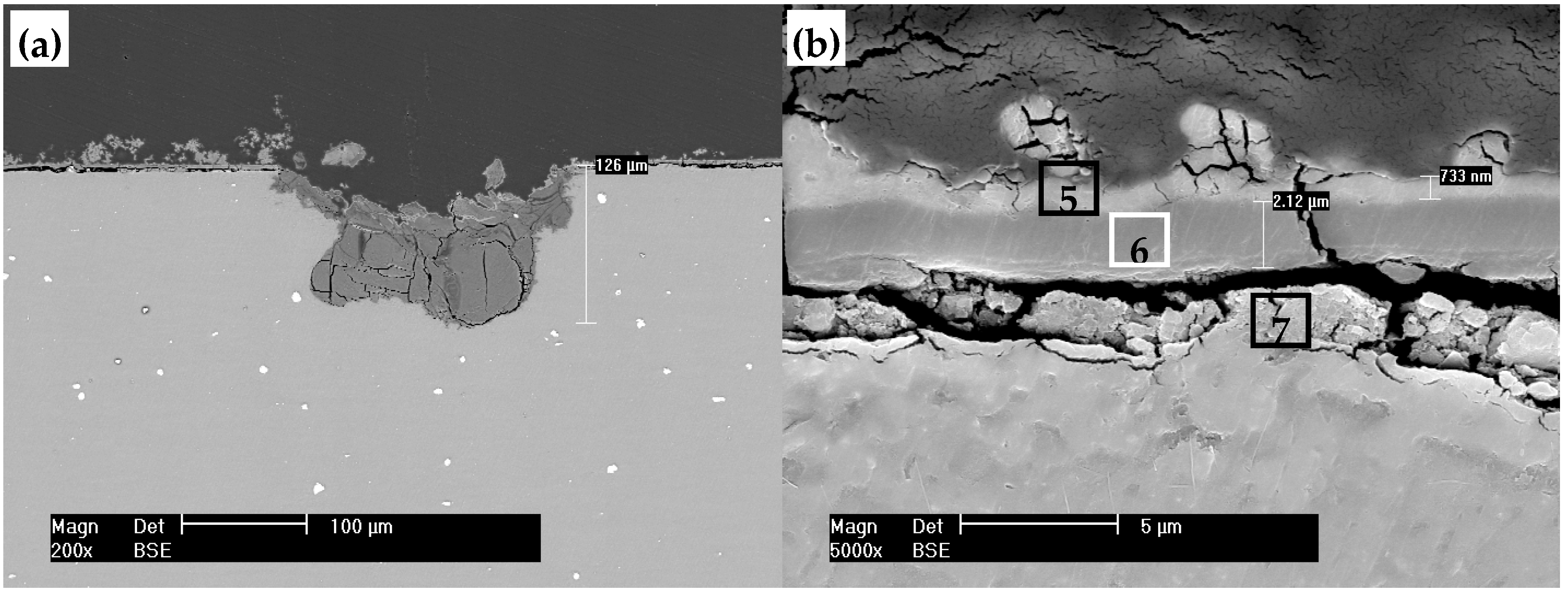
| Element (at. %) | C | O | Mg | Al | P | Ca |
|---|---|---|---|---|---|---|
| 1 | 15.67 | 21.00 | 59.54 | 03.80 | − | − |
| 2 | 16.53 | 42.96 | 31.85 | 8.66 | − | − |
| 3 | 19.36 | 42.94 | 04.02 | − | 12.57 | 21.11 |
| 4 | 20.09 | 48.88 | 16.91 | 05.19 | 04.99 | 03.94 |
| 5 | 37.12 | 40.36 | 06.18 | 00.62 | 09.04 | 06.67 |
| 6 | 21.84 | 48.57 | 17.17 | 05.49 | 04.65 | 02.28 |
| 7 | 25.35 | 40.90 | 23.78 | 02.60 | 05.85 | 01.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Kang, K.; Song, Y.; Han, E.-H.; Ma, S.; Ao, J. Corrosion Behavior of Hydrotalcite Film on AZ31 Alloy in Simulated Body Fluid. Coatings 2019, 9, 113. https://doi.org/10.3390/coatings9020113
Chen J, Kang K, Song Y, Han E-H, Ma S, Ao J. Corrosion Behavior of Hydrotalcite Film on AZ31 Alloy in Simulated Body Fluid. Coatings. 2019; 9(2):113. https://doi.org/10.3390/coatings9020113
Chicago/Turabian StyleChen, Jun, Kai Kang, Yingwei Song, En-Hou Han, Sude Ma, and Jinqing Ao. 2019. "Corrosion Behavior of Hydrotalcite Film on AZ31 Alloy in Simulated Body Fluid" Coatings 9, no. 2: 113. https://doi.org/10.3390/coatings9020113
APA StyleChen, J., Kang, K., Song, Y., Han, E.-H., Ma, S., & Ao, J. (2019). Corrosion Behavior of Hydrotalcite Film on AZ31 Alloy in Simulated Body Fluid. Coatings, 9(2), 113. https://doi.org/10.3390/coatings9020113




