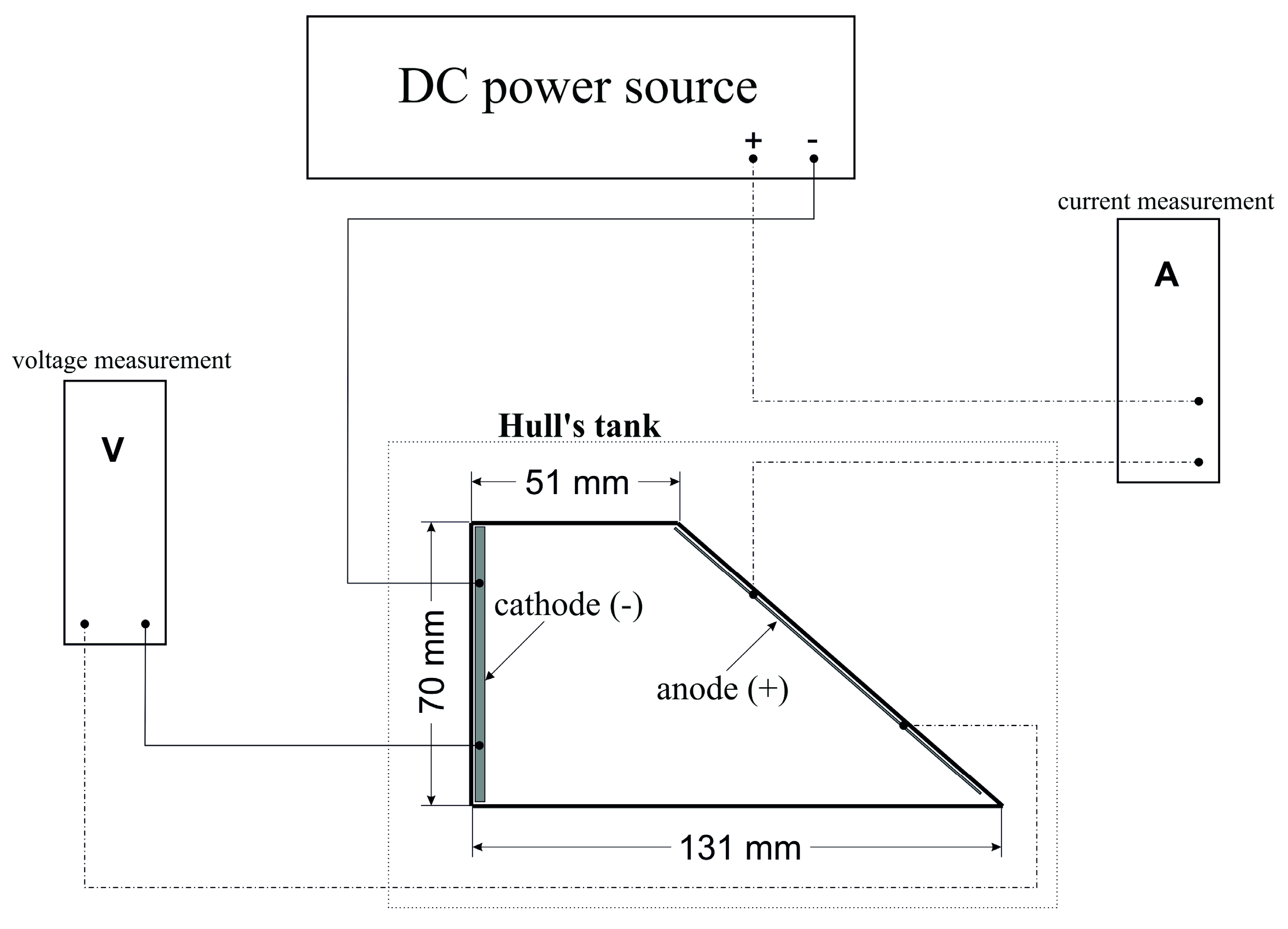
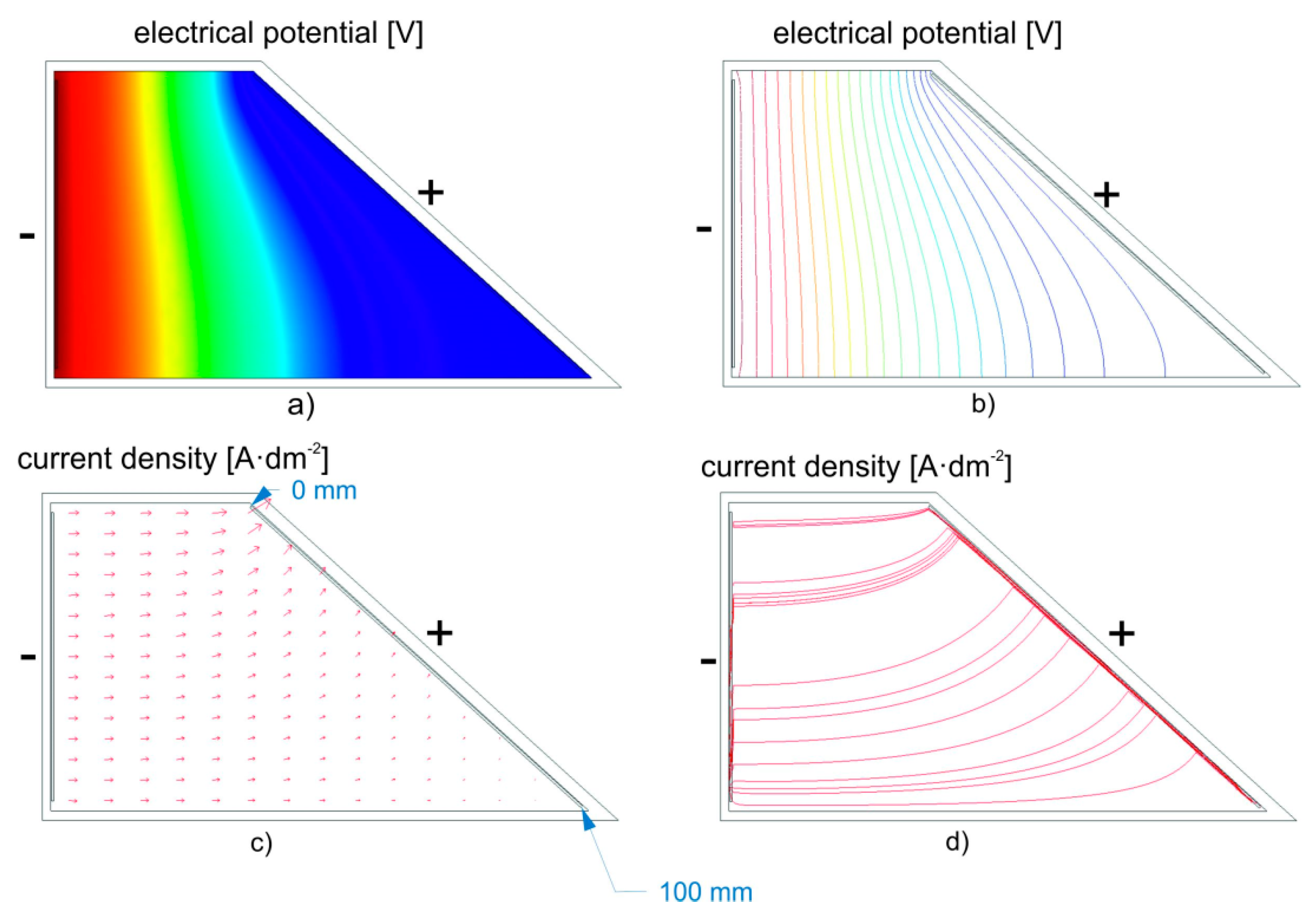
In general, theoretically, the current density (anode, cathode) significantly affects the way the deposited coatings are formed and their properties. Based on Faraday’s laws applicable to electrochemical processes, the thickness of the deposited coating is directly proportional to the current density and the deposition time. Surface treatment plants use DC power sources with automatic control so that the current density (Equation (4)) remains constant at different surface sizes of the treated products, over a wide range of selection options for surface area size (Equation (4)):
where
JC(A/K) is anode/cathode current density in the measurement unit A·dm
−2,
I is the total current in A, and
PA/K is the effective anode/cathode area in dm
2.
Practically, the actual, instantaneous, local anode current density (in the text briefly referred to as the current density) as determined in the individual anode locations may differ significantly from the general overall value and is influenced by the shape of the anode, its placement in the tank and also the distribution of the DC electric field in the tank. Also, the value of the total current changes during the anodic oxidation process, so the average value was used to calculate the current density.
3.1. Results of Statistical Analysis
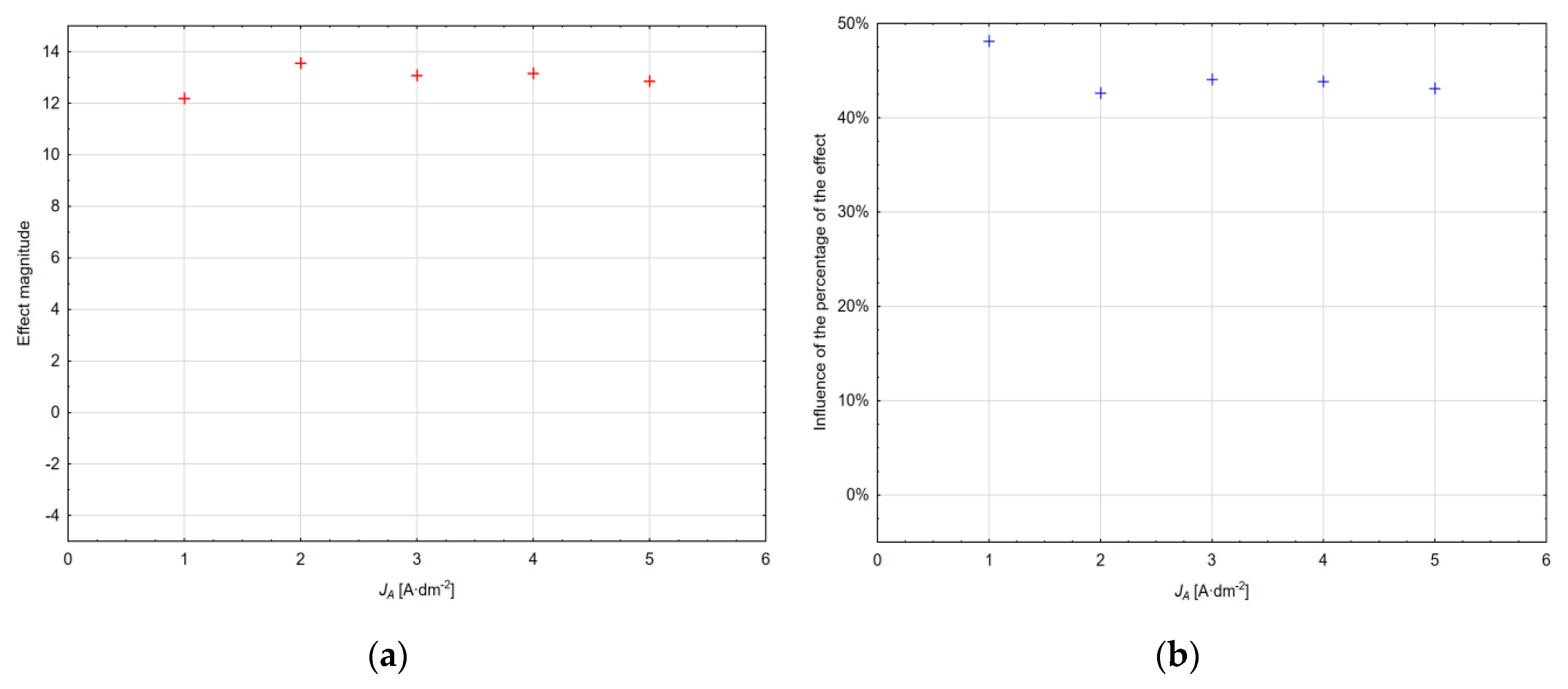
Based on the analysis performed at different current density values, it can be stated that with the change in the current density in the interval from 1 to 5 A·dm
−2, the magnitude of the individual effects of the technological models changes; this change for the absolute member values is shown in
Figure 7a, and its percentage within the total variability value can be seen in
Figure 7b. The above diagrams show that the magnitude of the effect is the lowest in the area of the current density of 1 A·dm
−2, it reaches the maximum at the current density of 2 A·dm
−2 and gradually decreases at higher current densities. The percentage (the absolute member represents all neglected factors) is influenced by the choice of the type of the experiment plan, the choice of the prediction model, the range of the factors considered, and the number of their levels; the highest share being at the current density of 1 A·dm
−2 and the lowest share at the current density of 2 A·dm
−2. The model created for this current density describes the experimental data best.
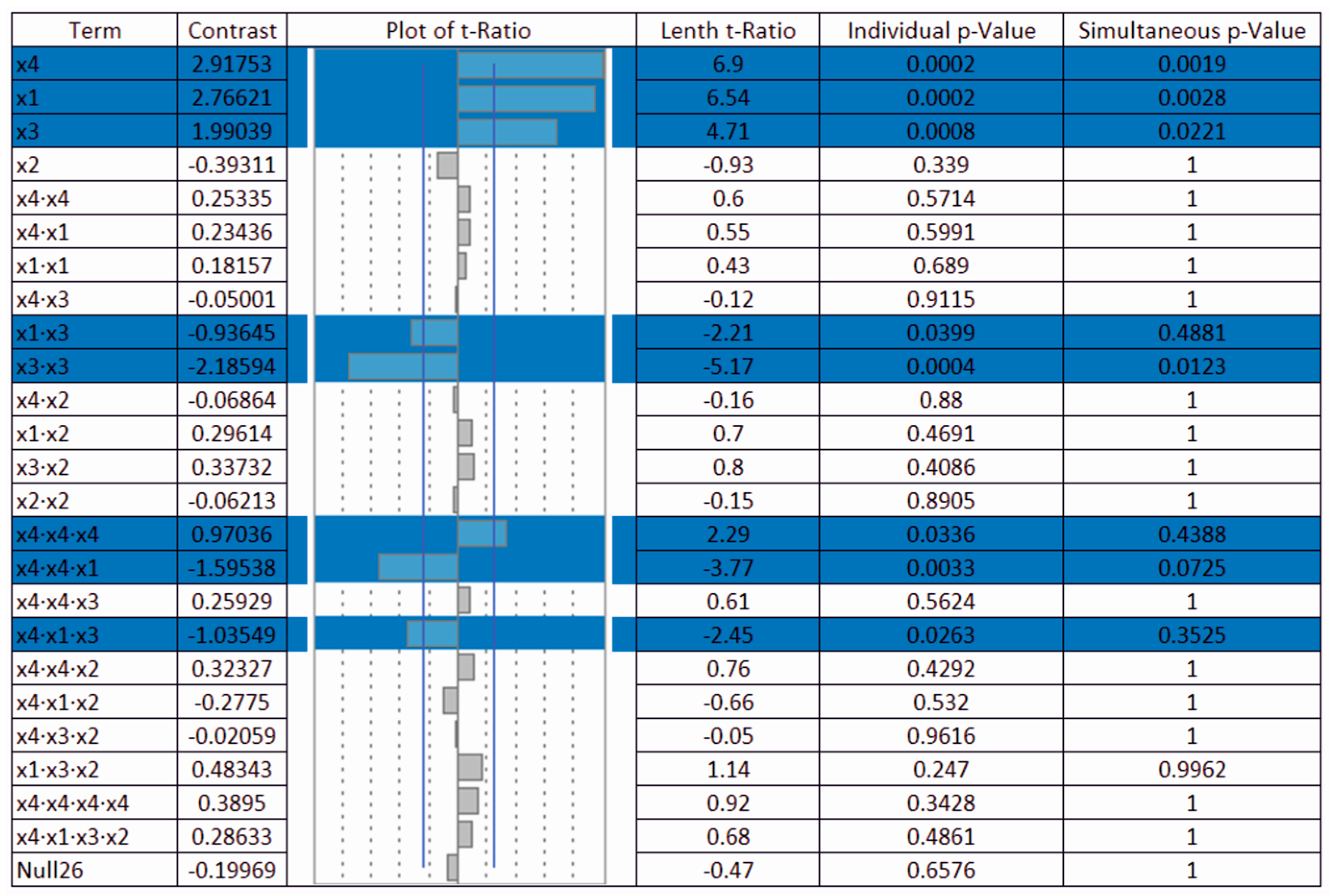
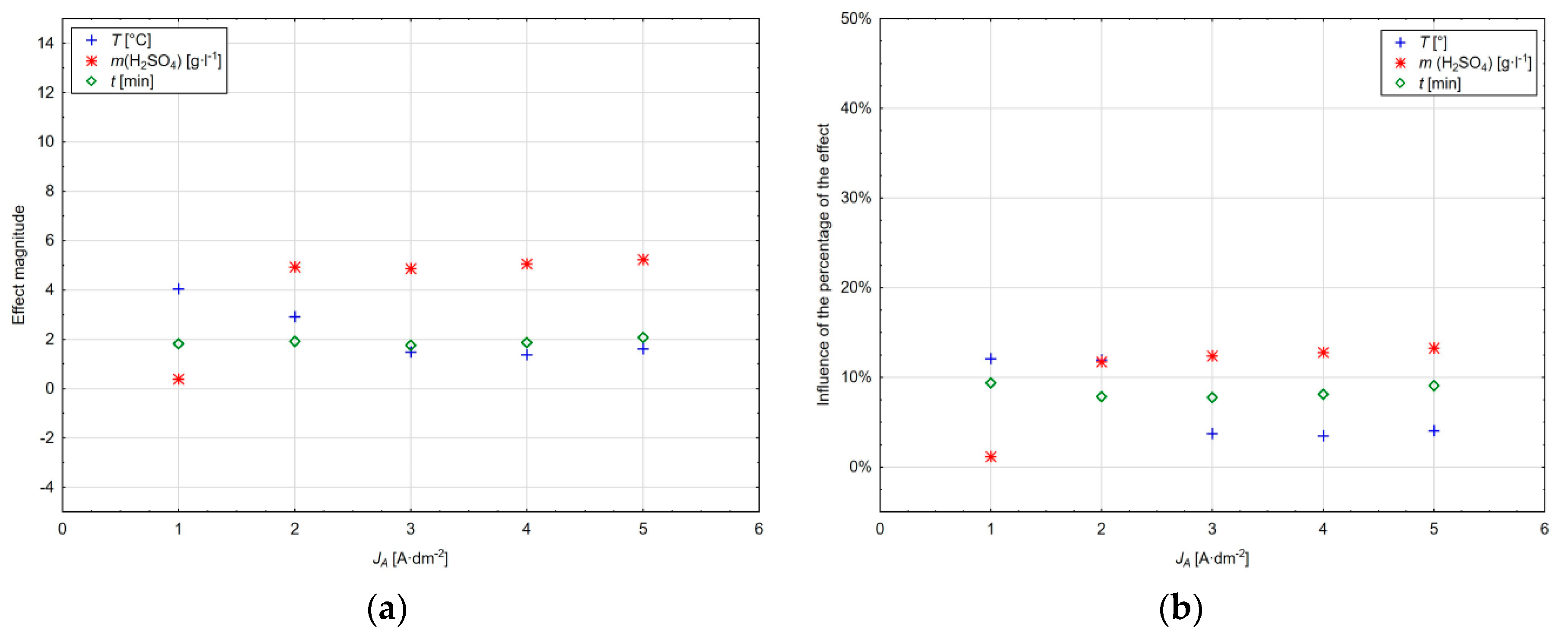
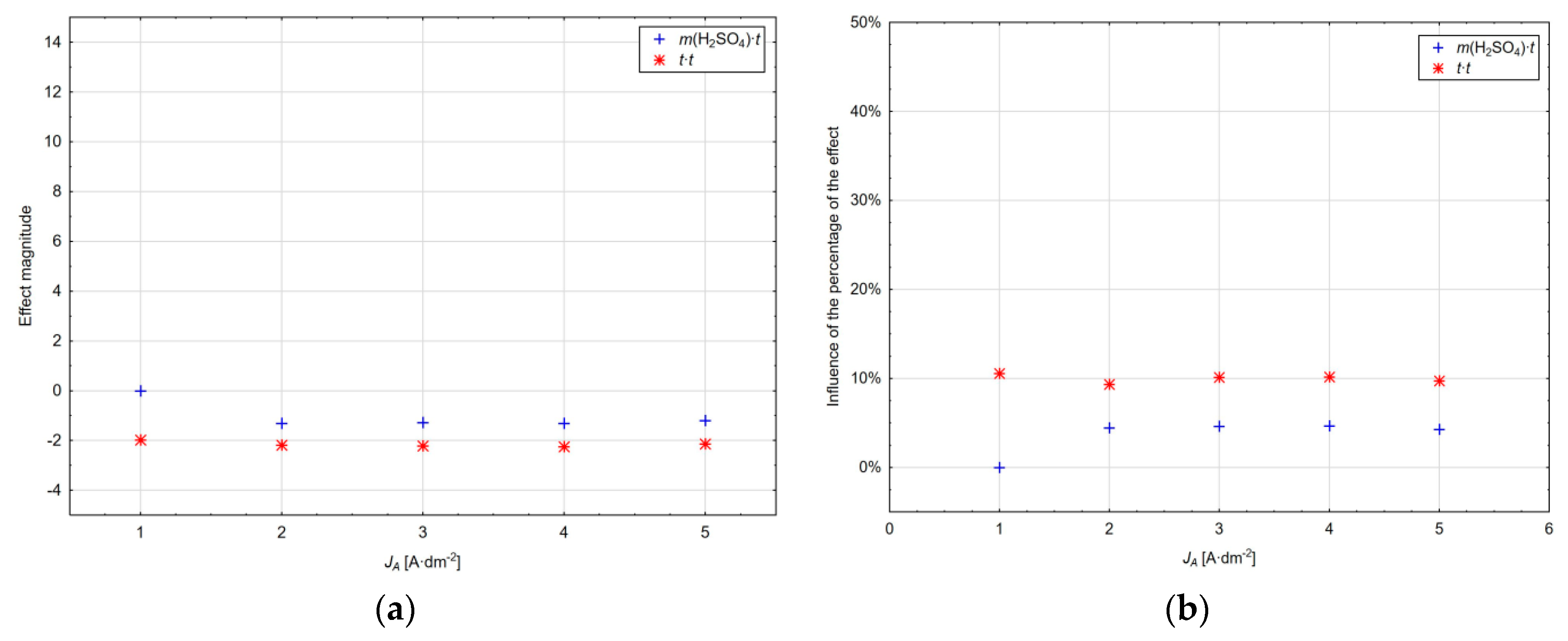
The value of the main effect magnitude within the range of current densities used as well as their percentage is shown in
Figure 8a,b. It can be seen that the magnitude of the effect of factor
x1 and the amount of sulphuric acid increases with the current density, whereas at 1 A·dm
−2 this factor is not significant at the significance level α = 0.05. The opposite trend can be observed for the
x4 factor and the anodic oxidation time, where the magnitude of this effect decreases with the increasing current density of up to 4 A·dm
−2 and its influence increases by 1% at the current density of 5 A·dm
−2. The electrolyte temperature represented by factor
x3 has an approximately constant effect with increasing the current density with a slight decrease at the current density of 3 A·dm
−2.
It is clear from the diagram in
Figure 8b that the amount of sulphuric acid at the current density of 1 A·dm
−2 represents a negligible value of 1%. At this current density, the influence of the anodic oxidation time and subsequently the temperature of the electrolyte is dominant. However, with the increasing current density, the effect of sulphuric acid also increases to the detriment of the anodic oxidation time of aluminium, with the influence of the electrolyte temperature being maintained within the range of the current densities in a relatively narrow range from 7.77% to 9.39%.
The influence of the interactions of the main effects is often more important than their isolated effect. For the two-level interactions of the created technological models, the magnitudes of the effects in dependence on the current densities are shown in
Figure 9a,b.
At the current density of 1 A·dm
−2, the thickness of the layer formed is not affected by the combined effect of the sulphuric acid amount and the anodic oxidation time; with its increasing value, the magnitude of this interaction is almost constant. At the same time, a negative influence on the thickness of the layer can be observed from its sign, which can be attributed above all to an increase in the electrolyte temperature as well as to the effect of the dissolution of the layer during the process of its formation. The second power of the electrolyte temperature over the entire range of current densities has a more pronounced effect on decreasing the thickness of the layer formed than in the previous interaction. This effect is also significant at the lowest value of the current density, where its value is maintained at a narrow interval over the entire range of current densities used. Therefore, it is necessary to consider this influence irrespective of the current density value used. Increasing the electrolyte temperature as the main effect causes the thickness of the layer to increase. However, since the quadratic effect of the electrolyte temperature on the thickness of the layer formed has the opposite trend, the thickness of the layer decreases. In accordance with the theoretical considerations, this effect is due to an increased level of chemical dissolution of the layer formed as the accompanying phenomenon of the electrochemical formation of the anode layer. This trend also follows the percentage effect of individual effects for the quadratic effect of the electrolyte temperature across the range of current densities ranging from 9.35% to 10.58% and the effect of the interaction of sulphuric acid and electrolyte temperature from 4.28% to 4.67%. The interaction of the amount of sulphuric acid and the electrolyte temperature is reflected in the reduction of the thickness of the layer formed, as demonstrated in the experimental section. Especially with an amount of sulphuric acid greater than 175 g·L
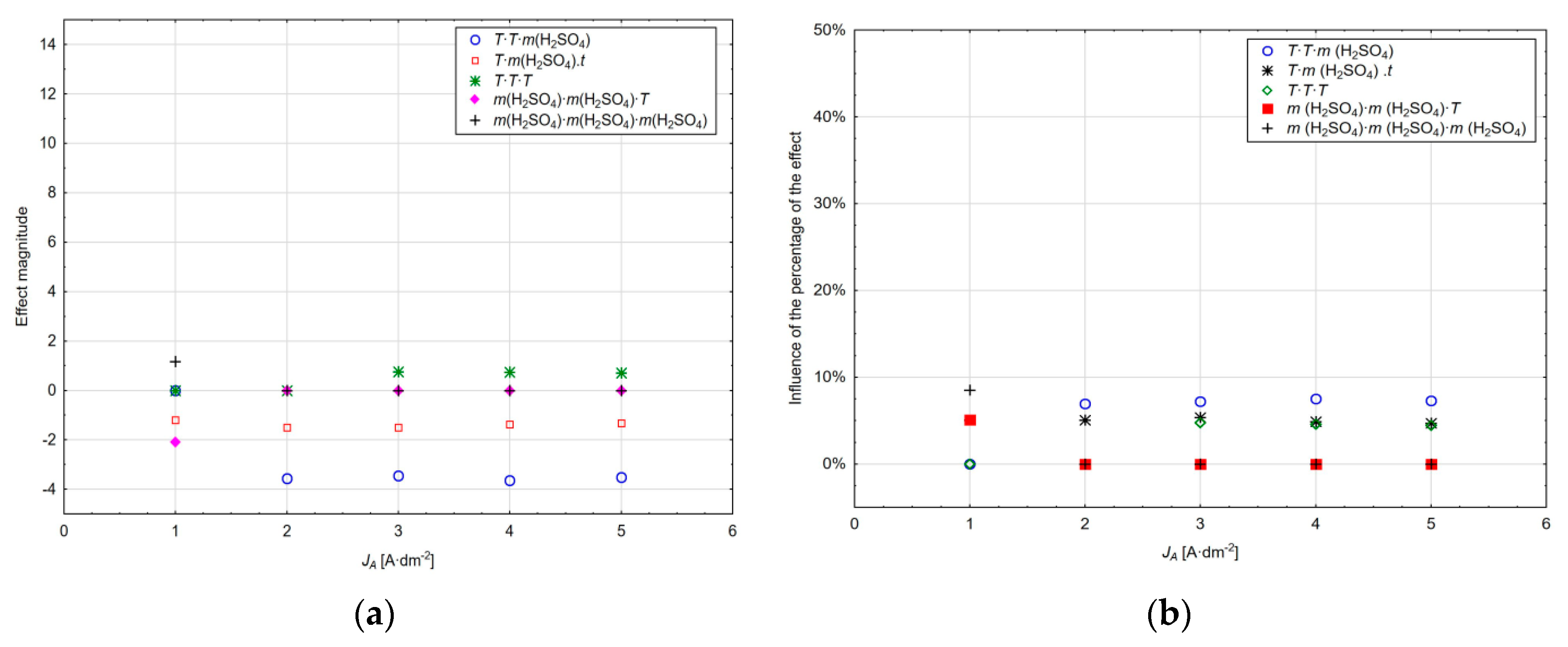
−1, a marked reduction in the thickness of the layer formed above the critical electrolyte temperature can be observed. The magnitude of the three-level effects for current densities of 1 to 5 A·dm
−2 and their influence on the percentage is shown in
Figure 10a,b. What is especially significant is the cubic action of sulphuric acid at the current density of only 1 A·dm
−2, with 8.49% impact, while at the other applied current densities, this effect does not act any more. Similarly, the interaction of the square amount of sulphuric acid and the time of anodic oxidation acts only at the current density of 1 A·dm
−2 with the influence of the percentage of 5.9%; the effect is reflected in the reduction of the thickness of the layer formed. The effect of the interaction of the square of the anodic oxidation time and the amount of sulphuric acid starts acting at the current density of 2 A·dm
−2, and the cubic effect of the time of anodic oxidation starts exerting its effect at the current density of 3 A·dm
−2. For a complete interpretation of the results, it should be added that the influence of the percentage of individual factors is also considered with the influence of the absolute model member.
Increasing the current density and increasing the amount of sulphuric acid in the solution and the anodic oxidation time causes a reduction in the thickness of the layer formed if the cubic value of the anodic oxidation time increases the conditional value of the layer thickness with the influence of the percentage on the change in the response variability from 4.44% to 4.74%. Interaction of all critical factors significantly influences the entire range of current density values, with the effect of reducing the thickness of the layer formed and the influence of the percentage from 4.72% to 5.38%.
Figure 11 shows a complex insight into the effect of various chemical and physical factors affecting the thickness of the layer formed; it shows the effect at values of the individual factors for current densities from 1 to 5 A·dm
−2 used in practice.
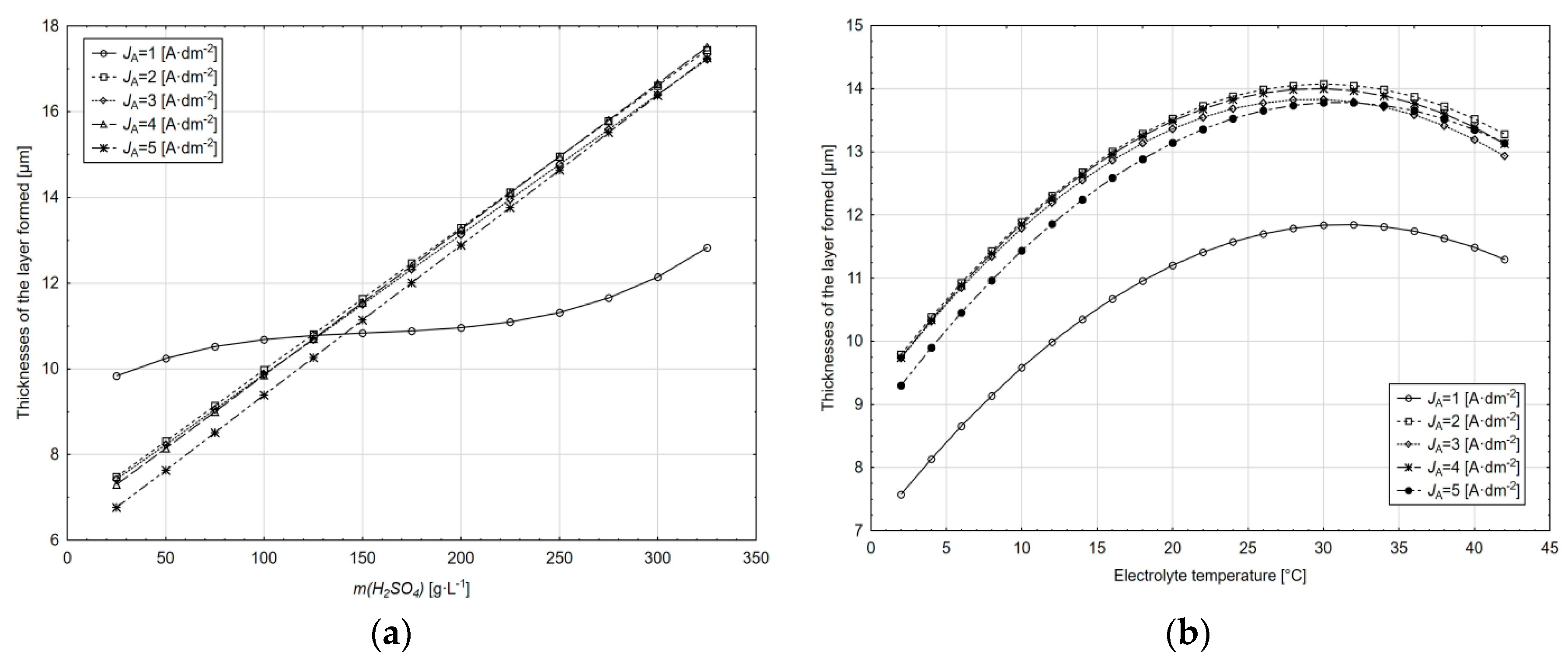
With the effect of the sulphuric acid amount in the electrolyte at the current density of 1 A·dm−2, only a slight change in the thickness of the layer formed can be observed with an increase in the sulphuric acid concentration; an increase in the layer thickness occurs in the area above 220 g·L−1.
At current densities of 2–5 A·dm−2, due to an increase in the sulphuric acid concentration, the thickness of the layer formed is increased over the entire interval; increasing the current density does not have a significant effect on the absolute thickness of the layer.
The same course can be observed for the dependence on the thickness of the layer formed on the electrolyte temperature irrespective of the current density value. The significant difference in the thickness of the layer formed is at the current density of 1 A·dm−2 relative to the other current densities. Two different areas of the electrolyte temperature influence the thickness of the layer formed, namely the area of growth of this thickness and the area of its decrease, with the areas separated by the critical temperature value. The critical temperature for anodic oxidation conditions ranges from 24 to 32 °C.
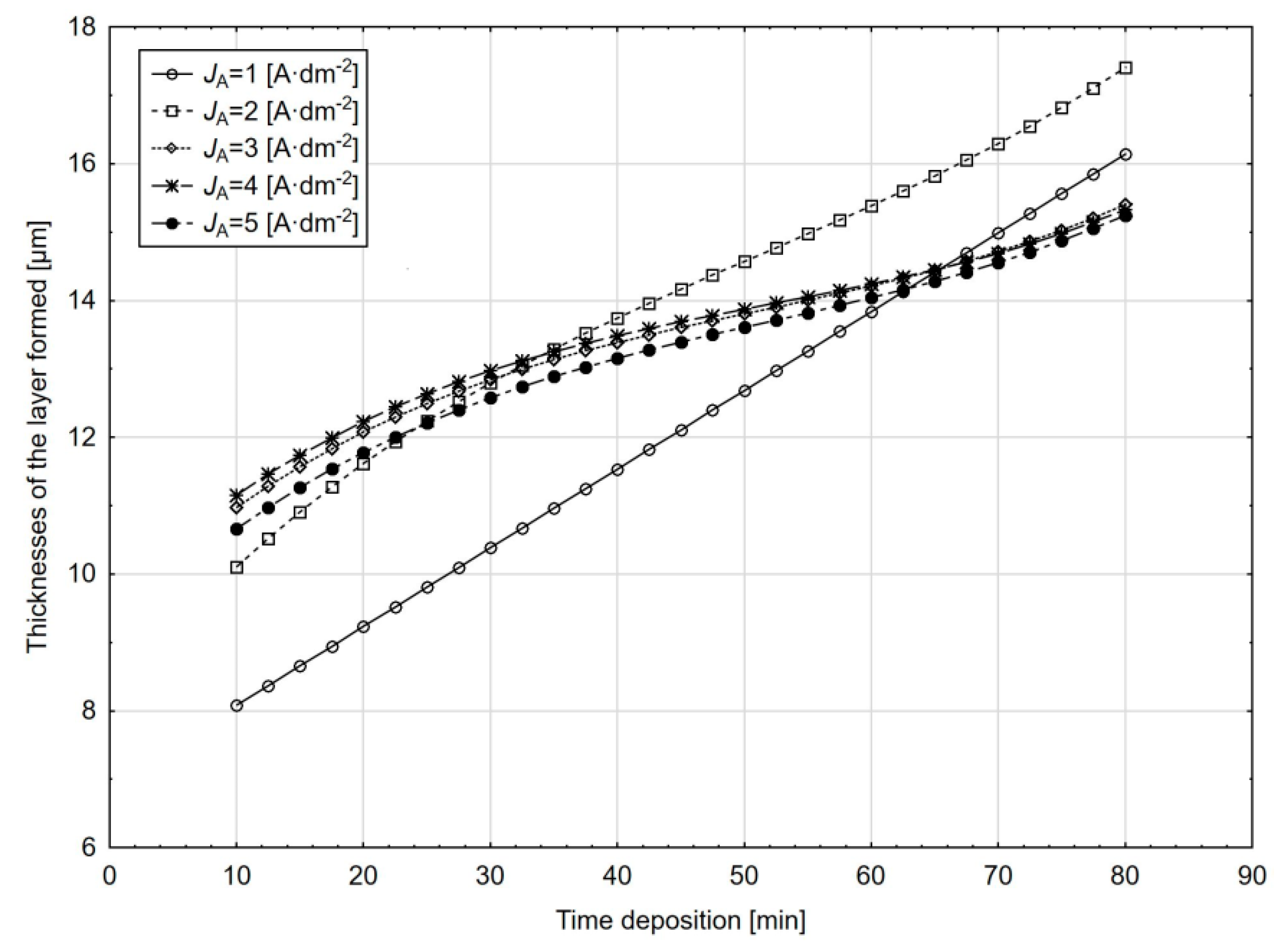
In the case of the dependence on the thickness of the layer formed on the anodic oxidation time with the amount of sulphuric acid of 200 g⋅L
−1 in the electrolyte and at the electrolyte temperature 18 °C (
Figure 12), the difference in the course of dependence at the current density of 1 A·dm
−2 is clearly visible compared to the others current densities. At the same time, we can observe that the highest thicknesses of the layers formed are achieved at the current density of 2 A·dm
−2 and the anodic oxidation time of more than 35 min. An increase in the current density over 2 A·dm
−2 does not have a significant effect on the thickness of the layer formed.
Current density mainly affects the way the inner structure of the aluminium oxide layer is formed and partly affects the thickness of the oxide layer formed. At low current densities (up to 0.5 A·dm
−2), an unarranged structure of the oxide layer is formed (pores pass through the wall of the cell and bind to the pores of neighbouring cells; the pores do not have a constant diameter; the cells do not have a hexagonal shape; the distances between the cells are not constant; the walls of the cells do not have constant thickness, etc.). At low current densities, oxide layers with a thickness of less than 1–3 μm are also formed. At high current densities (above 10 A·dm
−2), the formed aluminium oxide layer is burnt out to disturb the surface of the oxidized substrate [
9].
3.2. Verification of the Proposed Prediction Model in Industrial Practice
For predicting the thickness of the layer formed, an empirical relationship (Equation (5)) derived from the essential working factors and the area of the treated components is commonly used in practice:
where η is the degree of electrolyte efficiency (0.6–0.7),
I is the DC current in A,
t is the anodic oxidation time in min, and
S is the area of anodically oxidized components in dm
2.
For the practical verification of the technological models (
Figure 13) created in the framework of the experimental analysis in the real production conditions, anodically modified components were used in the order of the companies: Locker, s.r.o.; Sensor, s.r.o.; Proving, s.r.o. and Kovdan, s.r.o., wherein the known chemical composition of the electrolyte, voltage, average current density and the electrolyte temperature, the influence of the time of anodic oxidation on the thickness of the layer formed. The thickness of the layer formed was then compared with the technological model for the respective current density according to the empirical relationship (Equation (5)).
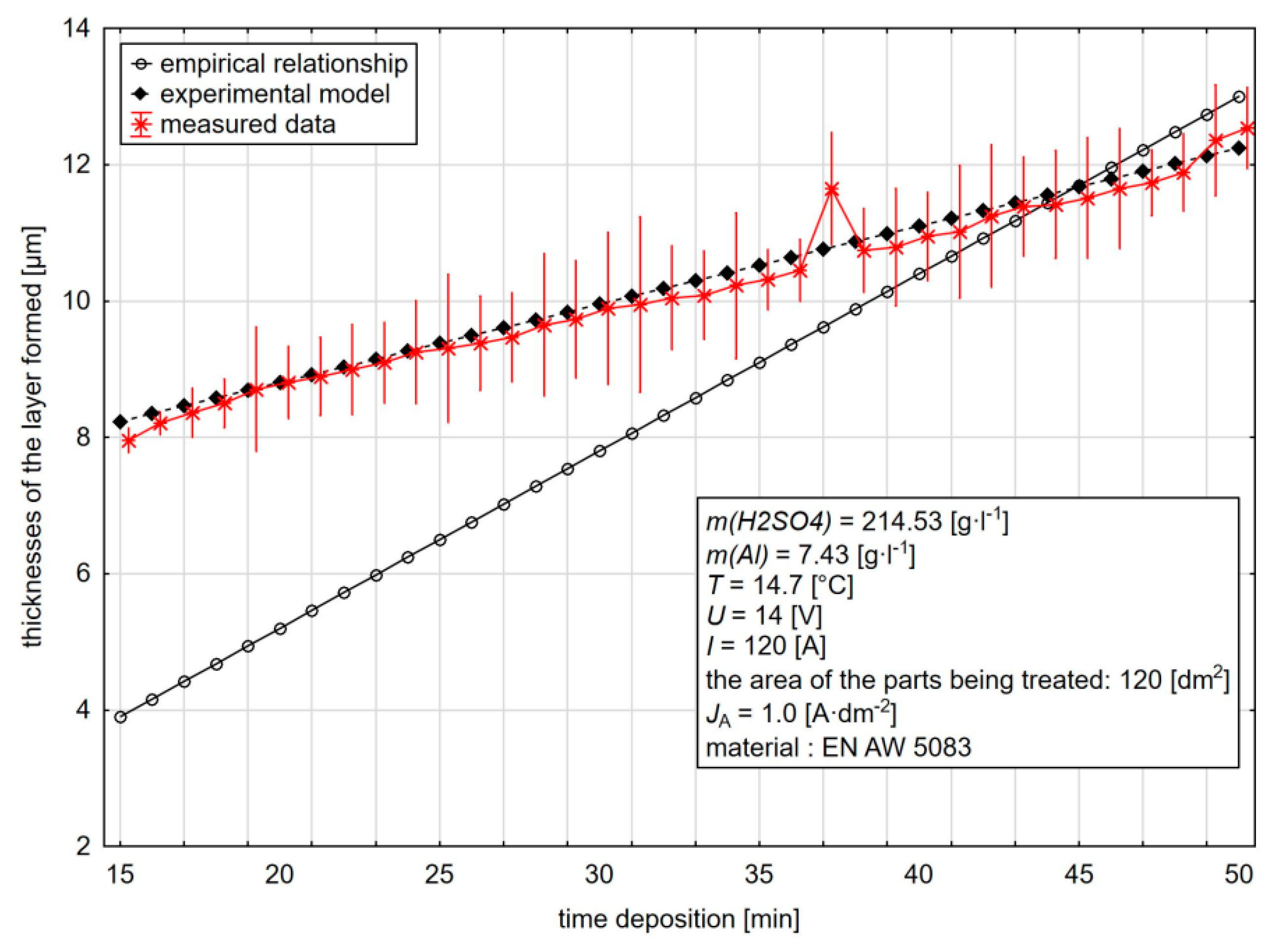
The results of the practical verification (
Figure 14) show that the model created on the basis of the experimental analysis for the current density of 1 A·dm
−2 represents the real measured thicknesses of the layer formed in the whole range, with an average relative uncertainty of 1.21% compared to the empirical relationship, which shows a difference at low values of the anodic oxidation time (15–35 min) compared to the measured values of 49.26%. If the 35 min duration of anodic oxidation is exceeded at the end of the observed interval, this mean deviation is reduced to 4.70%. For the whole time of anodic oxidation, the mean deviation between the actual thicknesses and the thicknesses predicted by the empirical relationship is 30.69%.
The mean deviation between the measured thicknesses of the layers formed and the experimental model for the current density of 2 A·dm
−2 is 4.89% for the difference between the real thicknesses and the empirical relationship of 20.29%. In the case of an increase in the current density above A·dm
−2, we can observe the change in the course of the actually measured thicknesses of the layer with the maximum anodic oxidation time of about 40 min and its subsequent gradual decrease down to the end of the monitored interval of the anodic oxidation time. Examples of components used in practical verification are shown in
Figure 15.
From the point of view of the time-consuming character of verifying the experimental relationship in practice, which took place in the operation of the private company of one of the authors, this verification took more than 7 months since this is a commercial operation of the surface treatments and it was necessary to adhere to the material. This verification was performed on real orders. The company‘s customers provided consent for experimental verification. For this reason, it was not possible to carry out practical verification also at other current densities.