Prospects of Low-Pressure Cold Spray for Superhydrophobic Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Powders Synthesis
2.2. Substrates Preparation and Spraying Parameters
2.3. Characterization Methods
3. Results and Discussion
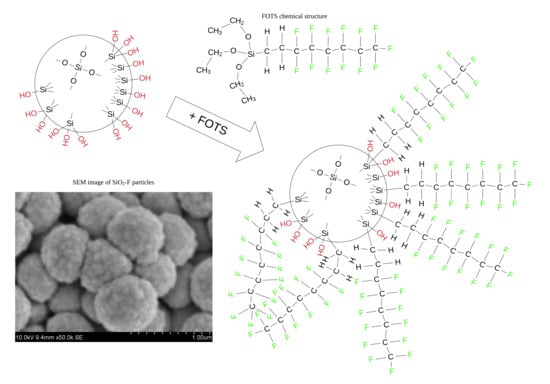
3.1. Selection of Functionalizing Reagent (FOTS) Content
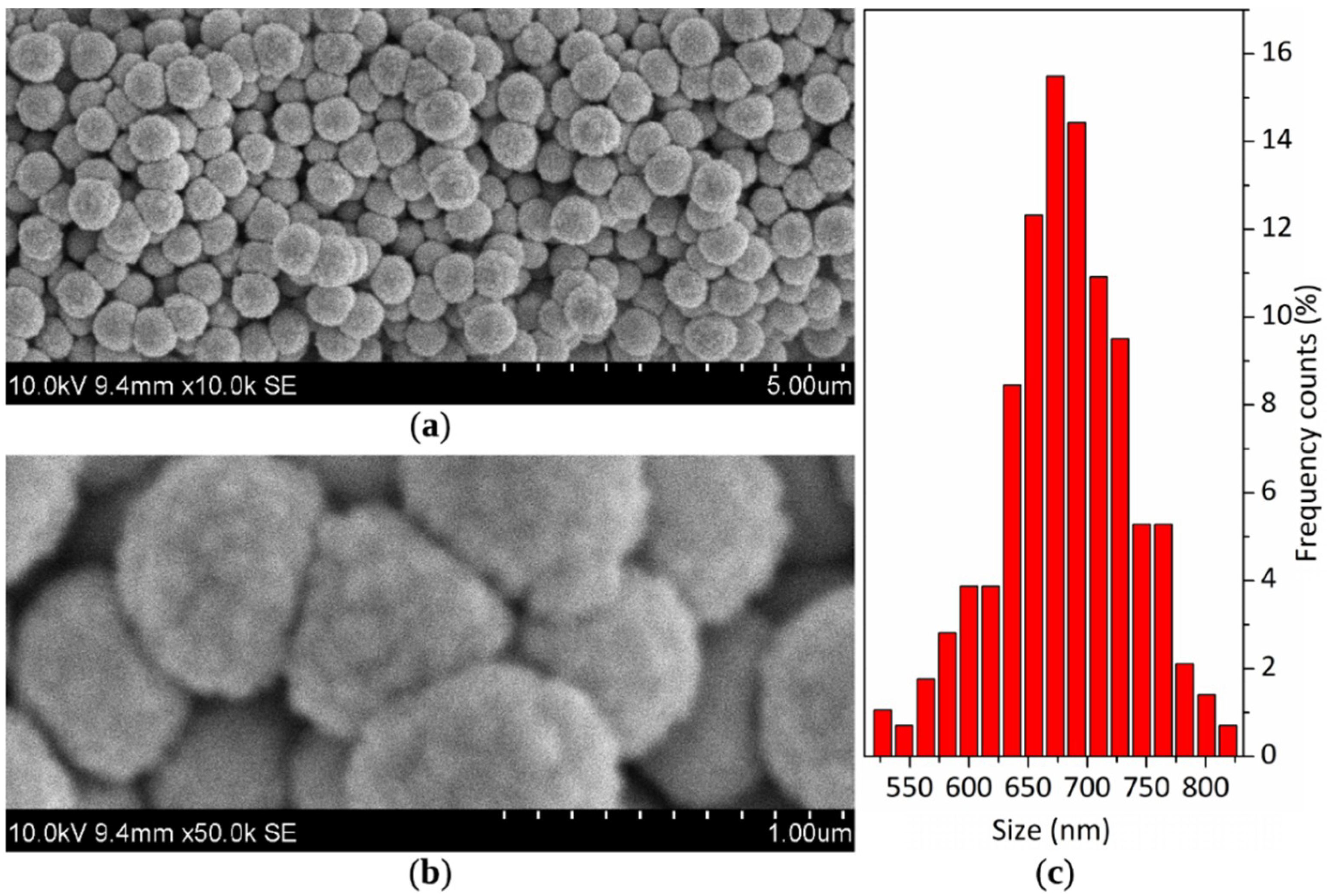
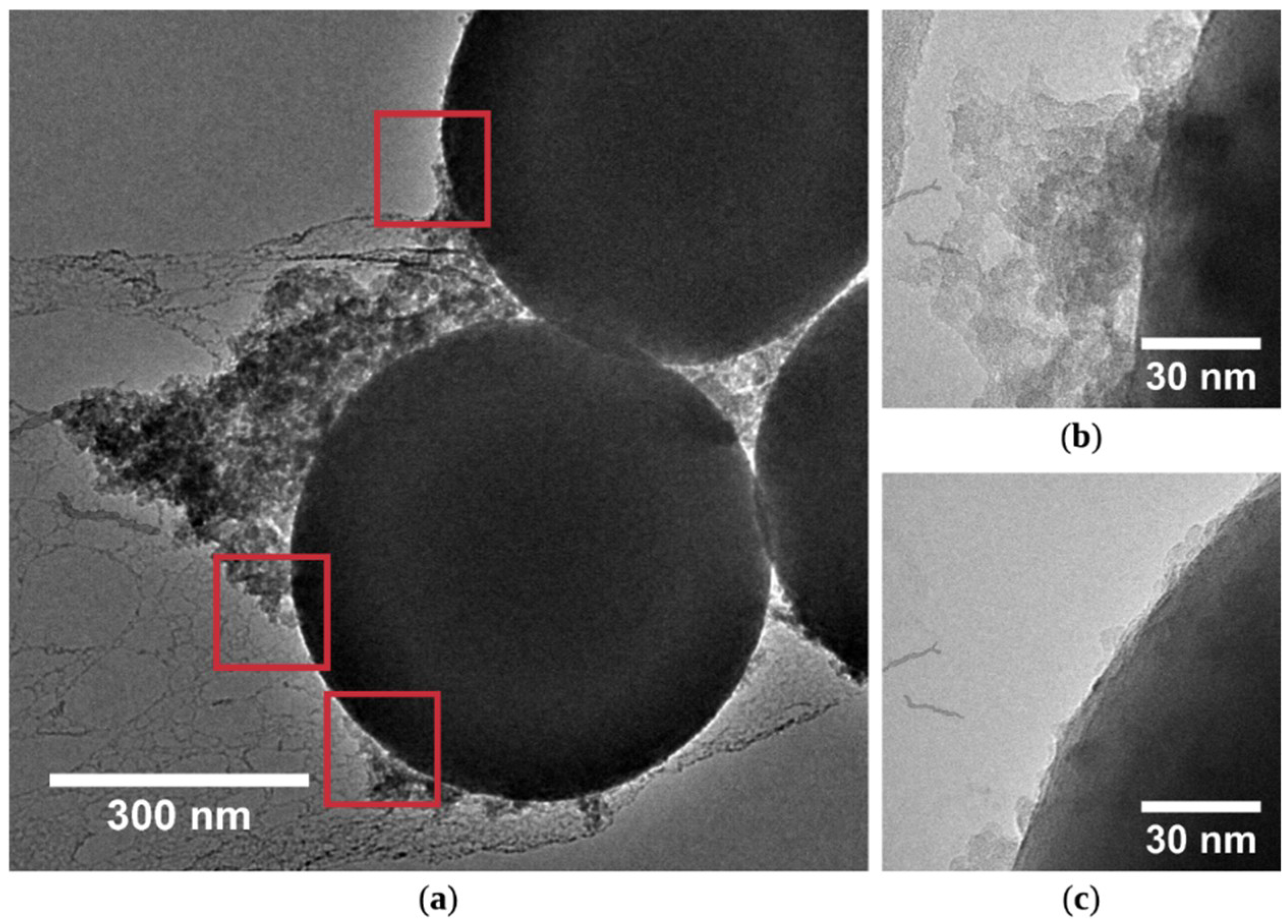
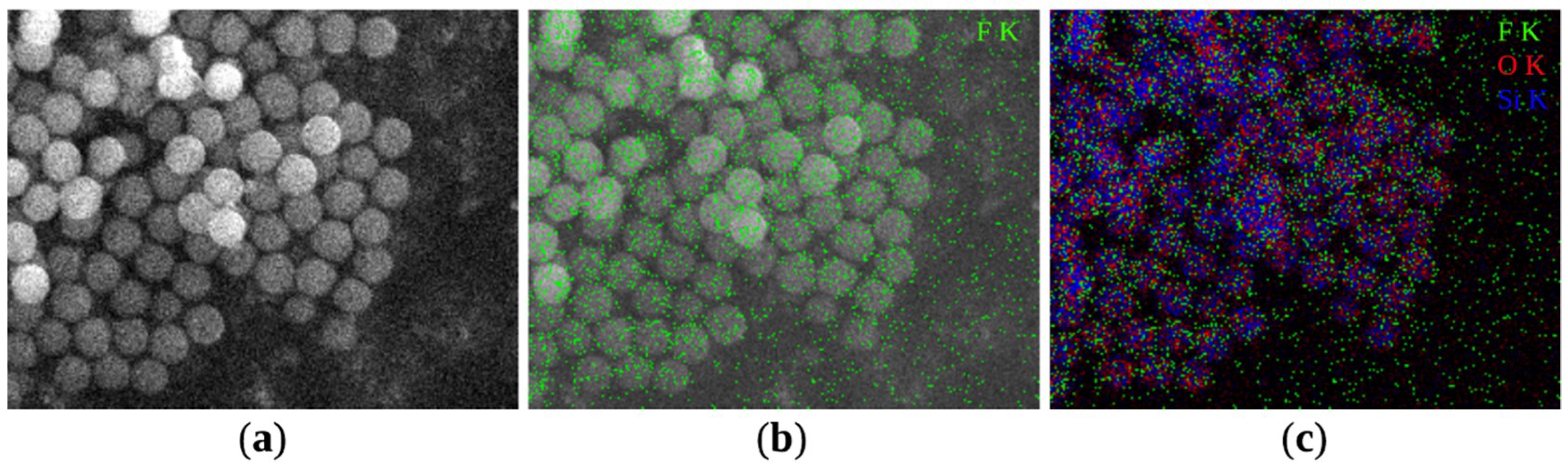
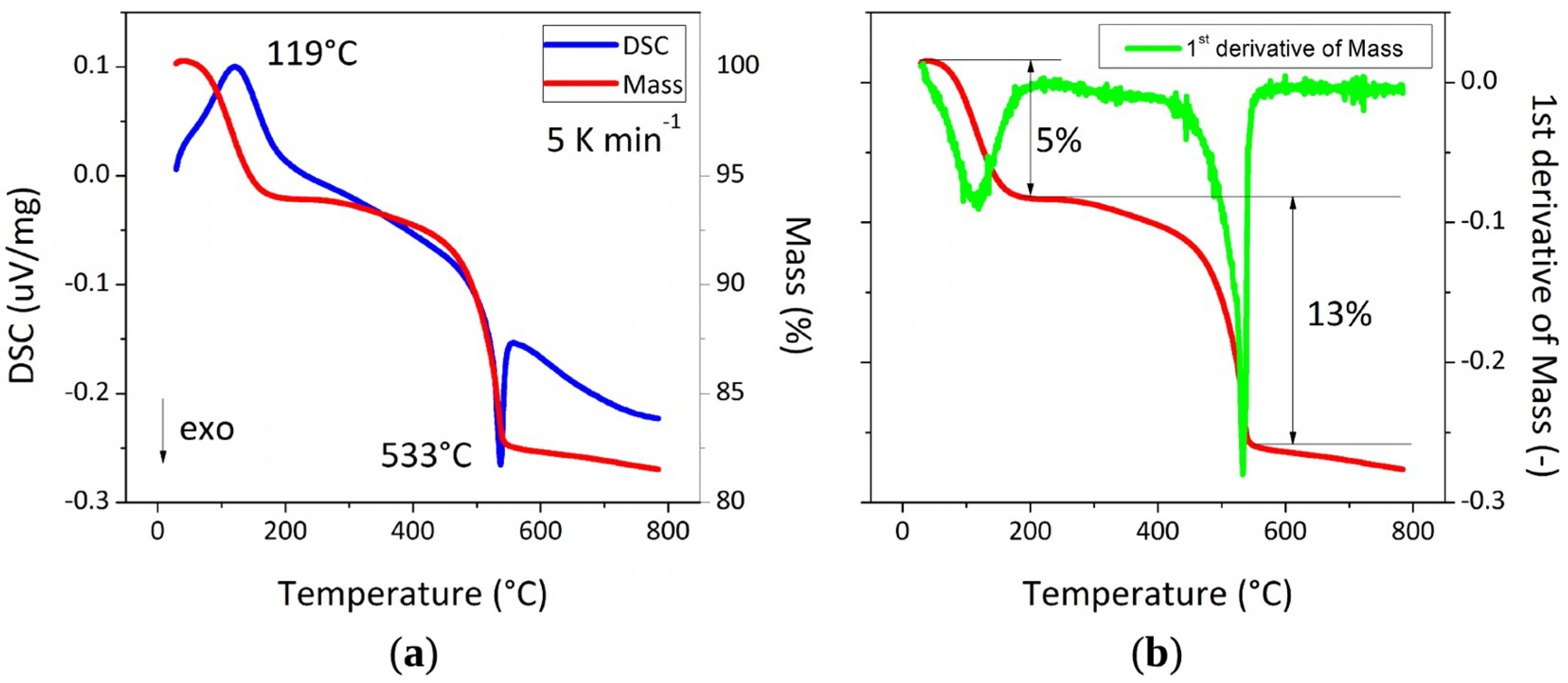
3.2. Characterization of Feedstock Powders
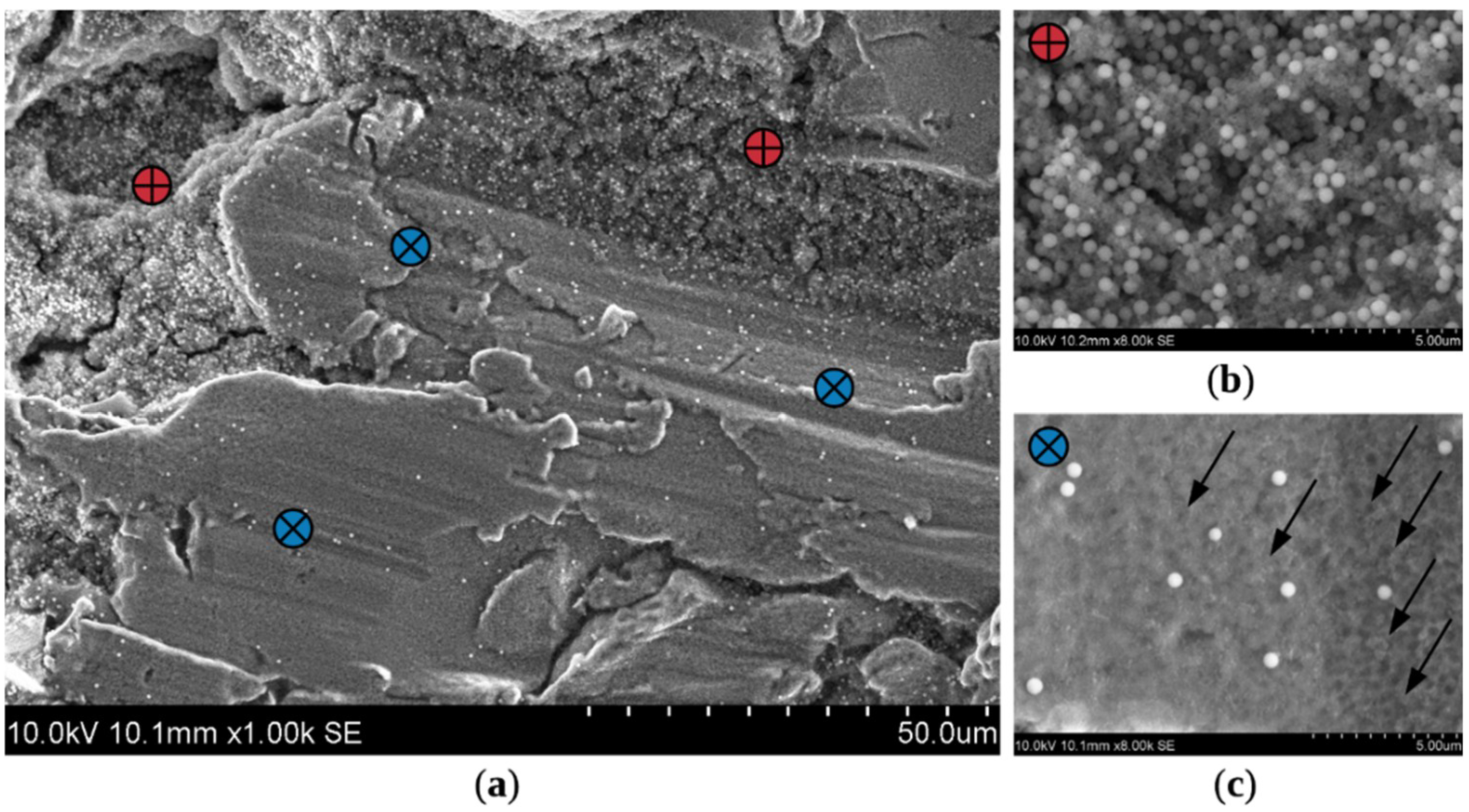
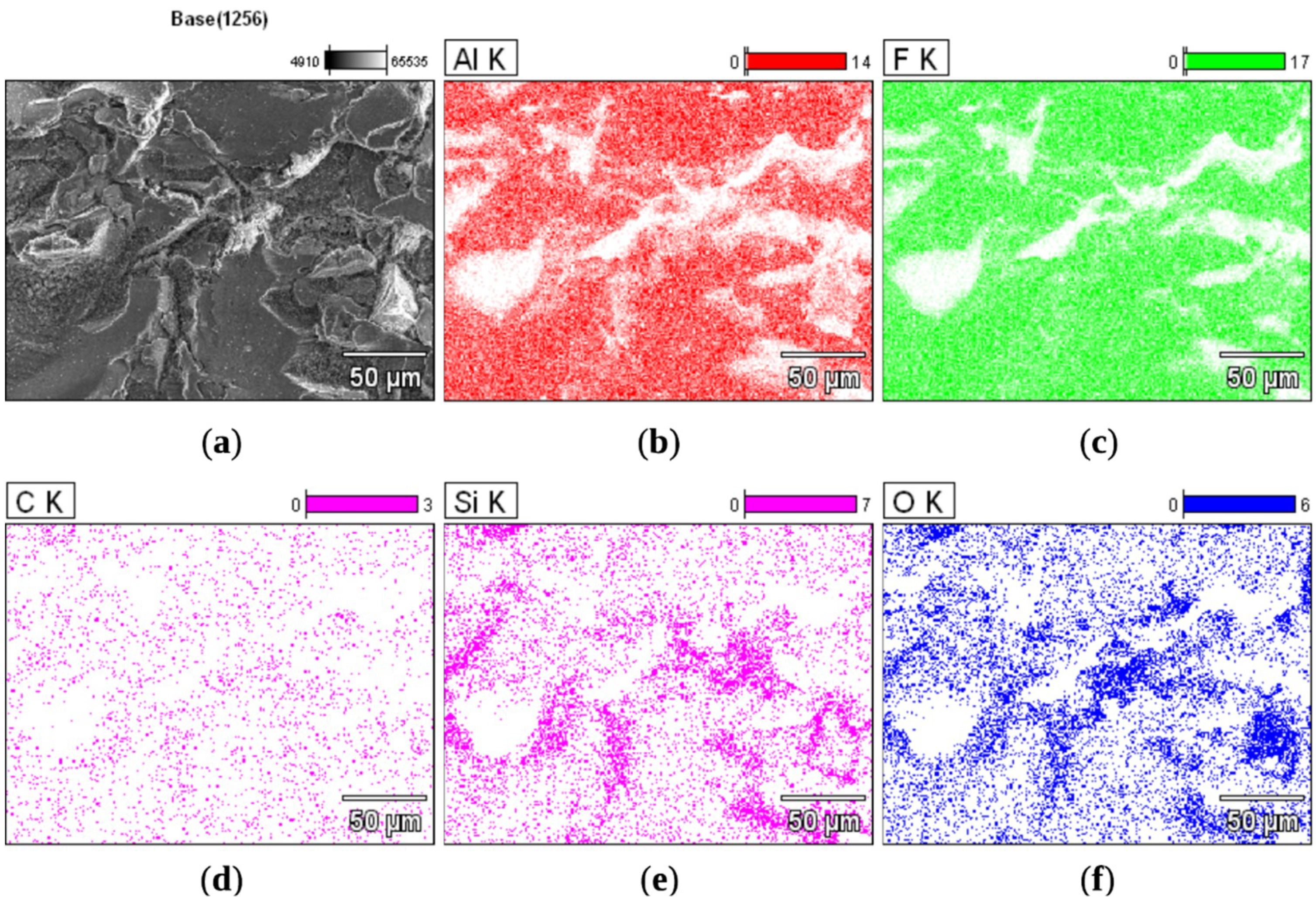
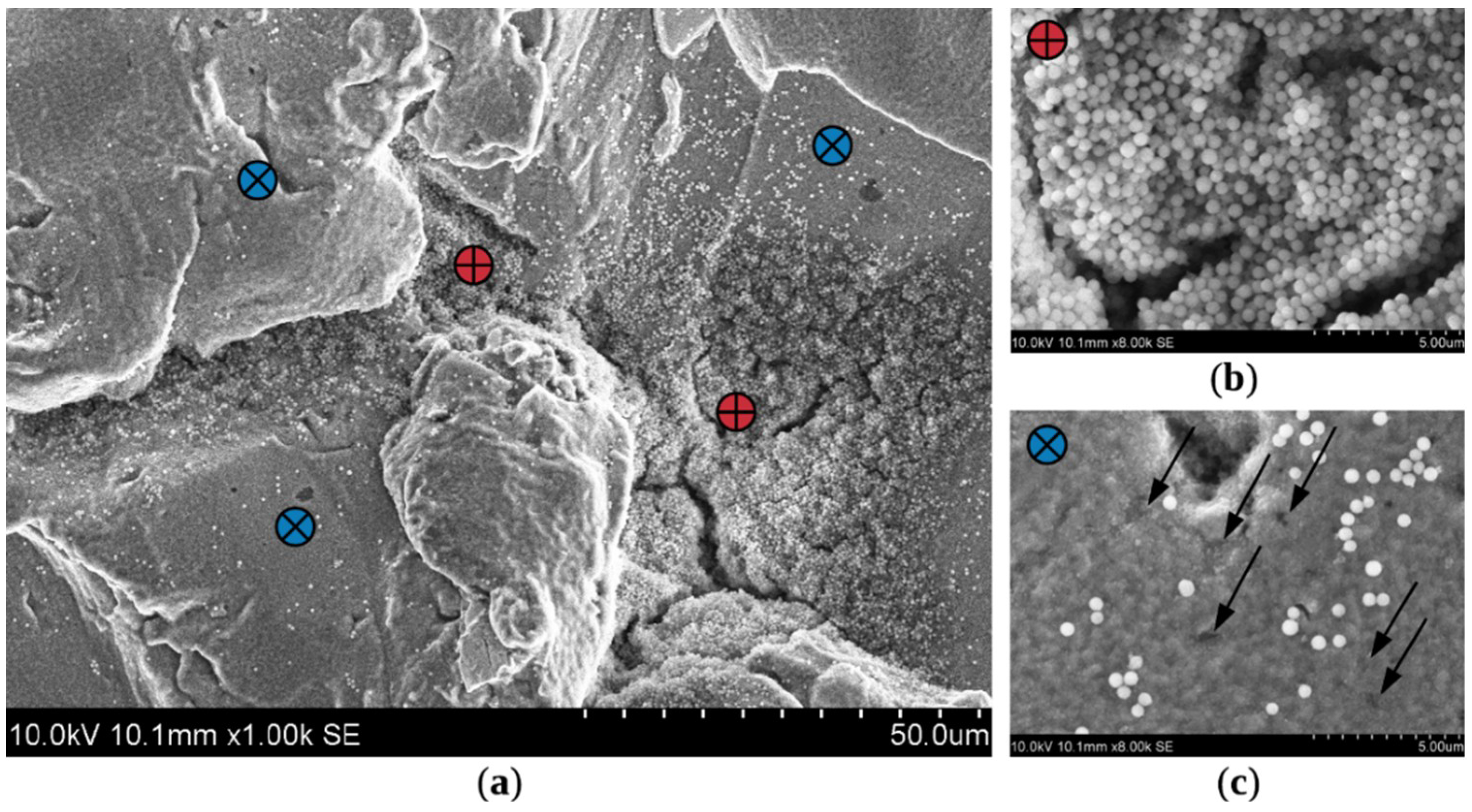
3.3. Characterization of Coatings
3.4. Characterization of Superhydrophobicity Durability of Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papyrin, A. Cold spray technology. Adv. Mater. Process. 2001, 159, 49–51. [Google Scholar]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 36, 369–395. [Google Scholar] [CrossRef]
- Tejero-Martin, D.; Rezvani Rad, M.; McDonald, A.; Hussain, T. Beyond traditional coatings: A review on thermal-sprayed functional and smart coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef]
- Irissou, E.; Legoux, J.-G.; Ryabinin, A.N.; Jodoin, B.; Moreau, C. Review on cold spray process and technology: Part I—intellectual property. J. Therm. Spray Technol. 2008, 17, 495–516. [Google Scholar] [CrossRef]
- Wank, A. Basics of Thermal Spray Technology; GTV Verschleiss-Schutz GmbH: Luckenbach, Germany, 2006. [Google Scholar]
- Nikbakht, R.; Seyedein, S.H.; Kheirandish, S.; Assadi, H.; Jodoin, B. The role of deposition sequence in cold spraying of dissimilar materials. Surf. Coat. Technol. 2019, 367, 75–85. [Google Scholar] [CrossRef]
- Lupoi, R.; O’Neill, W. Deposition of metallic coatings on polymer surfaces using cold spray. Surf. Coat. Technol. 2010, 205, 2167–2173. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, M.; Veysset, D.; Champagne, V.K.; Nelson, K.A.; Schuh, C.A. Adiabatic shear instability is not necessary for adhesion in cold spray. Acta Mater. 2018, 158, 430–439. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Li, H.; Fan, X. Modelling of alloying process of cold sprayed Ni/Al coating. Mech. Mater. 2019, 135, 129–143. [Google Scholar] [CrossRef]
- Bolelli, G.; Dosta, S.; Lusvarghi, L.; Manfredini, T.; Guilemany, J.M.; Cano, I.G. Building up WC-Co coatings by cold spray: A finite element simulation. Surf. Coatings Technol. 2019, 374, 674–689. [Google Scholar] [CrossRef]
- da Silva, F.S.; Cinca, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M.; Benedetti, A.V. Cold gas spray coatings: Basic principles corrosion protection and applications. Eclética Química J. 2017, 42, 9. [Google Scholar] [CrossRef]
- Winnicki, M.; Baszczuk, A.; Jasiorski, M.; Borak, B.; Małachowska, A. Preliminary studies of TiO2 nanopowder deposition onto metallic substrate by low pressure cold spraying. Surf. Coat. Technol. 2019, 371, 194–202. [Google Scholar] [CrossRef]
- Baszczuk, A.; Jasiorski, M.; Winnicki, M. Low-temperature transformation of amorphous sol–gel TiO2 powder to anatase during cold spray deposition. J. Therm. Spray Technol. 2018. [Google Scholar] [CrossRef]
- Ravi, K.; Sulen, W.L.; Bernard, C.; Ichikawa, Y.; Ogawa, K. Fabrication of micro-/nano-structured super-hydrophobic fluorinated polymer coatings by cold-spray. Surf. Coatings Technol. 2019, 373, 17–24. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Cinca, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M. Feasibility of using low pressure cold gas spray for the spraying of thick ceramic hydroxyapatite coatings. Int. J. Appl. Ceram. Technol. 2019, 16, 221–229. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Deng, Y.; Shi, Y.; Wang, K. Mechanically robust superhydrophobic steel surface with anti-icing, UV-durability, and corrosion resistance properties. ACS Appl. Mater. Interfaces 2015, 7, 6260–6272. [Google Scholar] [CrossRef] [PubMed]
- Hassani-Gangaraj, S.M.; Moridi, A.; Guagliano, M. Critical review of corrosion protection by cold spray coatings. Surf. Eng. 2015, 31, 803–815. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sudhagar, P.; Devadoss, A.; Kumar, A.M.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. A mechanically bendable superhydrophobic steel surface with self-cleaning and corrosion-resistant properties. J. Mater. Chem. A 2015, 3, 14263–14271. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Xiang, T.; Hu, W.; Wang, J.; Ding, S.; Liu, P.; Chen, Z. Fabrication of a micro-nanostructured superhydrophobic aluminum surface with excellent corrosion resistance and anti-icing performance. RSC Adv. 2016, 6, 79389–79400. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coatings Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Gąsiorek, J.; Szczurek, A.; Babiarczuk, B.; Kaleta, J.; Jones, W.; Krzak, J. Functionalizable sol-gel silica coatings for corrosion mitigation. Materials 2018, 11, 197. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Zhao, Y.; Lellig, P.; Müllen, K.; Li, C.; Butt, H.J.; Vollmer, D. Transparent, thermally stable and mechanically robust superhydrophobic surfaces made from porous silica capsules. Adv. Mater. 2011, 23, 2962–2965. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, Y.; Yin, W.; Tao, D.; Pesika, N.; Meng, Y.; Tian, Y. Propulsion principles of water striders in sculling forward through shadow method. J. Bionic Eng. 2018, 15, 516–525. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Gogolides, E.; Ellinas, K.; Tserepi, A. Hierarchical micro and nano structured, hydrophilic, superhydrophobic and superoleophobic surfaces incorporated in microfluidics, microarrays and lab on chip microsystems. Microelectron. Eng. 2015, 132, 135–155. [Google Scholar] [CrossRef]
- Li, L.; Tian, J.; Li, M.; Shen, W. Superhydrophobic surface supported bioassay—An application in blood typing. Colloids Surf. B Biointerfaces 2013, 106, 176–180. [Google Scholar] [CrossRef]
- Shiu, J.-Y.; Chen, P.L. Addressable protein patterning via switchable superhydrophobic microarrays. Adv. Funct. Mater. 2007, 17, 2680–2686. [Google Scholar] [CrossRef]
- Gentile, F.; Coluccio, M.L.; Coppedè, N.; Mecarini, F.; Das, G.; Liberale, C.; Tirinato, L.; Leoncini, M.; Perozziello, G.; Candeloro, P.; et al. Superhydrophobic surfaces as smart platforms for the analysis of diluted biological solutions. ACS Appl. Mater. Interfaces 2012, 4, 3213–3224. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Dong, J.; Zhang, J. Roles of silanes and silicones in forming superhydrophobic and superoleophobic materials. J. Mater. Chem. A 2016, 4, 13677–13725. [Google Scholar] [CrossRef]
- Matsubayashi, T.; Tenjimbayashi, M.; Manabe, K.; Kyung, K.-H.; Ding, B.; Shiratori, S. A facile method of synthesizing size-controlled hollow cyanoacrylate nanoparticles for transparent superhydrophobic/oleophobic surfaces. RSC Adv. 2016, 6, 15877–15883. [Google Scholar] [CrossRef]
- Yang, G.J.; Li, C.J.; Han, F.; Li, W.Y.; Ohmori, A. Low Temperature deposition and characterization of TiO2 photocatalytic film through cold spray. Appl. Surf. Sci. 2008, 254, 3979–3982. [Google Scholar] [CrossRef]
- Toibah, A.R.; Sato, M.; Yamada, M.; Fukumoto, M. Cold-sprayed TiO2 coatings from nanostructured ceramic agglomerated powders. Mater. Manuf. Process. 2016, 31, 1527–1534. [Google Scholar] [CrossRef]
- Rahim, T.A.; Takahashi, K.; Yamada, M.; Fukumoto, M. Effect of powder calcination on the cold spray titanium dioxide coating. Mater. Trans. 2016, 57, 1345–1350. [Google Scholar] [CrossRef]
- Salim, N.T.; Yamada, M.; Nakano, H.; Shima, K.; Isago, H.; Fukumoto, M. The effect of post-treatments on the powder morphology of titanium dioxide (TiO2) powders synthesized for cold spray. Surf. Coat. Technol. 2011, 206, 366–371. [Google Scholar] [CrossRef]
- Assadi, H.; Gärtner, F.; Stoltenhoff, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Hussain, T.; McCartney, D.G.; Shipway, P.H. Impact phenomena in cold-spraying of titanium onto various ferrous alloys. Surf. Coat. Technol. 2011, 205, 5021–5027. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Ma, K.; Pan, X.-D.; Li, C.-X.; Yang, G.-J.; Li, C.-J. Microstructure and transparent super-hydrophobic performance of vacuum cold-sprayed Al2O3 and SiO2 aerogel composite coating. J. Therm. Spray Technol. 2018, 27, 471–482. [Google Scholar] [CrossRef]
- Zou, X.; Tao, C.; Yang, K.; Yang, F.; Lv, H.; Yan, L.; Yan, H.; Li, Y.; Xie, Y.; Yuan, X.; et al. Rational design and fabrication of highly transparent, flexible, and thermally stable superhydrophobic coatings from raspberry-like hollow silica nanoparticles. Appl. Surf. Sci. 2018, 440, 700–711. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Cinca, N.; Cano, I.G.; Concustell, A.; Dosta, S.; Guilemany, J.M.; Estradé, S.; Ruiz-Caridad, A.; Peiró, F. Dense nanostructured calcium phosphate coating on titanium by cold spray. J. Eur. Ceram. Soc. 2017, 37, 1747–1755. [Google Scholar] [CrossRef]
- Cinca, N.; Vilardell, A.M.; Dosta, S.; Concustell, A.; Garcia Cano, I.; Guilemany, J.M.; Estradé, S.; Ruiz, A.; Peiró, F. A new alternative for obtaining nanocrystalline bioactive coatings: Study of hydroxyapatite deposition mechanisms by cold gas spraying. J. Am. Ceram. Soc. 2016, 99, 1420–1428. [Google Scholar] [CrossRef]
- Yin, S.; Cavaliere, P.; Aldwell, B.; Jenkins, R.; Liao, H.; Li, W.; Lupoi, R. Cold spray additive manufacturing and repair: Fundamentals and applications. Addit. Manuf. 2018, 21, 628–650. [Google Scholar] [CrossRef]
- Sun, W.; Bhowmik, A.; Tan, A.W.Y.; Li, R.; Xue, F.; Marinescu, I.; Liu, E. Improving microstructural and mechanical characteristics of cold-sprayed inconel 718 deposits via local induction heat treatment. J. Alloys Compd. 2019, 797, 1268–1279. [Google Scholar] [CrossRef]
- Petráčková, K.; Kondás, J.; Guagliano, M. Fixing a hole (with cold spray). Int. J. Fatigue 2018, 110, 144–152. [Google Scholar] [CrossRef]
- Huang, C.J.; Wu, H.J.; Xie, Y.C.; Li, W.Y.; Verdy, C.; Planche, M.P.; Liao, H.L.; Montavon, G. Advanced brass-based composites via cold-spray additive-manufacturing and its potential in component repairing. Surf. Coat. Technol. 2019, 371, 211–223. [Google Scholar] [CrossRef]
- Arole, V.M.; Munde, S.V. Fabrication of nanomaterials by top-down and bottom-up approaches—An overview. JAAST Mater. Sci. (Spec. Issue) 2014, 1, 89–93. [Google Scholar]
- Wang, X.D.; Shen, Z.X.; Sang, T.; Cheng, X.B.; Li, M.F.; Chen, L.Y.; Wang, Z.S. Preparation of spherical silica particles by stöber process with high concentration of tetra-ethyl-orthosilicate. J. Colloid Interface Sci. 2010, 341, 23–29. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Jasiorski, M.; Łuszczyk, K.; Baszczuk, A. Morphology and absorption properties control of silver nanoparticles deposited on two types of sol–gel spherical silica substrates. J. Alloys Compd. 2014, 588, 70–74. [Google Scholar] [CrossRef]
- Zhao, Z.-B.; Zhang, D.-M.; Meng, Y.-F.; Tai, L.; Jiang, Y. One-pot fabrication of fluoride-silica@silica raspberry-like nanoparticles for superhydrophobic coating. Ceram. Int. 2016, 42, 14601–14608. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y.; Tao, J.; He, Z.; Xie, Y.; Chen, H.; Jin, M.; Hou, W. Facile spraying fabrication of highly flexible and mechanically robust superhydrophobic F-SiO2@PDMS coatings for self-cleaning and drag-reduction applications. New J. Chem. 2018, 42, 18208–18216. [Google Scholar] [CrossRef]
- Brassard, J.D.; Sarkar, D.K.; Perron, J. Synthesis of monodisperse fluorinated silica nanoparticles and their superhydrophobic thin films. ACS Appl. Mater. Interfaces 2011, 3, 3583–3588. [Google Scholar] [CrossRef]
- Holmberg, K.; Matthews, A. Tribology series. Chapter 2 Surface coating methods. In Coating Tribology: Properties, Techniques and Applications in Surface Engineering; Elsevier Science Ltd: Amsterdam, The Netherlands, 1994; Volume 28, pp. 7–32. [Google Scholar]
- Boinovich, L.; Emelyanenko, A.M.; Pashinin, A.S. Analysis of long-term durability of superhydrophobic properties under continuous contact with water. ACS Appl. Mater. Interfaces 2010, 2, 1754–1758. [Google Scholar] [CrossRef]
- Davis, A.; Surdo, S.; Caputo, G.; Bayer, I.S.; Athanassiou, A. Environmentally benign production of stretchable and robust superhydrophobic silicone monoliths. ACS Appl. Mater. Interfaces 2018, 10, 2907–2917. [Google Scholar] [CrossRef]
- Stańczyk, B.; Dobrzański, L.; Góra, K.; Jach, K.; Jagoda, A. Hydrofobowe pokrycia organiczne na gładkich podłożach i na podłożach z rozwiniętą powierzchnią. Electron. Mater. 2015, 43, 25–34. [Google Scholar]
- Kim, E.K.; Lee, C.S.; Kim, S.S. Superhydrophobicity of electrospray-synthesized fluorinated silica layers. J. Colloid Interface Sci. 2012, 368, 599–602. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Novel superamphiphobic surfaces based on micro-nano hierarchical fluorinated Ag/SiO2 structures. Appl. Surf. Sci. 2018, 445, 262–271. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Huang, K.; Zou, H.; Dengguang, Y.; Li, Y.; Qiu, B.; Wang, X. Durable superamphiphobic nano-silica/epoxy composite coating via coaxial electrospraying method. Appl. Surf. Sci. 2018, 436, 283–292. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and engineering of superhydrophobic surfaces: Review of corrosion resistance, chemical and mechanical stability. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Jasiorski, M.; Maruszewski, K.; Stręk, W. Optical behaviour of sol-gel derived photonic structures formed by submicron silica spheres. Mater. Sci. 2002, 20, 51–56. [Google Scholar]
- Zhang, Z.H.; Wang, H.J.; Liang, Y.H.; Li, X.J.; Ren, L.Q.; Cui, Z.Q.; Luo, C. One-step fabrication of robust superhydrophobic and superoleophilic surfaces with self-cleaning and oil/water separation function. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Kim, J.M.; Chang, S.M.; Kong, S.M.; Kim, K.S.; Kim, J.; Kim, W.S. Control of hydroxyl group content in silica particle synthesized by the sol-precipitation process. Ceram. Int. 2009, 35, 1015–1019. [Google Scholar] [CrossRef]
- Ek, S.; Root, A.; Peussa, M.; Niinistö, L. Determination of the hydroxyl group content in silica by thermogravimetry and a comparison with 1H MAS NMR results. Thermochim. Acta 2001, 379, 201–212. [Google Scholar] [CrossRef]
- Lee, S.G.; Ham, D.S.; Lee, D.Y.; Bong, H.; Cho, K. Transparent superhydrophobic/translucent superamphiphobic coatings based on silica-fluoropolymer hybrid nanoparticles. Langmuir 2013, 29, 15051–15057. [Google Scholar] [CrossRef]
- Miao, B.; Song, L.; Chai, Y.; Wei, K.; Hu, J. The effect of sand blasting pretreatment on plasma nitriding. Vacuum 2017, 136, 46–50. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Kiani-Rashid, A.-R.; Babakhani, A. Surface nanocrystallization and gradient microstructural evolutions in the surface layers of 321 stainless steel alloy treated via severe shot peening. Vacuum 2017, 144, 152–159. [Google Scholar] [CrossRef]
- Trško, L.; Fintová, S.; Nový, F.; Bokůvka, O.; Jambor, M.; Pastorek, F.; Florková, Z.; Oravcová, M. Study of relation between shot peening parameters and fatigue fracture surface character of an AW 7075 aluminium alloy. Metals 2018, 8. [Google Scholar] [CrossRef]
- Wei, Y.-K.; Luo, X.-T.; Li, C.-X.; Li, C.-J. Optimization of in-situ shot-peening-assisted cold spraying parameters for full corrosion protection of Mg alloy by fully dense Al-based alloy coating. J. Therm. Spray Technol. 2017, 26, 173–183. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Okagaki, J.; Saji, T. Simple method for preparing superhydrophobic paper: Spray-deposited hydrophobic silica nanoparticle coatings exhibit high water-repellency and transparency. Langmuir 2012, 28, 4605–4608. [Google Scholar] [CrossRef]
- Fleming, R.A.; Zou, M. Silica nanoparticle-based films on titanium substrates with long-term superhydrophilic and superhydrophobic stability. Appl. Surf. Sci. 2013, 280, 820–827. [Google Scholar] [CrossRef]
- Michael, N.; Bhushan, B. Hierarchical roughness makes superhydrophobic states stable. Microelectron. Eng. 2007, 84, 382–386. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Hierarchical roughness optimization for biomimetic superhydrophobic surfaces. Ultramicroscopy 2007, 107, 969–979. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11. [Google Scholar] [CrossRef]
- Björklund, S.; Kocherbitov, V. Alcohols react with MCM-41 at room temperature and chemically modify mesoporous silica. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibas, A.; Baszczuk, A.; Jasiorski, M.; Winnicki, M. Prospects of Low-Pressure Cold Spray for Superhydrophobic Coatings. Coatings 2019, 9, 829. https://doi.org/10.3390/coatings9120829
Gibas A, Baszczuk A, Jasiorski M, Winnicki M. Prospects of Low-Pressure Cold Spray for Superhydrophobic Coatings. Coatings. 2019; 9(12):829. https://doi.org/10.3390/coatings9120829
Chicago/Turabian StyleGibas, Anna, Agnieszka Baszczuk, Marek Jasiorski, and Marcin Winnicki. 2019. "Prospects of Low-Pressure Cold Spray for Superhydrophobic Coatings" Coatings 9, no. 12: 829. https://doi.org/10.3390/coatings9120829
APA StyleGibas, A., Baszczuk, A., Jasiorski, M., & Winnicki, M. (2019). Prospects of Low-Pressure Cold Spray for Superhydrophobic Coatings. Coatings, 9(12), 829. https://doi.org/10.3390/coatings9120829