Atomic Oxygen Adaptability of Flexible Kapton/Al2O3 Composite Thin Films Prepared by Ion Exchange Method
Abstract
:1. Introduction
2. Experiment
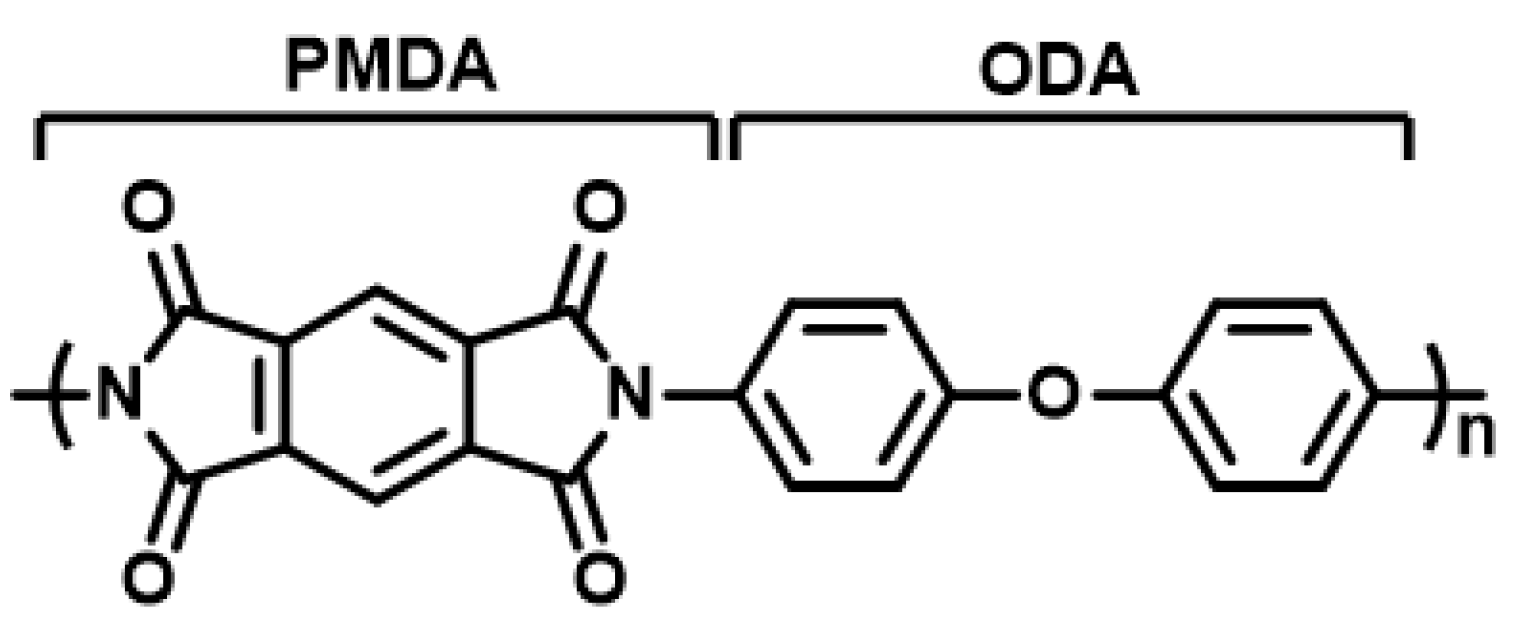
2.1. Experimental Materials
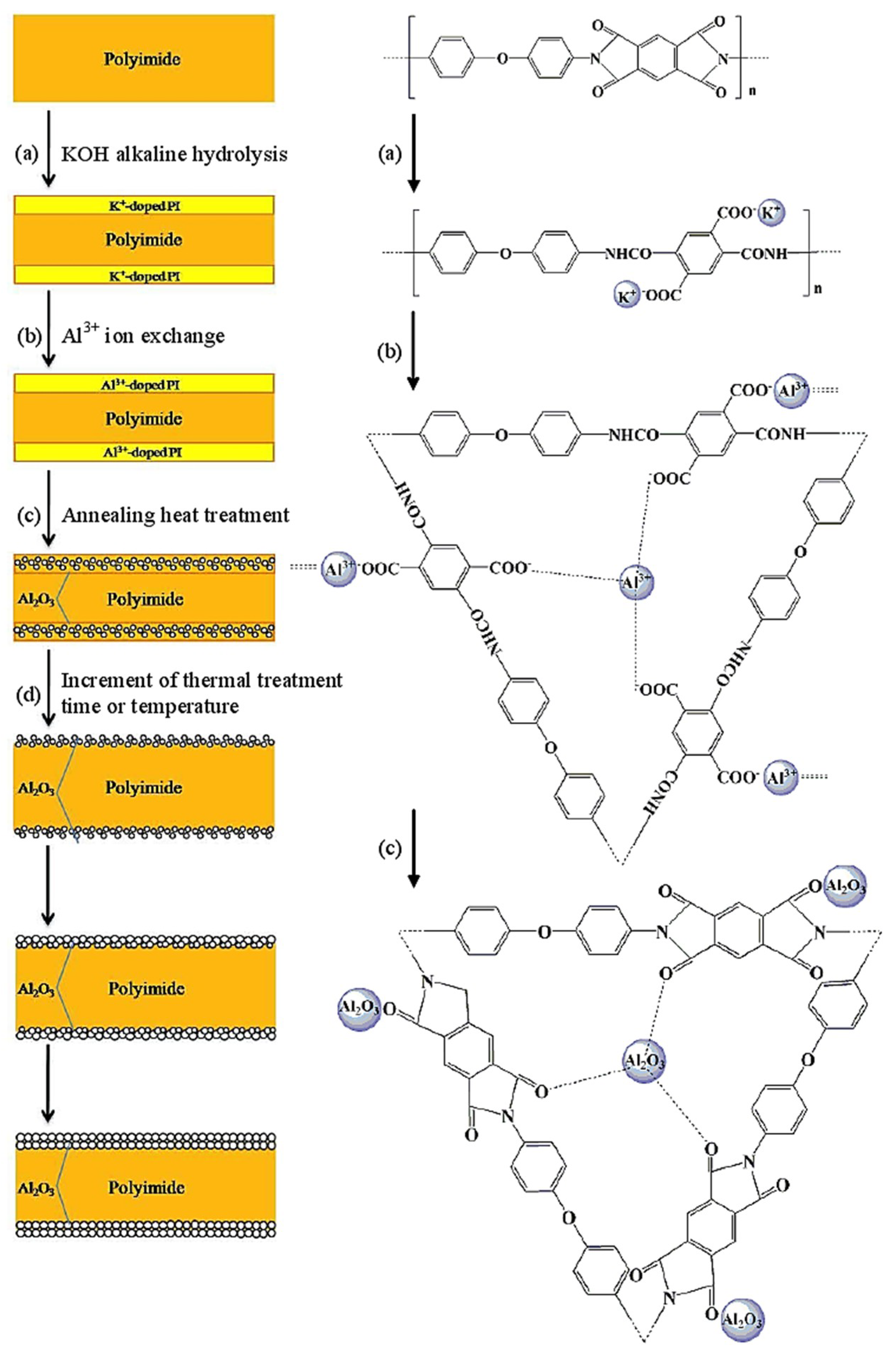
2.2. Preparation of Kapton/Al2O3 Composite Film
2.3. Atomic Oxygen Exposure Test
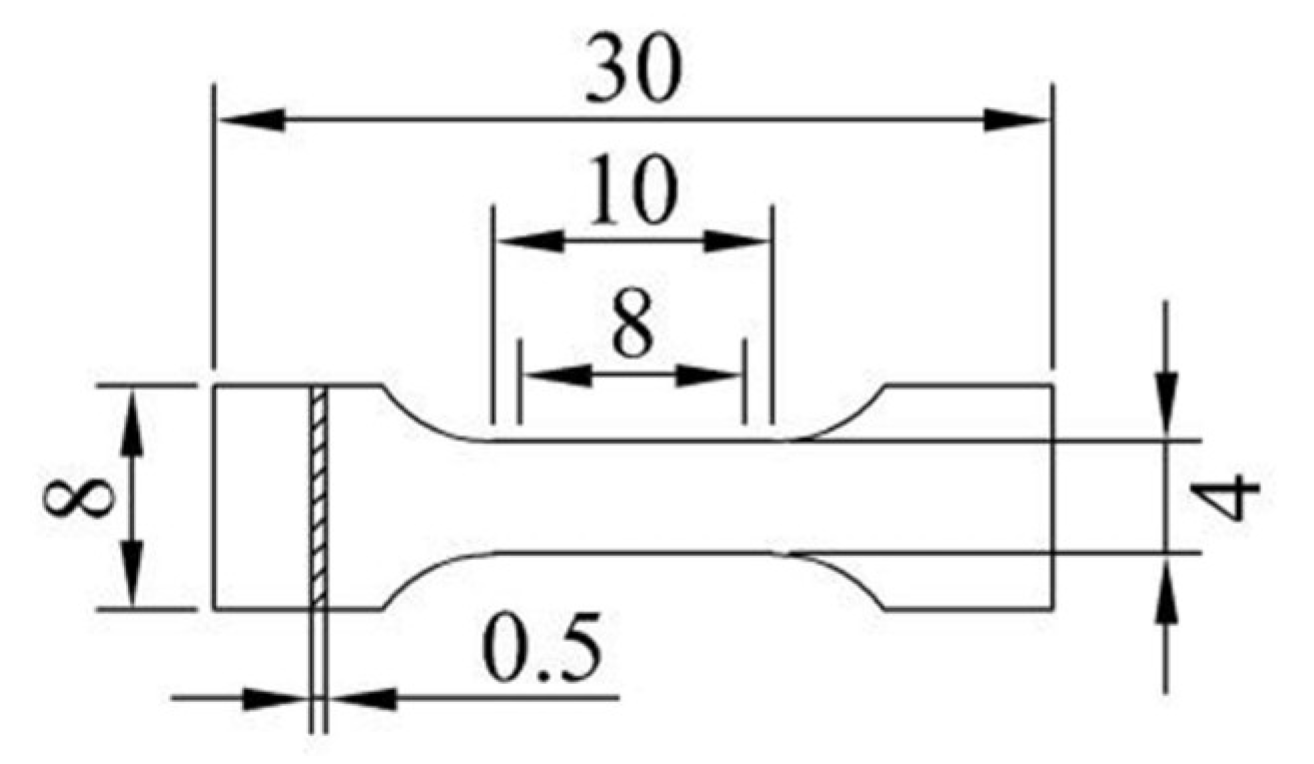
2.4. Testing and Characterization
3. Kapton/Al2O3 Nanocomposite Film
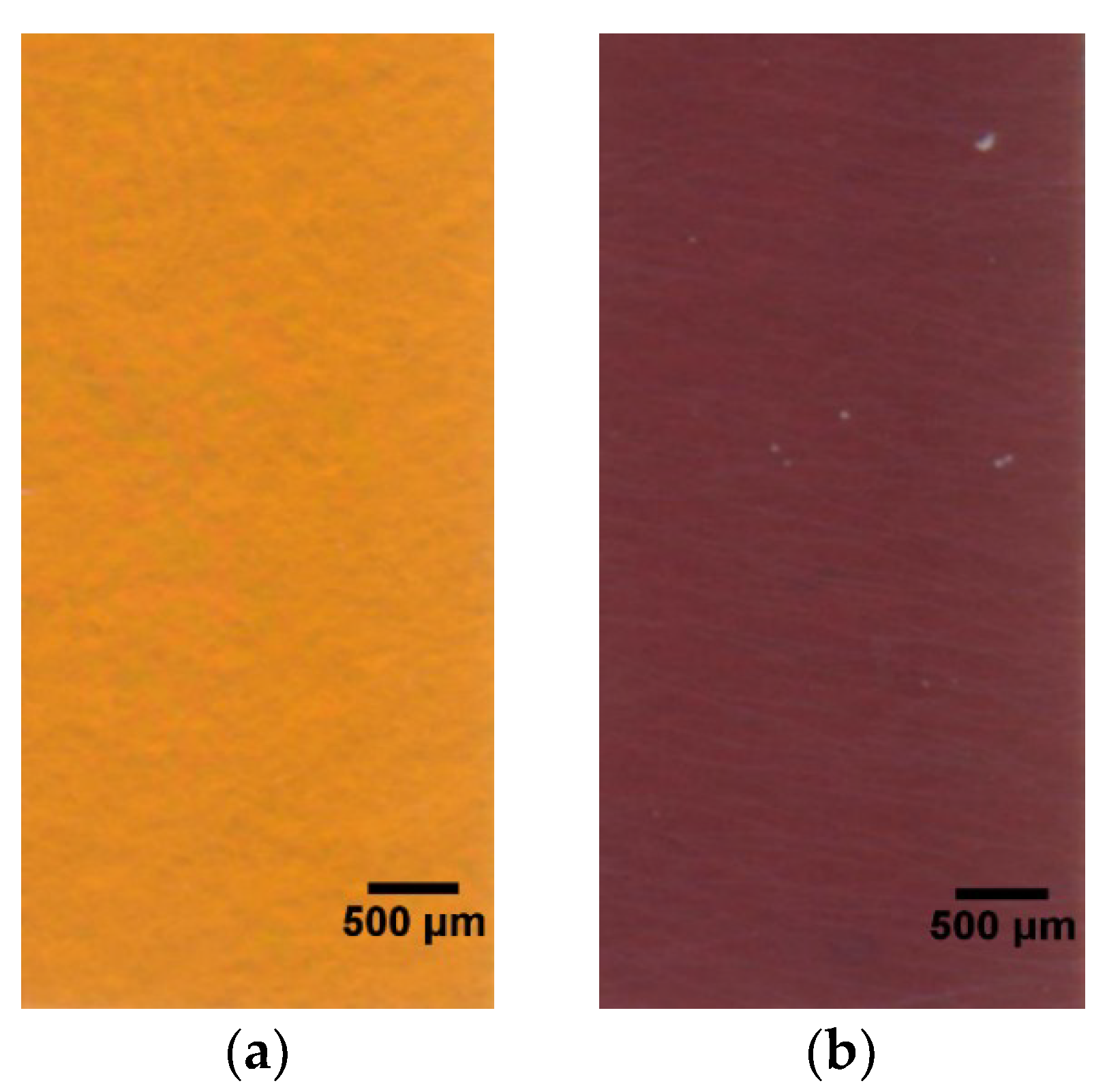
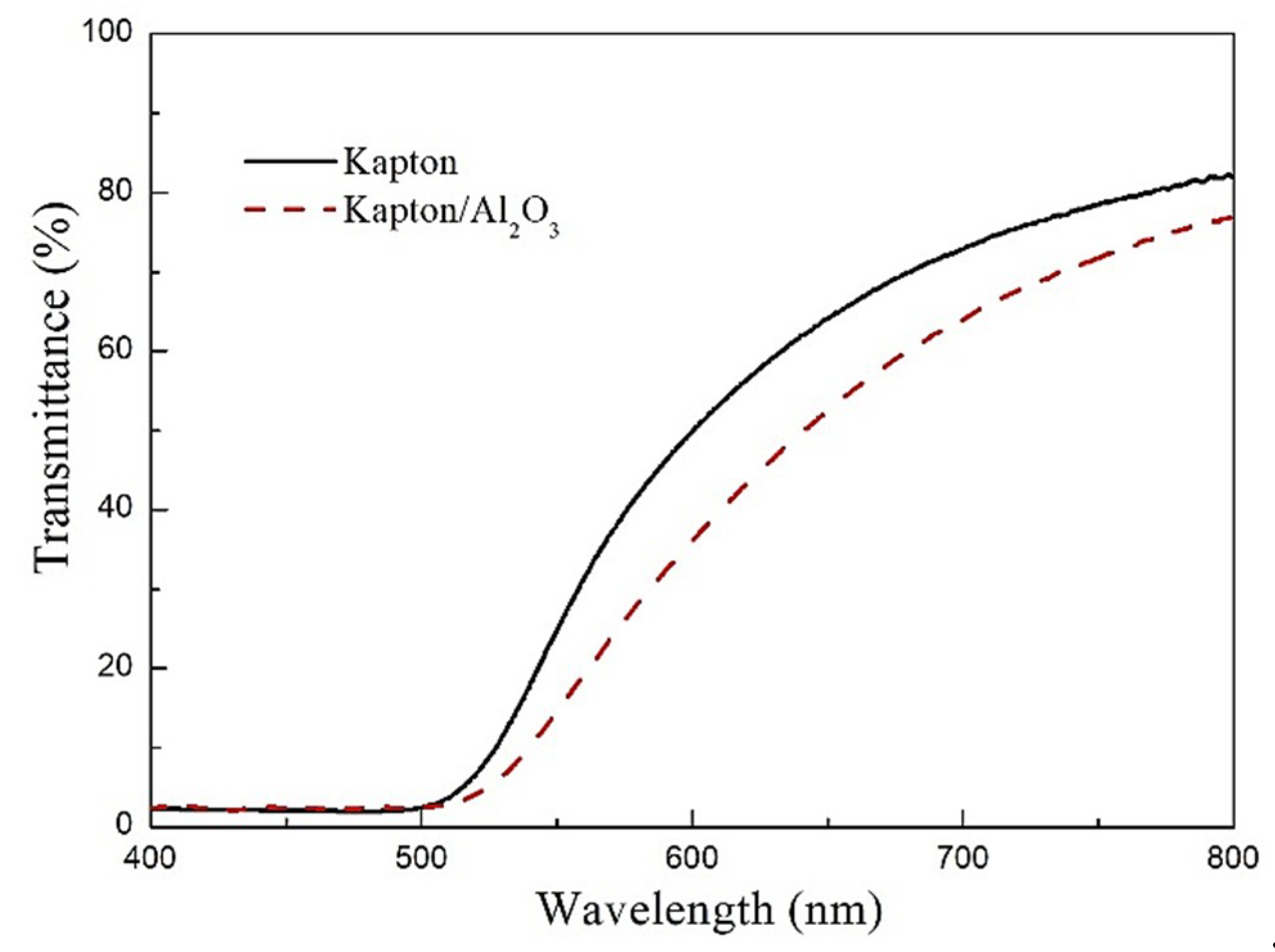
3.1. Macroscopic Observation and Optical Performance Characterization
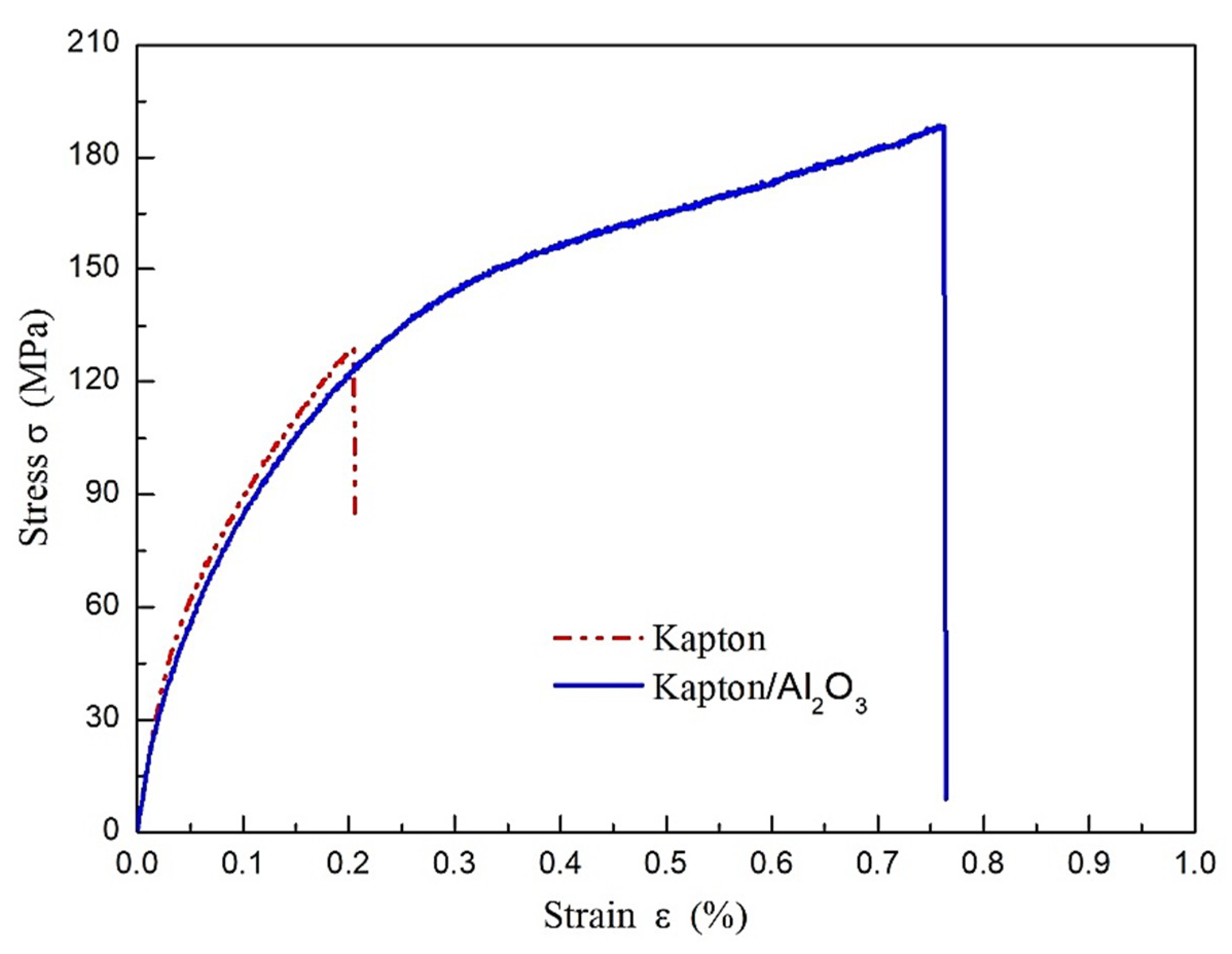
3.2. Characterization of Mechanical Properties
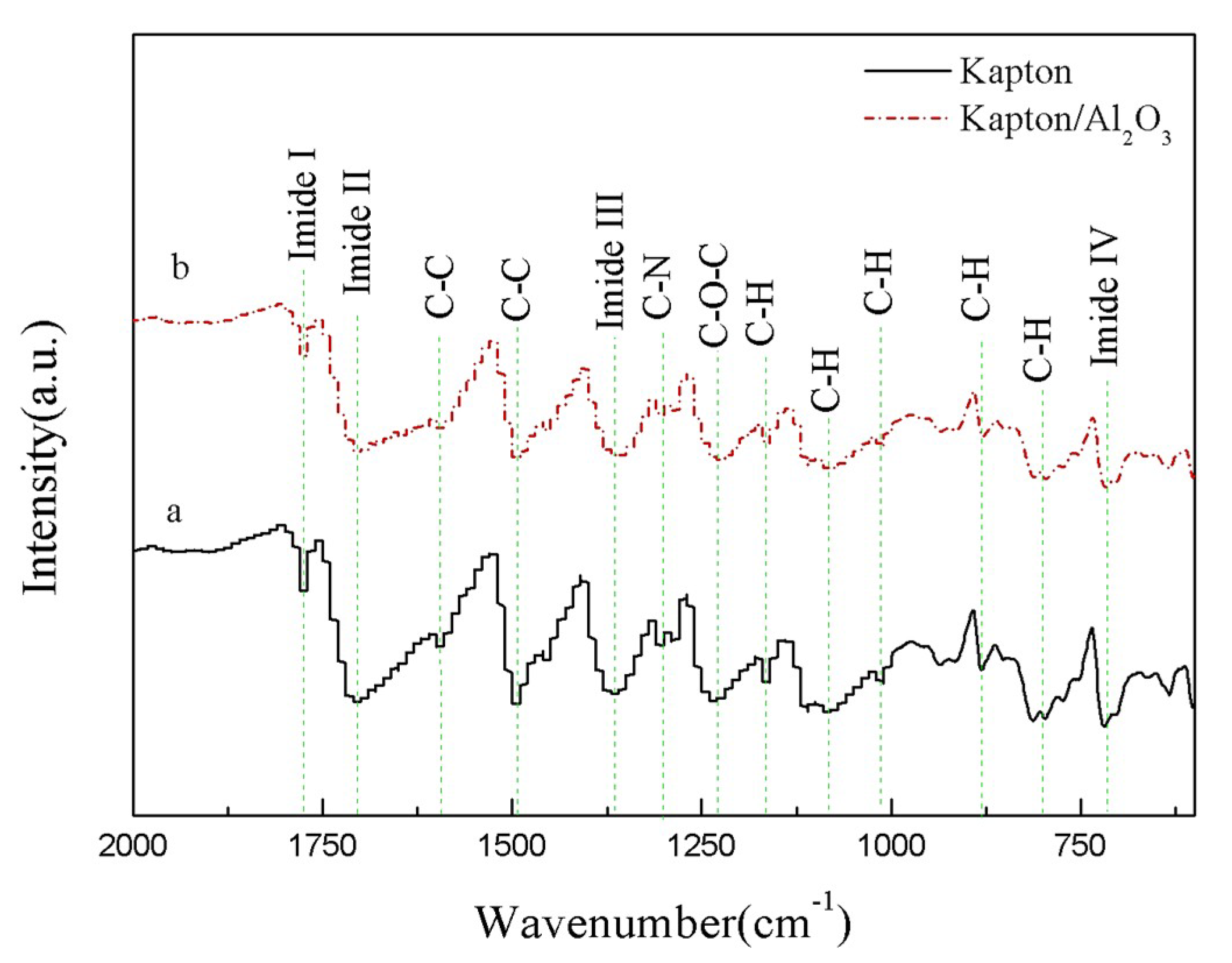
3.3. Infrared Spectroscopy Analysis
4. Atomic Oxygen Environment Adaptability Evaluation
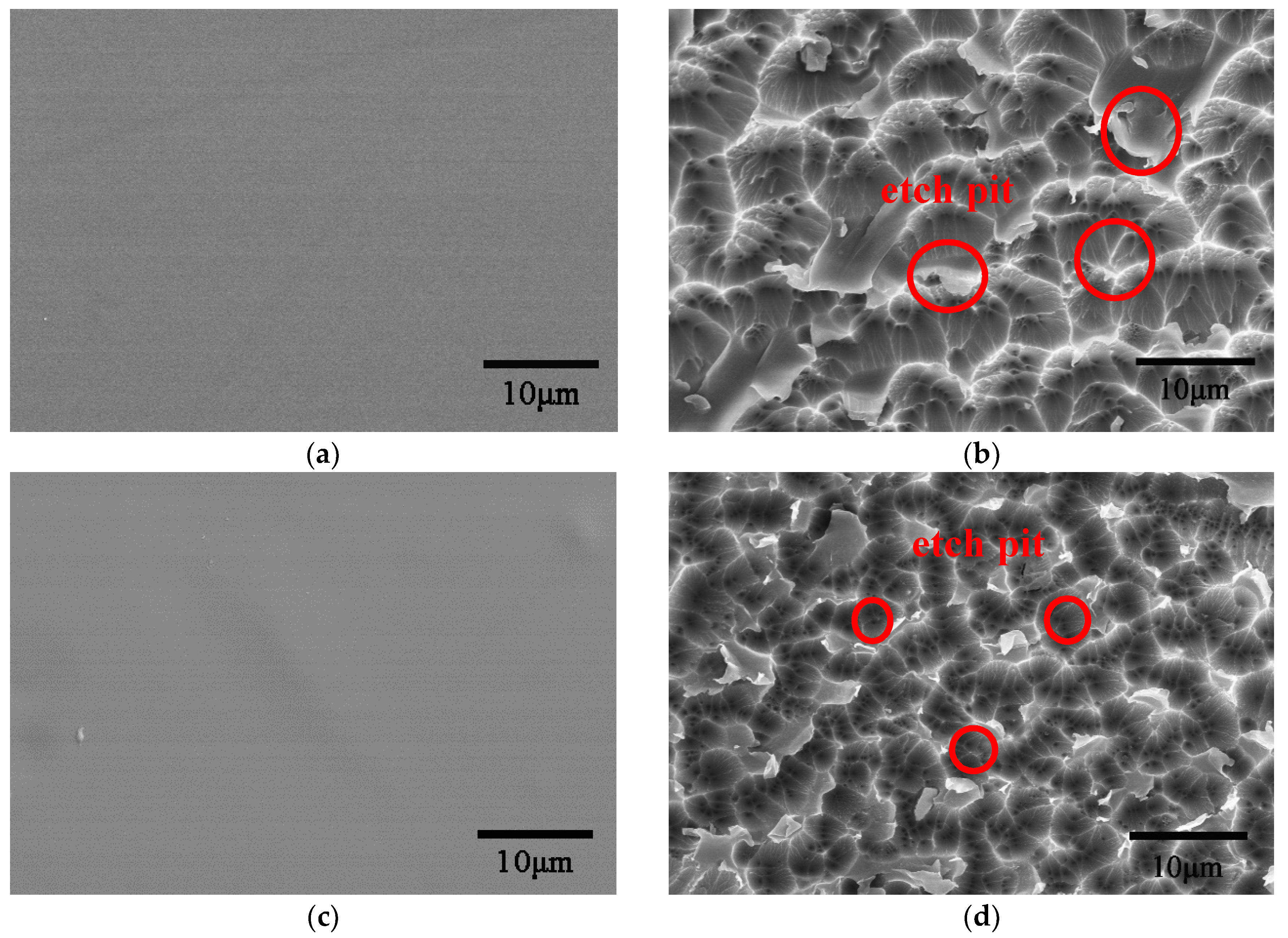
4.1. Morphology Analysis
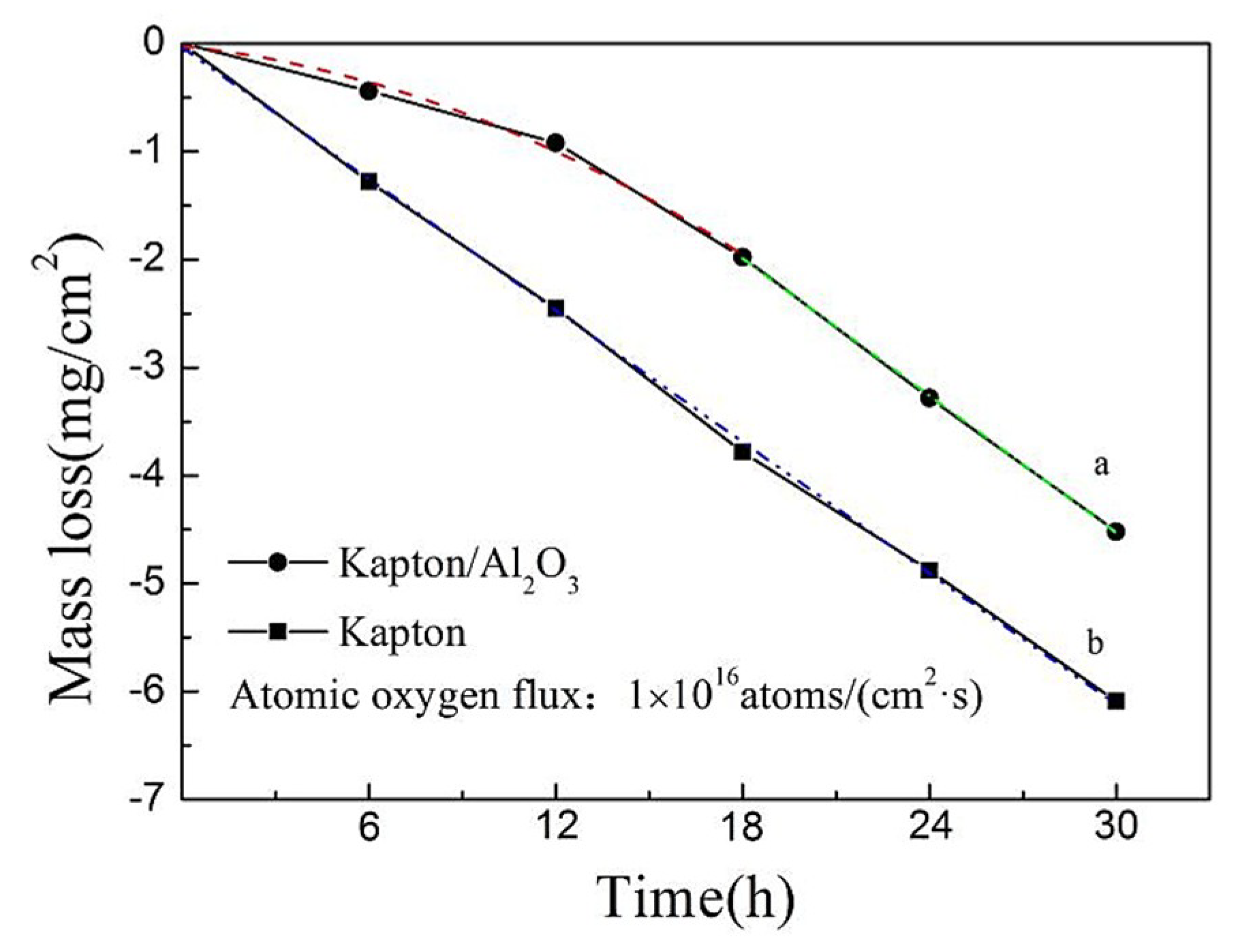
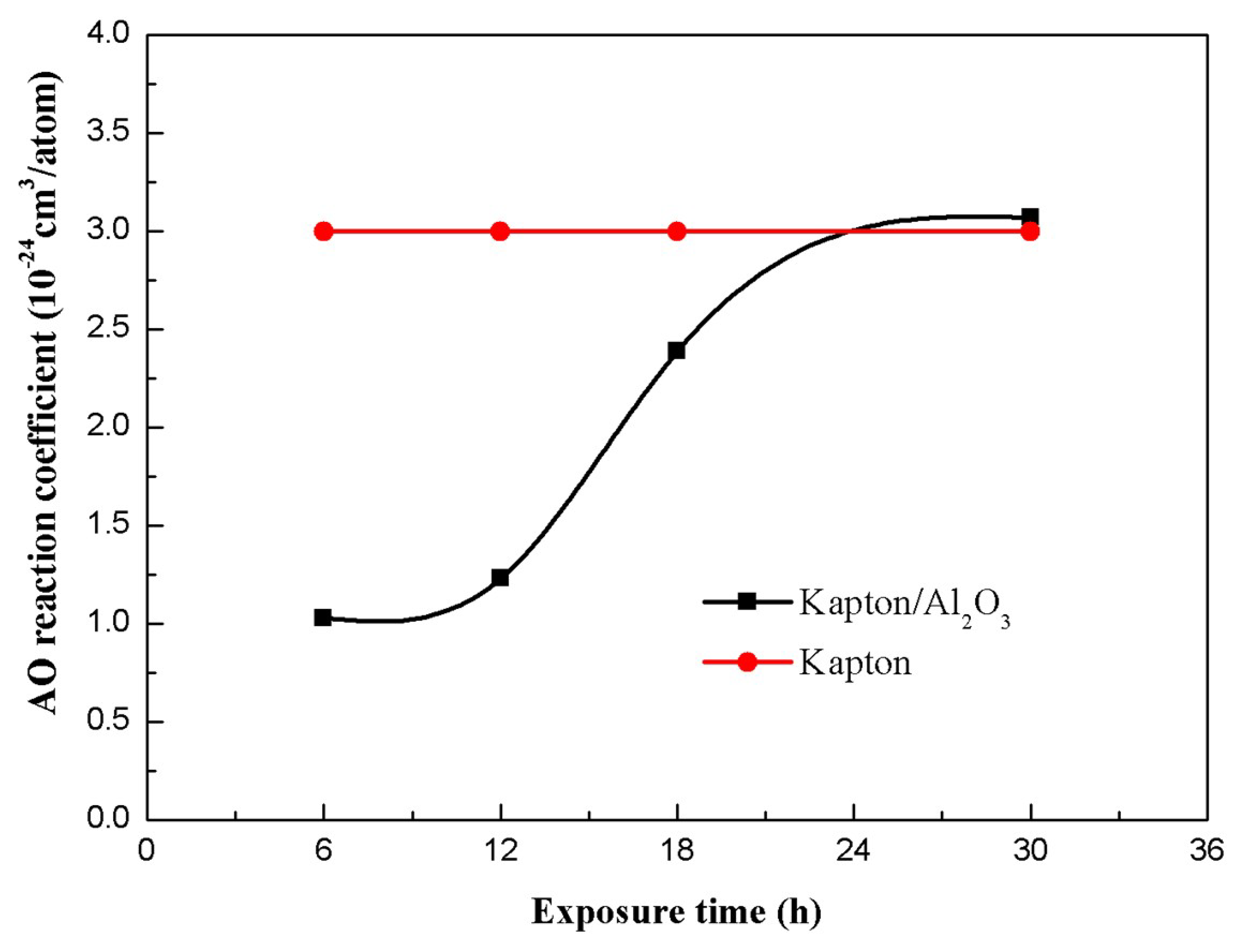
4.2. Atomic Oxygen Weight Loss Analysis
4.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
4.3.1. Kapton Film
4.3.2. Kapton/Al2O3 Composite Film
5. Conclusions
- Compared with the Kapton film, the Kapton/Al2O3 surface nanocomposite film prepared via the ion exchange method had no effect on the internal structure of the Kapton film matrix. However, the optical transmittance decreased by ~10% in the wavelength range of 500–800 nm for the enhancement of diffuse reflection caused by Al2O3 particles on the surface. The tensile strength and elongation were much higher than for the pure Kapton film and demonstrated its good flexibility, which is due to hindering initiation and propagation of surface cracks by Al2O3 particles in the subsurface layer for the existence of phase interface.
- Under the action of atomic oxygen erosion, the surface of the Kapton film and the Kapton/Al2O3 composite film showed a carpet-like morphology, and the surface of Kapton/ Al2O3 composite film had relatively small corrosion pits. In contrast to C–C bond rupture in ODA benzene of the Kapton film, the C=C bond in the PMDA benzene ring was mainly destroyed in the Kapton/Al2O3 nanocomposite film; finally, the carbon can be oxidized to form volatile small molecular substances, such as CO2 or CO.
- At short exposure times to the atomic oxygen environment, the Al2O3 layer on the surface of Kapton can inhibit the erosion and diffusion of atomic oxygen. The corrosion weight loss rate and the atomic oxygen reaction were lower. The Kapton/Al2O3 surface nanocomposite film demonstrated an improved resistance to atomic oxygen erosion. With increasing exposure time to atomic oxygen, the Al2O3 layer prepared by ion exchange became less dense. The atomic oxygen diffused into the Kapton matrix via the pores of the Al2O3 layer, causing detachment from the substrate. This results in a loss of the protective surface Al2O3 layer and exposure of the Kapton matrix, and the atomic oxygen reaction coefficient is consistent with the Kapton matrix.
Author Contributions
Funding
Conflicts of Interest
References
- Rose, M.F. Electrical insulation and dielectrics in the space environment. IEEE Trans. Electr. Insul. 1987, 5, 555–571. [Google Scholar] [CrossRef]
- Nishikawa, T.; Sonoda, K.; Nakanishi, K. Effect of atomic oxygen on polymers used as surface materials for spacecrafts, electrical insulating materials. In Proceedings of the Twenty-First Symposium on Electrical Insulating Materials, Nerima, Japan, 26 September 1988; pp. 191–194. [Google Scholar]
- Whiteside, J.B.; Kamykowski, E.; Rooney, W.D.; Schulte, R.; Stauber, M. Effects of 69 months in low earth orbit on Kapton antenna structures. J. Spacecr. Rockets 1994, 31, 860–865. [Google Scholar]
- Golub, M.A.; Wydeven, T. Reactions of atomic oxygen (O(3P)) with various polymer films. Polym. Degrad. Stabil. 1988, 22, 325–338. [Google Scholar] [CrossRef]
- Ueda, M.; Tan, I.H.; Dallaqua, R.S.; Rossi, J.O.; Barroso, J.J.; Tabacniks, M.H. Aluminum plasma immersion ion implantation in polymers. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 206, 760–766. [Google Scholar] [CrossRef]
- Iskanderova, Z.A.; Kleiman, J.I.; Gudimenko, Y.; Tkachenko, A.; Tennyson, R.C.; Brown, I.G.; Monteiro, O.R. Metal ion implantation and dynamic ion mixing for the protection of high-performance polymers from severe oxidative environment. Nucl. Instrum. Methods Phys. Res. Sect. B. 1999, 148, 1090–1096. [Google Scholar] [CrossRef]
- Banks, B.A.; Mirtich, M.J.; Rutledge, S.K.; Swec, D.M.; Nahra, H.K. Ion beam sputter-deposited thin film coatings for protection of spacecraft polymers in low earth orbit, NASA TM-87051. In Proceedings of the 23rd Aerospace Science Meeting, Reno, NV, USA, 14–17 January 1985; pp. 14–17. [Google Scholar]
- Cooper, R.; Upadhyaya, H.P.; Minton, T.K.; Berman, M.R.; Du, X.H.; George, S.M. Protection of polymer from atomic-oxygen erosion using Al2O3 atomic layer deposition coatings. Thin Solid Films 2008, 516, 4036–4039. [Google Scholar] [CrossRef]
- Reddy, M.R.; Srinivasamurthy, N.; Agrawal, B.L. Atomic oxygen protective coatings for Kapton film: A review. Surf. Coat. Technol. 1993, 58, 1–17. [Google Scholar] [CrossRef]
- Reddy, M.R. Effect of low earth orbit atomic oxygen on spacecraft materials. J. Mater. Sci. 1995, 30, 281–307. [Google Scholar] [CrossRef]
- Mutikainen, R. Multiple layer coating scheme to protect polymer films from atomic oxygen erosion. Thin Solid Films 1994, 238, 248–257. [Google Scholar] [CrossRef]
- Tong, J.Y.; Liu, X.P.; Zhang, C.; Xiang, S.H.; Duo, S.W.; Li, M.S. The space atomic oxygen environment erosion on spacecraft surfaces and its protection techniques. Spacecr. Environ. Eng. 2009, 26, 1–4. [Google Scholar]
- Dworak, D.P.; Banks, B.A.; Karniotis, C.A.; Soucek, M.D. Evaluation of protective Silicone/Siloxane coatings in simulated low-earth-orbit environment. J. Spacecr. Rockets 2006, 43, 393–401. [Google Scholar] [CrossRef]
- Gilman, J.W.; Schlitzer, D.S.; Lichtenhan, J.D. Low earth orbit resistant siloxane copolymers. J. Appl. Polym. Sci. 1996, 60, 591–596. [Google Scholar] [CrossRef]
- Hobson, S.T.; Shea, K.J. Bridged bisimide polysilsesquioxane xerogels: New hybrid organic-inorganic materials. Chem. Mater. 1997, 9, 616–623. [Google Scholar] [CrossRef]
- Duo, S.W.; Li, M.S.; Zhu, M.; Zhou, Y.C. Resistance of polyimide/silica hybrid films to atomic oxygen attack. Surf. Coat. Tech. 2006, 200, 6671–6677. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, C.W.; Qu, Q.; Li, M.S.; Cao, C.N.; Sun, G.; Tong, J.Y. Atomic oxygen irradiation induced degradation of polyimide without and with siloxane coating. Corros. Sci. Prot. Technol. 2002, 14, 78–81. [Google Scholar]
- Groh, K.K.D.; Banks, B.A. Atomic-oxygen undercutting of long duration exposure facility atomized-Kapton multilayer insulation. J. Spacecr. Rockets 1994, 31, 656–664. [Google Scholar]
- Zhang, X.; Wu, Y.Y.; Liu, G.; He, S.Y.; Yang, D.Z. Investigation on sol-gel boehmite-AlOOH films on Kapton and their erosion resistance to atomic oxygen. Thin Solid Films 2008, 516, 5020–5026. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, E.J.; Kwon, S.H. Influence of surface treatment of polyimide film on adhesion enhancement between polyimide and metal films. Bull. Korean Chem. Soc. 2007, 28, 188–192. [Google Scholar]
- Akamatsu, K.; Shinkai, H.; Ikeda, S.; Adachi, S.; Nawafune, H.; Tomita, S. Controlling interparticle spacing among metal nanoparticles through metal-catalyzed decomposition of surrounding polymer matrix. J. Am. Chem. Soc. 2005, 127, 7980–7981. [Google Scholar] [CrossRef]
- Mu, S.X.; Wu, Z.P.; Wang, Y.; Qi, S.L.; Yang, X.P.; Wu, D.Z. Formation and characterization of cobalt oxide layers on polyimide films via surface modification and ion-exchange technique. Thin Solid Films 2010, 518, 4175–4182. [Google Scholar] [CrossRef]
- Akamatsu, K.; Ikeda, S.; Nawafune, H. Site-Selective direct silver metallization on surface-modified polyimide Layers. Langmuir 2003, 19, 10366–10371. [Google Scholar] [CrossRef]
- Wu, Z.P.; Wu, D.Z.; Qi, S.L.; Zhang, T.; Jin, R.G. Preparation of surface conductive and highly reflective silvered polyimide films by surface modification and in situ self-metallization technique. Thin Solid Films 2005, 493, 179–184. [Google Scholar] [CrossRef]
- Mu, S.X.; Wu, D.Z.; Wang, Y.; Wu, Z.P.; Yang, X.P.; Yang, W.T. Fabrication of nickel oxide nanocomposite layer on a flexible polyimide substrate via ion exchange technique. ACS Appl. Mater. Inter. 2010, 2, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Akamatsu, K.; Nawafune, H.; Nishino, T.; Deki, S. Formation and growth of copper nanoparticles from ion-doped precursor polyimide layers. J. Phys. Chem. B. 2004, 108, 15599–15607. [Google Scholar] [CrossRef]
- Ikeda, S.; Yanagimoto, H.; Akamatsu, K.; Nawafune, H. Copper/polyimide heterojunctions: Controlling interfacial structures through an additive-based, all-wet chemical process using ion-doped precursors. Adv. Funct. Mater. 2007, 17, 889–897. [Google Scholar] [CrossRef]
- Mu, S.X.; Wu, D.Z.; Qi, S.L.; Wu, Z.P. Preparation of polyimide/zinc oxide nanocomposite films via an ion-exchange technique and their photoluminescence properties. J. Nanomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Wu, Z.P.; Wu, D.Z.; Yang, W.T.; Jin, R.G. Preparation of highly reflective and conductive metallized polyimide films through surface modification: Processing, morphology and properties. J. Mater. Chem. 2006, 16, 310–316. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Byon, C. Hydrothermally synthesized ternary heterostructured MoS2/Al2O3/g-C3N4 photocatalyst. Mater. Res. Bull. 2017, 96, 233–245. [Google Scholar] [CrossRef]
- National Standards of the People’s Republic of China, GB/T 1040.3-2006 Plastics—Determination of Tensile properties—Part 3: Test Conditions for Films and Sheet; China Standards Press: Beijing, China, 2007; pp. 1–4.
- Man, Y.R.; Li, Z.H.; Shu, M.; Liu, K.; Liu, H.T.; Gao, Y. Surface treatment of 25-μm Kapton film by ammonia for improvement of TiO2/SiO2 coating’s adhesion. Surf. Interface Anal. 2017, 49, 843–849. [Google Scholar] [CrossRef]
- Quinten, M. The color of finely dispersed nanoparticles. Appl. Phys. B. 2001, 73, 317–326. [Google Scholar] [CrossRef]
- Hulteen, J.C.; Patrissi, C.J.; Miner, D.L.; Crosthwait, E.R.; Oberhauser, E.B.; Martin, C.R. Changes in the shape and optical properties of gold nanoparticles contained within alumina membranes due to low-temperature annealing. J. Phys. Chem. B 1997, 101, 7727–7731. [Google Scholar] [CrossRef]
- Ishida, H.; Wellinghoff, S.T.; Baer, E.; Koenig, J.L. Spectroscopic studies of poly [N, N′-bis (phenoxy phenyl) pomalidomide]. 1. structures of the polyimide and three model compounds. Macromolecules 1980, 13, 826–834. [Google Scholar] [CrossRef]
- Jin, Y.; Zeng, G.B.; Zhu, D.Y.; Huang, Y.; Su, Z.H. Analysis of structure and imidization of poly (amic acid) using FTIR spectroscopy. Chin. J. Appl. Chem. 2011, 28, 258–262. [Google Scholar]
- Murphy, D.; Depinho, M.N. An ATR-FTIR study of water in cellulose acetate membranes prepared by phase inversion. J. Membrane Sci. 1995, 106, 245–257. [Google Scholar] [CrossRef]
- Banks, B.A.; Miller, S.K.; Groh, K.K.D. Low earth orbital atomic oxygen interactions with materials. In Proceedings of the 2nd International Energy Conversion Engineering Conference, Providence, RI, USA, 16–19 August 2004; pp. 2004–5638. [Google Scholar]
- Liang, W. Oxidation kinetics of siliconized TiAl-based alloy. Trans. Mater. Heat Treat. 2005, 26, 47–50. [Google Scholar]
- Guo, W.M.; Xiao, H.N.; Tian, R.Y.A. Kinetic model and mechanisms of oxidation of 2D 2C/C composite. Acta Mater. Compos. Sin. 2007, 24, 45–52. [Google Scholar]
- Du, H.; Ye, Z.H. LEO Spacecraft Space Environment Manua, 1st ed.; National Defense Industry Press: Beijing, China, 1996. [Google Scholar]
- Grossman, E.; Lifshitz, Y.; Wolan, J.T.; Mount, C.K.; Hoflund, G.B. In situ erosion study of Kapton using novel hyperthermal oxygen atom source. J. Spacecr. Rockets 1999, 36, 75–78. [Google Scholar] [CrossRef]
- Duo, S.W.; Li, M.S.; Zhang, Y.M.; Han, E.H.; Tong, J.Y.; Sun, G. Ground-based investigations of atomic oxygen erosion behaviors of Al2O3 coating on Kapton. J. Astronaut. 2002, 23, 68–72. [Google Scholar]
- Silverman, B.D.; Sanda, P.N.; Ho, P.S.; Rossi, A.R. Origin of the carbon 1s-core level shifts in polyimide model compounds. J. Polym. Sci. Pol. Chem. 1985, 23, 2857–2863. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons: Chichester, UK; New York, NY, USA, 1998; pp. 301–302. [Google Scholar]
- Yan, Y.L.; Helfand, M.A.; Clayton, C.R. Evaluation of the effect of surface roughness on thin film thickness measurements using variable angle XPS. Appl. Surf. Sci. 1989, 37, 395–405. [Google Scholar] [CrossRef]
- Kameshima, Y.; Yasumori, A.; Okada, K. XPS and X-ray AES (XAES) study of different aluminate compounds. Surf. Sci. 2000, 21, 481–487. [Google Scholar]
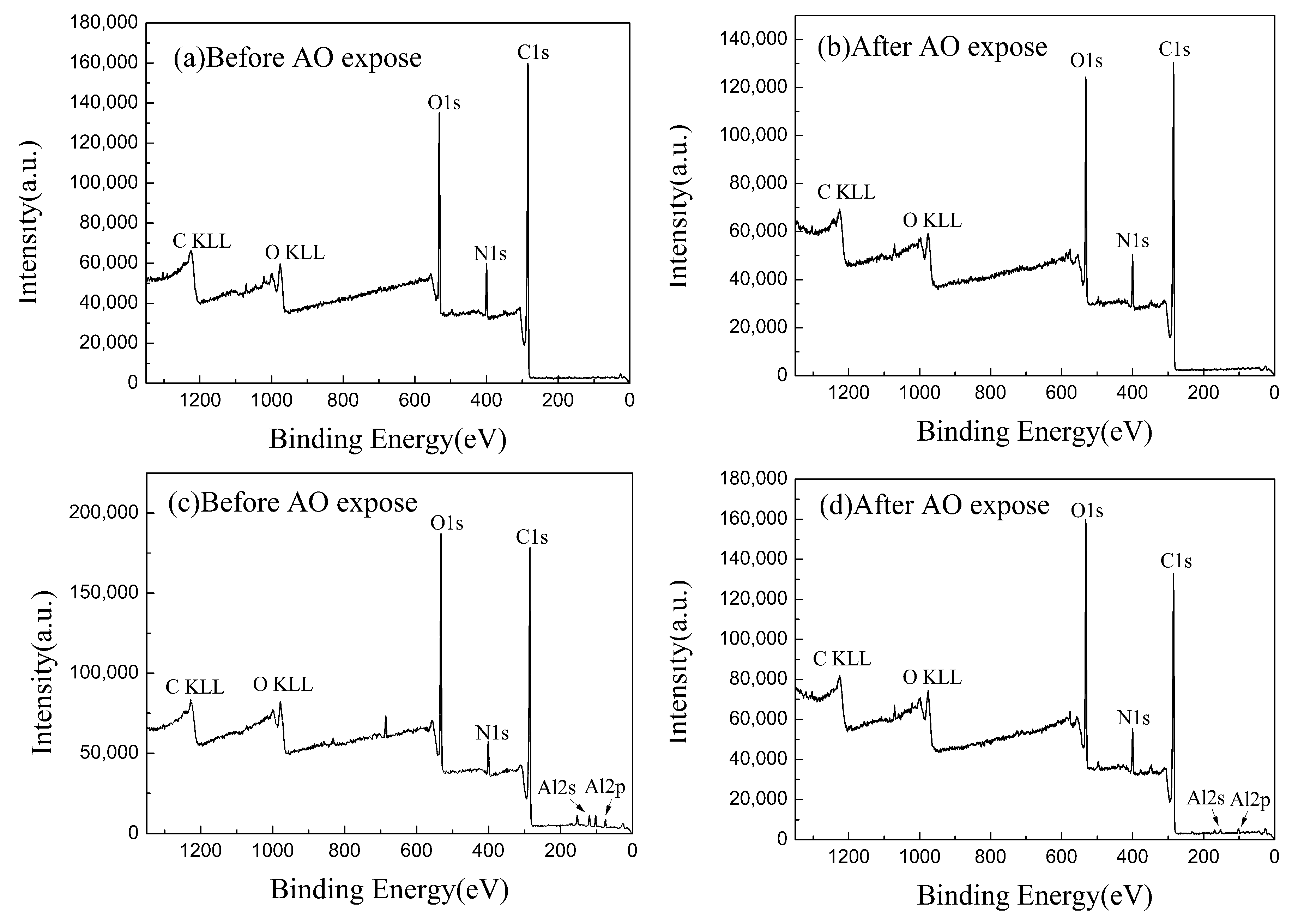

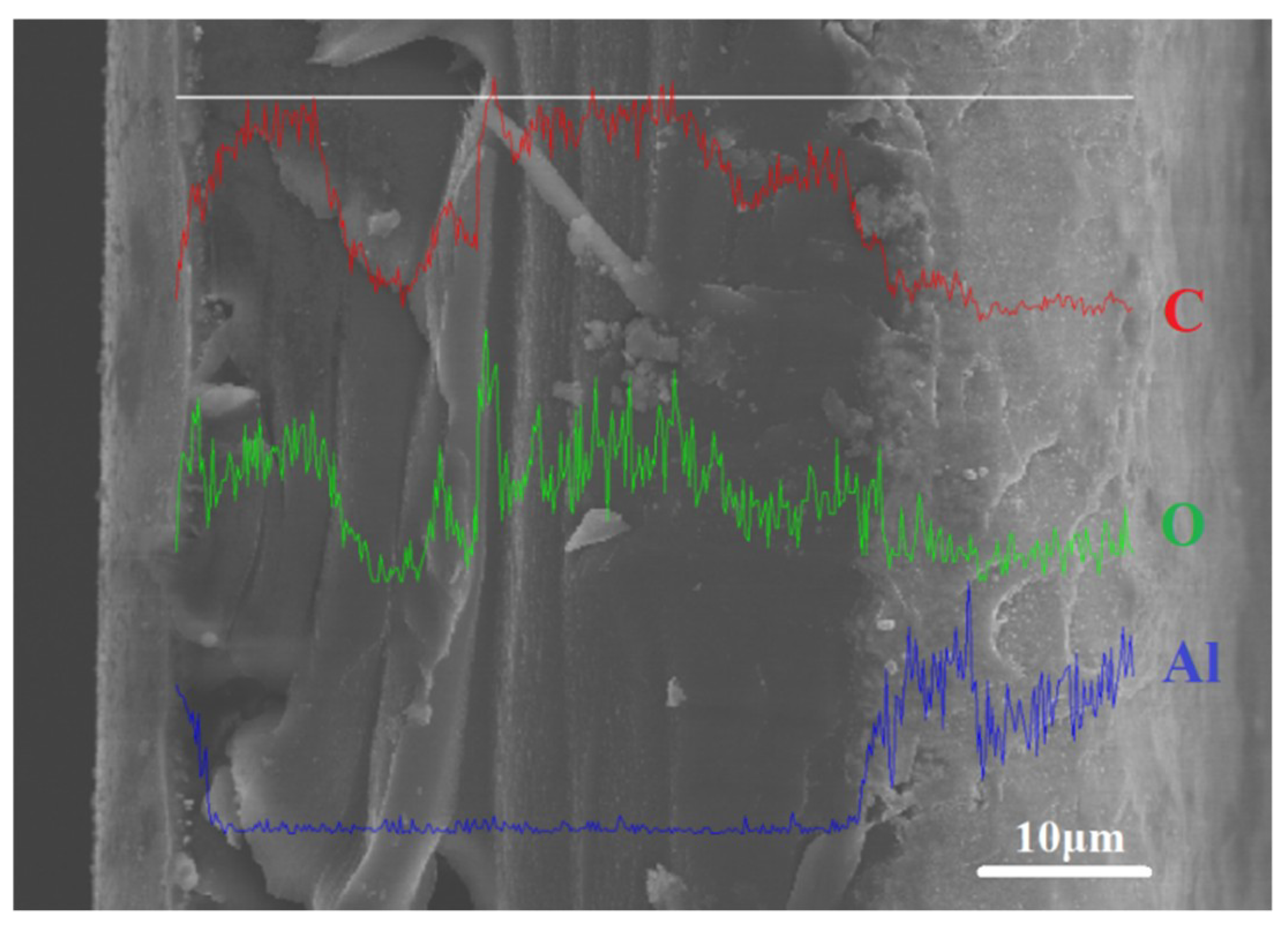
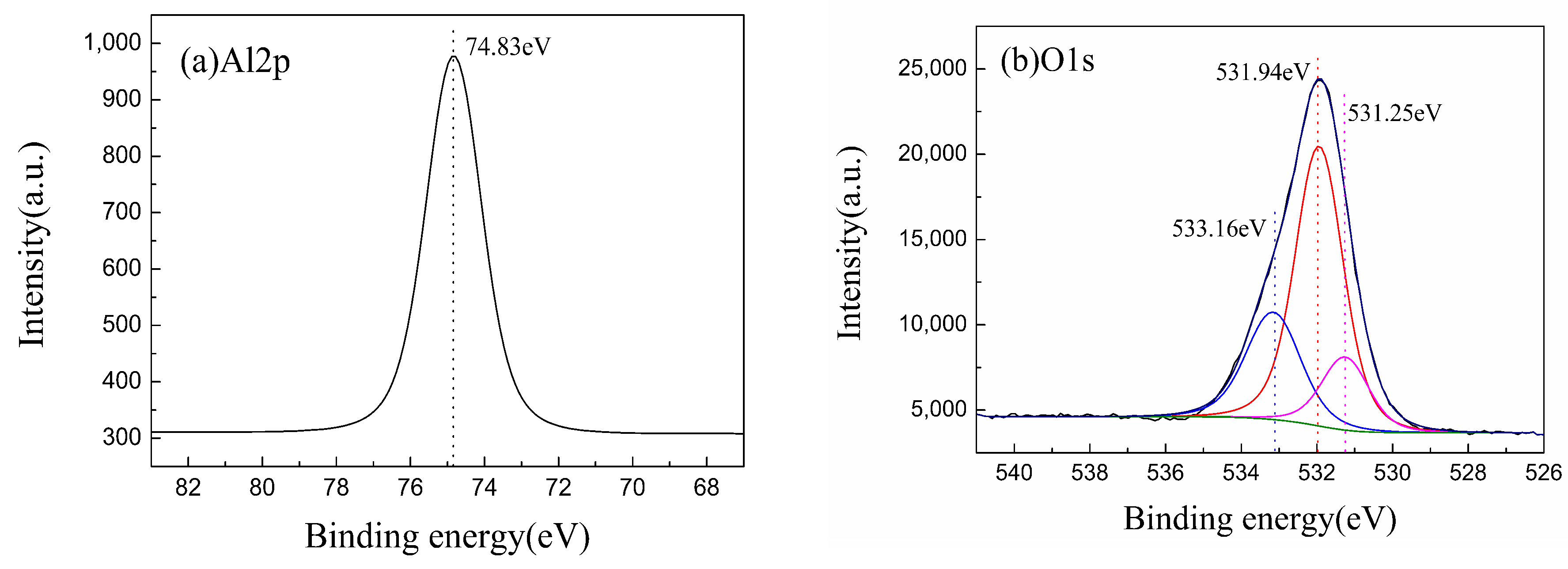
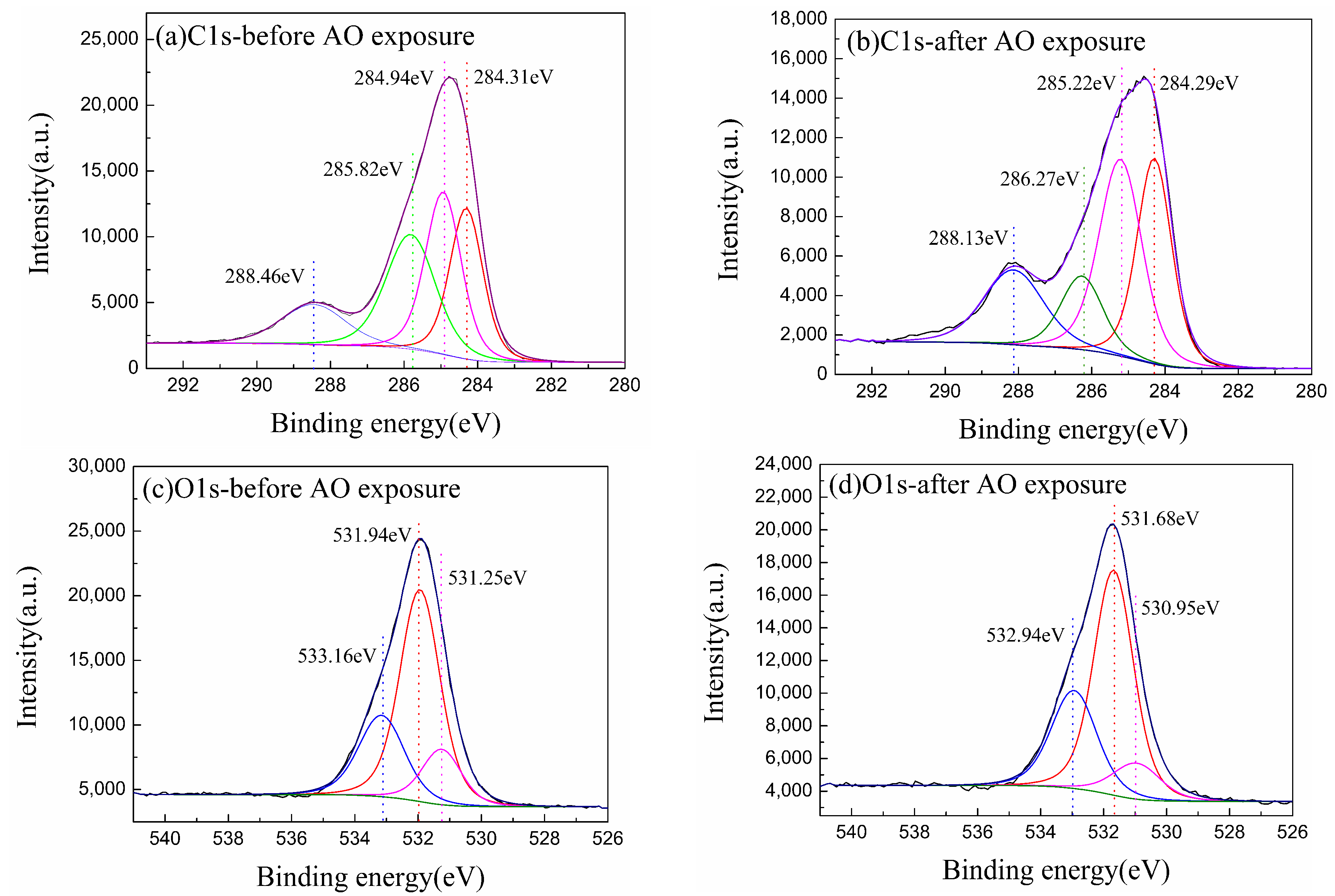
| Materials | Condition | C1s | N1s | O1s | Al2p |
|---|---|---|---|---|---|
| Kapton | Before exposure | 74.85 | 6.96 | 18.19 | – |
| After exposure | 71.98 | 7.2 | 20.83 | – | |
| Kapton/Al2O3 | Before exposure | 65.6 | 6.96 | 23.3 | 4.13 |
| After exposure | 61.63 | 10.87 | 26.45 | 1.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Wang, D.; Liu, G.; Wei, Q. Atomic Oxygen Adaptability of Flexible Kapton/Al2O3 Composite Thin Films Prepared by Ion Exchange Method. Coatings 2019, 9, 624. https://doi.org/10.3390/coatings9100624
Jiang D, Wang D, Liu G, Wei Q. Atomic Oxygen Adaptability of Flexible Kapton/Al2O3 Composite Thin Films Prepared by Ion Exchange Method. Coatings. 2019; 9(10):624. https://doi.org/10.3390/coatings9100624
Chicago/Turabian StyleJiang, Donghua, Dan Wang, Gang Liu, and Qiang Wei. 2019. "Atomic Oxygen Adaptability of Flexible Kapton/Al2O3 Composite Thin Films Prepared by Ion Exchange Method" Coatings 9, no. 10: 624. https://doi.org/10.3390/coatings9100624
APA StyleJiang, D., Wang, D., Liu, G., & Wei, Q. (2019). Atomic Oxygen Adaptability of Flexible Kapton/Al2O3 Composite Thin Films Prepared by Ion Exchange Method. Coatings, 9(10), 624. https://doi.org/10.3390/coatings9100624






