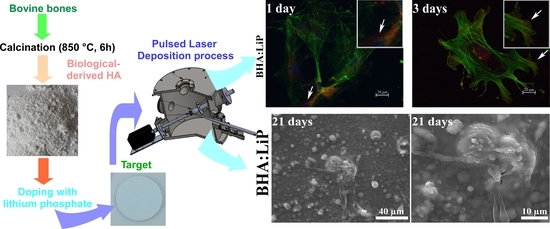
Lithium-Doped Biological-Derived Hydroxyapatite Coatings Sustain In Vitro Differentiation of Human Primary Mesenchymal Stem Cells to Osteoblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Powders Preparation
2.2. Target Preparation
2.3. PLD Experiment
2.4. Cell Culture
2.4.1. Cell Adhesion and Differentiation
Osteogenic Differentiation
ALP Activity Assay
Osteocalcin Detection
2.4.2. Mineralization Assay
2.4.3. SEM
2.4.4. Statistical Analysis
3. Results and Discussion
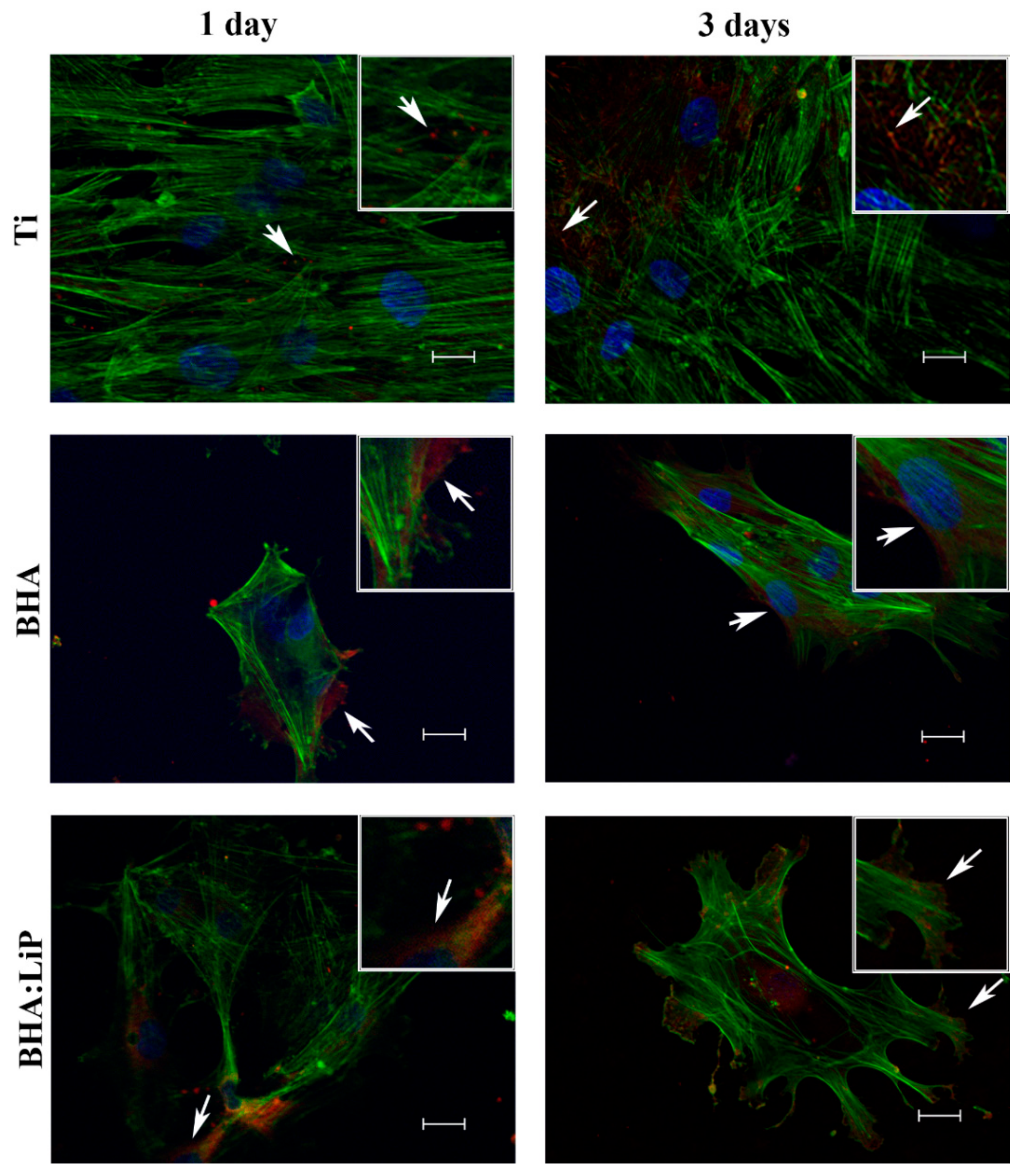
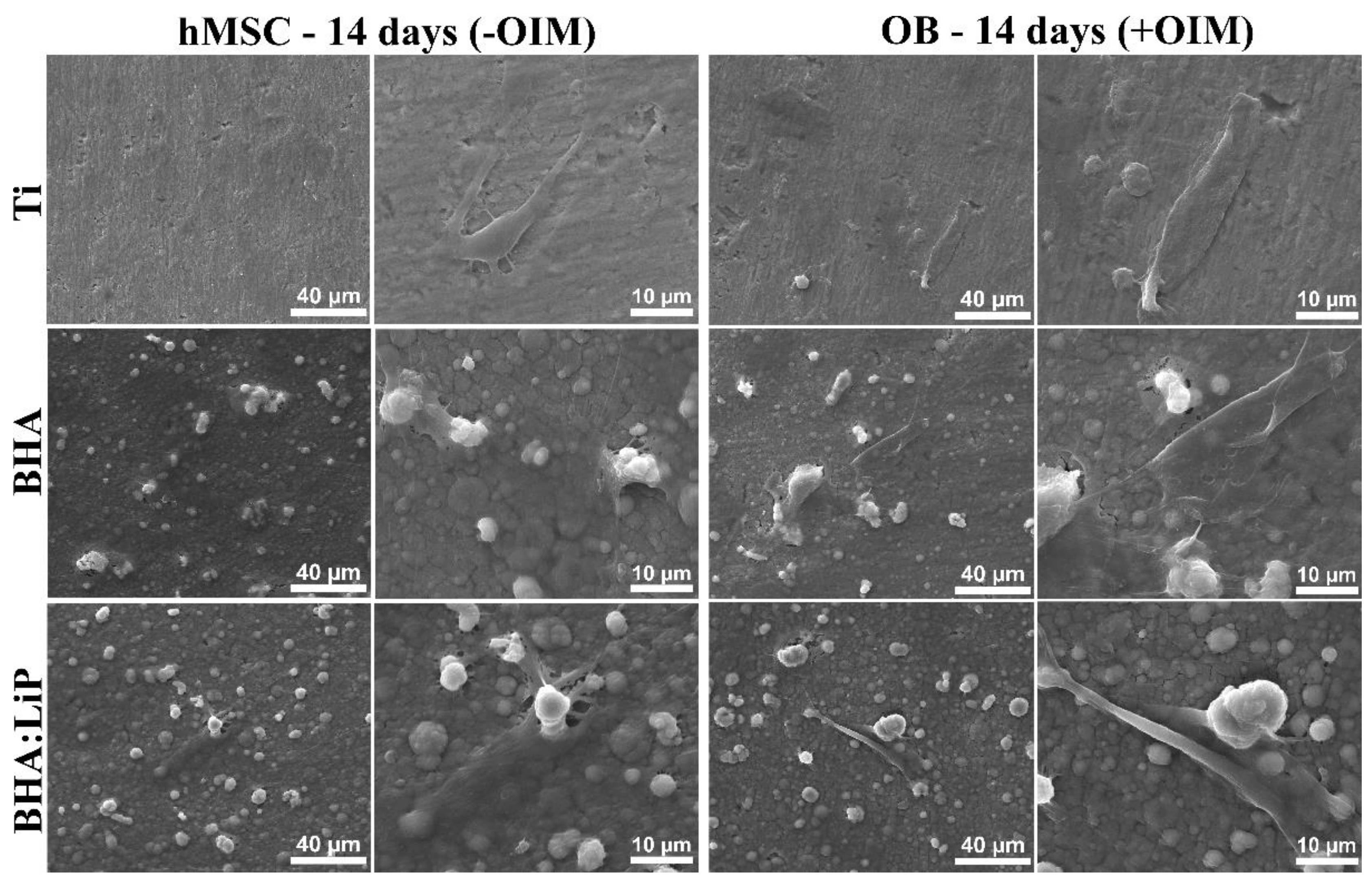
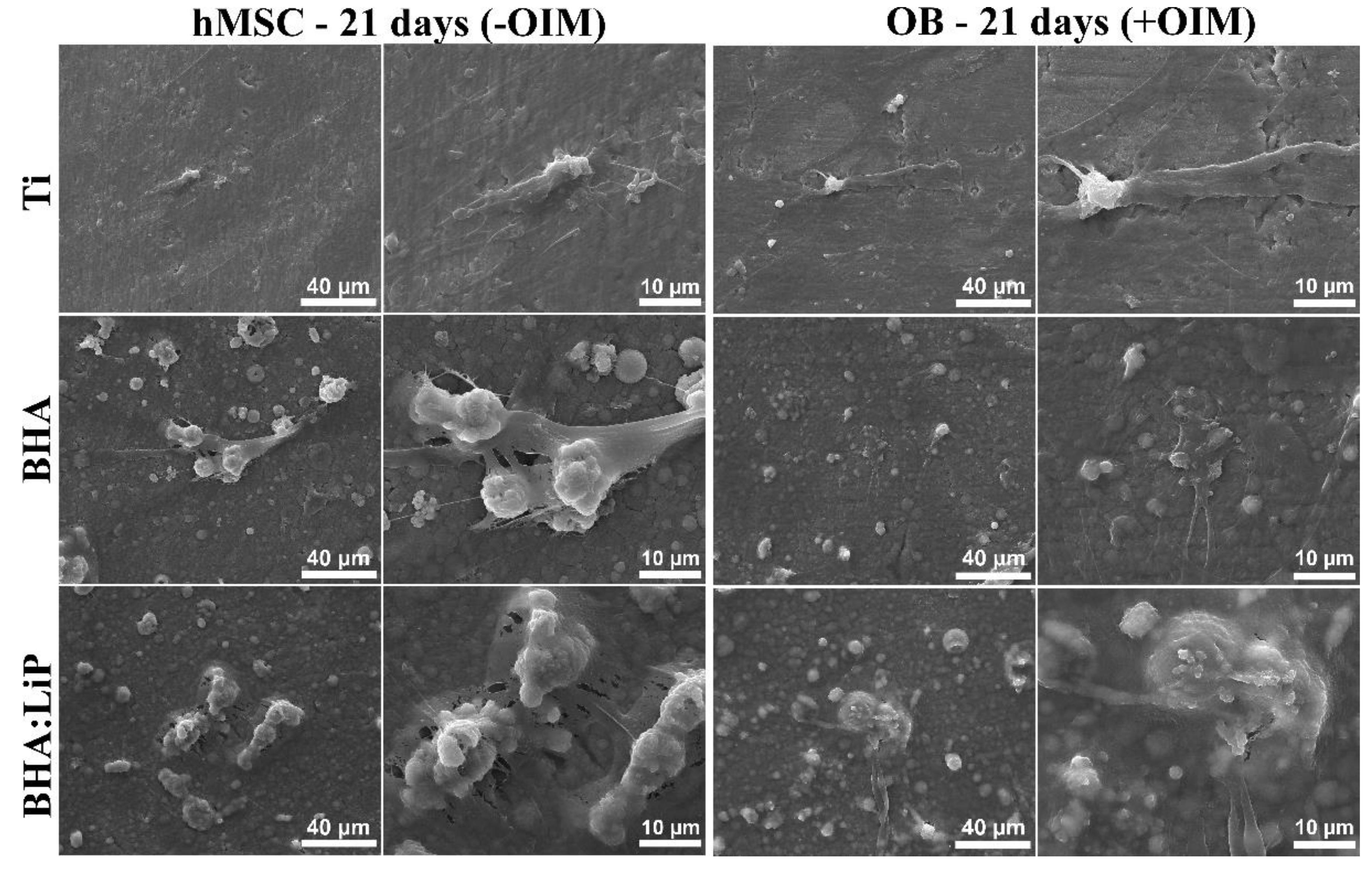
3.1. Cell Adhesion and Morphology
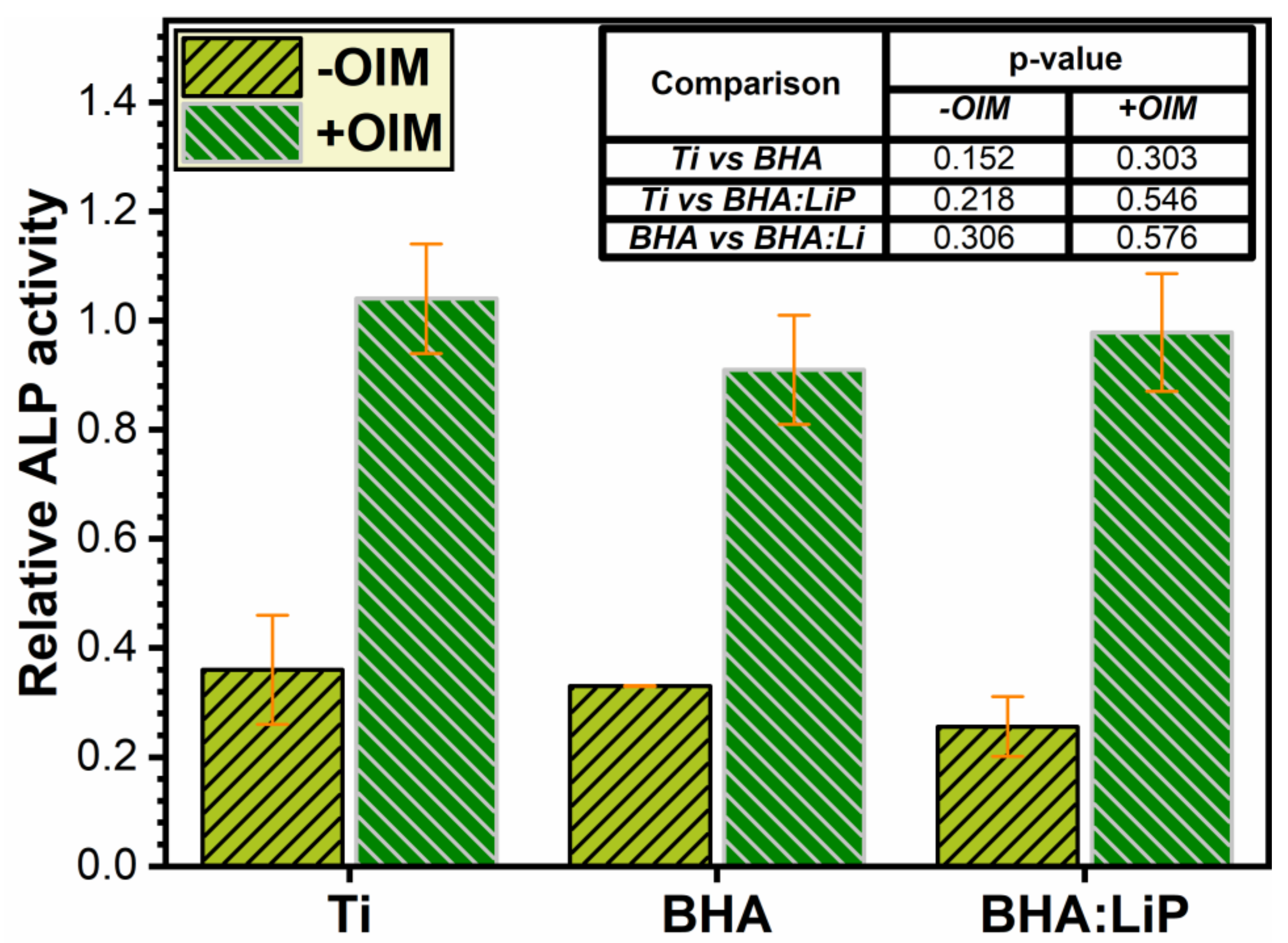
3.2. Analysis of ALP Activity
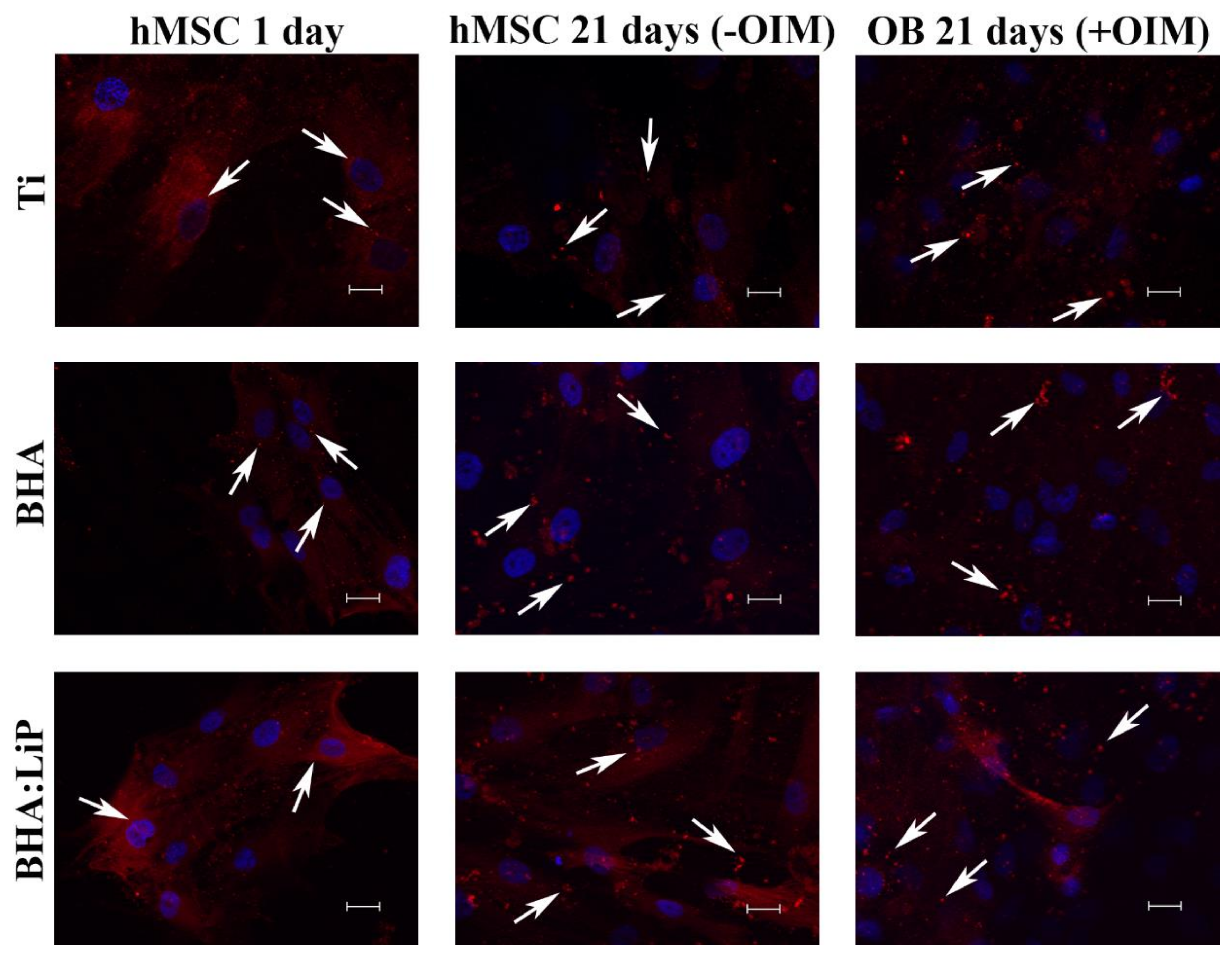
3.3. Expression of OB-Specific Differentiation Markers
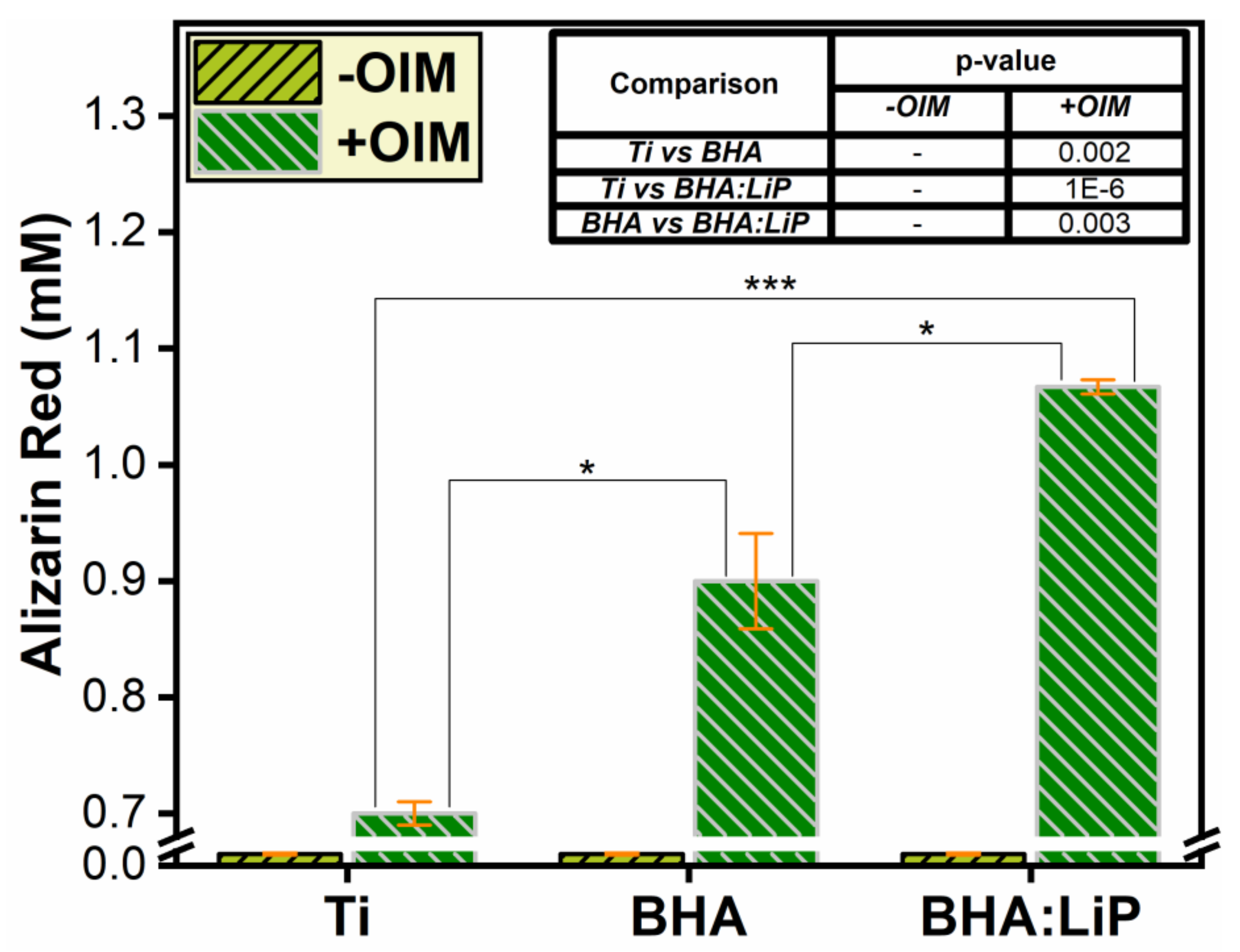
3.4. Mineralization
3.5. Structural Morphology of OB
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvarez, K.; Nakajima, H. Metallic Scaffolds for Bone Regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Anselme, K. Biomaterials and interface with bone. Osteoporos. Int. 2011, 22, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.S.; Sul, Y.T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Shah, F.A. Fluoride-containing bioactive glasses: Glass design, structure, bioactivity, cellular interactions, and recent developments. Mater. Sci. Eng. C 2016, 58, 1279–1289. [Google Scholar] [CrossRef]
- Bouler, J.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium phosphate-based coatings on titanium and its alloys. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 279–299. [Google Scholar] [CrossRef]
- Yang, Y.; Dennison, D.; Ong, J.L. Protein adsorption and osteoblast precursor cell attachment to hydroxyapatite of different crystallinities. Int. J. Oral Maxillofac. Implant 2005, 20, 187–192. [Google Scholar]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on ionic substitutions in hydroxyapatite thin films: towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Vlădescu, A.; Pârâu, A.; Pană, I.; Cotruț, C.M.; Constantin, L.R.; Braic, V.; Vrânceanu, D.M. In vitro activity assays of sputtered HAp coatings with SiC addition in various simulated biological fluids. Coatings 2019, 9, 389. [Google Scholar] [CrossRef]
- Jonauske, V.; Stanionyte, S.; Chen, S.-W.; Zarkov, A.; Juskenas, R.; Selskis, A.; Matijosius, T.; Yang, T.C.K.; Ishikawa, K.; Ramanauskas, R.; et al. Characterization of sol-gel derived calcium hydroxyapatite coatings fabricated on patterned rough stainless steel surface. Coatings 2019, 9, 334. [Google Scholar] [CrossRef]
- León, B.; Jansen, J.A. Thin Calcium Phosphate Coatings for Medical Implants; Springer: New York, NY, USA, 2009. [Google Scholar]
- Batebi, K.; Khazaei, B.A.; Afshar, A. Characterization of sol-gel derived silver/fluor-hydroxyapatite composite coatings on titanium substrate. Surf. Coat. Technol. 2018, 352, 522–528. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef]
- Duta, L.; Popescu, A.C. Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources. Coatings 2019, 9, 335. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Hubler, G.K. Pulsed Laser Deposition of Thin Films, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 1–649. [Google Scholar]
- Eason, R. Pulsed Laser Deposition of Thin Films-Applications-Led Growth of Functional Materials; Wiley-Interscience: Hoboken, NJ, USA, 2006; pp. 1–682. [Google Scholar]
- Christen, H.M.; Eres, G. Recent advances in pulsed-laser deposition of complex oxides. J. Phys. Condens. Matter 2008, 20, 264005. [Google Scholar] [CrossRef]
- Popescu, A.C.; Florian, P.E.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Enculescu, M.; Oktar, F.N.; Trusca, R.; Sima, L.E.; Roseanu, A.; et al. Physical-chemical characterization and biological assessment of simple and lithium-doped biological-derived hydroxyapatite thin films for a new generation of metallic implants. Appl. Surf. Sci. 2018, 439, 724–735. [Google Scholar] [CrossRef]
- Duta, L.; Chifiriuc, M.C.; Popescu-Pelin, G.; Bleotu, C.; (Pircalabioru) Gradisteanu, G.; Anastasescu, M.; Achim, A.; Popescu, A. Pulsed Laser Deposited Biocompatible Lithium-Doped Hydroxyapatite Coatings with Antimicrobial Activity. Coatings 2019, 9, 54. [Google Scholar] [CrossRef]
- Vats, A.; Bielby, R.C.; Tolley, N.S.; Nerem, R.; Polak, J.M. Stem cells. Lancet 2005, 366, 592–602. [Google Scholar] [CrossRef]
- Vohra, S.; Hennessy, K.M.; Sawyer, A.A.; Zhuo, Y.; Bellis, S.L. Comparison of mesenchymal stem cell and osteosarcoma cell adhesion to hydroxyapatite. J. Mater. Sci. Mater. Med. 2008, 19, 3567–3574. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Mihailescu, N.; Popescu, A.C.; Luculescu, C.R.; Mihailescu, I.N.; Cetin, G.; Gunduz, O.; Oktar, F.N.; Popa, A.C.; Kuncser, A.; et al. Comparative physical, chemical and biological assessment of simple and titanium-doped ovine dentine-derived hydroxyapatite coatings fabricated by pulsed laser deposition. Appl. Surf. Sci. 2017, 413, 129–139. [Google Scholar] [CrossRef]
- Duta, L.; Oktar, F.N.; Stan, G.E.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I.N. Novel doped hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Eisenhart, S. EU Regulation 722. In New EU Animal Tissue Regulations in Effect for Some Medical Devices; Emergo: Hong Kong, China, 2013; Available online: https://www.emergobyul.com/blog/2013/09/new-eu-animaltissue-regulations-effect-some-medical-devices (accessed on 29 August 2019).
- ISO 22442-1. Medical Devices Utilizing Animal Tissues and Their Derivatives—Part 1: Application of Risk Management; International Organization for Standardization: Berlin, Germany, 2015. [Google Scholar]
- Negroiu, G.; Piticescu, R.M.; Chitanu, G.C.; Mihailescu, I.N.; Zdrentu, L.; Miroiu, M. Biocompatibility evaluation of a novel hydroxyapatite-polymer coating for medical implants (in vitro tests). J. Mater. Sci. 2008, 19, 1537–1544. [Google Scholar] [CrossRef]
- Sima, L.E.; Filimon, A.; Piticescu, R.M.; Chitanu, G.C.; Suflet, D.M.; Miroiu, M.; Socol, G.; Mihailescu, I.N.; Neamtu, J.; Negroiu, G. Specific biofunctional performances of the hydroxyapatite–sodium maleate copolymer hybrid coating nanostructures evaluated by in vitro studies. J. Mater. Sci. Mater. Med. 2009, 20, 2305–2316. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Sima, L.E.; Stan, G.E.; Morosanu, C.O.; Melinescu, A.; Ianculescu, A.; Melinte, R.; Neamtu, J.; Petrescu, S.M. Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films. J. Biomed. Mater. Res. A 2010, 95, 1203–1214. [Google Scholar] [CrossRef]
- Brakebusch, C.; Fassler, R. The integrin-actin connection, an eternal love affair. EMBO J. 2003, 22, 2324–2333. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Okumura, A.; Goto, M.; Goto, T.; Yoshinari, M.; Masuko, S.; Katsuki, T.; Tanaka, T. Substrate affects the initial attachment and subsequent behavior of human osteoblastic cells (Saos-2). Biomaterials 2001, 22, 2263–2271. [Google Scholar] [CrossRef]
- Hoylaerts, M.F.; Manes, T.; Millan, J.L. Mammalian alkaline phosphatases are allosteric enzymes. J. Biol. Chem. 1997, 272, 22781–22787. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Weiss, M.J.; Henthorn, P.S.; Lafferty, M.A.; Slaughter, C.; Raducha, M.; Harris, H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc. Natl. Acad. Sci. USA 1986, 83, 7182–7186. [Google Scholar] [CrossRef]
- Gronthos, S.; Zannettino, A.C.; Graves, S.E.; Ohta, S.; Hay, S.J.; Simmons, P.J. Differential cell surface expression of the STRO-1 and alkaline phosphatase antigens on discrete developmental stages in primary cultures of human bone cells. J. Bone Miner. Res. 1999, 14, 47–56. [Google Scholar] [CrossRef]
- Dos Santos, E.A.; Farina, M.; Soares, G.A.; Anselme, K. Chemical and topographical influence of hydroxyapatite and beta-tricalcium phosphate surfaces on human osteoblastic cell behavior. J. Biomed. Mater. Res. A 2009, 89, 510–520. [Google Scholar] [CrossRef]
- Hamilton, D.W.; Chehroudi, B.; Brunette, D.M. Comparative response of epithelial cells and osteoblasts to microfabricated tapered pit topographies in vitro and in vivo. Biomaterials 2007, 28, 2281–2293. [Google Scholar] [CrossRef]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef]
- Van de Peppel, J.; van Leeuwen, J.P.T.M. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014, 5, 137. [Google Scholar] [CrossRef]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell... and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.C.; Sima, F.; Duta, L.; Popescu, C.; Mihailescu, I.N.; Capitanu, D.; Mustata, R.; Sima, L.E.; Petrescu, S.M.; Janackovic, D. Biocompatible and bioactive nanostructured glass coatings synthesized by pulsed laser deposition: In vitro biological tests. Appl. Surf. Sci. 2009, 255, 5486–5490. [Google Scholar] [CrossRef]
- Sun, Q.; Choudhary, S.; Mannion, C.; Kissin, Y.; Zilberberg, J.; Lee, W.Y. Ex vivo construction of human primary 3D-networked osteocytes. Bone 2017, 105, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dosier, C.R.; Park, J.H.; De, S.; Guldberg, R.E.; Boyan, B.D.; Schwartz, Z. Mineralization of three-dimensional osteoblast cultures is enhanced by the interaction of 1α,25-dihydroxyvitamin D3 and BMP2 via two specific vitamin D receptors. J. Tissue Eng. Regen. Med. 2016, 10, 40–51. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florian, P.E.; Duta, L.; Grumezescu, V.; Popescu-Pelin, G.; Popescu, A.C.; Oktar, F.N.; Evans, R.W.; Roseanu Constantinescu, A. Lithium-Doped Biological-Derived Hydroxyapatite Coatings Sustain In Vitro Differentiation of Human Primary Mesenchymal Stem Cells to Osteoblasts. Coatings 2019, 9, 781. https://doi.org/10.3390/coatings9120781
Florian PE, Duta L, Grumezescu V, Popescu-Pelin G, Popescu AC, Oktar FN, Evans RW, Roseanu Constantinescu A. Lithium-Doped Biological-Derived Hydroxyapatite Coatings Sustain In Vitro Differentiation of Human Primary Mesenchymal Stem Cells to Osteoblasts. Coatings. 2019; 9(12):781. https://doi.org/10.3390/coatings9120781
Chicago/Turabian StyleFlorian, Paula E., Liviu Duta, Valentina Grumezescu, Gianina Popescu-Pelin, Andrei C. Popescu, Faik N. Oktar, Robert W. Evans, and Anca Roseanu Constantinescu. 2019. "Lithium-Doped Biological-Derived Hydroxyapatite Coatings Sustain In Vitro Differentiation of Human Primary Mesenchymal Stem Cells to Osteoblasts" Coatings 9, no. 12: 781. https://doi.org/10.3390/coatings9120781
APA StyleFlorian, P. E., Duta, L., Grumezescu, V., Popescu-Pelin, G., Popescu, A. C., Oktar, F. N., Evans, R. W., & Roseanu Constantinescu, A. (2019). Lithium-Doped Biological-Derived Hydroxyapatite Coatings Sustain In Vitro Differentiation of Human Primary Mesenchymal Stem Cells to Osteoblasts. Coatings, 9(12), 781. https://doi.org/10.3390/coatings9120781