Effect of Graphene Oxide Concentration in Electrolyte on Corrosion Behavior of Electrodeposited Zn–Electrochemical Reduction Graphene Composite Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Zn–ERGO Composite Coatings
2.2. Electrochemical Tests
2.3. Coating Characterization
3. Results
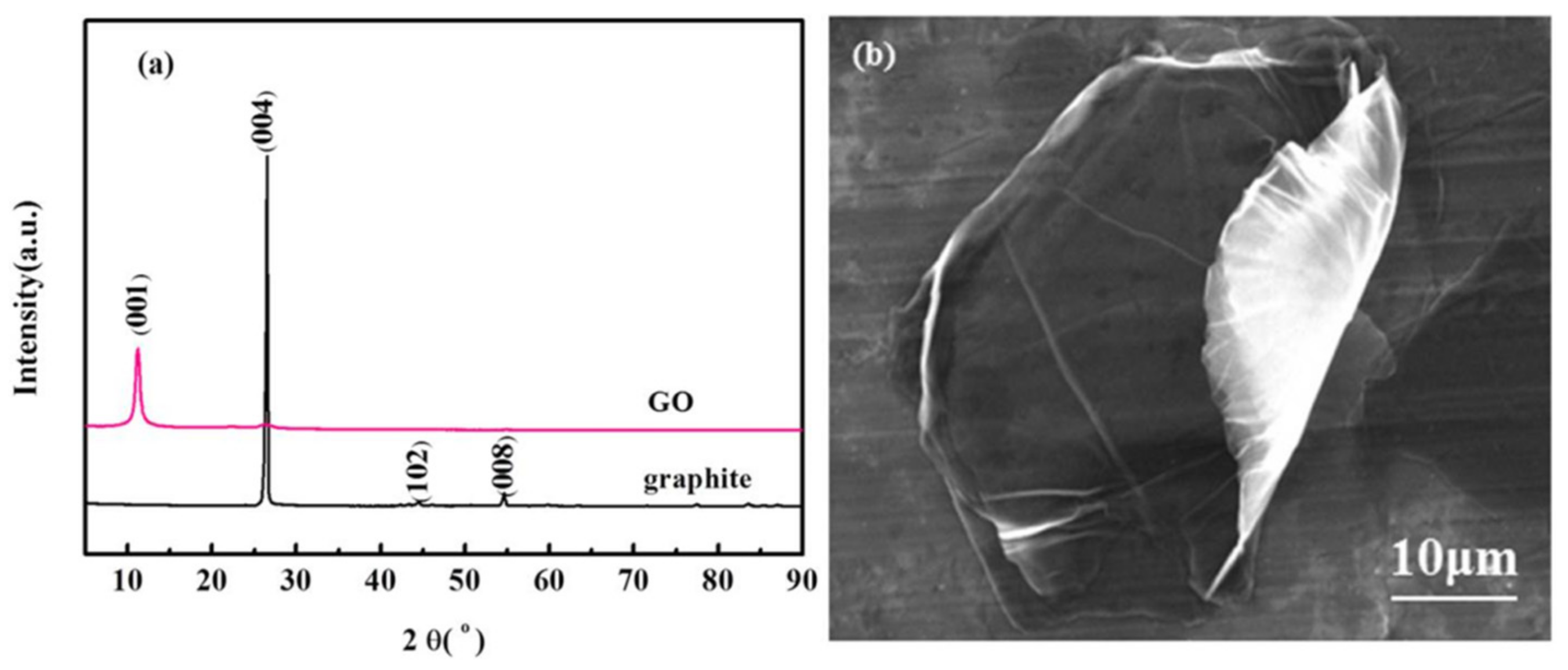
3.1. The XRD and SEM Analysis of GO Sheets
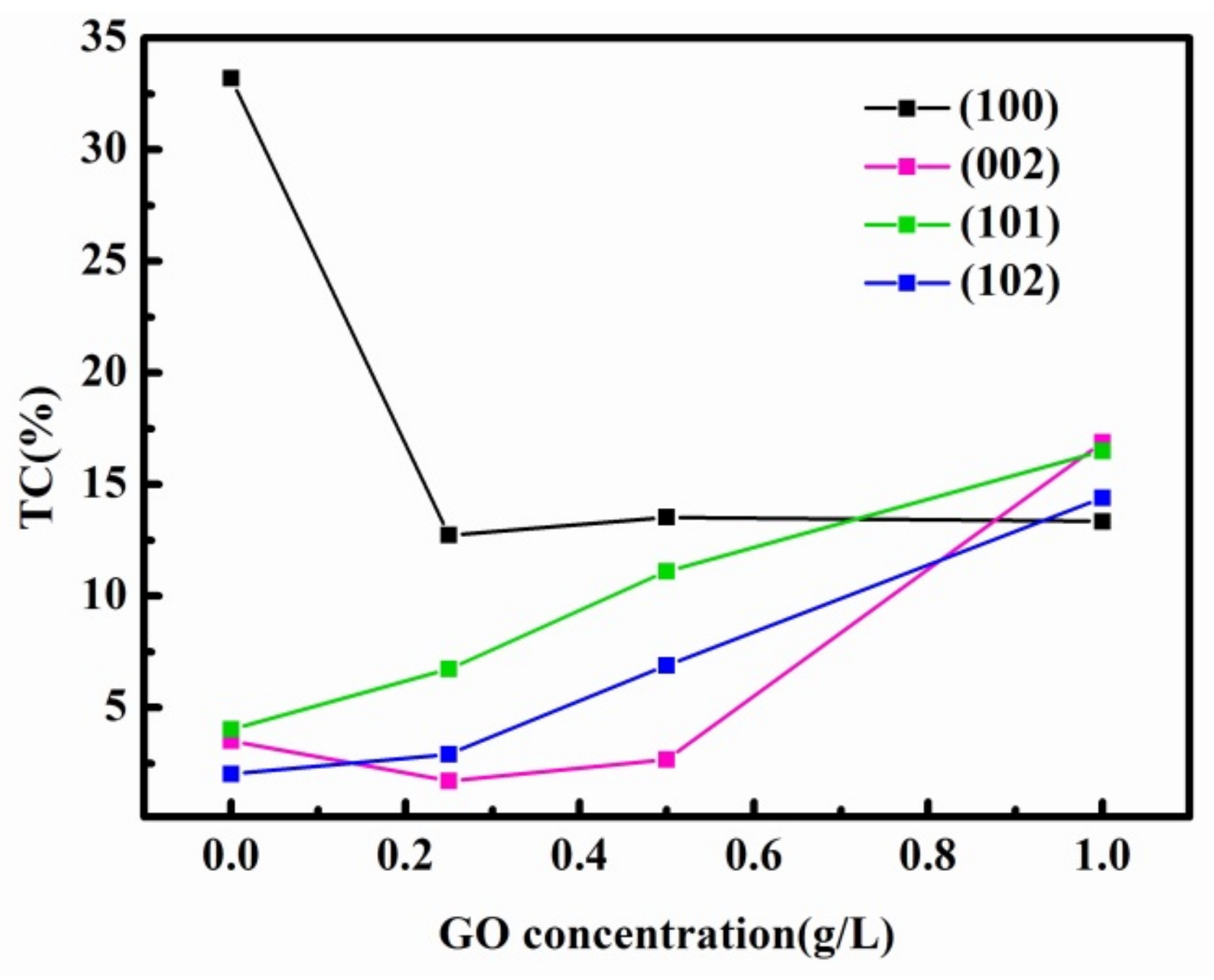
3.2. XRD and Raman Analysis of Zn and Zn–ERGO Composite Coatings
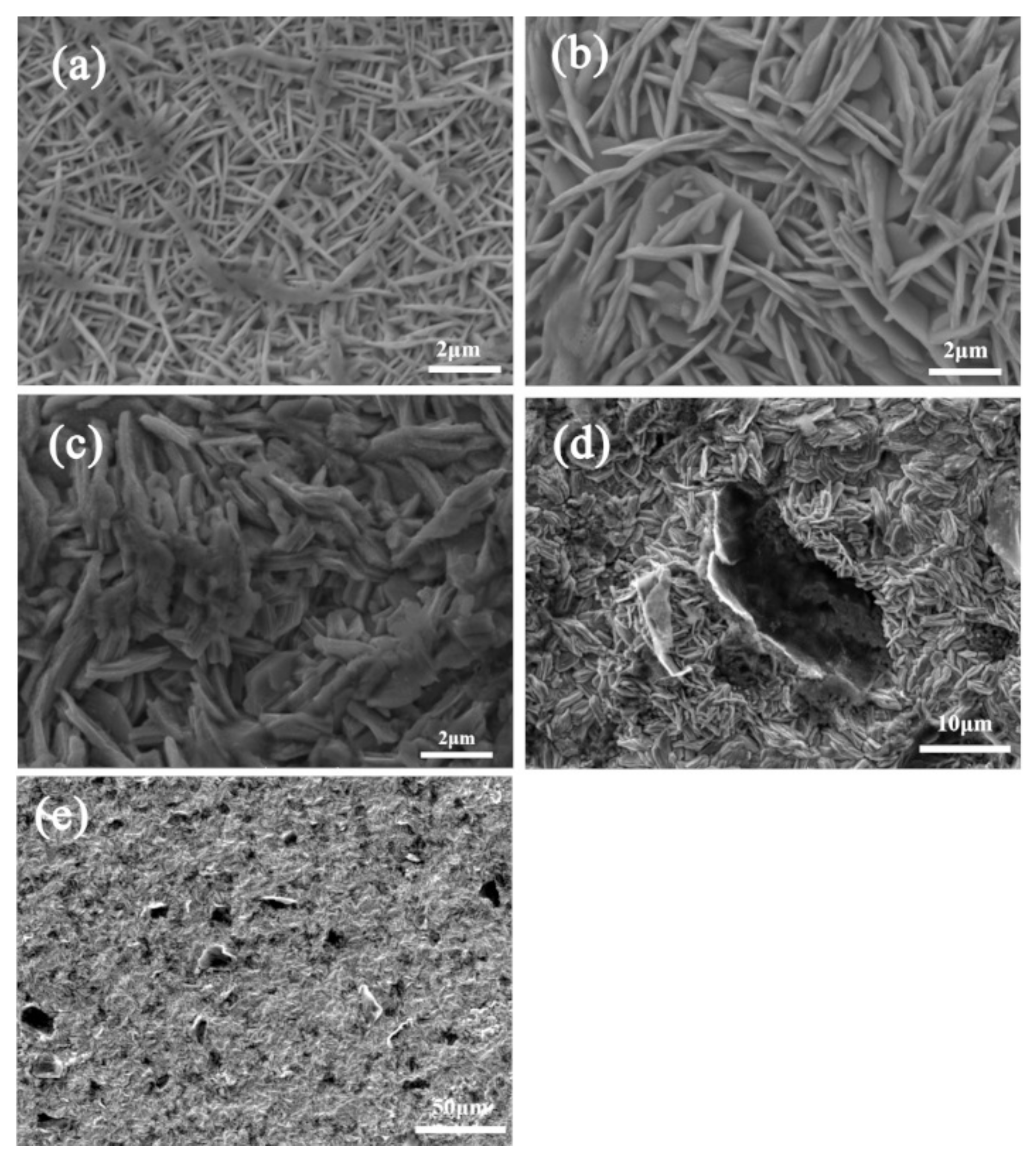
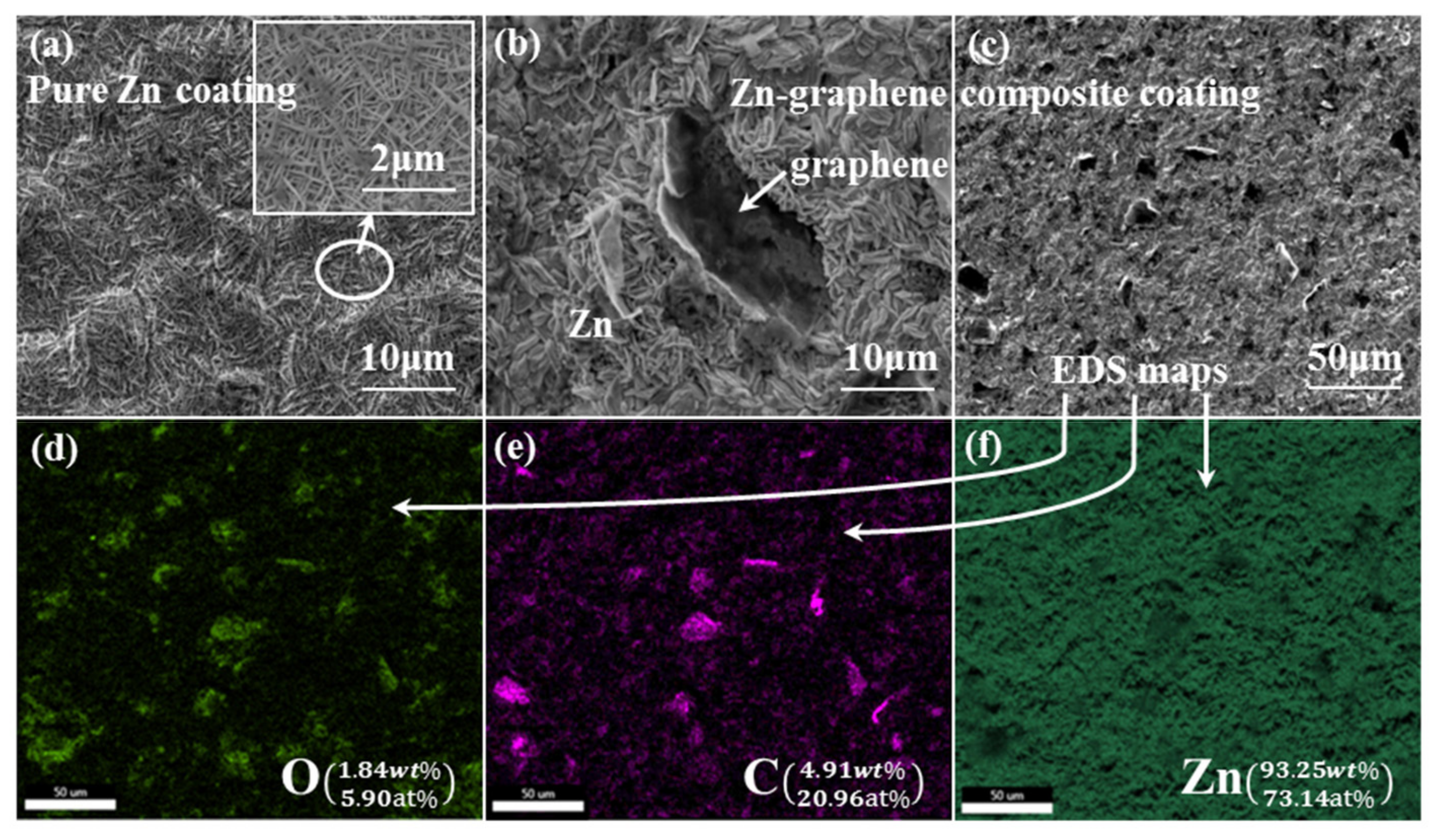
3.3. Morphologies of Zn and Zn–ERGO Composite Coatings
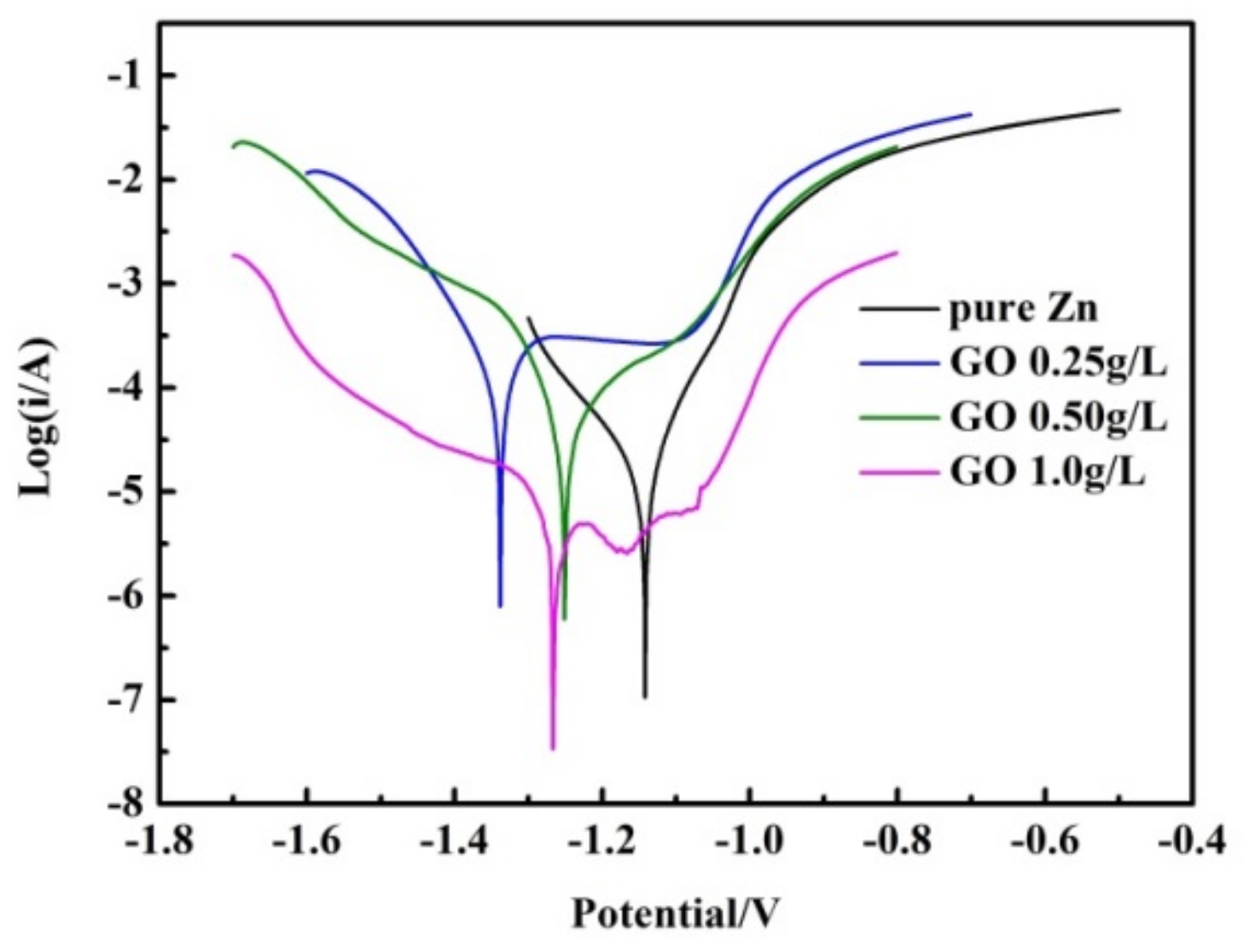
3.4. Electrochemical Corrosion Behavior of Zn and Zn–ERGO Composite Coatings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prabhu, R.A.; Venkatesha, T.V.; Shanbhag, A.V.; Praveen, B.M.; Kulkarni, G.M.; Kalkhambar, R.G. Quinol-2-thione compounds as corrosion inhibitors for mild steel in acid solution. Mater. Chem. Phys. 2008, 108, 283–289. [Google Scholar] [CrossRef]
- Vathsala, K.; Venkatesha, T.V. Zn-ZO2 nanocomposite coatings: Elecrodeposition and evaluation of corrosion resistance. Appl. Surf. Sci. 2011, 257, 8929–8936. [Google Scholar] [CrossRef]
- Paramonov, V.A.; Filatova, N.G. Passivation of chrome-plated sheet iron in electrolytes based on chromium(III). Prot. Met. 2002, 38, 475–478. [Google Scholar] [CrossRef]
- Shihab, M.S.; Al-Doori, H.H. Experimental and theoretical study of [N-substituted] paminoazobenzene derivatives as corrosion inhibitors for mild steel in sulfuric acid solution. J. Mol. Struct. 2014, 1076, 658–663. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodepositionof Ni-SiC nanocomposite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. 2008, A315, 176–182. [Google Scholar] [CrossRef]
- Sancakoglu, O.; Culha, O.; Toparli, M.; Agaday, B.C.; Clik, E. Co-deposited Zn-submicron sized Al2O3 composite coatings: Production, characterization and micromechanical properties. Mater. Des. 2011, 32, 4054–4061. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Gao, W. Synthesis of Zn-Bi nanocomposite coatings by an ionic co-discharge process. Chem. Eng. 2012, 192, 242–245. [Google Scholar] [CrossRef]
- Kumar, M.K.; Venkatesha, T.V.; Pavithra, M.K.; Nithyanandashetty, A. A study on corrosion behavior of electrodeposited Zn-Rutile TiO2 composite coatings. Synth. React. Inorg. Metal Org. Nano Met. Chem. 2012, 42, 1426–1434. [Google Scholar] [CrossRef]
- Erten, U.; Unal, H.I.; Zor, S.; Atapek, S.H. Structural and electrochemical characterization of Zn-TiO2 and Zn-WO3 nano composite coatings electrodeposited on St 37 steel. J. Appl. Electrochem. 2015, 45, 991–1003. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Mozafari, A.; Omidvar, H.; Javanbakht, M. Preparation and corrosion resistance of pulse electrodeposited Zn and Zn-SiC nanocomposite coatings. Appl. Surf. Sci. 2014, 300, 1–7. [Google Scholar] [CrossRef]
- Chandrappa, K.G.; Venkatesha, T.V.; Nayana, K.O.; Punithkumar, M.K. Generation of nanocrystalline NiO particles by solution combustion method and its Zn–NiO composite coating for corrosion protection. Mater. Corros. 2012, 63, 445–455. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V. Fabrication and electrochemical characterization of Zn-halloysite nanotubes composite coatings. RSC Adv. 2014, 4, 31230–31238. [Google Scholar] [CrossRef]
- Punithkumar, M.K.; Mahander, P.S.; Chandan, S. Electrochemical behavior of Zn-grapheme composite coatings. RSC Adv. 2015, 5, 25603–25608. [Google Scholar] [CrossRef]
- Scarpa, F.; Adhikari, S.; Phani, A.S. Effective elastic mechanical properties of single layer graphene sheets. Nanotechnology 2009, 20, 065709. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, N.T.; Schiller, T.; Medhekar, N.; Birbilis, N. Exploring graphene as a corrosion protection barrier. Corros. Sci. 2012, 56, 1–4. [Google Scholar] [CrossRef]
- Singhraman, R.K.; Banerjee, P.C.; Lobo, D.E.; Gullapalli, H.; Sumandasa, M.; Kumar, A.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Protecting copper from electrochemical degradation by graphene coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zett, A. Graphene as a long-term metal oxidation barrier: Worse than nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Brown, L.; Levendorf, M. Oxidation resistance of graphene-coated Cu and Cu/Ni Alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.M.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Praveen kumar, C.P.; Venkatsha, T.V.; Shabadi, R. Preparation and corrosion behavior of Ni and Ni-graphene composite coatings. Mater. Res. Bull. 2013, 48, 1477–1483. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.J.; Geng, H.Z.; Zhang, X.; Du, B.; Ding, E.X.; Wang, J.; Lu, Z.; Sun, B.; Wang, J.; Liu, J. A timesaving, low-cost, high-yield method for the synthesis of ultrasmall uniform graphene oxide nanosheets and their application in surfactants. Nanotechnology 2016, 27, 055601–055609. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.T.; Lu, Y.; Somers, A.L.; Johnson, A.T.C. High yield preparation of macroscopic graphene oxide membranes. J. Am. Chem. Soc. 2009, 131, 898–899. [Google Scholar] [CrossRef]
- Moussa, S.; Siamaki, A.R.; Gupton, B.F.; Eishall, M.S. Pd-partially reduced graphene oxide catalysts(Pd/PRGO): Laser synthesis of Pd nanoparticles supported on PRGO nanosheets for carbon-carbon cross coupling reactions. ACS Catal. 2012, 2, 145–154. [Google Scholar] [CrossRef]
- Maharana, H.S.; Rai, P.K.; Basu1, A. Surface-mechanical and electrical properties of pulse electrodeposited Cu–graphene oxide composite coating for electrical contacts. J. Mater. Sci. 2017, 52, 1089–1105. [Google Scholar] [CrossRef]
- Li, R.Q.; Liang, J.; Hou, Y.Y.; Chu, Q.W. Enhanced corrosion performance of Zn coating by incorporating graphene oxide electrodeposited from deep eutectic solvent. RSC Adv. 2015, 5, 60698–60707. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J. Producing cobalt-graphene composite coating by pulse electrodeposition with excellent wear and corrosion resistance. Appl. Surf. Sci. 2015, 351, 889–896. [Google Scholar] [CrossRef]
- Raghupathy, Y.; Kamboj, A.; Rekha, M.Y.; Narasimha, N.P.; Chandan, S. Copper-graphene oxide composite coatings for corrosion protection of mild steel in 3.5% NaCl. Thin Solid Films 2017, 636, 107–115. [Google Scholar] [CrossRef]
- Qiu, Z.Z.; Wang, R.; Wu, J.Z.; Zhang, Y.S.; Qu, Y.F.; Wu, X.H. Graphene oxide as a corrosion-inhibitive coating on magnesium alloys. RSC. Adv. 2015, 5, 44149–44159. [Google Scholar] [CrossRef]
- Du, D.; Liu, J.; Zhang, X.; Cui, X.; Lin, Y. One-step electrochemical deposition of a graphene–ZrO2 nanocomposite: Preparation, characterization and application for detection of organophosphorus agents. J. Mater. Chem. 2011, 21, 8032–8037. [Google Scholar] [CrossRef]
- Perret, P.G.; Malenfant, P.R.; Bock, C.; Macdougall, B. Electro-deposition and dissolution of MnO2 on a grapheme composite electrode for its utilization in an aqueous based hybrid supercapacitor. J. Electrochem. Soc. 2012, 159, A1554–A1557. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; Wu, Q.; Zhao, Y.; Jiao, Q. Synthesis of TiO2 nanowire/reduced graphene oxide nanocomposites and their photocatalytic performances. Chem. Eng. J. 2015, 263, 144–150. [Google Scholar] [CrossRef]
- Cui, P.; Lee, J.; Wang, E.H.; Lee, H. One-pot reduction of graphene oxide at subzero temperatures. Chem. Commun. 2011, 47, 12370–12372. [Google Scholar] [CrossRef] [PubMed]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K.; Punithkumar, M.K. Electrochemical studies on Zn/nano-CeO2 electrodeposited composite coatings. Surf. Coat. Technol. 2012, 208, 64–72. [Google Scholar] [CrossRef]
- Adriana, V.; Simona, V.; Aurel, P.; Caius, B. Electrodeposited Zn-TiO2 nanocomposite coatings and their corrosion behavior. J. Appl. Electrochem. 2010, 40, 1519–1527. [Google Scholar] [CrossRef]
- Gomes, A.; Pereira, M.I. Pulsed electrodeposition of Zn in the presence of surfactants. Electrochim. Acta. 2006, 51, 1342–1350. [Google Scholar] [CrossRef]
- Tu, W.Y.; Xu, B.S.; Dong, S.Y.; Wang, H.D.; Bin, J. Chemical and electrocatalytical interaction: Influence of nano-electroactive ceramic nanopartic on nickel electrodeposition and composite coating. J. Mater. Sci. 2008, 43, 1102–1108. [Google Scholar] [CrossRef]


| Chemical Composition | Concentration |
|---|---|
| ZnSO4 | 0.5 mol/L |
| Na2SO4 | 1 mol/L |
| H3BO4 | 20 g/L |
| CTAB | 1.4 g/L |
| GO | 0, 0.25, 0.50, 1.0 g/L, respectively |
| Parameters | Values |
|---|---|
| Current density | 25 A/cm2 |
| Depositing time | 30 min |
| pH | 3.5 |
| Temperature | 25 ± 2 ℃ |
| Stirring speed | 150 rpm |
| Samples | Ecorr (V vs. Hg/Hg2Cl2) | icorr (A) |
|---|---|---|
| Pure Zn | –1.143 | 1.699 × 10−5 |
| Zn-0.25 g/L ERGO | –1.352 | 3.931 × 10−4 |
| Zn-0.50 g/L ERGO | –1.252 | 8.887 × 10−5 |
| Zn-1.0 g/L ERGO | –1.267 | 1.225 × 10−6 |
| Samples | Rs (Ω) | Icorr (A) | Rct (Ω) | Cf (F) | Rf (Ω) | W (S-secΛ.5) | |
|---|---|---|---|---|---|---|---|
| Q-YO (S-secΛn) | Q-n | ||||||
| Pure Zn | 10.94 | 2.431 × 10−3 | 7.464 × 10−1 | 413.8 | 2.206 × 10−5 | 24.76 | – |
| Zn-0.25 g/L ERGO | 3.08 | 3.697 × 10−4 | 7.864 × 10−1 | 121.6 | 3.979 × 10−7 | 12.29 | 3.184 × 10−2 |
| Zn-0.50 g/L ERGO | 2.586 | 4.449 × 10−4 | 7.642 × 10−1 | 188.4 | 5.334 × 10−7 | 19.21 | 1.190 × 10−2 |
| Zn-1.0 g/L ERGO | 7.347 | 1.446 × 10−3 | 6.631 × 10−1 | 638.4 | 1.511 × 10−5 | 30.82 | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Zhang, P.; Wang, G.; Wang, A.; Chen, X.; Wei, S.; Xie, J. Effect of Graphene Oxide Concentration in Electrolyte on Corrosion Behavior of Electrodeposited Zn–Electrochemical Reduction Graphene Composite Coatings. Coatings 2019, 9, 758. https://doi.org/10.3390/coatings9110758
Yang B, Zhang P, Wang G, Wang A, Chen X, Wei S, Xie J. Effect of Graphene Oxide Concentration in Electrolyte on Corrosion Behavior of Electrodeposited Zn–Electrochemical Reduction Graphene Composite Coatings. Coatings. 2019; 9(11):758. https://doi.org/10.3390/coatings9110758
Chicago/Turabian StyleYang, Bin, Pengfei Zhang, Guangxin Wang, Aiqin Wang, Xiaofang Chen, Shizhong Wei, and Jingpei Xie. 2019. "Effect of Graphene Oxide Concentration in Electrolyte on Corrosion Behavior of Electrodeposited Zn–Electrochemical Reduction Graphene Composite Coatings" Coatings 9, no. 11: 758. https://doi.org/10.3390/coatings9110758
APA StyleYang, B., Zhang, P., Wang, G., Wang, A., Chen, X., Wei, S., & Xie, J. (2019). Effect of Graphene Oxide Concentration in Electrolyte on Corrosion Behavior of Electrodeposited Zn–Electrochemical Reduction Graphene Composite Coatings. Coatings, 9(11), 758. https://doi.org/10.3390/coatings9110758





