Surface Modification of Graphene for Use as a Structural Fortifier in Water-Borne Epoxy Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene (G)
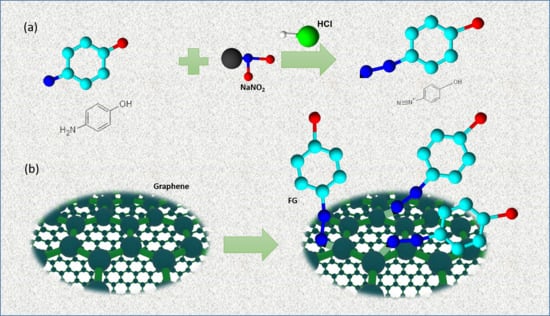
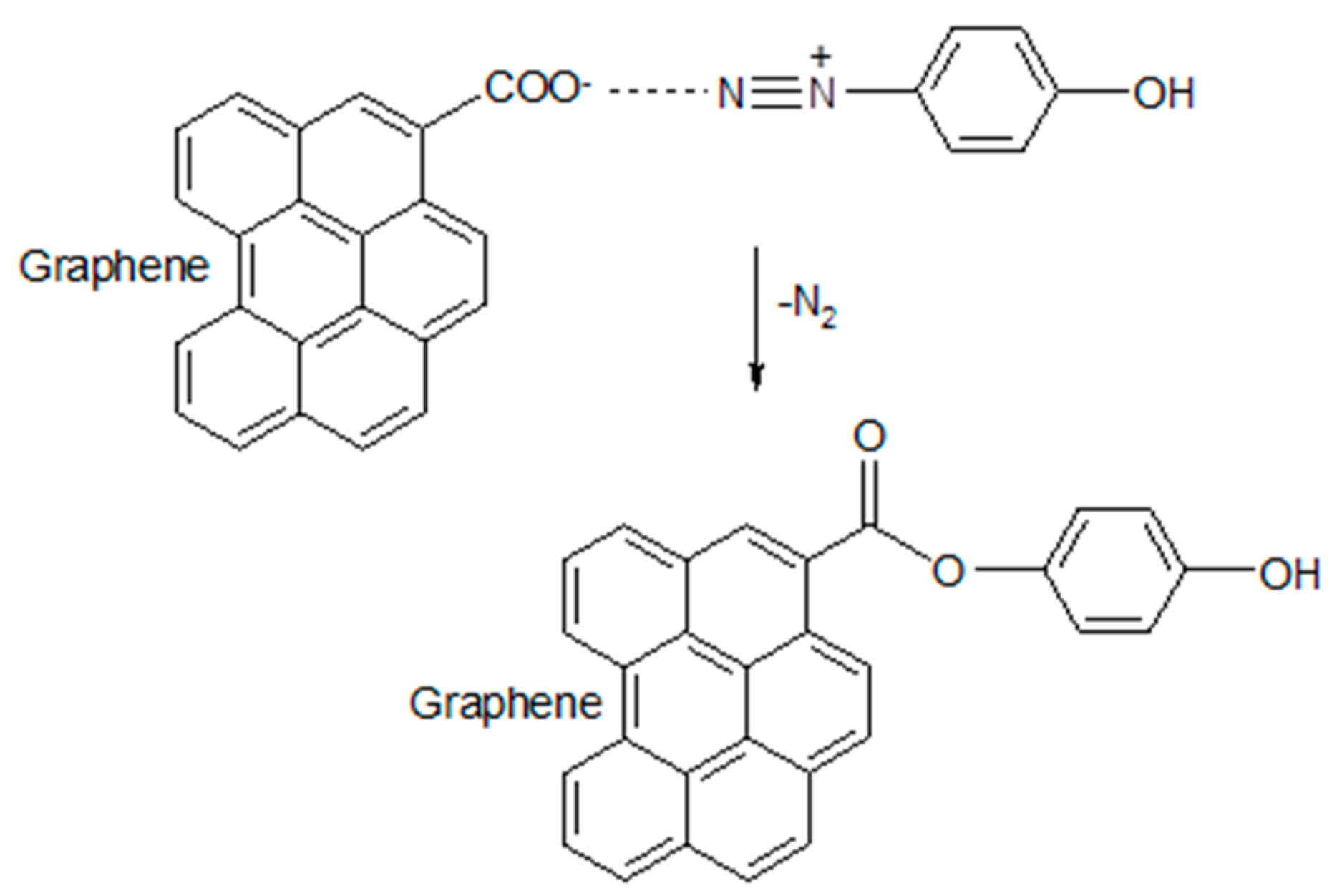
2.2. Preparation of Diazonium Salt (DS)
2.3. Preparation of Functionalized Graphene
2.3.1. Preparation of Water-Borne Coatings Containing Functionalized Graphene
2.3.2. Characterization
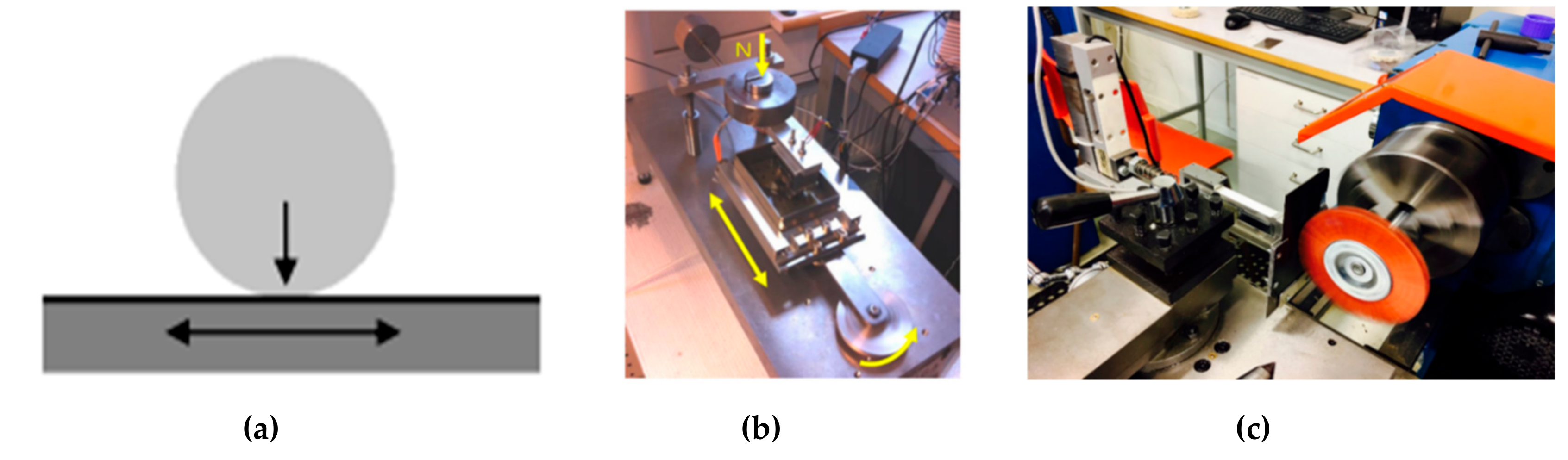
2.3.3. Tribological Tests
3. Results and Discussion
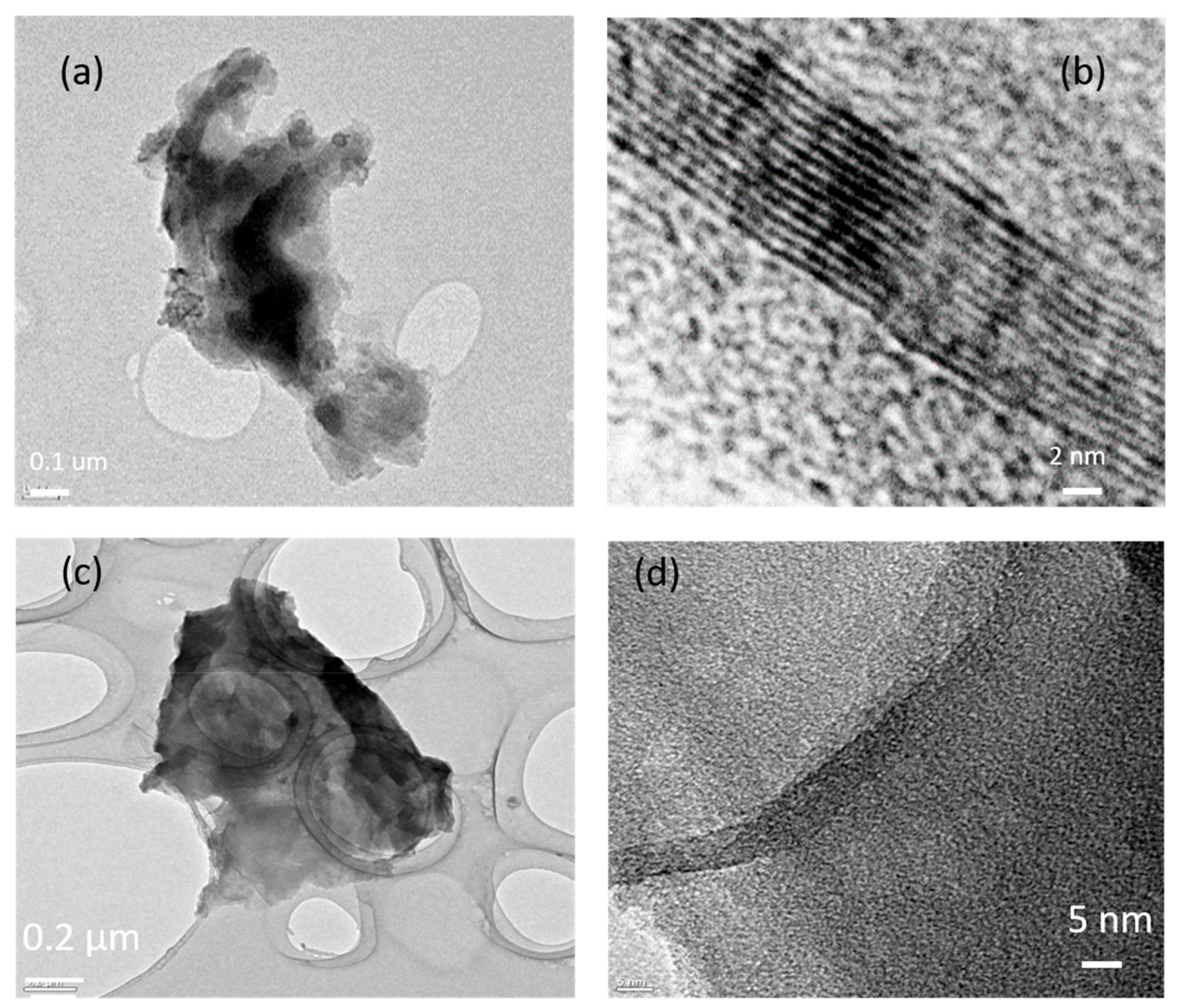
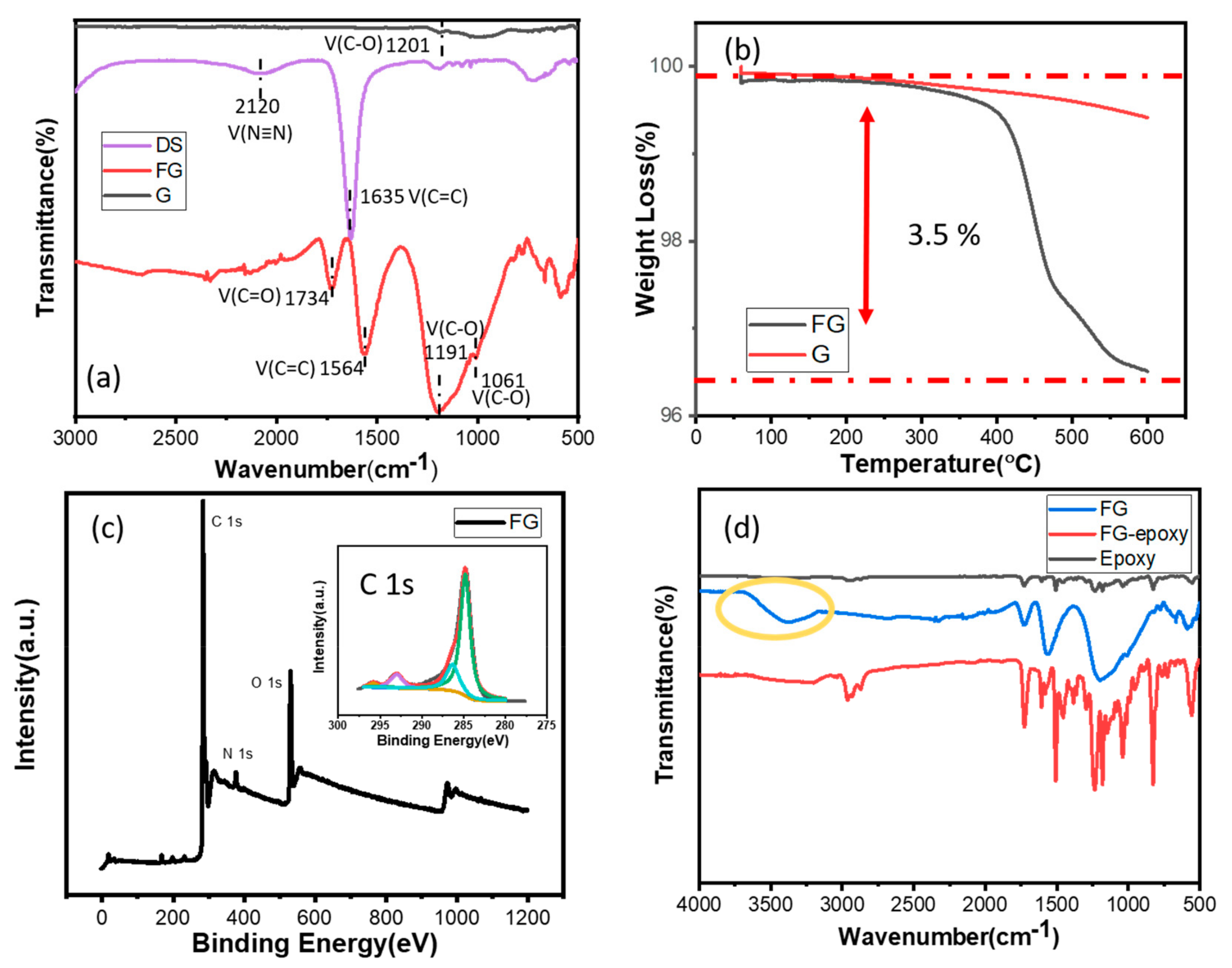
3.1. Characterization of Functional Graphene
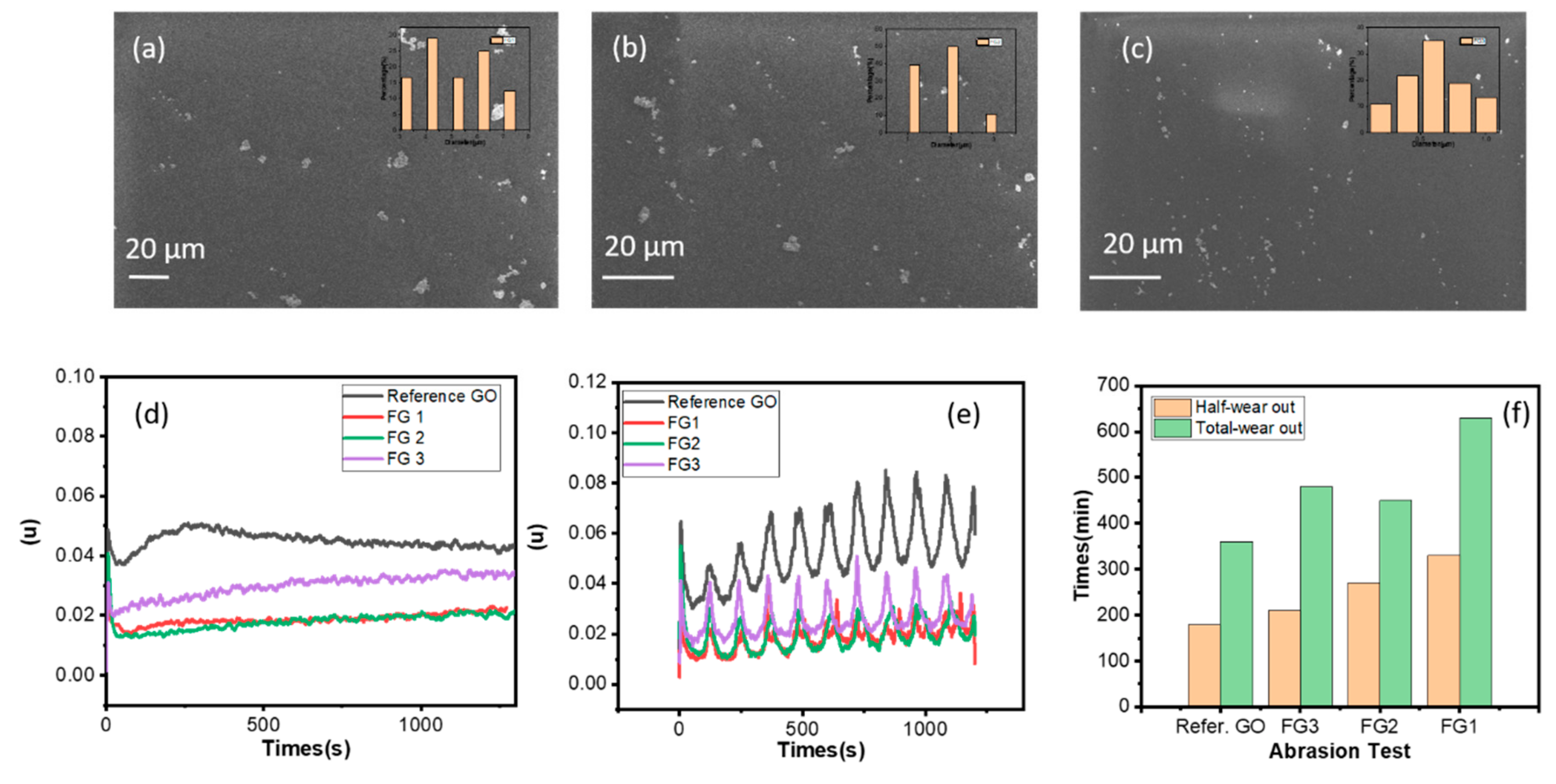
3.2. Tribological Performance of FG Enhanced Epoxy Coating
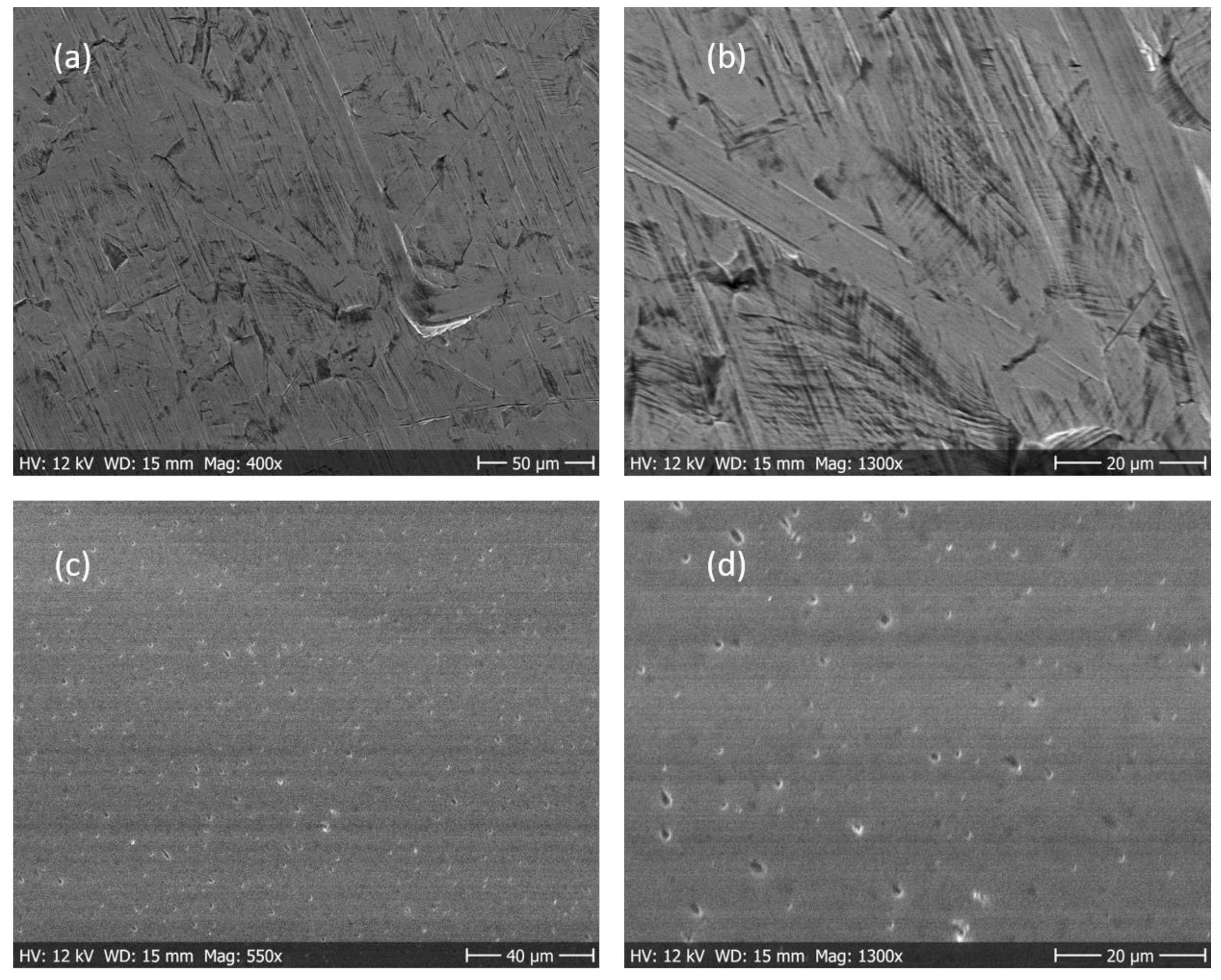
3.3. Coating Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cui, M.J.; Ren, S.M.; Zhao, H.C.; Xue, Q.J.; Wang, L.P. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Guo, Q.B.; Lau, K.T.; Zheng, B.F.; Rong, M.Z.; Zhang, M.Q. Imparting Ultra-Low Friction and Wear Rate to Epoxy by the Incorporation of Microencapsulated Lubricant? Macromol. Mater. Eng. 2009, 294, 20–24. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, S.J.; Wang, Q. Enhancement of tribological properties of polymer composites reinforced by functionalized graphene. Compos. Part B Eng. 2017, 120, 83–91. [Google Scholar] [CrossRef]
- Lv, T.; Huang, S.Q.; Hu, X.D.; Ma, Y.L.; Xu, X.F. Tribological and machining characteristics of a minimum quantity lubrication (MQL) technology using GO/SiO2 hybrid nanoparticle water-based lubricants as cutting fluids. Int. J. Adv. Manuf. Technol. 2018, 96, 2931–2942. [Google Scholar] [CrossRef]
- Wu, P.; Chen, X.C.; Zhang, C.H.; Luo, J.B. Synergistic tribological behaviors of graphene oxide and nanodiamond as lubricating additives in water. Tribol. Int. 2019, 132, 177–184. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.B.; Jia, Y.H.; Li, X.F.; Yang, J.; Li, C.S.; Yan, F.Y. Enhanced tribological properties of epoxy-based lubricating coatings using carbon nanotubes-ZnS hybrid. Surf. Coat. Technol. 2018, 344, 154–162. [Google Scholar] [CrossRef]
- Li, H.Y.; Shi, N.Q.; Ji, J.; Wang, H.Y. Preparation of microcapsules containing double-component lubricant and self-lubricating performance of polymer composites. Mater. Res. Express 2018, 5, 8. [Google Scholar] [CrossRef]
- Bandeira, P.; Monteiro, J.; Baptista, A.M.; Magalhaes, F.D. Influence of oxidized graphene nanoplatelets and DMIM NTf2 ionic liquid on the tribological performance of an epoxy-PTFE coating. Tribol. Int. 2016, 97, 478–489. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Qi, Y.Z.; Liu, J.; Zhang, J.; Dong, Y.L.; Li, Q.Y. Wear Resistance Limited by Step Edge Failure: The Rise and Fall of Graphene as an Atomically Thin Lubricating Material. ACS Appl. Mater. Interfaces 2017, 9, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Tambe, N.S.; Bhushan, B. Scale dependence of micro/nano-friction and adhesion of MEMS/NEMS materials, coatings and lubricants. Nanotechnology 2004, 15, 1561–1570. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Song, J.; Dai, Z.; Li, J.; Zhao, H.; Wang, L. Silane coupling agent modified BN–OH as reinforcing filler for epoxy nanocomposite. High Perform. Polym. 2019, 31, 116–123. [Google Scholar] [CrossRef]
- Rasheed, A.K.; Khalid, M.; Rashmi, W.; Gupta, T.; Chan, A. Graphene based nanofluids and nanolubricants-Review of recent developments. Renew. Sustain. Energy Rev. 2016, 63, 346–362. [Google Scholar] [CrossRef]
- Presser, A.; Hüfner, A. Trimethylsilyldiazomethane–a mild and efficient reagent for the methylation of carboxylic acids and alcohols in natural products. Monatshefte Für Chem. Chem. Mon. 2004, 135, 1015–1022. [Google Scholar]
- Li, Z.; Young, R.J.; Wang, R.; Yang, F.; Hao, L.; Jiao, W.; Liu, W. The role of functional groups on graphene oxide in epoxy nanocomposites. Polymer 2013, 54, 5821–5829. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xia, C.; Zehri, A.; Ye, L.; Wang, N.; Zhmud, B.; Lu, H.; Liu, J. Surface Modification of Graphene for Use as a Structural Fortifier in Water-Borne Epoxy Coatings. Coatings 2019, 9, 754. https://doi.org/10.3390/coatings9110754
Liu Y, Xia C, Zehri A, Ye L, Wang N, Zhmud B, Lu H, Liu J. Surface Modification of Graphene for Use as a Structural Fortifier in Water-Borne Epoxy Coatings. Coatings. 2019; 9(11):754. https://doi.org/10.3390/coatings9110754
Chicago/Turabian StyleLiu, Ya, Chao Xia, Abdelhafid Zehri, Lilei Ye, Nan Wang, Boris Zhmud, Hongbin Lu, and Johan Liu. 2019. "Surface Modification of Graphene for Use as a Structural Fortifier in Water-Borne Epoxy Coatings" Coatings 9, no. 11: 754. https://doi.org/10.3390/coatings9110754
APA StyleLiu, Y., Xia, C., Zehri, A., Ye, L., Wang, N., Zhmud, B., Lu, H., & Liu, J. (2019). Surface Modification of Graphene for Use as a Structural Fortifier in Water-Borne Epoxy Coatings. Coatings, 9(11), 754. https://doi.org/10.3390/coatings9110754