Preparation of Fluoroalkyl-Acrylate-Modified Polysiloxane Nanocomposite and Its Surface Properties as a Superhydrophobic Coating Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
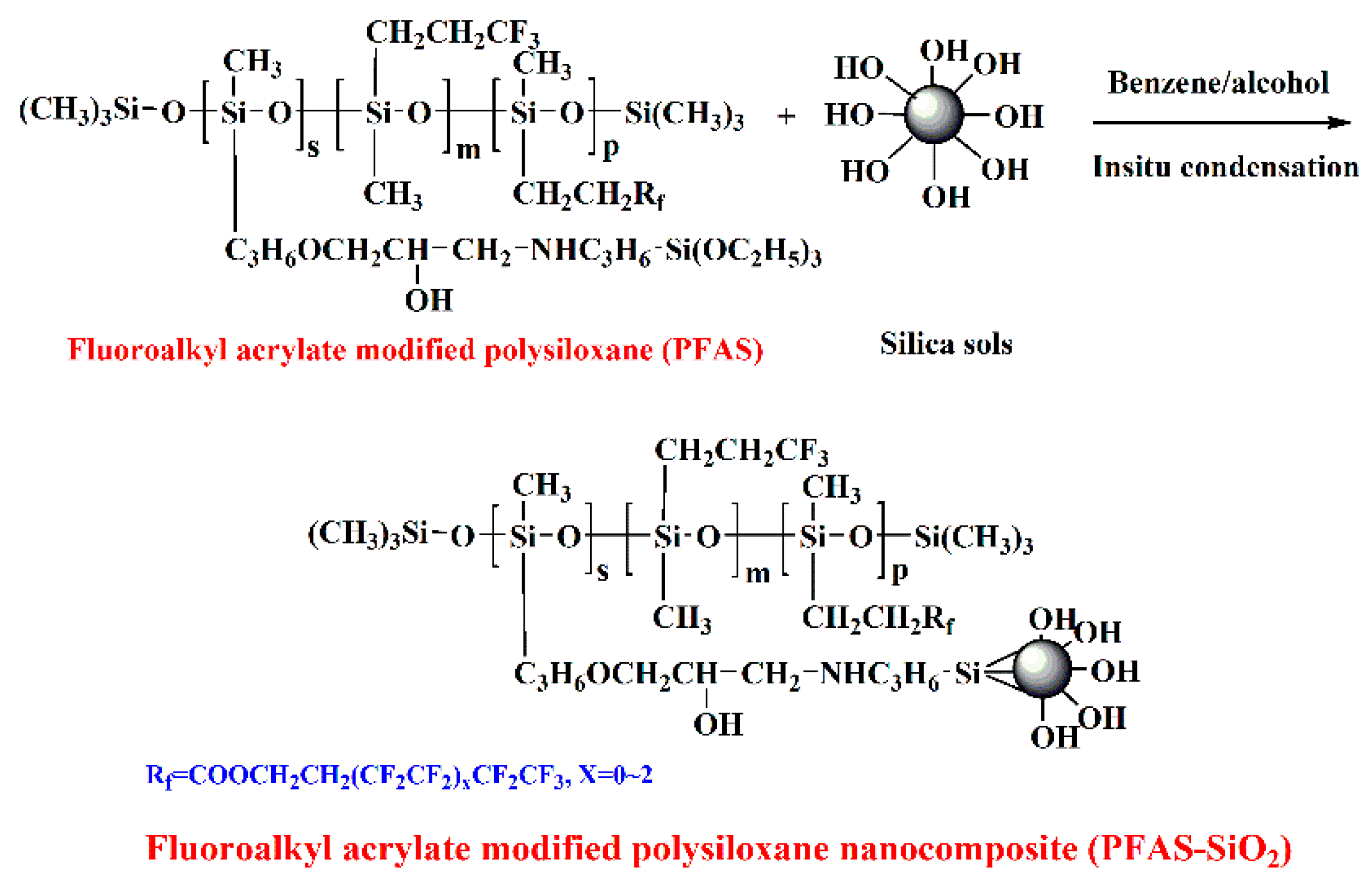
2.2. Synthesis of Fluoroalkyl-Acrylate-Modified Polysiloxane Nanocomposites
2.3. Finishing Process of Cotton Fabric
2.4. Characterization
2.4.1. Structural Characterization
2.4.2. Thermal Stability
2.4.3. SEM Observation
2.4.4. Fine Film Morphology of PFAS-SiO2-3
2.4.5. Measurement of the Performance Properties of the Fabrics
3. Results and Discussion
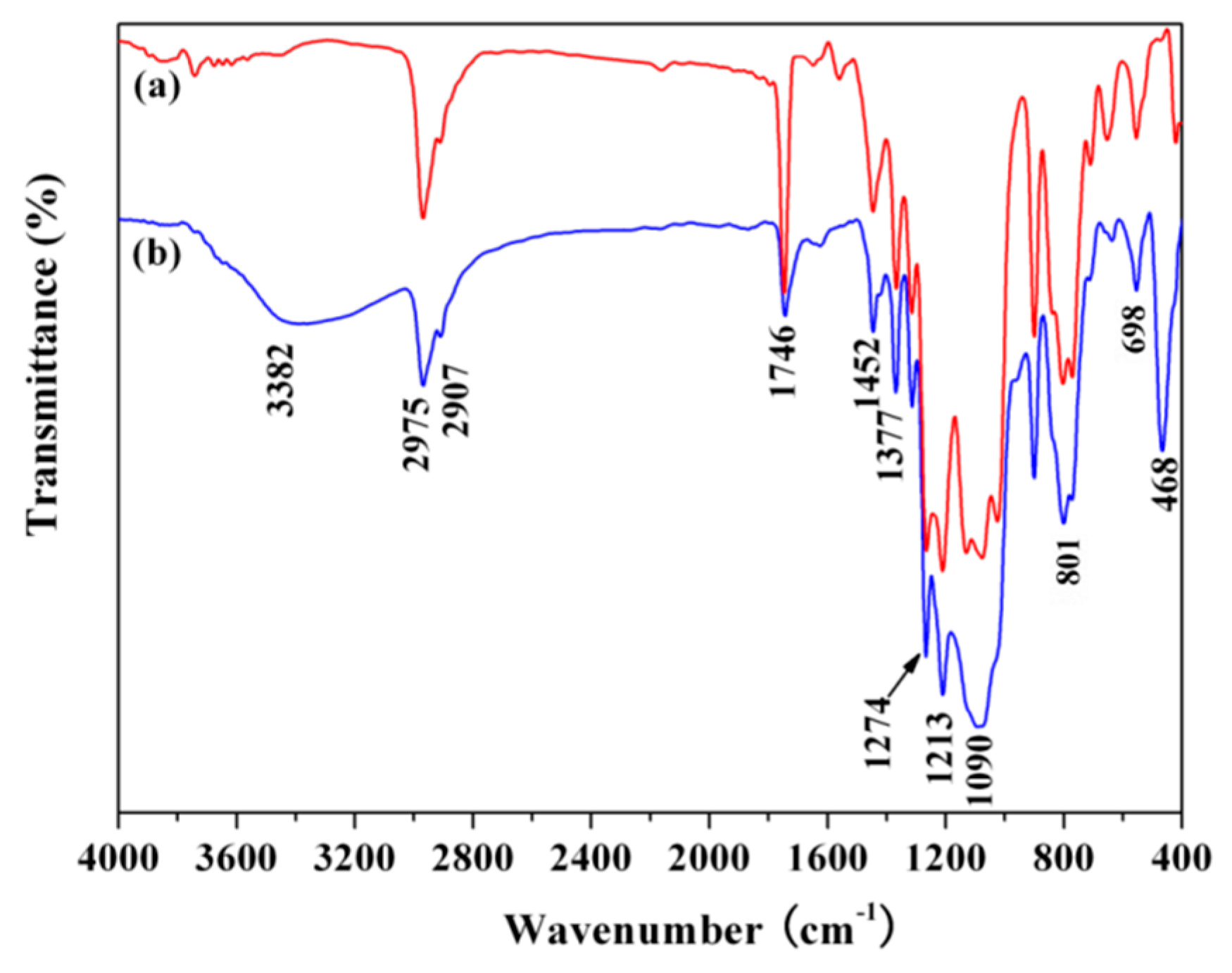
3.1. Infrared Spectrum Analysis
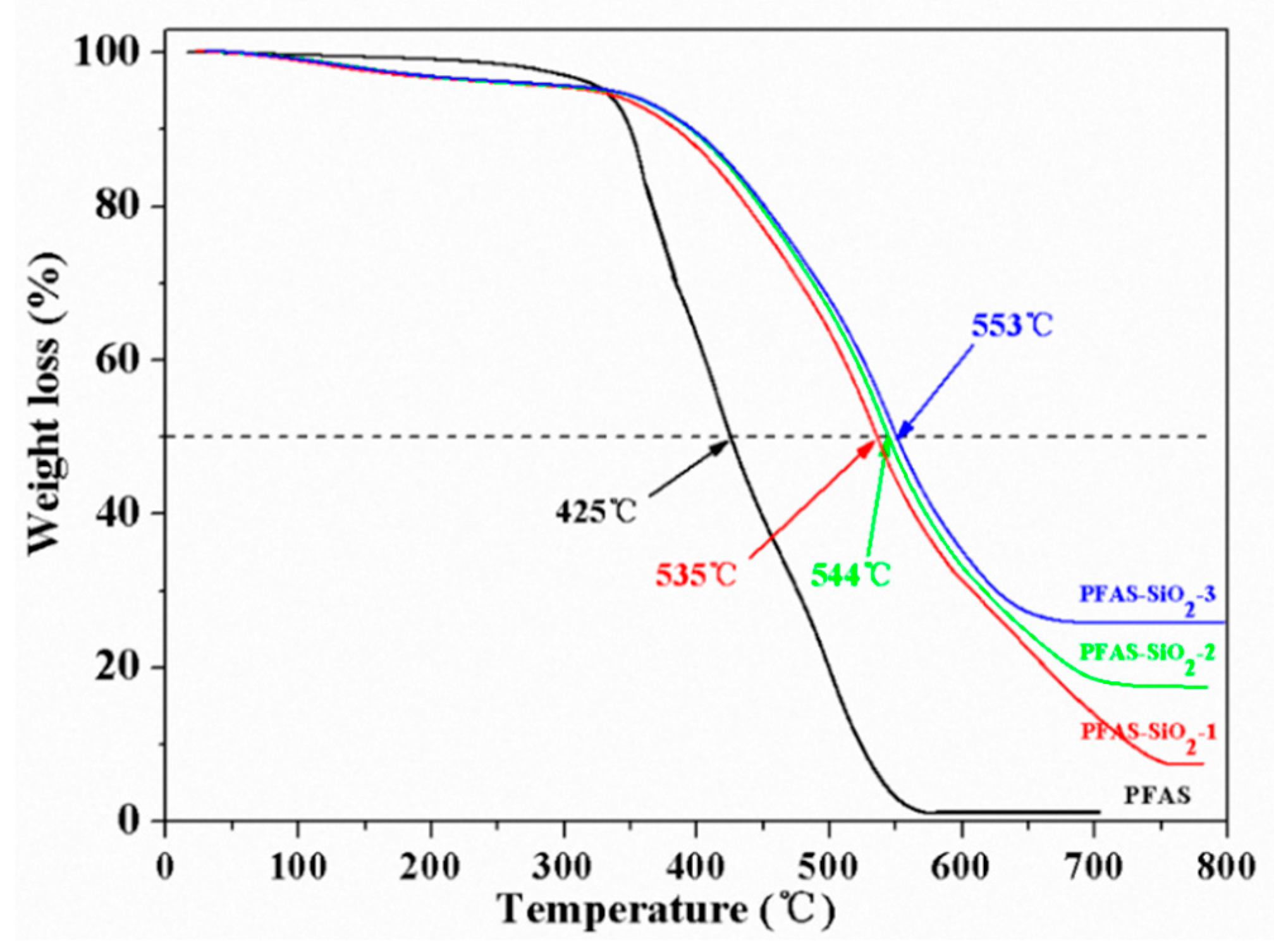
3.2. Thermal Properties
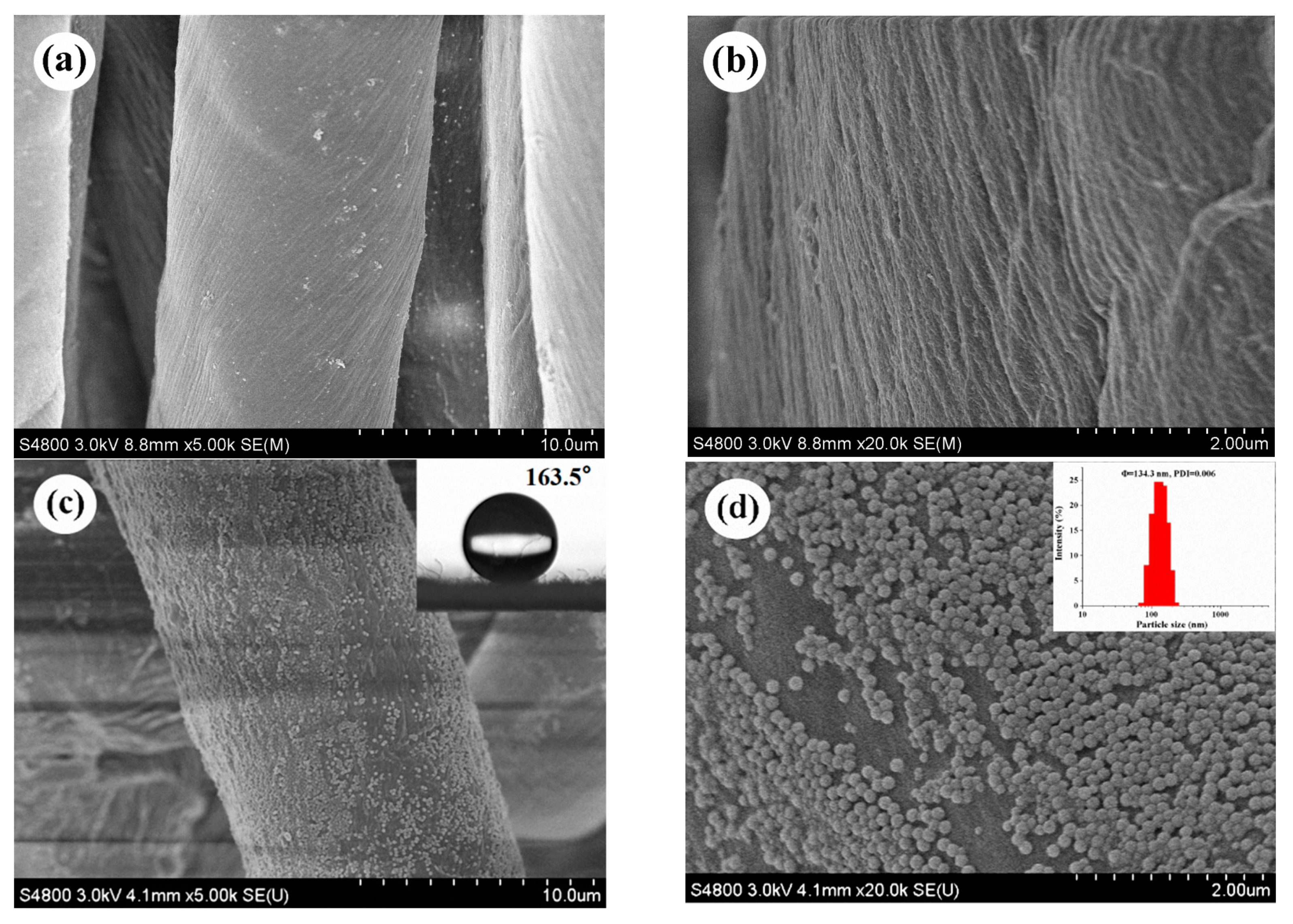
3.3. SEM Observation
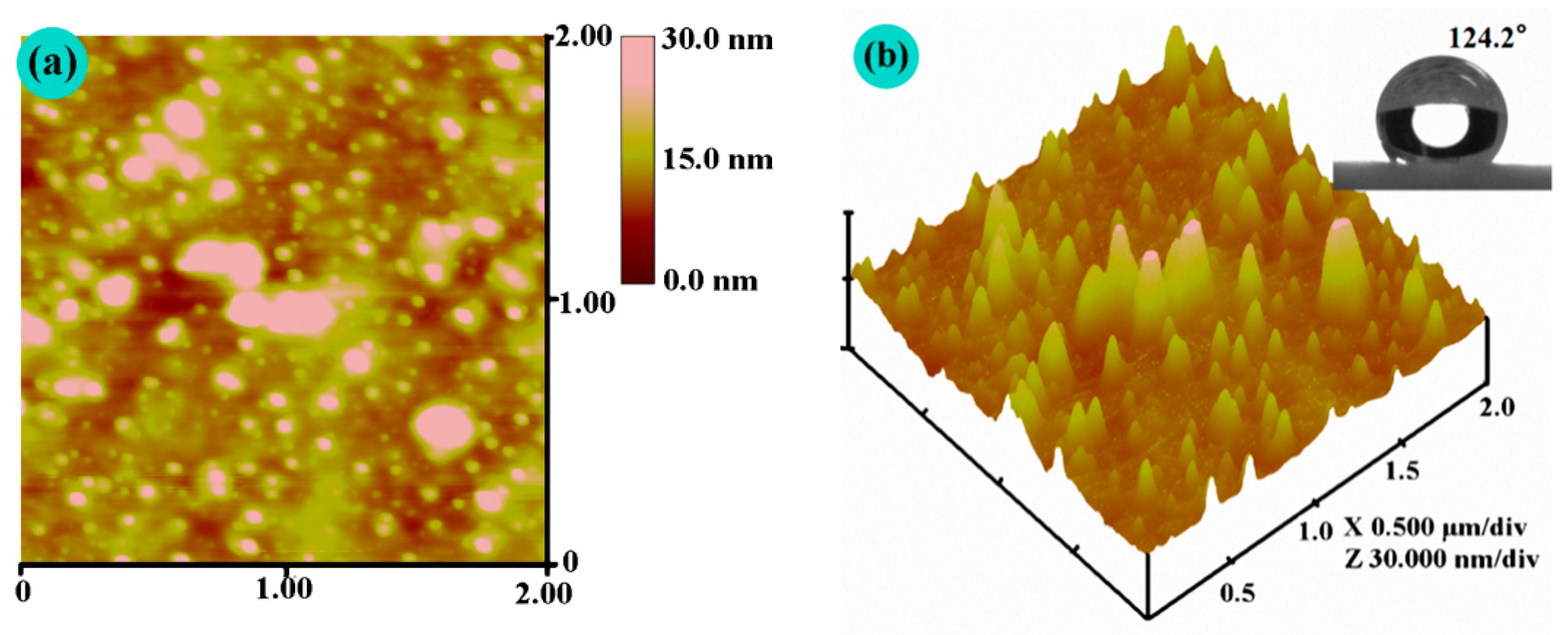
3.4. AFM Observation
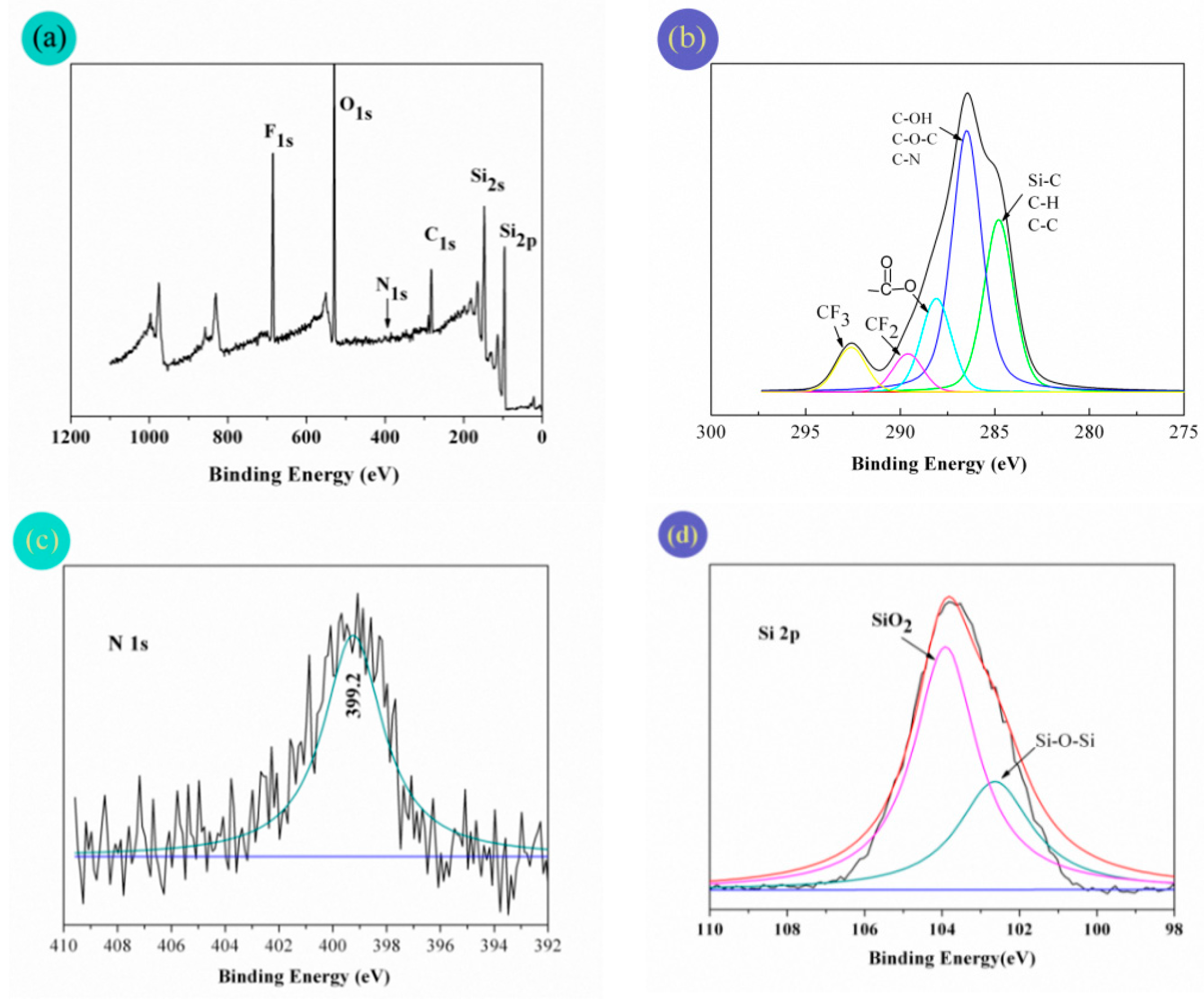
3.5. Surface Analysis
3.6. Performance Properties of the Finished Fabrics
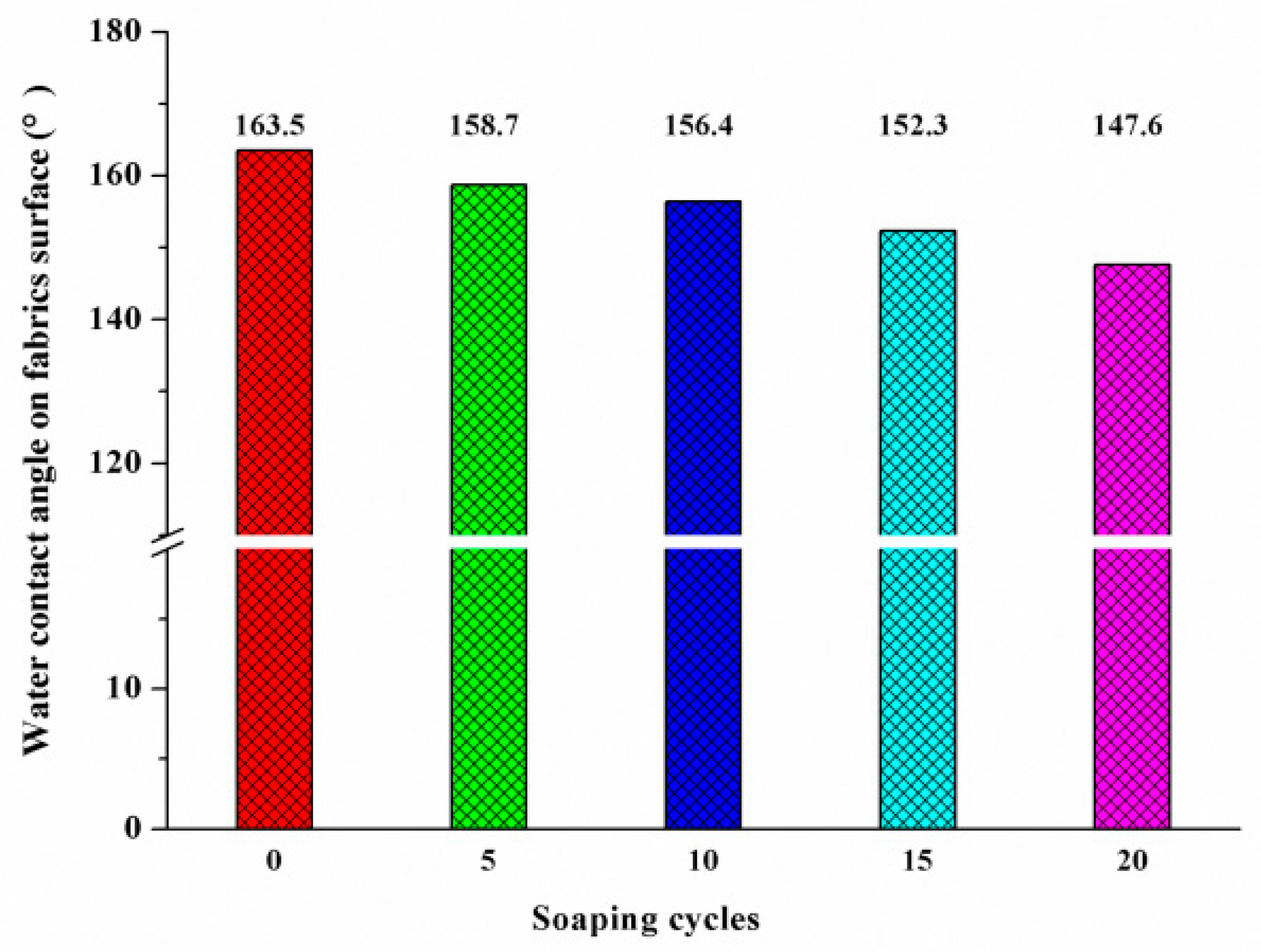
3.7. Washing Durability of the Treated Fabric
3.8. Theoretical Model of the Superhydrophobic System
4. Conclusions
- (1)
- The synthesized product had the desired structure and good thermal stability.
- (2)
- The finished fabric had a static contact angle of 163.5° and a lag angle of 7°. The whiteness and softness were basically the same as those before the finish was applied.
- (3)
- After the superhydrophobic fabric was washed for 15 cycles, the static contact angle on the fabric surface was still 152.3°, showing good water resistance.
Author Contributions
Funding
Conflicts of Interest
References
- An, Q.F.; Li, L.S.; Huang, L.X.; Chen, K.C. Film morphology and characterization of functional polysiloxane softeners. AATCC Rev. 2006, 6, 39–43. [Google Scholar]
- Bao, L.H.; Lan, Y.J. Silicone softener for stain repellent stain release and wrinkle resistance fabric finishing. J. Eng. Fibers Fabr. 2018, 13, 1–5. [Google Scholar] [CrossRef]
- Kang, T.J.; Kim, M.S. Effects of silicone treatments dimensional properties of wool fabric. Text. Res. J. 2001, 71, 295–300. [Google Scholar] [CrossRef]
- Zhang, G.D.; Hu, Y.Q.; Wu, J.R.; Li, J.Y.; Lai, G.Q.; Zhong, M.Q. Improved synthesis and properties of hydroxyl-terminated liquid fluorosilicone. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Wang, B.; Qian, T.; Zhang, Q.H.; Zhan, X.L.; Chen, F.Q. Heat resistance and surface properties of polyester resin modified with fluorosilicone. Surf. Coat. Technol. 2016, 304, 31–39. [Google Scholar] [CrossRef]
- Yan, Z.L.; Liu, W.Q.; Gao, N.; Wang, H.L.; Su, K. Synthesis and properties of a novel UV-cured fluorinated siloxane graft copolymer for improved surface, dielectric and tribological properties of epoxy acrylate coating. Appl. Surf. Sci. 2013, 284, 683–691. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Zang, D.L.; Lu, Y.; Gao, Z.X.; Shi, J.Y.; Wang, C.Y. Preparation and characterization of cotton fabric with potential use in UV resistance and oil reclaim. Carbohyd. Polym. 2016, 137, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhai, S.R.; Xiao, Z.Y.; An, Q.D. One-step fabrication of highly stable, superhydrophobic composites from controllable and low-cost PMHS/TEOS sols for efficient oil cleanup. J. Colloid Interface Sci. 2015, 446, 155–162. [Google Scholar] [CrossRef]
- Xue, C.H.; Yin, W.; Jia, S.T.; Ma, J.Z. UV-durable superhydrophobic textiles with UV-shielding properties by coating fibers with ZnO/SiO2 core/shell particles. Nanotechnology 2011, 22, 1–8. [Google Scholar] [CrossRef]
- Gonçalves, G.; Marques, P.A.A.P.; Trindade, T.; Neto, C.P.; Gandini, A. Superhydrophobic cellulose nanocomposites. J. Colloid Interface Sci. 2008, 324, 42–46. [Google Scholar] [CrossRef]
- Daoud, W.A.; Xin, J.H.; Zhang, Y.H.; Mak, C.L. Pulsed laser deposition of superhydrophobic thin Teflon films on cellulosic fibers. Thin Solid Films 2006, 515, 835–837. [Google Scholar] [CrossRef]
- Zimmermann, J.; Reifler, F.A.; Fortunato, G.; Gerhardt, L.C.; Seeger, S. A simple, one-step approach to durable and robust superhydrophobic textiles. Adv. Funct. Mater. 2008, 18, 3662–3669. [Google Scholar] [CrossRef]
- Hoefnagels, H.F.; Wu, D.; With, G.D.; Ming, W. Biomimetic superhydrophobic and highly oleophobic cotton textiles. Langmuir 2007, 23, 13158–13163. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.X.; Niu, H.T.; Gestos, A.; Wang, X.G.; Lin, T. Fluoroalkyl silane modified silicone rubber/ nanoparticle composite: A super durable, robust superhydrophobic fabric coating. Adv. Mater. 2012, 24, 2409–2412. [Google Scholar] [CrossRef]
- Li, K.Q.; Zeng, X.R.; Li, H.Q.; Lai, X.J. Fabrication and characterization of stable superhydrophobic fluorinated-polyacrylate/silica hybrid coating. Appl. Surf. Sci. 2014, 298, 214–220. [Google Scholar] [CrossRef]
- Li, B.C.; Zhang, J.P. Polysiloxane/multiwalled carbon nanotubes nanocomposites and their applications as ultrastable, healable and superhydrophobic coatings. Carbon 2015, 93, 648–658. [Google Scholar] [CrossRef]
- Hao, L.F.; An, Q.F.; Xu, W. Facile Fabrication of superhydrophobic cotton fabric from stearyl methacrylate modified polysiloxane/silica nanocomposite. Fiber Polym. 2012, 13, 1145–1153. [Google Scholar] [CrossRef]
- Hao, L.F.; Gao, T.T.; Xu, W.; Wang, X.C.; Yang, S.Q.; Liu, X.G. Preparation of crosslinked polysiloxane/SiO2 nanocomposite via in-situ condensation and its surface modification on cotton fabrics. Appl. Surf. Sci. 2016, 371, 281–288. [Google Scholar] [CrossRef]
- Jin, H.; Xu, W. The preparation and properties of fluoroacrylate-modified polysiloxane as a fabric coating agent. Coatings 2018, 8, 31. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.F.; Hao, L.F.; Zhang, D.; Zhang, M. Synthesis and characterization of self-crosslinking fluorinated polyacrylate soap-free latices with core-shell structure. Appl. Surf. Sci. 2013, 268, 373–380. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.F.; Hao, L.F.; Huang, L.X. Synthesis, film morphology and performance of cationic fluorinated polyacrylate emulsion with core-shell structure. J. Appl. Polym. Sci. 2012, 125, 2376–2383. [Google Scholar] [CrossRef]
- Hao, L.-F.; An, Q.F.; Xu, W.; Huang, L.X. Synthesis, film morphology and hydrophobicity of novel fluorinated polyacrylate emulsion and solution on silicon wafer. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 83–89. [Google Scholar] [CrossRef]
- Shang, S.-M.; Li, Z.X.; Xing, Y.J.; Xin, J.H.; Tao, X.M. Preparation of durable hydrophobic cellulose fabric from water glass and mixed organosilanes. Appl. Surf. Sci. 2010, 257, 1495–1499. [Google Scholar] [CrossRef]
- Liu, J.Y.; Huang, W.Q.; Xing, Y.J.; Li, R.; Dai, J.J. Preparation of durable superhydrophobic surface by sol–gel method with water glass and citric acid. J. Sol-Gel Sci. Technol. 2011, 58, 18–23. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
| Fabric Weight (g/m2) | Yarn Count | Fabric Density | ||
|---|---|---|---|---|
| Warp | Weft | End/cm | Picks/cm | |
| 119 | 40 | 40 | 133 | 72 |
| Element | F 1s/% (mass) | O 1s/% (mass) | C 1s/% (mass) | Si 2s/% (mass) | N 1s/% (mass) |
|---|---|---|---|---|---|
| Experimental value | 15.23 | 25.52 | 21.68 | 33.47 | 4.10 |
| Theoretical value | 11.24 | 29.94 | 16.86 | 41.85 | 0.11 |
| Dose of Finishing Agent (g/100 g Ethyl Acetate) | WCA (°) | RCA (°) | Whiteness (°) | BR (mN) | |
|---|---|---|---|---|---|
| w | f | ||||
| 0 | 0 | – | 76.89 | 239 | 175 |
| 0.1 | 153.6 ± 1.1 | 15 | 77.04 | 235 | 152 |
| 0.2 | 157.9 ± 1.5 | 10 | 77.11 | 230 | 149 |
| 0.3 | 161.8 ± 1.3 | 9 | 76.98 | 227 | 155 |
| 0.4 | 163.4 ± 0.9 | 7 | 76.88 | 237 | 163 |
| 0.5 | 163.5 ± 1.1 | 7 | 76.85 | 241 | 170 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, F.; Jin, H.; Xu, W. Preparation of Fluoroalkyl-Acrylate-Modified Polysiloxane Nanocomposite and Its Surface Properties as a Superhydrophobic Coating Material. Coatings 2019, 9, 616. https://doi.org/10.3390/coatings9100616
Deng F, Jin H, Xu W. Preparation of Fluoroalkyl-Acrylate-Modified Polysiloxane Nanocomposite and Its Surface Properties as a Superhydrophobic Coating Material. Coatings. 2019; 9(10):616. https://doi.org/10.3390/coatings9100616
Chicago/Turabian StyleDeng, Fuquan, Hua Jin, and Wei Xu. 2019. "Preparation of Fluoroalkyl-Acrylate-Modified Polysiloxane Nanocomposite and Its Surface Properties as a Superhydrophobic Coating Material" Coatings 9, no. 10: 616. https://doi.org/10.3390/coatings9100616
APA StyleDeng, F., Jin, H., & Xu, W. (2019). Preparation of Fluoroalkyl-Acrylate-Modified Polysiloxane Nanocomposite and Its Surface Properties as a Superhydrophobic Coating Material. Coatings, 9(10), 616. https://doi.org/10.3390/coatings9100616




