Effect of Transglutaminase Cross-Linking in Protein Isolates from a Mixture of Two Quinoa Varieties with Chitosan on the Physicochemical Properties of Edible Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Extraction of Quinoa Protein
2.2.2. Moisture and Protein Content
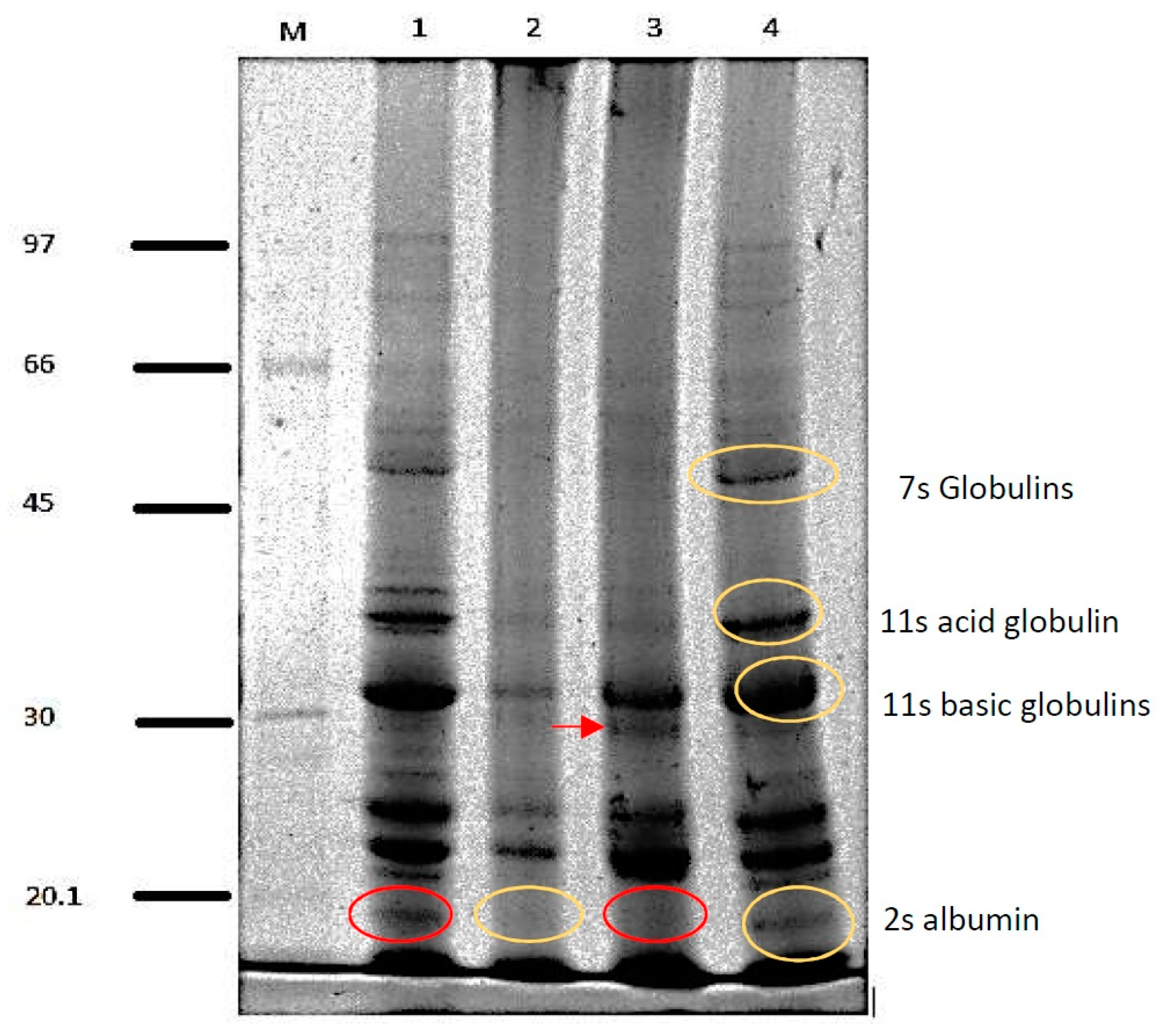
2.2.3. Electrophoresis (SDS-PAGE)
2.2.4. Quinoa Protein Edible Films
2.2.5. Chitosan Edible Films
2.2.6. Chitosan-Quinoa Protein Films
2.2.7. Determination of the Extent of Cross-Linking
2.2.8. Edible Film Characterization
2.2.9. Simulated Gastric and Duodenal Digestion
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Protein Isolate Characterization
3.2. Cross-Linking
3.3. Physical Properties
3.4. Edible Film Sorption Isotherms
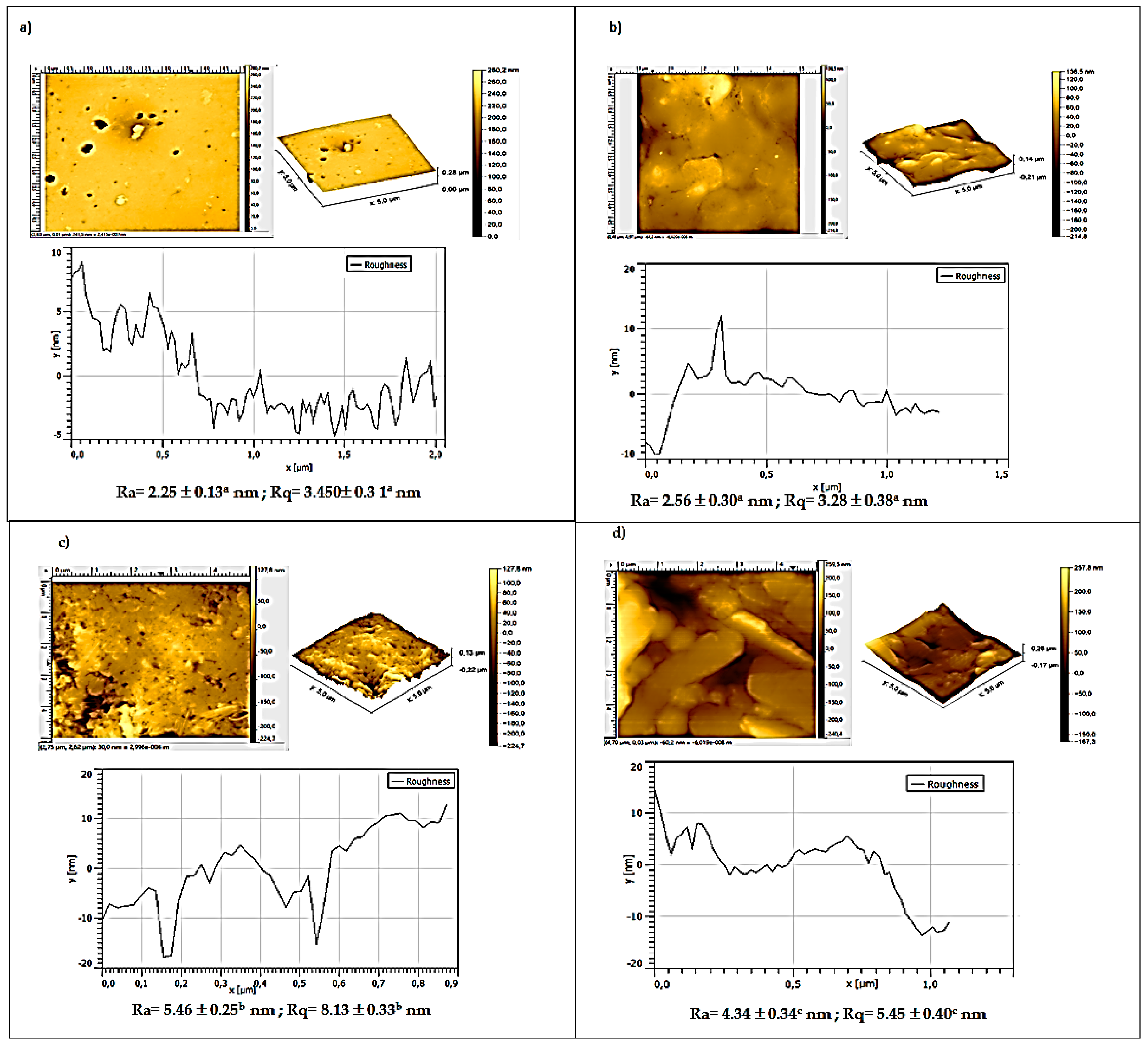
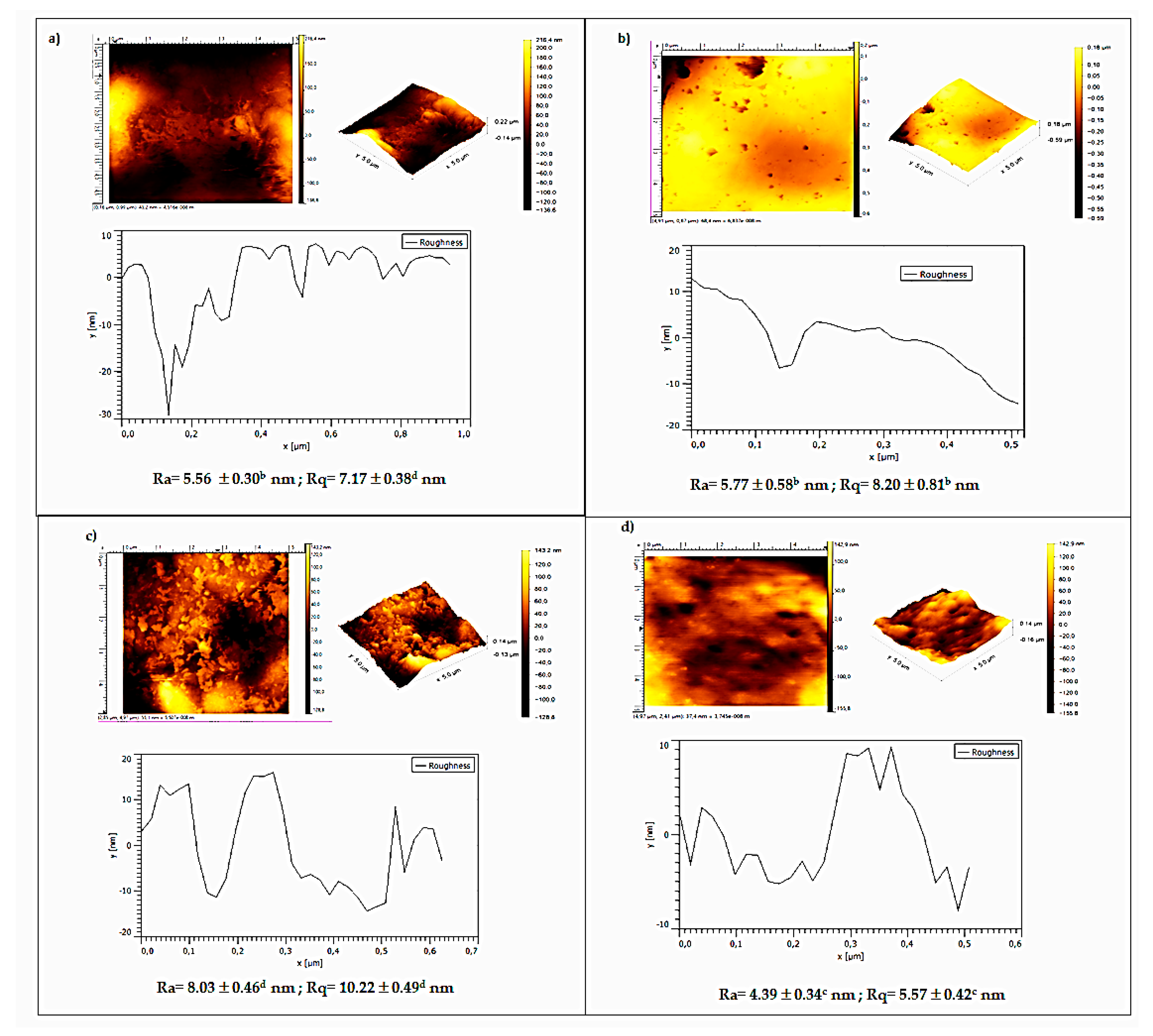
3.5. Atomic Force Microscopy
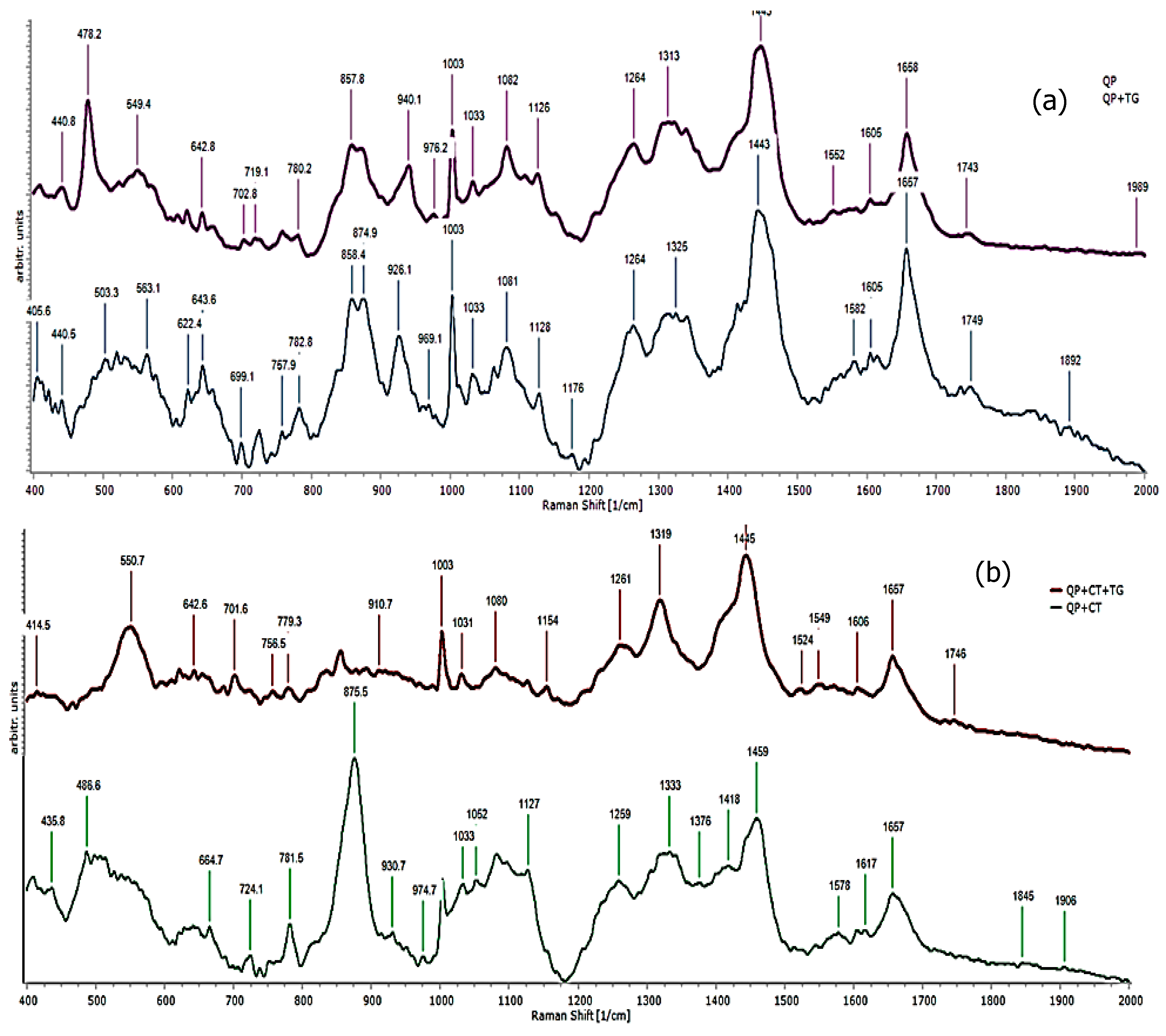
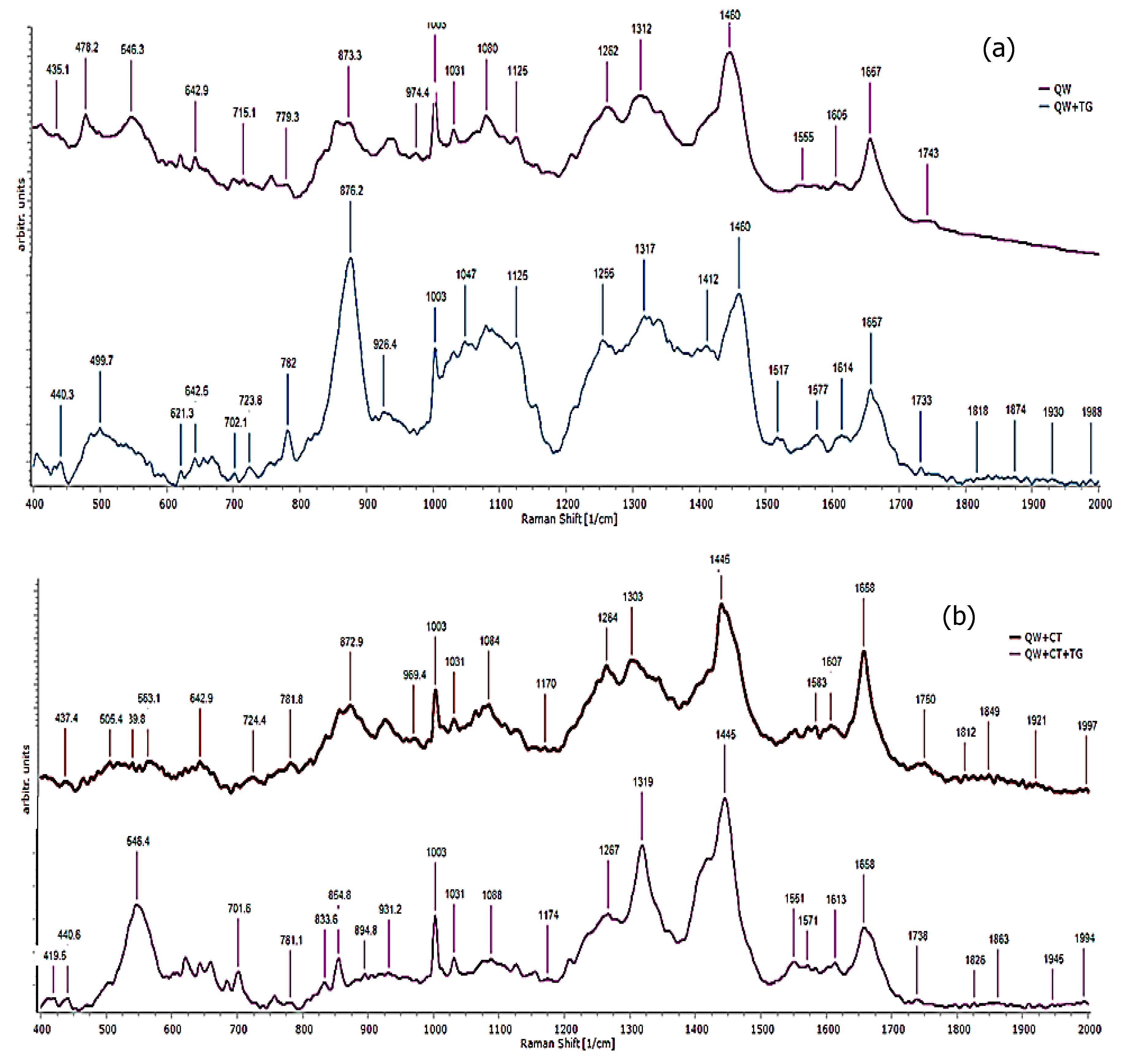
3.6. Raman Spectroscopy
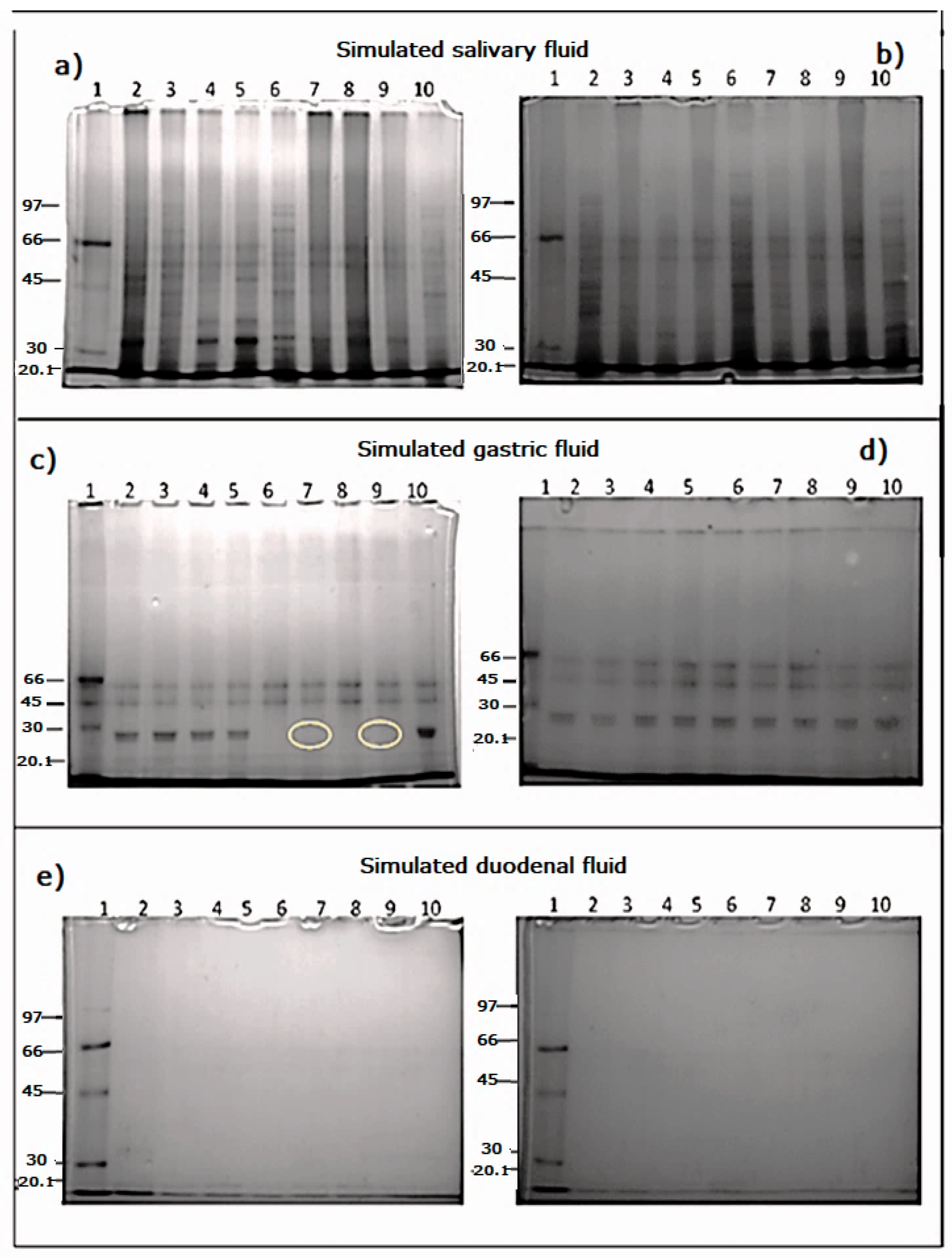
3.7. Simulated Gastric and Duodenal Digestion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Chiralt, A.; González-Martínez, C.; Vargas, M.; Atarés, L. 18-Edible films and coatings from proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, MA, USA, 2018; pp. 477–500. ISBN 978-0-08-100722-8. [Google Scholar]
- Elsohaimy, S.A.; Refaay, T.M.; Zaytoun, M.A.M. Physicochemical and functional properties of quinoa protein isolate. Ann. Agric. Sci. 2015, 60, 297–305. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. (In press). [CrossRef]
- Caro, N.; Medina, E.; Díaz-Dosque, M.; López, L.; Abugoch, L.; Tapia, C. Novel active packaging based on films of chitosan and chitosan/quinoa protein printed with chitosan-tripolyphosphate-thymol nanoparticles via thermal ink-jet printing. Food Hydrocoll. 2016, 52, 520–532. [Google Scholar] [CrossRef]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Fernandez-Bats, I.; Di Pierro, P.; Villalonga-Santana, R.; Garcia-Almendarez, B.; Porta, R. Bioactive mesoporous silica nanocomposite films obtained from native and transglutaminase-crosslinked bitter vetch proteins. Food Hydrocoll. 2018, 82, 106–115. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Romeih, E.; Walker, G. Recent advances on microbial transglutaminase and dairy application. Trends Food Sci. Technol. 2017, 62, 133–140. [Google Scholar] [CrossRef]
- Ruiz, G.A.; Xiao, W.; van Boekel, M.; Minor, M.; Stieger, M. Effect of extraction pH on heat-induced aggregation, gelation and microstructure of protein isolate from quinoa (Chenopodium quinoa Willd). Food Chem. 2016, 209, 203–210. [Google Scholar] [CrossRef] [PubMed]
- AACC. International Approved Methods—AACC Method 46-10.01. Crude Protein—Improved Kjeldahl Method. Available online: http://methods.aaccnet.org/summaries/46-10-01.aspx (accessed on 19 April 2018).
- Brunelle, J.L.; Green, R. Chapter Twelve—One-dimensional SDS-Polyacrylamide Gel Electrophoresis (1D SDS-PAGE). In Methods in Enzymology; Lorsch, J., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 541, pp. 151–159. ISBN 0076-6879. [Google Scholar]
- Sharma, L.; Singh, C. Sesame protein based edible films: Development and characterization. Food Hydrocoll. 2016, 61, 139–147. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Calderón-Domínguez, G.; Chanona-Pérez, J.J.; Mendoza-Madrigal, A.G.; Di Pierro, P.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Physical, structural, barrier, and antifungal characterization of chitosan–zein edible films with added essential oils. Int. J. Mol. Sci. 2017, 18, 2370. [Google Scholar] [CrossRef] [PubMed]
- AL-Hassan, A.A.; Norziah, M.H. Effect of transglutaminase induced crosslinking on the properties of starch/gelatin films. Food Packag. Shelf Life 2017, 13, 15–19. [Google Scholar] [CrossRef]
- Prasertsung, I.; Mongkolnavin, R.; Kanokpanont, S.; Damrongsakkul, S. The effects of pulsed inductively coupled plasma (PICP) on physical properties and biocompatibility of crosslinked gelatin films. Int. J. Biol. Macromol. 2010, 46, 72–78. [Google Scholar] [CrossRef]
- Soo, P.Y.; Sarbon, N.M. Preparation and characterization of edible chicken skin gelatin film incorporated with rice flour. Food Packag. Shelf Life 2018, 15, 1–8. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of Citrus pectin edible films containing transglutaminase-modified phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef]
- Abugoch, L.E.; Tapia, C.; Villamán, M.C.; Yazdani-Pedram, M.; Díaz-Dosque, M. Characterization of quinoa protein–chitosan blend edible films. Food Hydrocoll. 2011, 25, 879–886. [Google Scholar] [CrossRef]
- Kaspchak, E.; de Oliveira, M.A.S.; Simas, F.F.; Franco, C.R.C.; Silveira, J.L.M.; Mafra, M.R.; Igarashi-Mafra, L. Determination of heat-set gelation capacity of a quinoa protein isolate (Chenopodium quinoa) by dynamic oscillatory rheological analysis. Food Chem. 2017, 232, 263–271. [Google Scholar] [CrossRef]
- Castellión, M.; Matiacevich, S.; Buera, P.; Maldonado, S. Protein deterioration and longevity of quinoa seeds during long-term storage. Food Chem. 2010, 121, 952–958. [Google Scholar] [CrossRef]
- Steffolani, M.E.; Villacorta, P.; Morales-Soriano, E.R.; Repo-Carrasco, R.; León, A.E.; Pérez, G.T. Physicochemical and functional characterization of protein isolated from different quinoa varieties (Chenopodium quinoa Willd). Cereal Chem. 2016, 93, 275–281. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, protein profile and rheological properties of pseudocereal-based protein-rich ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Burrieza, H.P.; Rizzo, A.J.; Moura Vale, E.; Silveira, V.; Maldonado, S. Shotgun proteomic analysis of quinoa seeds reveals novel lysine-rich seed storage globulins. Food Chem. 2019, 293, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jiao, X.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H. Catalytic effect of transglutaminase mediated by myofibrillar protein crosslinking under microwave irradiation. Food Chem. 2019, 284, 45–52. [Google Scholar] [CrossRef]
- Basman, A.; Köksel, H.; Ng, P.K. Effects of increasing levels of transglutaminase on the rheological properties and bread quality characteristics of two wheat flours. Eur. Food Res. Tech. 2002, 215, 419–424. [Google Scholar] [CrossRef]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C.F.R. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W.; Li, Y.; Gao, G.; Zhang, K.; Zhou, J.; Wu, Z. Cross-linking and film-forming properties of transglutaminase-modified collagen fibers tailored by denaturation temperature. Food Chem. 2019, 271, 527–535. [Google Scholar] [CrossRef]
- Tan, W.; Dong, F.; Zhang, J.; Zhao, X.; Li, Q.; Guo, Z. Physical and antioxidant properties of edible chitosan ascorbate films. J. Agric. Food Chem. 2019, 67, 2530–2539. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Z.; Hu, Q. Difference between chitosan hydrogels via alkaline and acidic solvent systems. Sci. Rep. 2016, 6, 36053. [Google Scholar] [CrossRef]
- Sk, B.; Awasthi, S.; Utiye, A.S.; Mishra, B. Investigation of moisture sorption properties of gelatin/poly (aniline)/films. SOJ Mater. Sci. Eng. 2017, 5, 1–6. [Google Scholar]
- Aguirre-Loredo, R.Y.; Rodríguez-Hernández, A.I.; Velazquez, G. Modelling the effect of temperature on the water sorption isotherms of chitosan films. Food Sci. Technol. 2017, 37, 112–118. [Google Scholar] [CrossRef]
- Phan, T.D.; Debeaufort, F.; Luu, D.; Voilley, A. Functional properties of edible agar-based and starch-based films for food quality preservation. J. Agric. Food Chem. 2005, 53, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Water sorption isotherm of pea starch edible films and prediction models. Foods 2016, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Shih, F.F.; Daigle, K.W.; Champagne, E.T. Effect of rice wax on water vapour permeability and sorption properties of edible pullulan films. Food Chem. 2011, 127, 118–121. [Google Scholar] [CrossRef]
- Opazo-Navarrete, M.; Schutyser, M.A.I.; Boom, R.M.; Janssen, A.E.M. Effect of pre-treatment on in vitro gastric digestion of quinoa protein (Chenopodium quinoa Willd.) obtained by wet and dry fractionation. Int. J. Food Sci. Nutr. 2018, 69, 1–11. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef]
- Mariniello, L.; Giosafatto, C.V.L.; Di Pierro, P.; Sorrentino, A.; Porta, R. Synthesis and resistance to in vitro proteolysis of transglutaminase cross-linked phaseolin, the major storage protein from Phaseolus vulgaris. J. Agric. Food Chem. 2007, 55, 4717–4721. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Zannini, E.; Arendt, E.K. Modifying the cold gelation properties of quinoa protein isolate: Influence of Heat-Denaturation pH in the alkaline range. Plant Foods Hum. Nutr. 2015, 70, 250–256. [Google Scholar] [CrossRef]
- Deleu, L.J.; Lambrecht, M.A.; de Vondel, J.V.; Delcour, J.A. The impact of alkaline conditions on storage proteins of cereals and pseudo-cereals. Curr. Opin. Food Sci. 2019, 25, 98–103. [Google Scholar] [CrossRef]
| Parameter | QW | QP |
|---|---|---|
| Moisture (%, w/w) | 12.42 ± 0.72 | 10.07 ± 0.54 |
| Protein (N × 6.25, %, w/w) | 71.49 ± 1.99 | 67.93 ± 2.01 |
| Edible Film | %Cross-Linking | Edible Film | %Cross-Linking |
|---|---|---|---|
| QW | 40.55 ± 3.62 a | QP | 61.45 ± 3.73 a |
| CT:QW 1:5 | 25.79 ± 3.67 b | CT:QP 1:5 | 11.45 ± 2.70 b |
| CT:QW 1:10 | 62.02 ± 1.97 c | CT:QP 1:10 | 44.28 ± 1.13 c |
| CT:QW 1:15 | 67.61 ± 3.28 c | CT:QP 1:15 | 39.31 ± 2.18 d |
| CT:QW 1:20 | 44.59 ± 3.21 a | CT:QP 1:20 | 32.63 ± 2.69 e |
| Edible Films without TG | |||
|---|---|---|---|
| Edible Film | Thickness (mm) | WVP × 10−13 (g cm Pa−1 cm−2 s−1) | %Solubility |
| QP | 0.155 ± 0.051 a | 2.85 ± 0. 78 a | 71.84 ± 1.58 a |
| CT:QP 1:5 | 0.231 ± 0.025 b | 4.97± 0.82 b | 27.98 ± 1.07 b |
| CT:QP 1:10 | 0.258 ± 0.014 b | 3.64 ± 0.64 be | 32.28 ± 1.09 c |
| CT QP 1:15 | 0.214 ± 0.057 b | 3.74 ± 0.37 be | 47.38 ± 1.07 d |
| CT:QP 1:20 | 0.173 ± 0.011 a | 3.26 ± 0.96 be | 56.91 ± 1.50 e |
| QW | 0.184 ± 0.016 a | 3.40 ± 0.68 be | 67.92 ± 1.58 f |
| CT:QW 1:5 | 0.253 ± 0.050 b | 9.95 ± 0.29 c | 17.5 ± 1.87 g |
| CT:QW 1:10 | 0.266 ± 0.003 b | 6.74 ± 0.83 d | 26.73 ± 2.54 b |
| CT:QW 1:15 | 0.223 ± 0.004 b | 5.56 ± 0.50 bd | 43.52 ± 1.22 h |
| CT:QW 1:20 | 0.181 ± 0.001 a | 4.77 ± 0.79 b | 55.24 ± 2.36 e |
| Edible Films with TG | |||
| QP | 0.179 ± 0.064 a | 2.87 ± 0.68 a | 68.82 ± 1.01 f |
| CT:QP 1:5 | 0.266 ± 0.003 b | 3.23 ± 0.43 e | 26.14 ± 1.82 b |
| CT:QP 1:10 | 0.205 ± 0.007 b | 2.83 ± 0.58 a | 26.83 ± 1.9 b |
| CT:QP 1:15 | 0.205 ± 0.003 b | 2.99 ± 0.62 ae | 42.95 ± 1.75 h |
| CT:QP 1:20 | 0.179 ± 0.011 a | 2.42 ± 0.5 ae | 55.25 ± 1.33 e |
| QW | 0.205 ± 0.003 b | 3.07 ± 0.29 ae | 55.24 ± 1.49 e |
| CT:QW 1:5 | 0.276 ± 0.068 b | 3.88 ± 0.12 ae | 14.02 ± 2.17 g |
| CT:QW 1:10 | 0.275 ± 0.003 b | 4.69 ± 0.52 b | 25.81 ± 2.95 b |
| CT:QW 1:15 | 0.225 ± 0.035 b | 3.40 ± 0.69 e | 42.26 ± 2.13 h |
| CT: QW 1:20 | 0.227 ± 0.007 b | 3.64 ± 0.49 be | 53.48 ± 1.57 e |
| Edible Films without TG | ||||
|---|---|---|---|---|
| Edible Film | K | C | Xm | r2 |
| QP | 0.95 ± 0.02 a | 11.73 ± 1.94 a | 0.03 ± 0.001 a | 0.95 ± 0.058 a |
| CT:QP 1:5 | 0.98 ± 0.07 a | 15.45 ± 2.16 a | 0.03 ± 0.001 a | 0.99 ± 0.004 a |
| CT:QP 1:10 | 0.74 ± 0.14 bc | 7.89 ± 0.67 b | 0.08± 0.003 b | 0.99 ± 0.004 a |
| CT:QP 1:15 | 0.70 ± 0.08 b | 7.07 ± 0.08 b | 0.11 ± 0.05 c | 0.98 ± 0.02 a |
| CT:QP 1:20 | 0.82 ± 0.03 c | 1.36 ± 0.23 c | 0.13 ± 0.02 c | 0.97 ± 0.01 a |
| QW | 0.78 ± 0.06 b | 7.27 ± 0.35 b | 0.06 ± 0.001 b | 0.93 ± 0.08 a |
| CT:QW 1:5 | 0.81 ± 0.06 c | 3.82 ± 1.14 d | 0.03 ± 0.001 a | 0.90 ± 0.07 a |
| CT:QW 1:10 | 0.75 ±0.12 b | 4.41 ± 0.46 d | 0.03 ± 0.007 a | 0.98 ± 0.02 a |
| CT:QW 1:15 | 0.53 ± 0.18 d | 4.91 ± 1.37 d | 0.04 ± 0.005 a | 0.96 ± 0.03 a |
| CT:QW 1:20 | 0.61 ± 0.17 d | 3.33 ± 0.68 d | 0.07 ± 0.008 d | 0.99 ± 0.01 a |
| Edible Films with TG | ||||
| QP | 0.95 ± 0.03 a | 78.72 ± 4.94 e | 0.02 ± 0.001 e | 0.92 ± 0.04 a |
| CT:QP 1:5 | 0.93 ± 0.08 a | 7.28 ± 1.22 b | 0.05 ± 0.001 f | 0.94 ± 0.05 a |
| CT:QP 1:10 | 0.90 ± 0.11 a | 7.07 ± 0.06 b | 0.07 ± 0.002 d | 0.94 ± 0.08 a |
| CT:QP 1:15 | 0.94 ± 0.07 a | 7.39 ± 0.7 b | 0.08 ± 0.003 g | 0.99 ± 0.12 a |
| CT:QP 1:20 | 0.74 ± 0.02 b | 9.79 ± 0.18 a | 0.06 ± 0.001 b | 0.98 ± 0.01 a |
| QW | 0.79 ± 0.18 b | 2.82 ± 0.82 f | 0.03 ± 0.007 a | 0.97 ± 0.03 a |
| CT:QW 1:5 | 0.88 ± 0.06 c | 6.80 ± 2.48 bf | 0.06 ± 0.008b | 0.96 ± 0.01 a |
| CT:QW 1:10 | 0.81 ± 0.16 c | 5.19 ± 0.83 d | 0.03 ± 0.002 a | 0.99 ± 0.01 a |
| CT:QW 1:15 | 0.69 ±0.18 b | 2.95 ± 0.93 f | 0.13 ± 0.02 c | 0.98 ± 0.01 a |
| CT:QW 1:20 | 0.62 ± 0.04 b | 2.31 ± 0.53 f | 0.18 ± 0.01 h | 0.99 ± 0.01 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla-García, M.; Delgado-Sánchez, L.F.; Ríos-Romo, R.A.; García-Almendárez, B.E.; Calderón-Domínguez, G.; Méndez-Méndez, J.V.; Amaro-Reyes, A.; Di Pierro, P.; Regalado-González, C. Effect of Transglutaminase Cross-Linking in Protein Isolates from a Mixture of Two Quinoa Varieties with Chitosan on the Physicochemical Properties of Edible Films. Coatings 2019, 9, 736. https://doi.org/10.3390/coatings9110736
Escamilla-García M, Delgado-Sánchez LF, Ríos-Romo RA, García-Almendárez BE, Calderón-Domínguez G, Méndez-Méndez JV, Amaro-Reyes A, Di Pierro P, Regalado-González C. Effect of Transglutaminase Cross-Linking in Protein Isolates from a Mixture of Two Quinoa Varieties with Chitosan on the Physicochemical Properties of Edible Films. Coatings. 2019; 9(11):736. https://doi.org/10.3390/coatings9110736
Chicago/Turabian StyleEscamilla-García, Monserrat, Luis Felipe Delgado-Sánchez, Raquel Adriana Ríos-Romo, Blanca E. García-Almendárez, Georgina Calderón-Domínguez, Juan Vicente Méndez-Méndez, Aldo Amaro-Reyes, Prospero Di Pierro, and Carlos Regalado-González. 2019. "Effect of Transglutaminase Cross-Linking in Protein Isolates from a Mixture of Two Quinoa Varieties with Chitosan on the Physicochemical Properties of Edible Films" Coatings 9, no. 11: 736. https://doi.org/10.3390/coatings9110736
APA StyleEscamilla-García, M., Delgado-Sánchez, L. F., Ríos-Romo, R. A., García-Almendárez, B. E., Calderón-Domínguez, G., Méndez-Méndez, J. V., Amaro-Reyes, A., Di Pierro, P., & Regalado-González, C. (2019). Effect of Transglutaminase Cross-Linking in Protein Isolates from a Mixture of Two Quinoa Varieties with Chitosan on the Physicochemical Properties of Edible Films. Coatings, 9(11), 736. https://doi.org/10.3390/coatings9110736