YAlO3—A Novel Environmental Barrier Coating for Al2O3/Al2O3–Ceramic Matrix Composites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
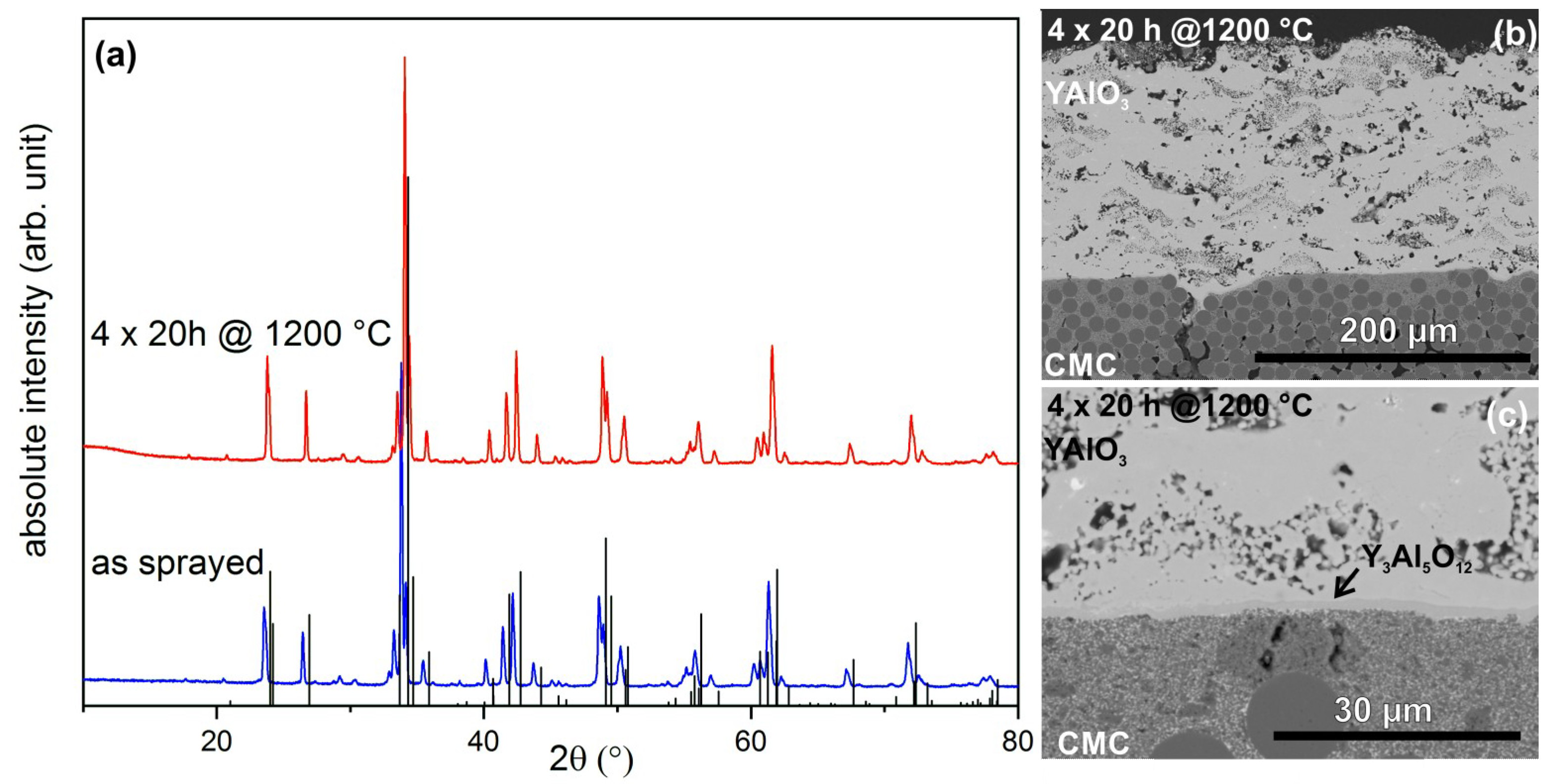
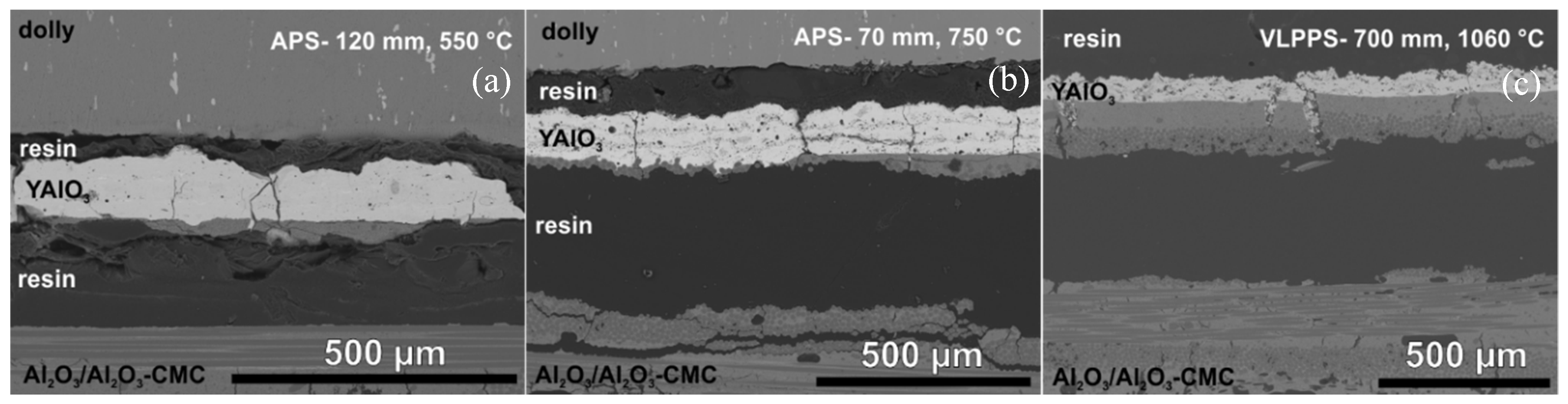
3.1. Coating Formation
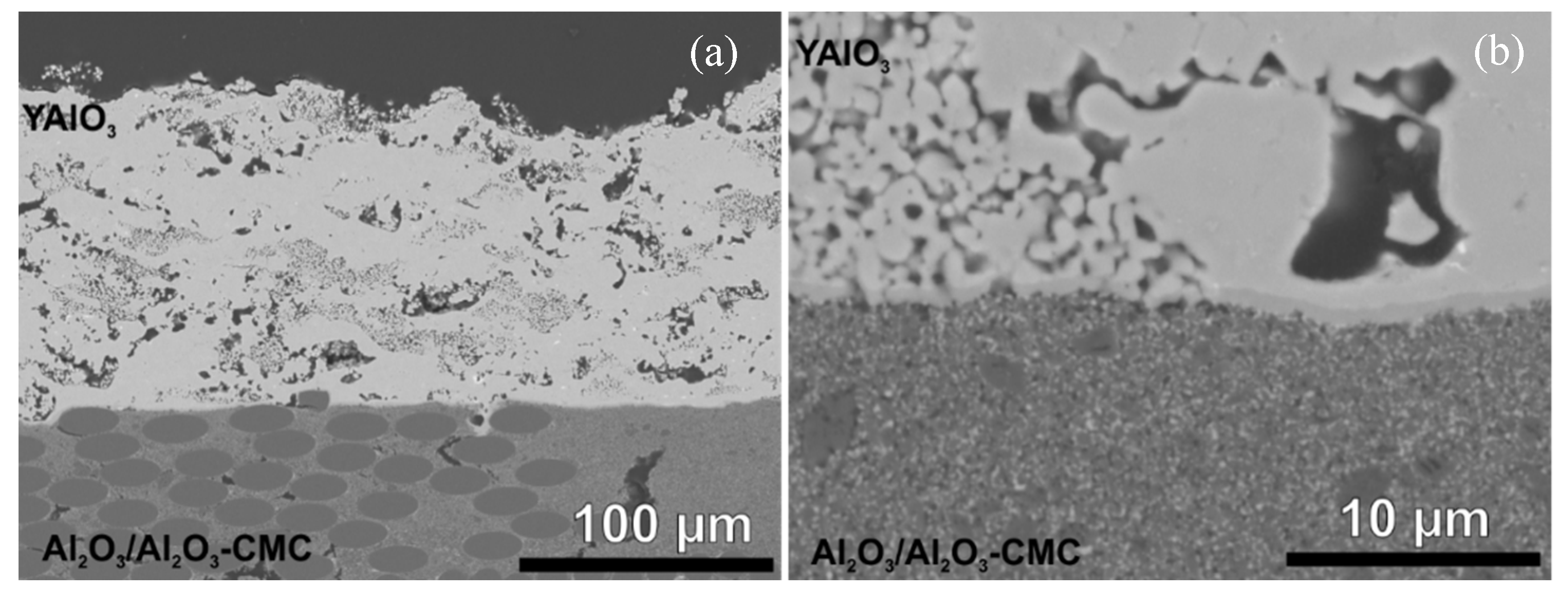
3.1.1. Atmospheric Plasma Spraying
3.1.2. Very Low Pressure Plasma Spraying
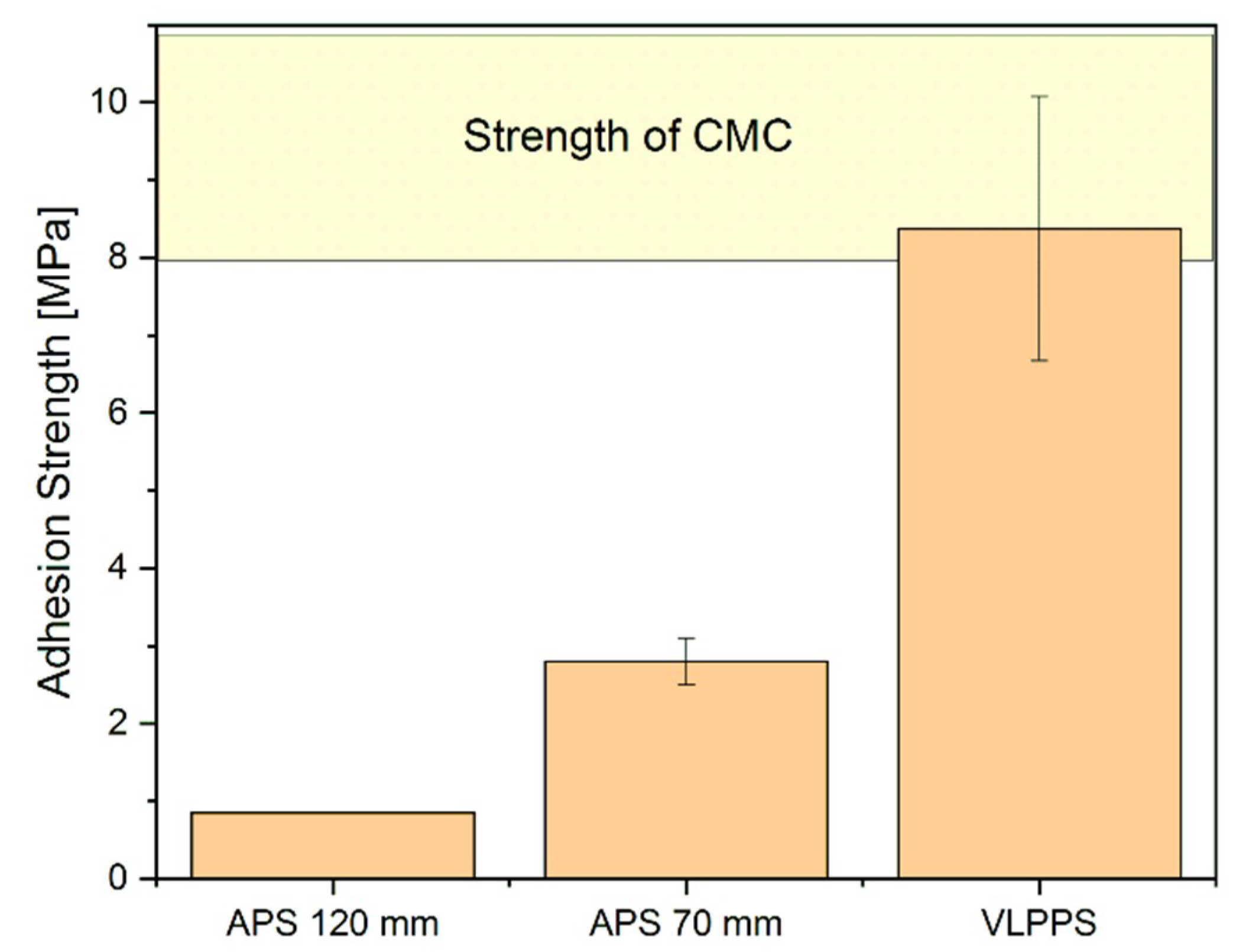
3.2. Pull–Adhesion Tests
3.3. Burner Rig Tests
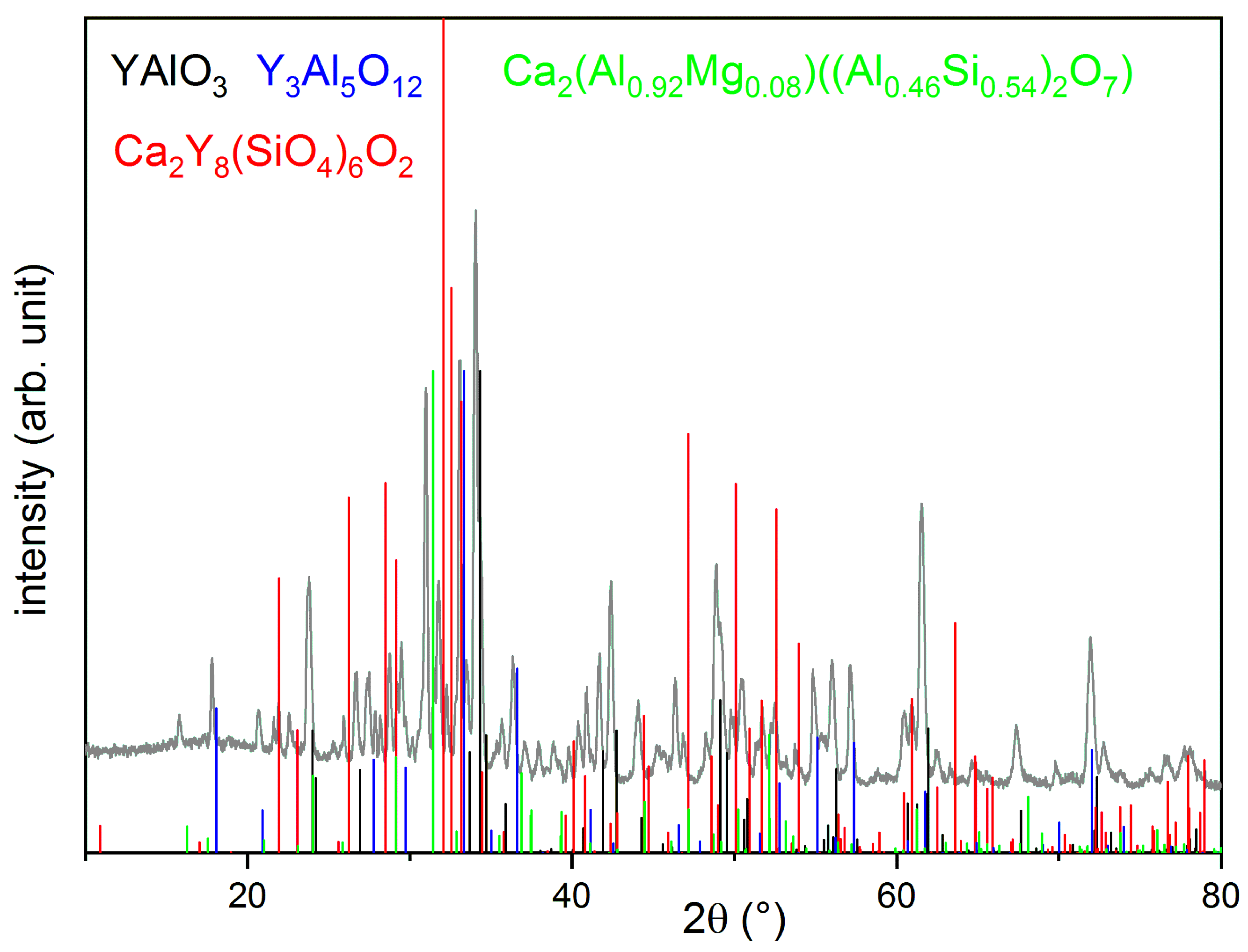
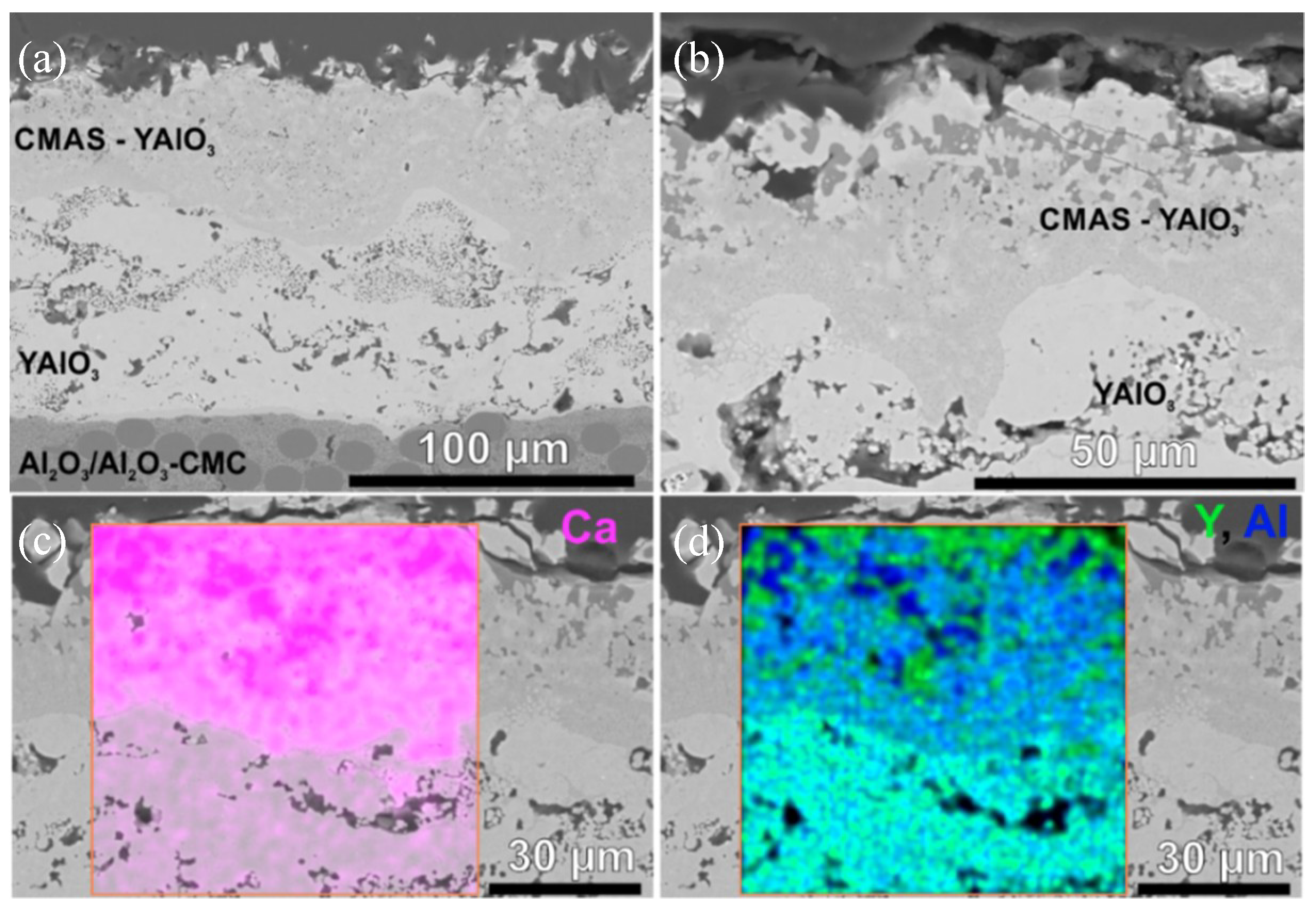
3.4. CMAS Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perepezko, J.H. The hotter the engine, the better. Science 2009, 326, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q. Diesel Engine System Design; Woodhead Publishing: Oxford, UK, 2013; pp. 860–908. [Google Scholar]
- Krenkel, W. Keramische Verbundwerkstoffe; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Opila, E.J.; Myers, D.L. Alumina volatility in water vapor at elevated temperatures. J. Am. Ceram. Soc. 2004, 87, 1701–1705. [Google Scholar] [CrossRef]
- Fritsch, M. Heißgaskorrosion Keramischer Werkstoffe in H2O-Haltigen Rauchgasatmosphären; Fraunhofer IRB Verlag, TU Dresden: Dresden, Germany, 2007. [Google Scholar]
- Fritsch, M.; Klemm, H. The water vapor hot gas corrosion of MGC materials with Al2O3 as a phase constituent in a combustion atmosphere. J. Eur. Ceram. Soc. 2008, 28, 2353–2358. [Google Scholar] [CrossRef]
- Fritsch, M.; Klemm, H. The water-Vapour hot gas corrosion behavior of Al2O3-Y2O3 materials, Y2SiO5 and Y3Al5O12-Coated alumina in A combustion environment. Adv. Ceram. Eng. Sci. Proc. 2006, 27, 149–159. [Google Scholar]
- Fritsch, M.; Klemm, H.; Herrmann, M.; Schenk, B. Corrosion of selected ceramic materials in hot gas environment. J. Eur. Ceram. Soc. 2006, 26, 3557–3565. [Google Scholar] [CrossRef]
- Herrmann, M.; Klemm, H. Corrosion of ceramic materials. Compr. Hard Mater. 2014, 2, 413–446. [Google Scholar]
- Bakan, E.; Marcano, D.; Zhou, D.; Sohn, Y.J.; Mauer, G.; Vaßen, R. Yb2Si2O7 environmental barrier coatings deposited by various thermal spray techniques: A preliminary comparative study. J. Therm. Spray Technol. 2017, 26, 1011–1024. [Google Scholar] [CrossRef]
- Richards, B.T.; Wadley, H.N.G. Plasma spray deposition of tri-layer environmental barrier coatings. J. Eur. Ceram. Soc. 2014, 34, 3069–3083. [Google Scholar] [CrossRef]
- Göring, J.; Kanka, B.; Schmücker, M.; Schneider, H. A Potential Oxide/Oxide Ceramic Matrix Composite for Gas Turbine Applications. In Proceedings of the ASME Turbo Expo 2003, collocated with the 2003 International Joint Power Generation Conference, Atlanta, GA, USA, 16–19 June 2003; pp. 621–624. [Google Scholar]
- Braue, W.; Mechnich, P. Tailoring protective coatings for all-oxide ceramic matrix composites in high temperature-/high heat flux environments and corrosive media. Mater. Werkst. 2007, 38, 690–697. [Google Scholar] [CrossRef]
- Braue, W.; Mechnich, P. Schutzschichtkonzepte für Oxidische Faserverbundwerkstoffe; DLR Kolloquium: Köln, Germany, 2004. [Google Scholar]
- Singh, D.; Zhu, D.; Zhou, Y.; Singh, M. Design, Development, and Applications of Engineering Ceramics and Composites: Ceramic Transactions; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Mechnich, P.; Braue, W.; Schneider, H. Multifunctional reaction-bonded alumina coatings for porous continuous fiber-reinforced oxide composites. Int. J. Appl. Ceram. Technol. 2004, 1, 343–350. [Google Scholar] [CrossRef]
- Vaßen, R.; Bakan, E.; Gatzen, C.; Kim, S.; Mack, D.E.; Guillon, O. Environmental barrier coatings made by different thermal spray technologies. Coatings 2019. in Preparation. [Google Scholar]
- Fritsch, M.; Klemm, H.; Herrmann, M.; Michaelis, A.; Schenk, B. The water vapour hot gas corrosion of ceramic materials. Ceram. Forum Int. 2010, 87, 11–12. [Google Scholar]
- Mechnich, P.; Braue, W. Air plasma-Sprayed Y2O3 coatings for Al2O3/Al2O3 ceramic matrix composites. J. Eur. Ceram. Soc. 2013, 33, 2645–2653. [Google Scholar] [CrossRef]
- Gatzen, C.; Mack, D.E.; Guillon, O.; Vaßen, R. Surface roughening of Al2O3/Al2O3-ceramic matrix composites by nanosecond laser ablation prior to thermal spraying. J. Laser Appl. 2019, 31, 022018. [Google Scholar] [CrossRef]
- Gerendás, M.; Cadoret, Y.; Wilhelmi, C.; Machry, T.; Knoche, R.; Behrendt, T.; Aumeier, T.; Denis, S.; Göring, J.; Koch, D.; et al. Improvement of Oxide/Oxide CMC and Development of combustor and turbine components in the HiPOC program. ASME Turbo Expo 2011, 1, 477–490. [Google Scholar]
- Fabrichnaya, O.; Seifert, H.J.; Weiland, R.; Ludwig, T.; Aldinger, F.; Navrotsky, A. Phase equilibria and thermodynamics in the Y2O3–Al2O3–SiO2 system. Z. Metallkd. 2001, 92, 1083–1097. [Google Scholar]
- Weyant, C.M.; Faber, K.T. Processing–microstructure relationships for plasma-sprayed yttrium aluminum garnet. Surf. Coat. Technol. 2008, 202, 6081–6089. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- ASTM D4541-17, Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- Traeger, F.; Vaßen, R.; Rauwald, K.H.; Stöver, D. Thermal cycling setup for testing thermal barrier coatings. Adv. Eng. Mater. 2003, 5, 429–433. [Google Scholar] [CrossRef]
- Steinke, T.; Sebold, D.; Mack, D.E.; Vaßen, R.; Stöver, D. A novel test approach for plasma-Sprayed coatings tested simultaneously under CMAS and thermal gradient cycling conditions. Surf. Coat. Technol. 2010, 205, 2287–2295. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-Spray Coating; Weinheim VCH: New York, NY, USA, 1996. [Google Scholar]
- Chandra, S.; Fauchais, P. Formation of solid splats during thermal spray deposition. J. Therm. Spray Technol. 2009, 18, 148–180. [Google Scholar] [CrossRef]
- Schulz, U.; Saruhan, B.; Fritscher, K.; Leyens, C. Review on advanced EB-PVD ceramic topcoats for TBC applications. Int. J. Appl. Ceram. Technol. 2004, 1, 302–315. [Google Scholar] [CrossRef]
- Aasland, S.; McMillan, P.F. Density-driven liquid–liquid phase separation in the system Al2O3–Y2O3. Nature 1994, 369, 633–636. [Google Scholar] [CrossRef]
- Srivastava, A.K. Oxide Nanostructures: Growth, Microstructures, and Properties; Pan Stanford Publishing: Singapore, 2014. [Google Scholar]
- Watanabe, Y.; Masuno, A.; Inoue, H. Glass formation of rare earth aluminates by containerless processing. J. Non-Cryst. Solids 2012, 358, 3563–3566. [Google Scholar] [CrossRef]
- Ching, W.Y.; Xu, Y.N. Nonscalability and nontransferability in the electronic properties of the Y–Al–O system. Phys. Rev. B 1999, 59, 12815–12821. [Google Scholar] [CrossRef]
- Fabrichnaya, O.; Seifert, H.J.; Ludwig, T.; Aldinger, F.; Navrotsky, A. The assessment of thermodynamic parameters in the Al2O3–Y2O3 system and phase relations in the Y–Al–O system. Scand. J. Metall. 2001, 30, 175–183. [Google Scholar] [CrossRef]
- Medraj, M.; Hammond, R.; Parvez, M.A.; Drew, R.A.L.; Thompson, W.T. High temperature neutron diffraction study of the Al2O3–Y2O3 system. J. Eur. Ceram. Soc. 2006, 26, 3515–3524. [Google Scholar] [CrossRef]
- Ahlborg, N.L.; Zhu, D. Calcium–Magnesium aluminosilicate (CMAS) reactions and degradation mechanisms of advanced environmental barrier coatings. Surf. Coat. Technol. 2013, 237, 79–87. [Google Scholar] [CrossRef]
- Turcer, L.R.; Krause, A.R.; Garces, H.F.; Zhang, L.; Padture, N.P. Environmental-Barrier coating ceramics for resistance against attack by molten calcia-magnesia-aluminosilicate (CMAS) glass: Part I, YAlO3 and γ-Y2Si2O7. J. Eur. Ceram. Soc. 2018, 38, 3905–3913. [Google Scholar] [CrossRef]
- Bansal, N.P.; Choi, S.R. Properties of Desert Sand and CMAS Glass; NASA Glenn Research Center: Cleveland, OH, USA, August 2014.
- Reddy, A.A.; Goel, A.; Tulyaganov, D.U.; Kapoor, S.; Pradeesh, K.; Pascual, M.J.; Ferreira, J.M.F. Study of calcium–magnesium–aluminum–silicate (CMAS) glass and glass-ceramic sealant for solid oxide fuel cells. J. Power Sources 2013, 231, 203–212. [Google Scholar] [CrossRef]
- Chaix-Pluchery, O.; Chenevier, B.; Robles, J.J. Anisotropy of thermal expansion in YAlO3 and NdGaO3. Appl. Phys. Lett. 2005, 86, 251911. [Google Scholar] [CrossRef]

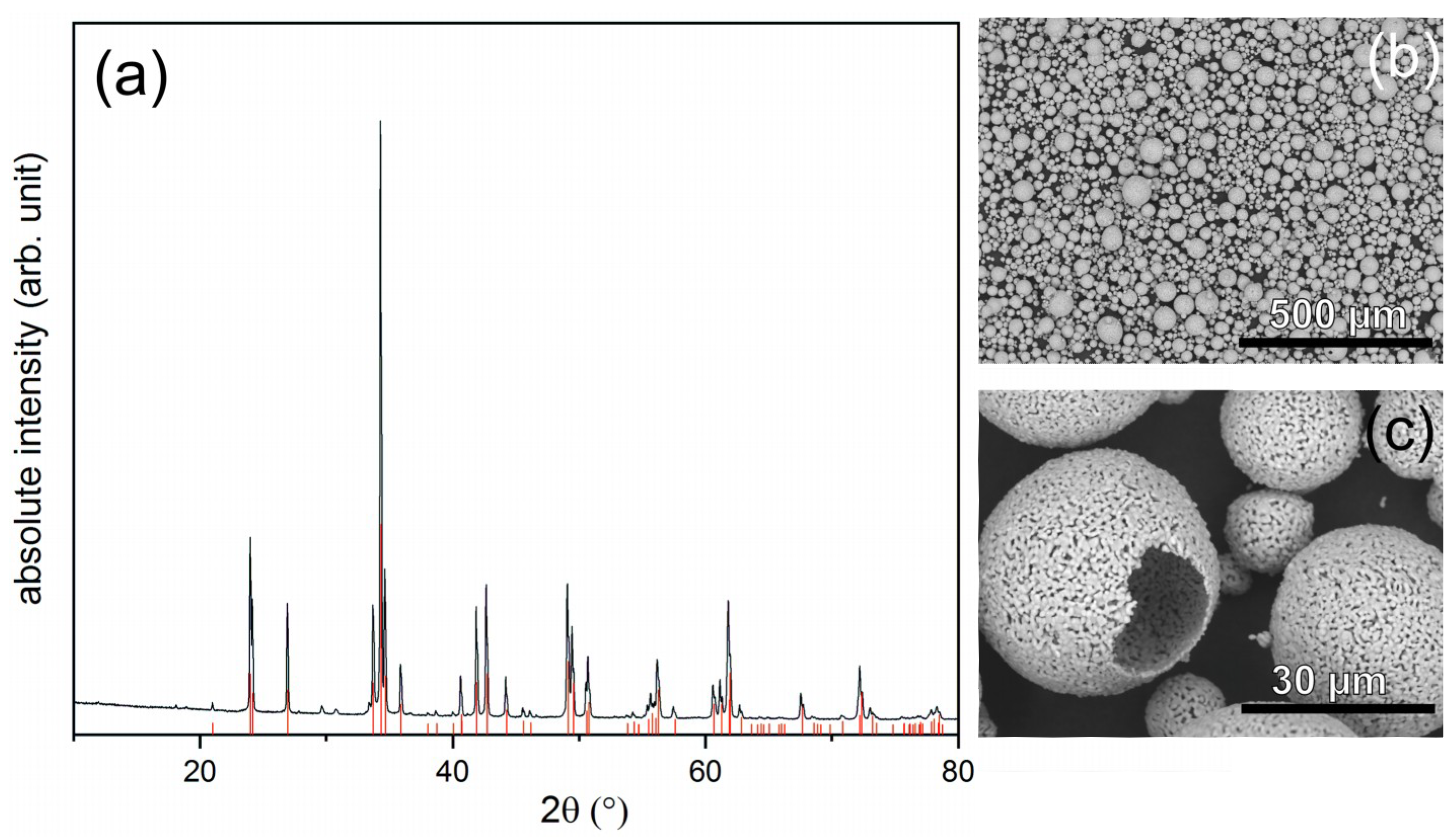

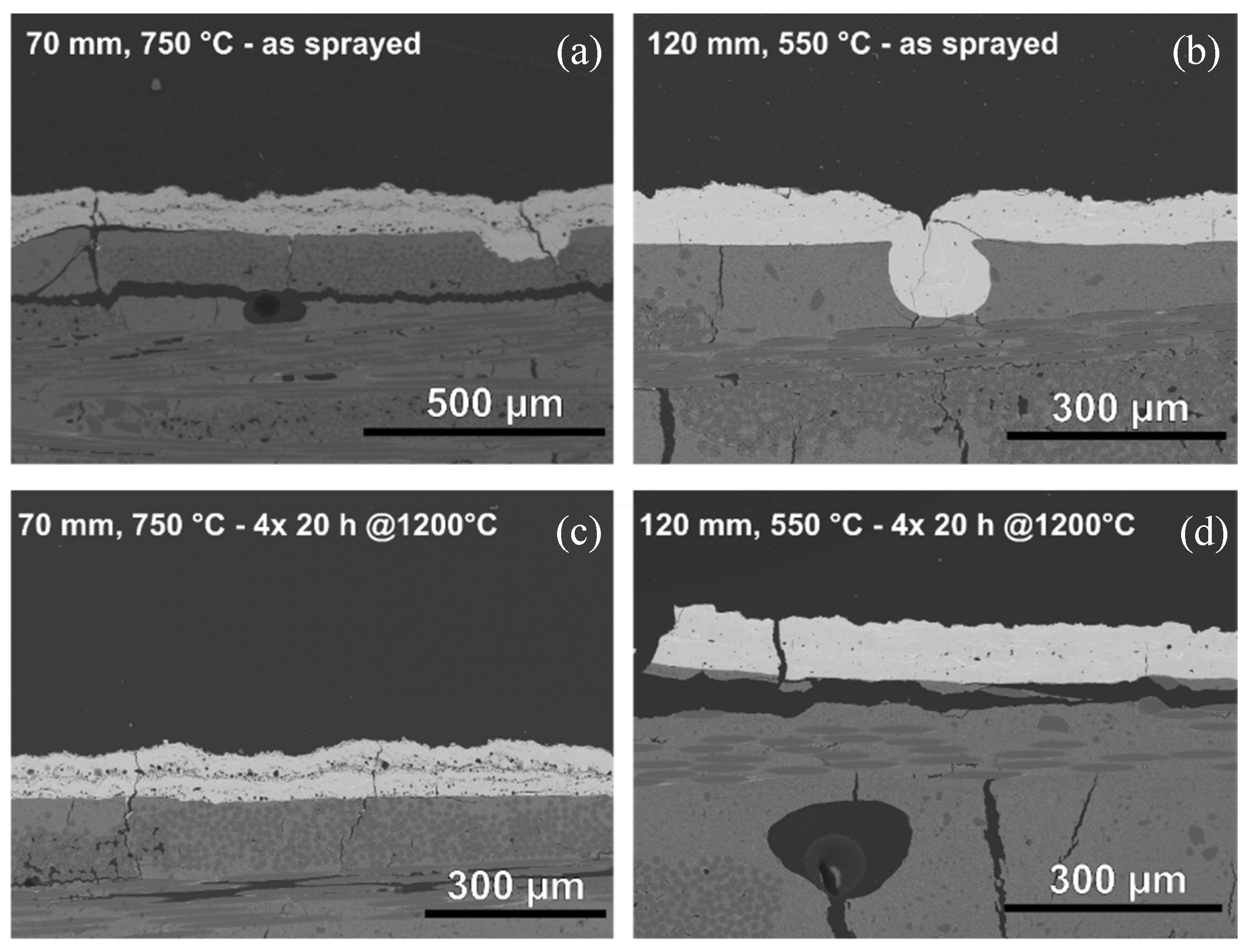



| Process | Distance (mm) | TPreheat (°C) | TCoating (°C) | Current (A) | Ar (SLPM) | He (SLPM) | Carrier Gas (SLPM) | Passes | Thickness (µm) |
|---|---|---|---|---|---|---|---|---|---|
| APS | 70 200 | 600 | 750 550 | 520 | 50 | 4 | 3 | 2 | 120 |
| VLPPS | 700 | 1025 | 1060 | 2150 | 110 | 20 | 11 | 2 min | 120 |
| Phase | 70 mm As-Sprayed | 70 mm Sintered | 120 mm As-Sprayed | 120 mm Sintered |
|---|---|---|---|---|
| YAlO3 | 74% | 90% | – | 39% |
| Y4Al2O9 | 14% | 6% | – | 32% |
| Y3Al5O12 | 12% | 4% | – | 23% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatzen, C.; Mack, D.E.; Guillon, O.; Vaßen, R. YAlO3—A Novel Environmental Barrier Coating for Al2O3/Al2O3–Ceramic Matrix Composites. Coatings 2019, 9, 609. https://doi.org/10.3390/coatings9100609
Gatzen C, Mack DE, Guillon O, Vaßen R. YAlO3—A Novel Environmental Barrier Coating for Al2O3/Al2O3–Ceramic Matrix Composites. Coatings. 2019; 9(10):609. https://doi.org/10.3390/coatings9100609
Chicago/Turabian StyleGatzen, Caren, Daniel Emil Mack, Olivier Guillon, and Robert Vaßen. 2019. "YAlO3—A Novel Environmental Barrier Coating for Al2O3/Al2O3–Ceramic Matrix Composites" Coatings 9, no. 10: 609. https://doi.org/10.3390/coatings9100609
APA StyleGatzen, C., Mack, D. E., Guillon, O., & Vaßen, R. (2019). YAlO3—A Novel Environmental Barrier Coating for Al2O3/Al2O3–Ceramic Matrix Composites. Coatings, 9(10), 609. https://doi.org/10.3390/coatings9100609






