Influence of Different Heat Treatment Temperatures on the Microstructure, Corrosion, and Mechanical Properties Behavior of Fe-Based Amorphous/Nanocrystalline Coatings
Abstract
1. Introduction
2. Experimental
2.1. Coatings Preparation
2.2. Coatings Characterization
2.3. Corrosion Test
2.4. Microhardness Test
3. Results and Discussion
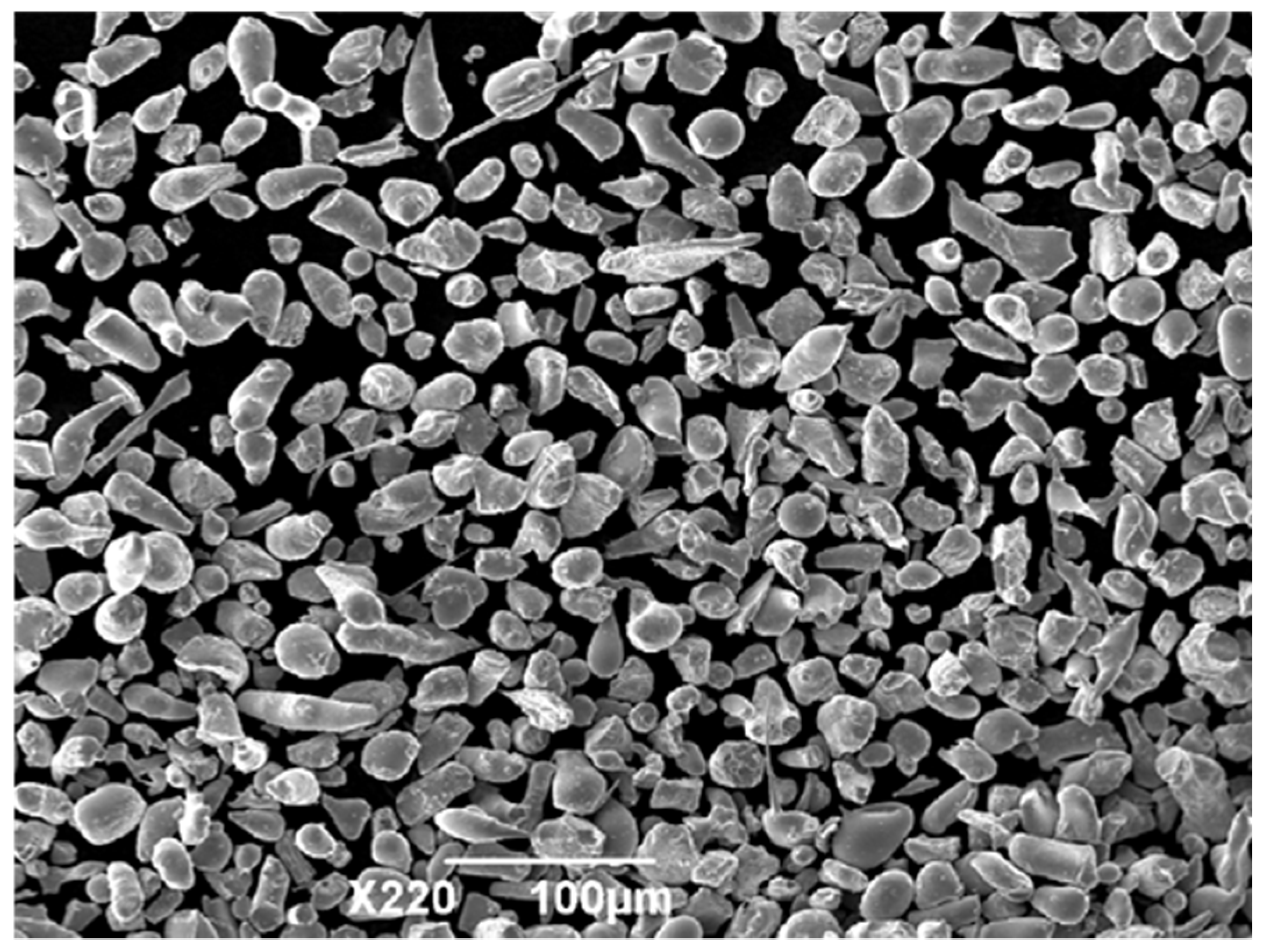
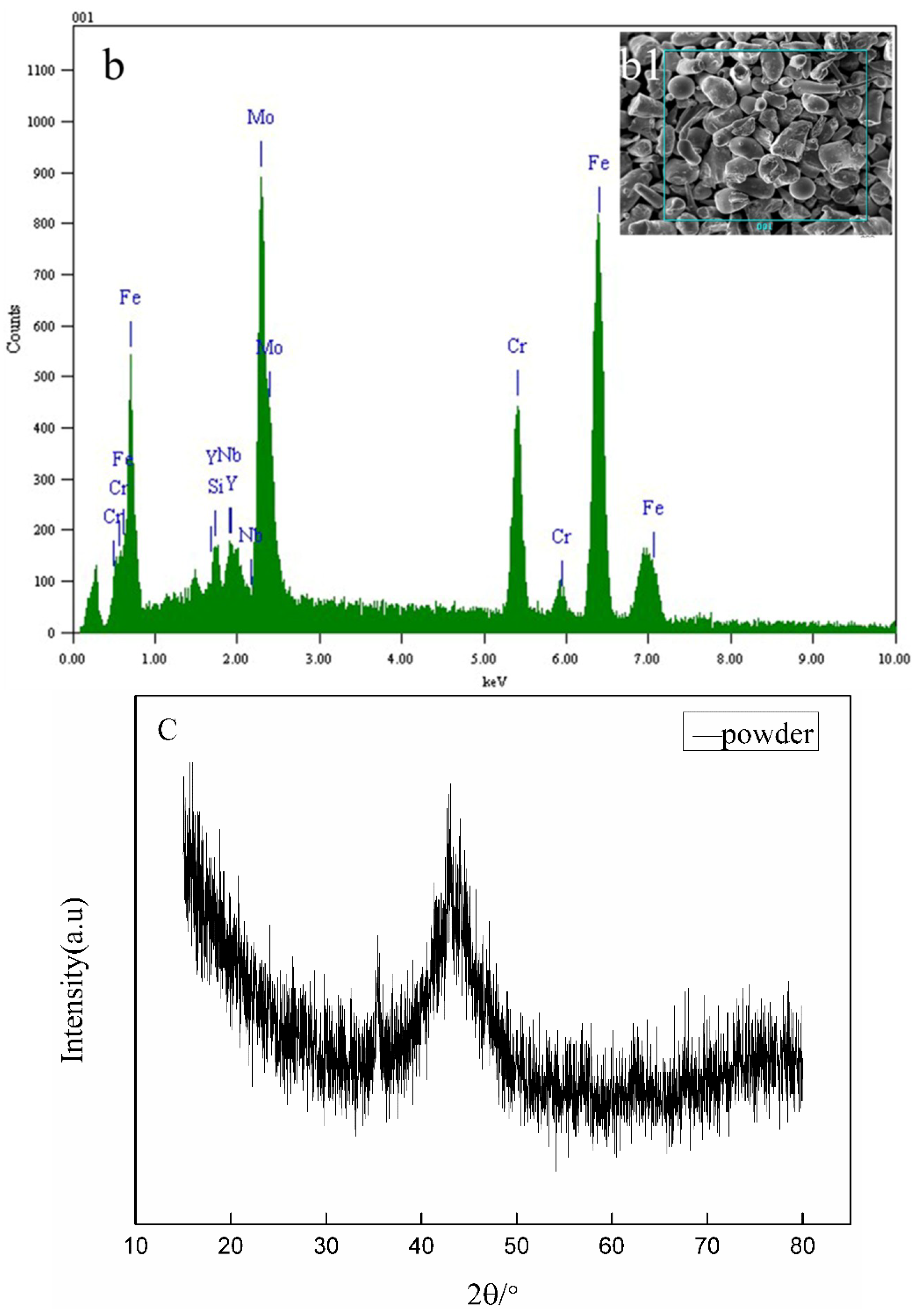
3.1. Powder Characteristics
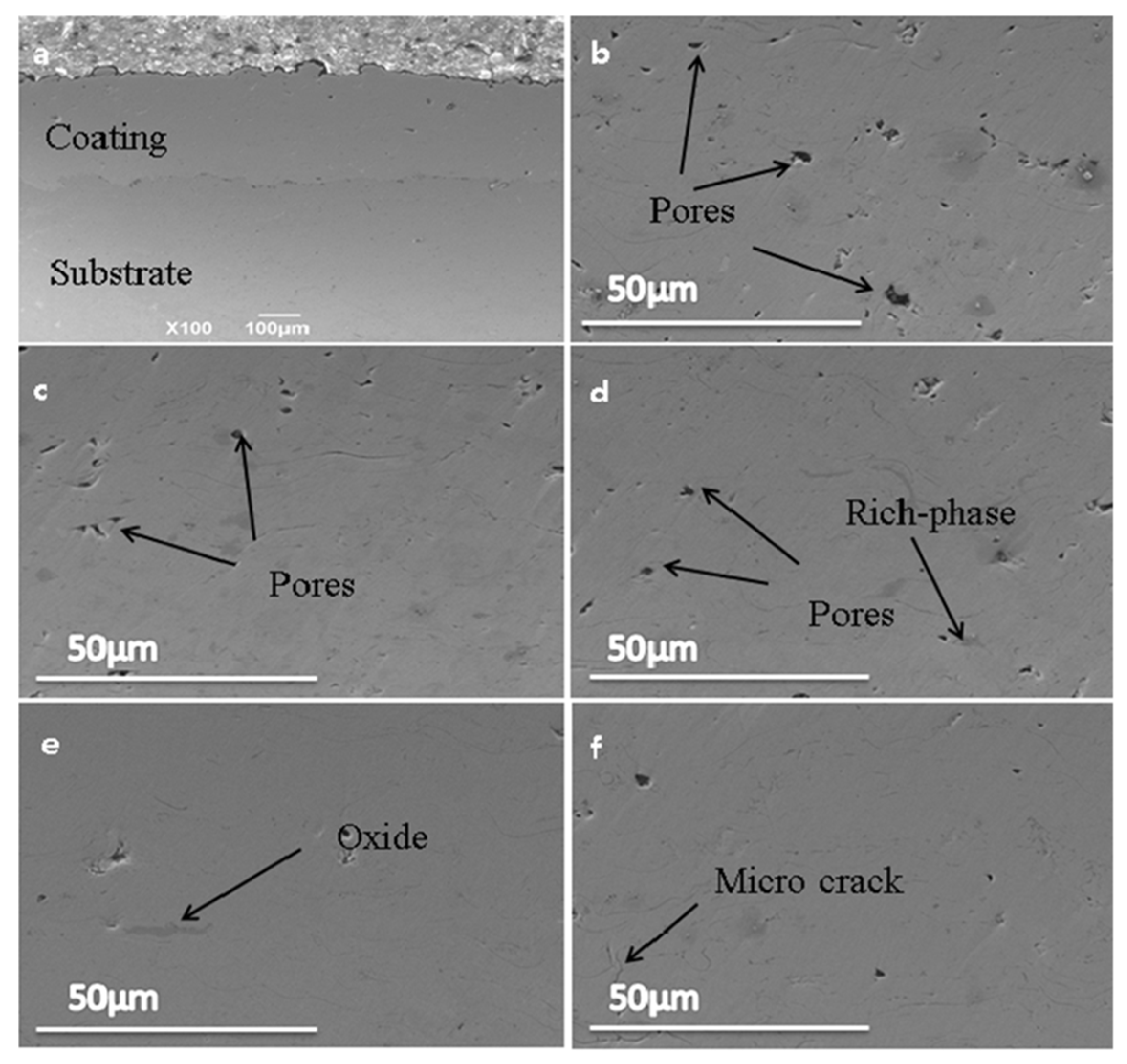
3.2. The Microstructure of the As-Sprayed Coating and Annealed Coatings
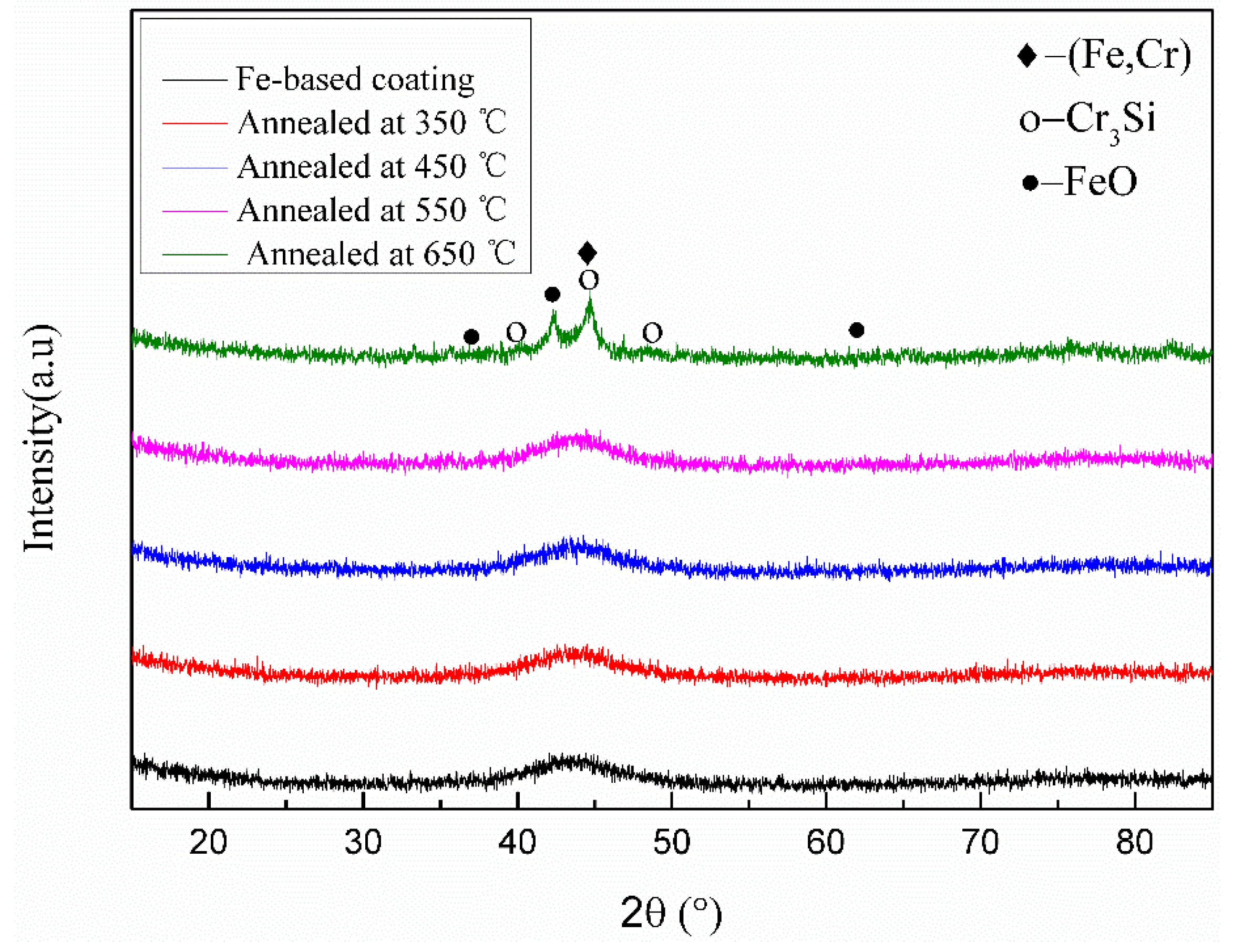
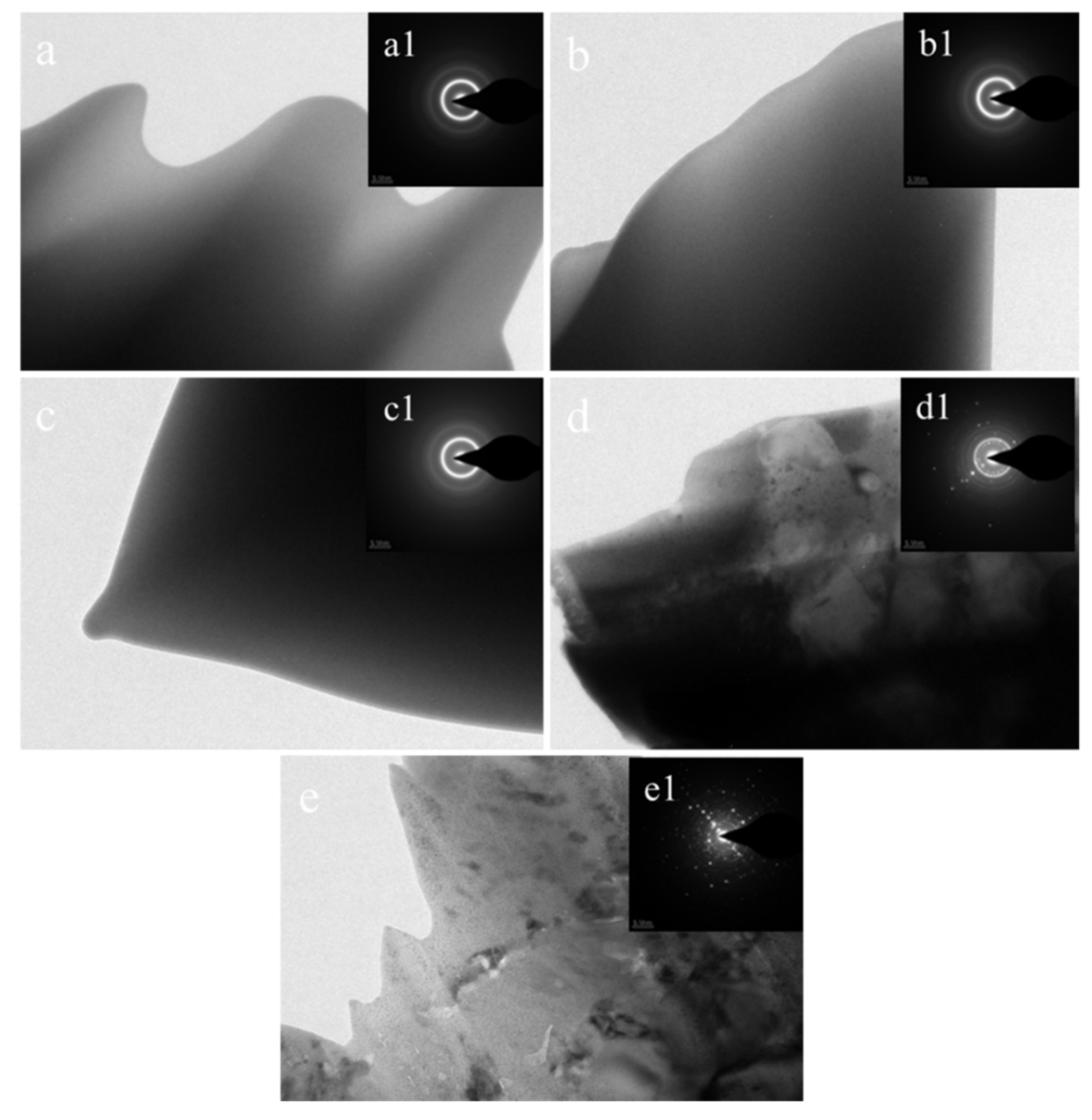
3.3. XRD and TEM Analysis
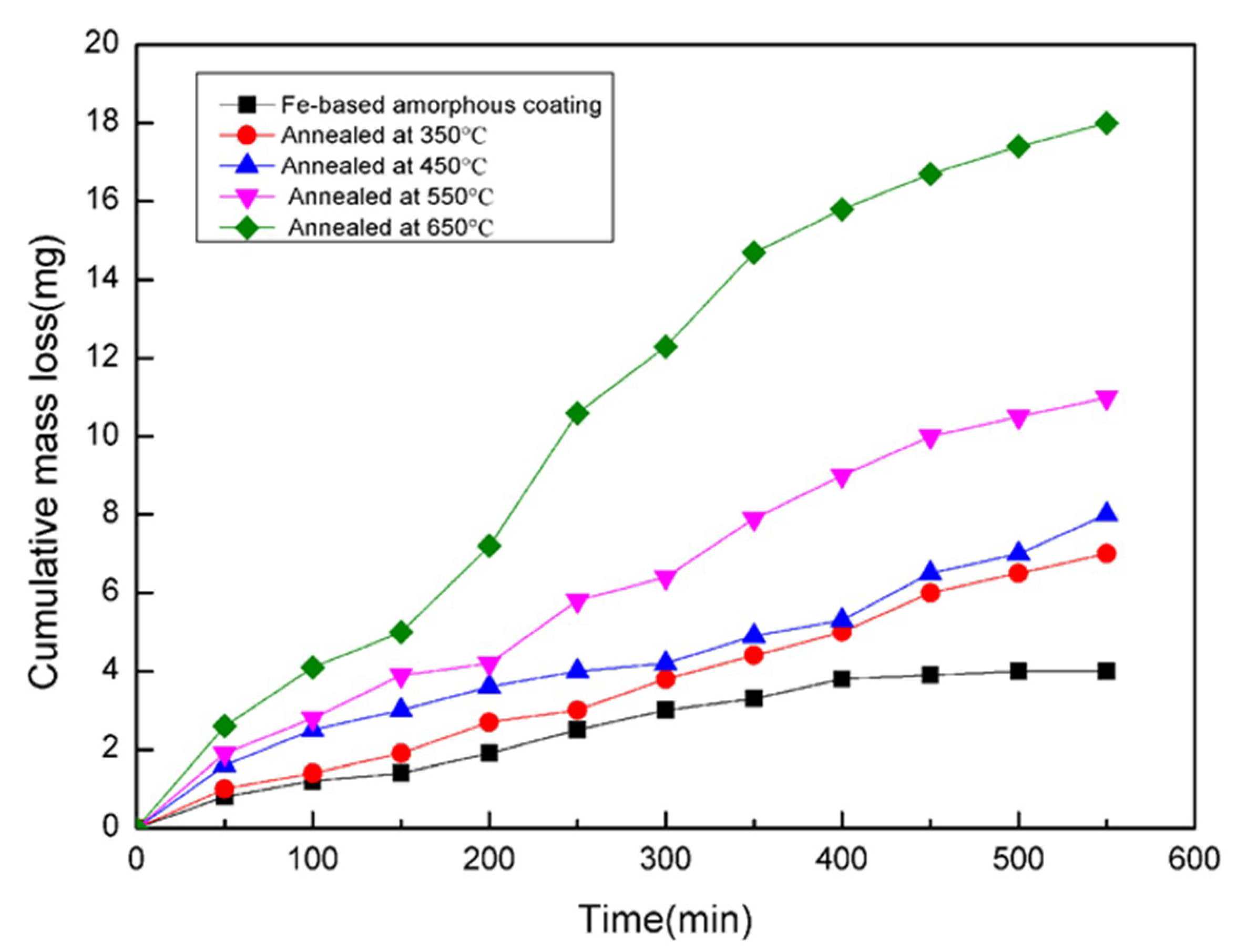
3.4. Influence of Annealing Temperature on the Corrosion Resistance
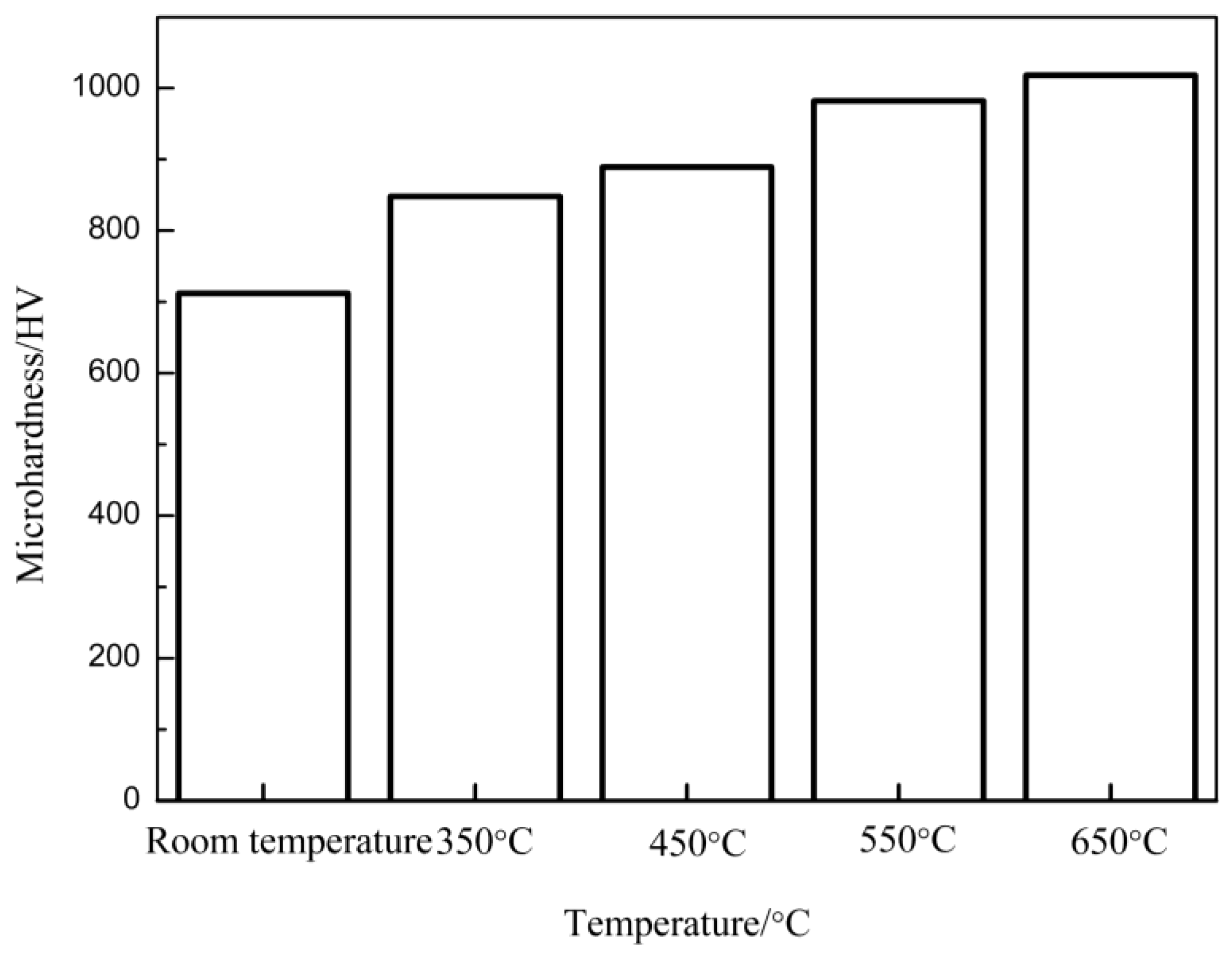
3.5. Influence of Annealing Temperature on the Microhardness
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, Y.J.; Wu, Y.P.; Zhang, J.F.; Guo, W.M.; Hong, S.; Chen, L.Y. Long-term corrosion behavior of HVOF sprayed FeCrSiBMn amorphous/nanocrystalline coating, Tran. Nonferrous Met. Soc. China 2015, 25, 1144–1150. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, C.; Zhou, H.; Liu, L. On the bonding strength in thermally sprayed Fe-based amorphous coatings. Surf. Coat. Technol. 2013, 218, 17–22. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Xu, P.; Yasir, M.; Liu, L. Enhancement of oxidation and wear resistance of Fe-based amorphous coatings by surface modification of feedstock powders. Mater. Des. 2015, 73, 35–41. [Google Scholar] [CrossRef]
- Kishitake, K.; Era, H.; Otsubo, F. Characterization of plasma sprayed Fe–17Cr–38Mo–4C amorphous coatings crystallizing at extremely high temperature. J. Therm. Spray Techn. 1996, 5, 283–288. [Google Scholar] [CrossRef]
- Otsubo, F.; Kishitake, K. Corrosion resistance of Fe–16%Cr–30%Mo–(C,B,P) amorphous coatings sprayed by HVOF and APS processes. Mater. Trans. 2005, 46, 80–83. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, D.; Liang, X.; Chen, Y. Wear behaviors of arc-sprayed FeBSiNb amorphous coatings. Tribol. Let. 2015, 60, 1–7. [Google Scholar] [CrossRef]
- Cherigui, M.; Fenineche, N.E.; Coddet, C. Structural study of iron-based microstructured and nanostructured powders sprayed by HVOF thermal spraying. Surf. Coat. Technol. 2005, 192, 19–26. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, P.; Xie, G.; Hu, J.; Cao, M. Formation of amorphous and nanocrystalline phasesin high velocity oxy-fuel thermally sprayed a Fe–Cr–Si–B–Mn alloy. Mater. Sci. Eng. A 2006, 430, 34–39. [Google Scholar] [CrossRef]
- Guo, Y.; Koga, G.Y.; Jorge, A.M., Jr.; Savoie, S.; Schulz, R.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Microstructural investigation of Fe–Cr–Nb–B amorphous/nanocrystalline coating produced by HVOF. Mater. Des. 2016, 110, 608–615. [Google Scholar] [CrossRef]
- Botta, W.J.; Berger, J.E.; Kiminami, C.S.; Roche, V.; Nogueira, R.P.; Bolfarini, C. Corrosion resistance of Fe-based amorphous alloys. J. Alloys Compd. 2014, 586, s105–s110. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Ma, G.Z.; Wang, H.D.; Li, G.L.; Chen, S.Y.; Wang, H.J. Fabrication and characterization of supersonic plasma sprayed Fe-based amorphous metallic coatings. Mater. Des. 2016, 110, 332–339. [Google Scholar] [CrossRef]
- Yasir, M.; Zhang, C.; Wang, W.; Xu, P.; Liu, L. Wear behaviors of Fe-based amorphous composite coatings reinforced by Al2O3 particles in air and in NaCl solution. Mater. Des. 2015, 88, 207–213. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, J.; Kim, B.D.; Lee, C. Tribological behavior of B4C reinforced Fe-base bulk metallic glass composite coating. Surf. Coat. Technol. 2010, 205, 1962–1968. [Google Scholar] [CrossRef]
- Terajima, T.; Takeuchi, F.; Nakata, K.; Adachi, S.; Nakashima, K.; Igarashi, T. Composite coating containing WC/12Co cermet and Fe-based metallic glass deposited by high-velocity oxygen fuel spraying. J. Alloy. Compd. 2010, 504, S288–S291. [Google Scholar] [CrossRef]
- Yugeswaran, S.; Kobayashi, A.; Suresh, K.; Subramanian, B. Characterization of gas tunnel type plasma sprayed TiN reinforced Fe-based metallic glass coatings. J. Alloy. Compd. 2013, 551, 168–175. [Google Scholar] [CrossRef]
- Do, J.; Jeon, C.; Kim, C.P.; Lee, B.J.; Lee, S.; Lee, E.S.; Yoon, T.S.; Shin, Y.S. Effects of (Cr,Fe)2B borides on hardness in powder-injection-molded product fabricated with Fe-based alloy powders. Mater. Sci. Eng. A 2012, 556, 366–372. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Z.; Lin, P.; Cheng, J.; Zhang, X.; Hong, S. Effects of post annealing on the microstructure, mechanical properties and cavitation erosion behavior of arc-sprayed FeNiCrBSiNbW coatings. Mater. Des. 2015, 65, 1035–1040. [Google Scholar] [CrossRef]
- Lin, J.R.; Wang, Z.H.; Lin, P.H.; Cheng, J.B.; Zhang, X.; Hong, S. Effect of crystallization on electrochemical properties of arc sprayed FeNiCrBSiNbW coatings. Surf. Eng. 2014, 30, 683–687. [Google Scholar] [CrossRef]
- Goedjen, J.G.; Shores, D.A. The effect of alloy grain size on the transient oxidation behavior of an alumina-forming alloy. Oxid. Met. 1992, 37, 125–142. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Y.; Zhang, J.; Yuan, W. Effect of the long-term heat treatmenton the cyclic oxidation behavior of Fe-based amorphous/nanocrystalline coatings prepared by high-velocity arc spray process. Surf. Coat. Technol. 2016, 307, 392–398. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, H.; Xu, P.; Lu, Q.; Li, C.; Yu, Z. Influence of different annealing temperatures and cooling rates on amorphous and crystalline composite coating. Surf. Coat. Technol. 2012, 206, 4981–4987. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.; Hui, X.; Wang, J.; Jin, P.; Li, H. Effect of amorphicity of HVOF sprayed Fe-based coatings on their corrosion performances and contacting osteoblast behavior. Surf. Coat. Technol. 2017, 310, 207–213. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Peng, Y.; Yu, Y.; Liu, L. Effects of crystallization on the corrosion resistance of Fe-based amorphous coatings. Corros. Sci. 2012, 59, 10–19. [Google Scholar] [CrossRef]
- Fu, B.Y.; He, D.Y.; Zhao, L.D. Effect of heat treatment on the microstructure and mechanical properties of Fe-based amorphous coatings. J. Alloy. Compd. 2009, 480, 422–427. [Google Scholar] [CrossRef]
- Thakur, L.; Arora, N. A study on erosive wear behavior of HVOF sprayed nanostructured WC–CoCr coatings. J. Mech. Sci. Technol. 2013, 27, 1461–1467. [Google Scholar] [CrossRef]
- Lin, J.R.; Wang, Z.H.; Lin, P.H.; Cheng, J.B.; Zhang, J.J.; Zhang, X. Microstructureand corrosion resistance of Fe-based coatings prepared by twin wires arc spraying process. J. Therm. Spray Technol. 2014, 23, 333–339. [Google Scholar] [CrossRef]
- Lee, C.H.; Min, K.O. Effects of heat treatment on the microstructure and properties of HVOF-sprayed Ni–Cr–W–Mo–B alloy coatings. Surf. Coat. Technol. 2000, 132, 49–57. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; He, D.Y.; Wang, F.C.; Liu, Y.B. Microstructure and electrochemical behavior of Fe-based amorphous metallic coatings fabricated by atmospheric plasma spraying. J. Therm. Spray Technol. 2011, 20, 344–350. [Google Scholar] [CrossRef]
- Guo, R.Q.; Zhang, C.; Yang, Y.; Peng, Y.; Liu, L. Corrosion and wear resistance of a Fe-based amorphous coating in underground environment. Intermetallics 2012, 30, 94–99. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, X.; Geng, G. Dry sliding wear behavior and corrosion resistance of NiCrBSi coating deposited by activated combustion-high velocity air fuel spray process. Mater. Des. 2010, 31, 913–917. [Google Scholar] [CrossRef]
- Jinran, L.; Zehua, W.; Jianguo, C.; Min, K.; Xiuqing, F.; Sheng, H. Evaluation of cavitation erosion resistance of are-sprayed Fe-based amorphous/nanocrystalline coatings in NaCl solution. Results Phys. 2019, 12, 597–602. [Google Scholar]
- Wang, Z.H.; Zhang, X.; Cheng, J.B.; Lin, J.R.; Zhou, Z.H. Cavitation erosion resistance of Fe-based amorphous/nanocrystal coatings prepared by high-velocity arc spraying. J. Therm. Spray Technol. 2014, 23, 742–749. [Google Scholar] [CrossRef]
- Bakare, M.S.; Voisey, K.T.; Chokethawai, K.; McCartney, D.G. Corrosion behavior of crystalline and amorphous forms of the glass forming alloy Fe43Cr16Mo16C15B10. J. Alloy. Compd. 2012, 527, 210–218. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.H. A study on cavitation erosion and corrosion behavior of Al-, Zn-, Cu-, and Fe-based coatings prepared by arc spraying. J. Therm. Spray Technol. 2010, 19, 1224–1230. [Google Scholar] [CrossRef]
- Chokethawai, K.; McCartney, D.G.; Shipway, P.H. Microstructure evolution and thermal stability of an Fe-based amorphous alloy powder and thermally sprayed coatings. J. Alloy. Compd. 2009, 480, 351–359. [Google Scholar] [CrossRef]
- Parthasarathi, N.L.; Duraiselvam, M.; Borah, U. Effect of plasma spraying parameter on wear resistance of NiCrBSiCFe plasma coatings on austenitic stainless steel stainless steel ay elevated temperatures at various loads. Mater. Des. 2012, 36, 141–151. [Google Scholar] [CrossRef]


| Spray Parameters | Values |
|---|---|
| Power (kW) | 41.4 |
| Argon flow (L/min) | 80 |
| Hydrogen flow (L/min) | 6 |
| Powder flow rate (g/min) | 40 |
| Standoff spray distance (mm) | 110 |
| Coating thickness (μm) | 350 ± 25 |
| Fe | Mo | Cr | Y | Si | Nb |
|---|---|---|---|---|---|
| 57.1422 | 20.4500 | 19.0378 | 2.1617 | 0.7391 | 0.4692 |
| Coatings | Ecorr (V) | Icorr (A) |
|---|---|---|
| As-sprayed | −0.34521 | 2.2626 × 10−5 |
| Annealed at 350 °C | −0.43964 | 8.695 × 10−5 |
| Annealed at 450 °C | −0.45435 | 2.5059 × 10−4 |
| Annealed at 550 °C | −0.46617 | 3.1165 × 10−4 |
| Annealed at 650 °C | −0.52495 | 3.1392 × 10−4 |
| Coatings | As-Sprayed Coating | Annealed at 350 °C | Annealed at 450 °C | Annealed at 550 °C | Annealed at 650 °C |
|---|---|---|---|---|---|
| Microhardness/HV | 712 | 848 | 889 | 982 | 1018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhu, Y.; Lai, X.; Zheng, X.; Jia, R.; Yuan, X. Influence of Different Heat Treatment Temperatures on the Microstructure, Corrosion, and Mechanical Properties Behavior of Fe-Based Amorphous/Nanocrystalline Coatings. Coatings 2019, 9, 858. https://doi.org/10.3390/coatings9120858
Liu S, Zhu Y, Lai X, Zheng X, Jia R, Yuan X. Influence of Different Heat Treatment Temperatures on the Microstructure, Corrosion, and Mechanical Properties Behavior of Fe-Based Amorphous/Nanocrystalline Coatings. Coatings. 2019; 9(12):858. https://doi.org/10.3390/coatings9120858
Chicago/Turabian StyleLiu, Shenglin, Yongsheng Zhu, Xinyue Lai, Xueping Zheng, Runnan Jia, and Xiangsheng Yuan. 2019. "Influence of Different Heat Treatment Temperatures on the Microstructure, Corrosion, and Mechanical Properties Behavior of Fe-Based Amorphous/Nanocrystalline Coatings" Coatings 9, no. 12: 858. https://doi.org/10.3390/coatings9120858
APA StyleLiu, S., Zhu, Y., Lai, X., Zheng, X., Jia, R., & Yuan, X. (2019). Influence of Different Heat Treatment Temperatures on the Microstructure, Corrosion, and Mechanical Properties Behavior of Fe-Based Amorphous/Nanocrystalline Coatings. Coatings, 9(12), 858. https://doi.org/10.3390/coatings9120858




